Abstract
The mitochondrial presequence translocase transports preproteins to either matrix or inner membrane. Two different translocase forms have been identified: the matrix transport form, which binds the heat-shock protein 70 (Hsp70) motor, and the inner membrane–sorting form, which lacks the motor but contains translocase of inner mitochondrial membrane 21 (Tim21). The sorting form interacts with the respiratory chain in a Tim21-dependent manner. It is unknown whether the respiratory chain–bound translocase transports preproteins and how the switch between sorting form and motor form occurs. We report that the respiratory chain–bound translocase contains preproteins in transit and, surprisingly, not only sorted but also matrix-targeted preproteins. Presequence translocase-associated motor (Pam) 16 and 18, two regulatory components of the six-subunit motor, interact with the respiratory chain independently of Tim21. Thus, the respiratory chain–bound presequence translocase is not only active in preprotein sorting to the inner membrane but also in an early stage of matrix translocation. The motor does not assemble en bloc with the translocase but apparently in a step-wise manner with the Pam16/18 module before the Hsp70 core.
Introduction
Most mitochondrial matrix proteins are synthesized with N-terminal presequences in the cytosol. The presequences function as targeting signals that direct the preproteins via the translocase of outer mitochondrial membrane (TOM) complex and the presequence translocase of inner mitochondrial membrane 23 (TIM23) complex into the matrix (Jensen and Johnson, 2001; Hoogenraad et al., 2002; Koehler, 2004; Dolezal et al., 2006; Bohnert et al., 2007; Neupert and Herrmann, 2007). Two energy sources drive protein import. The electrochemical gradient (proton-motive force [Δp]) is required for translocation of the presequence through the channel-forming protein Tim23 (Huang et al., 2002). ATP drives the matrix heat-shock protein 70 (Hsp70 [mtHsp70]) that binds to the unfolded preproteins and completes translocation into the matrix (Krayl et al., 2007). The presequences are cleaved off by the mitochondrial processing peptidase (MPP). Several presequence-carrying proteins possess a hydrophobic sorting signal that arrests translocation in the TIM23 complex and induces lateral release of the preprotein into the lipid phase of the inner membrane. This inner membrane sorting can be driven by Δp alone without a requirement for the mtHsp70 motor (Chacinska et al., 2005; Neupert and Herrmann, 2007).
The TIM23 complex contains three core subunits: the channel Tim23, the regulatory protein Tim17, and Tim50, which exposes a domain to the intermembrane space and binds preproteins during transfer from TOM to TIM23 (Geissler et al., 2002; Yamamoto et al., 2002; Chacinska et al., 2005). The presequence translocase-associated motor (PAM) consists of six subunits. In addition to the matrix chaperone mtHsp70, four cochaperones—Tim44, Pam16, Pam17, and Pam18—are bound to the inner membrane and interact with the TIM23 complex. These cochaperones recruit mtHsp70 to the protein translocation channel and regulate its activity. Tim44 provides a transient binding site for mtHsp70 at the inner membrane, where the J-protein Pam18 stimulates its ATPase activity. Pam16 forms a complex with Pam18 and controls its activity (Li et al., 2004; D'Silva et al., 2005; Mokranjac et al., 2006). Pam17 stimulates formation of the Pam16/18 module (van der Laan et al., 2005). The nucleotide exchange factor Mge1 completes the reaction cycle of mtHsp70.
Originally, it has been assumed that the TIM23 complex and PAM are permanently associated. However, the identification of a fourth subunit of the TIM23 complex, Tim21, suggested the existence of two different forms of the presequence translocase (Chacinska et al., 2005; Mokranjac et al., 2005; Oka and Mihara, 2005; van der Laan et al., 2006). (1) One form, TIM23Motor, is responsible for protein transport into the matrix. This TIM23 complex is associated with the six-subunit motor but does not contain Tim21. (2) The other TIM23 form, termed sorting translocase TIM23SORT, contains Tim21 but lacks the motor. TIM23SORT is involved in protein transfer from TOM to TIM23 and Δp-driven lateral sorting of proteins into the inner membrane. Different views have been discussed as to whether two forms of the TIM23 complex exist or whether one form containing all subunits is responsible for both matrix transport and inner membrane sorting (Chacinska et al., 2005; Meinecke et al., 2006; Tamura et al., 2006; van der Laan et al., 2006; Mokranjac et al., 2007; Neupert and Herrmann, 2007). Reconstitution of purified TIM23SORT into proteoliposomes demonstrated that the motor-free translocase functions as a minimal unit for membrane insertion of preproteins (van der Laan et al., 2007).
In mitochondria, Tim21 interacts with two different membrane protein complexes in an alternating manner. First, Tim21 transiently binds to the TOM complex and cooperates with Tim50 in the transfer of preproteins from TOM to TIM23 (Geissler et al., 2002; Yamamoto et al., 2002; Chacinska et al., 2005; Mokranjac et al., 2005; Albrecht et al., 2006). Second, Tim21 interacts with a supercomplex of the mitochondrial respiratory chain that contains complex III (bc1 complex) and complex IV (cytochrome c oxidase). Thus, Tim21 stimulates the Δp-driven insertion of preproteins into the inner membrane under conditions in which the overall Δp of the mitochondrial inner membrane is reduced (van der Laan et al., 2006); possible explanations are a higher Δp in the direct vicinity of proton-pumping complexes or a transfer of protons from respiratory chain complexes to the presequence translocase. However, these studies did not show whether an active sorting translocase (i.e., a translocase containing a preprotein in transit) interacts with the respiratory chain.
For this study, we established a system to study the interaction of a preprotein-carrying translocase with the respiratory chain. Surprisingly, we found that not only inner membrane–sorted but also matrix-targeted preproteins were associated with the respiratory chain. The identification of a regulatory PAM module at the respiratory chain suggests a new mechanism of switching between TIM23SORT and TIM23Motor.
Results and discussion
Respiratory chain supercomplex interacts with TIM-accumulated preprotein in organello
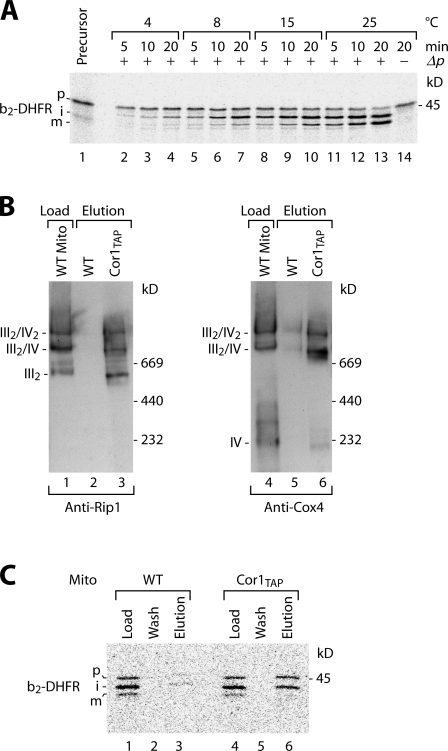
To test for the interaction of a translocation-active TIM23 complex with the respiratory chain, we established conditions for efficient accumulation of an inner membrane–sorted preprotein in mitochondrial import sites. We used a fusion protein between the N-terminal portion of cytochrome b2 and dihydrofolate reductase (DHFR; Chacinska et al., 2005). The presequence of b2-DHFR contains both a matrix-targeting signal and a hydrophobic sorting signal. Thus, the preprotein is arrested in the inner membrane, and MPP cleaves off the matrix-targeting signal, yielding the intermediate form. A second processing step by the inner membrane protease removes the sorting signal, and the protein is released to the intermembrane space. By addition of methotrexate, the DHFR moiety is stabilized and impaired in unfolding by the mitochondrial import machinery. Therefore, DHFR remains on the cytosolic side of the TOM complex. The intermediate-sized b2-DHFR is thus accumulated across both outer and inner membranes. We tested different import conditions to accumulate a large fraction of intermediate and selected import at low temperature into fully energized mitochondria (+Δp; Fig. 1 A) to probe for an interaction with the respiratory chain.
Figure 1.
Copurification of preprotein in transit with tagged respiratory chain supercomplex. (A) 35S-labeled b2(220)-DHFR was imported into isolated yeast mitochondria at different temperatures in the presence of methotrexate. Mitochondrial proteins were analyzed by SDS-PAGE and digital autoradiography. p, precursor (20%); i, intermediate; m, mature. (B) Isolated wild-type (WT) and Cor1TAP mitochondria were solubilized in digitonin and subjected to IgG chromatography. Bound complexes were eluted by tobacco etch virus protease cleavage and analyzed by blue native electrophoresis and immunodecoration. Load, 40%; elution, 100%. III2/IV and III2/IV2, respiratory chain supercomplexes. (C) b2-DHFR was imported into wild-type and Cor1TAP mitochondria at 8°C in the presence of methotrexate followed by IgG chromatography. Load and wash, 2%; elution, 100%. Samples were analyzed by SDS-PAGE and autoradiography. The yield of copurification of b2-DHFR with Cor1TAP was ∼25% of that of Tim23.
We used a yeast strain carrying a tandem affinity purification (TAP) tag at the core 1 subunit of complex III to copurify the respiratory chain supercomplex and associated complexes (van der Laan et al., 2006). In yeast wild-type mitochondria, complexes III and IV associate to the supercomplexes III2/IV and III2/IV2 that remain stable upon lysis with digitonin and blue native electrophoresis (Cruciat et al., 2000; Schägger and Pfeiffer, 2000; van der Laan et al., 2006). To exclude that the TAP tag interfered with formation or stability of the supercomplexes, IgG affinity chromatography was performed, and the eluate was separated by blue native electrophoresis. Immunodecoration with antibodies against the Rieske iron-sulfur protein (complex III) and subunit 4 of cytochrome c oxidase demonstrated that the supercomplexes were efficiently copurified (Fig. 1 B, lanes 3 and 6). The 35S-labeled preprotein b2-DHFR was accumulated in the import sites of wild-type and Cor1TAP mitochondria in the presence of methotrexate (Fig. 1 C, lanes 1 and 4). The mitochondria were lysed with digitonin and subjected to IgG affinity chromatography. Precursor and intermediate forms of b2-DHFR were copurified with the supercomplexes from Cor1TAP mitochondria (Fig. 1 C, lane 6), whereas only background signal was obtained with wild-type mitochondria lacking the TAP tag (Fig. 1 C, lane 3). Thus, the two forms of accumulated b2-DHFR, which still contain the hydrophobic sorting signal, were copurified with the respiratory chain.
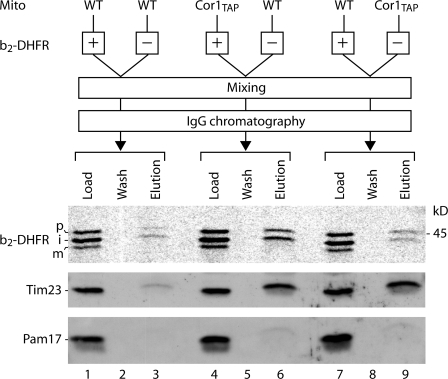
The possibility was of concern that the translocase with accumulated preprotein was not associated with the respiratory chain in organello but was bound to the supercomplexes after lysis of mitochondria. To compare the copurification yield of a postlysis interaction with that of an intramitochondrial interaction, we performed a mixing experiment with wild-type and Cor1TAP mitochondria. Radiolabeled b2-DHFR was imported into wild-type mitochondria. The mitochondria were subsequently mixed with Cor1TAP mitochondria not containing radiolabeled preproteins. Purification of the III/IV supercomplexes by IgG affinity chromatography led to efficient copurification of Tim23 as expected, whereas only a low amount of b2-DHFR was found in the eluate (Fig. 2, lane 9), which is comparable with the background signal obtained when only wild-type mitochondria were used (Fig. 2, lane 3). In contrast, the reverse experiment (i.e., import of radiolabeled b2-DHFR into Cor1TAP mitochondria and mixing with wild-type mitochondria) yielded an efficient copurification of b2-DHFR (Fig. 2, lane 6). Pam17 of the import motor was not copurified. Thus, the preprotein has to be imported into mitochondria with tagged complex III to be copurified with the respiratory chain, demonstrating that interaction of the preprotein-carrying translocase with the respiratory chain occurred within mitochondria and not after lysis.
Figure 2.
TIM-accumulated preprotein interacts with respiratory chain in organello. [35S]b2-DHFR precursor was imported into wild-type and Cor1TAP mitochondria in the presence of methotrexate. After import, the mitochondria were mixed with the same amount of wild-type and Cor1TAP mitochondria as indicated and were subjected to IgG chromatography. Load and wash, 1%; elution, 100%. The samples were analyzed by SDS-PAGE and autoradiography for b2-DHFR and immunodecoration for Tim23 and Pam17.
Yeast complex III is not required for preprotein processing
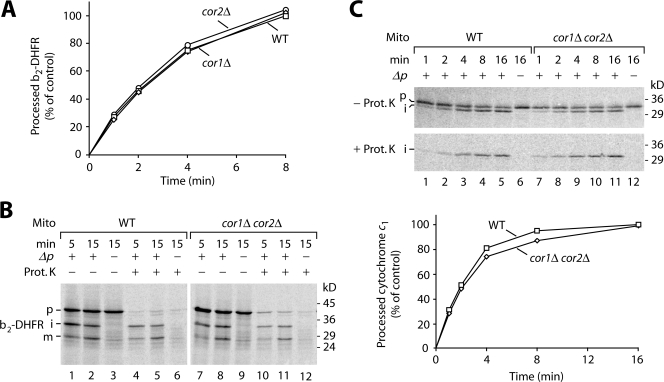
van der Laan et al. (2006) reported that interaction of the respiratory chain with TIM23 influenced the energetics of preprotein import. The demonstration that a preprotein in transit was associated with III/IV supercomplexes raised an additional explanation based on the finding that two subunits of mitochondrial complex III, core 1 and 2, are homologous to the subunits of MPP. For plant and bovine mitochondria, it was indeed shown that the core subunits of complex III can proteolytically remove presequences of some preproteins (Dessi et al., 2000; Deng et al., 2001). Thus, we asked whether complex III of yeast mitochondria was required for the processing of sorted preproteins and generated yeast strains lacking Cor1 or Cor2, respectively. Mitochondria isolated from these strains processed b2-DHFR with wild-type efficiency (Fig. 3 A). To exclude that Cor1 and Cor2 substituted for each other in preprotein processing, we generated a double mutant lacking both core subunits. b2-DHFR was processed to the intermediate- and mature-sized forms by cor1Δ cor2Δ mitochondria with wild-type efficiency (Fig. 3 B). We used the precursor of cytochrome c1 as further substrate. cor1Δ cor2Δ mitochondria efficiently processed the protein (Fig. 3 C). Thus, processing of preproteins by yeast mitochondria does not depend on the core 1 and 2 subunits of complex III, excluding the alternative possibility that preprotein processing would represent the functional reason for a TIM–respiratory chain interaction.
Figure 3.
Processing of preproteins does not require Cor1 and Cor2 of complex III. (A) [35S]b2-DHFR was imported into wild-type, cor1Δ, and cor2Δ mitochondria. Samples were treated with 50 μg/ml proteinase K and analyzed by SDS-PAGE. Processed b2-DHFR was quantified using ImageQuant software (GE Healthcare). Import in wild-type mitochondria after the longest incubation time was set to 100% (control). (B) b2-DHFR was imported into wild-type and cor1Δ cor2Δ mitochondria. Samples were treated with proteinase K (Prot. K) as indicated and were analyzed by SDS-PAGE. Processing of b2-DHFR did not require Cor1/Cor2 independently of the length of the b2 part (85–167 amino acid residues). (C) [35S]Cytochrome c1 was imported into wild-type and cor1Δ cor2Δ mitochondria. Samples were treated as described for A and B.
Interaction of TIM-accumulated preprotein with respiratory chain partially depends on Tim21
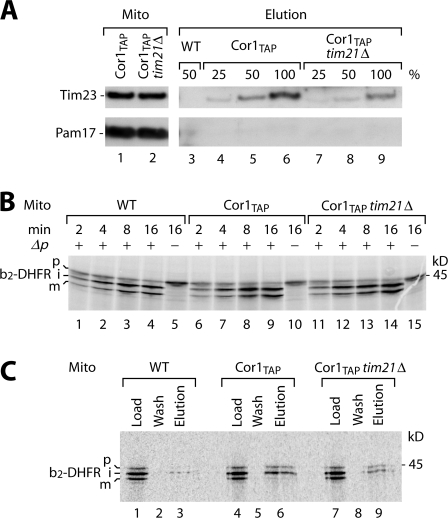
The intermembrane space domain of Tim21 binds to III/IV supercomplexes. Upon the deletion of Tim21, the interaction between TIM23 complex and respiratory chain is diminished but not blocked (van der Laan et al., 2006). For a quantitative assessment, we compared the copurification efficiency of Tim23 with Cor1TAP in the presence and absence of Tim21 in a Western blot titration. Fig. 4 A shows that the lack of Tim21 decreased the yield of copurification by ∼50%. To analyze the role of Tim21 in the interaction of a translocation-active TIM23 complex with the respiratory chain, it was important to load the mitochondria with comparable amounts of preprotein. Therefore, we determined the efficiency of b2-DHFR import into three types of mitochondria: Cor1TAP mitochondria lacking Tim21, Cor1TAP mitochondria containing Tim21, and wild-type mitochondria. When the mitochondria were fully energized, the three types of mitochondria imported the preprotein with comparable efficiency (Fig. 4 B).
Figure 4.
Lack of Tim21 impairs but does not block preprotein copurification with respiratory chain. (A) IgG chromatography was performed with wild-type, Cor1TAP, and Cor1TAP tim21Δ mitochondria. The samples were analyzed by SDS-PAGE and immunodecoration. (B) [35S]b2(220)-DHFR was imported into isolated mitochondria and analyzed by SDS-PAGE. (C) b2- DHFR was imported in the presence of methotrexate. IgG chromatography was performed, and samples were analyzed by autoradiography. Load and wash, 2%; elution, 100%.
b2-DHFR was accumulated in the mitochondrial import sites in the presence of methotrexate. IgG affinity chromatography revealed that the lack of Tim21 decreased the yield of copurification of the preprotein intermediate by ∼50% (Fig. 4 C, lane 9 vs. lane 6). We conclude that Tim21 is involved in interaction of the translocation-active TIM23 complex with the respiratory chain but is not the only interaction element.
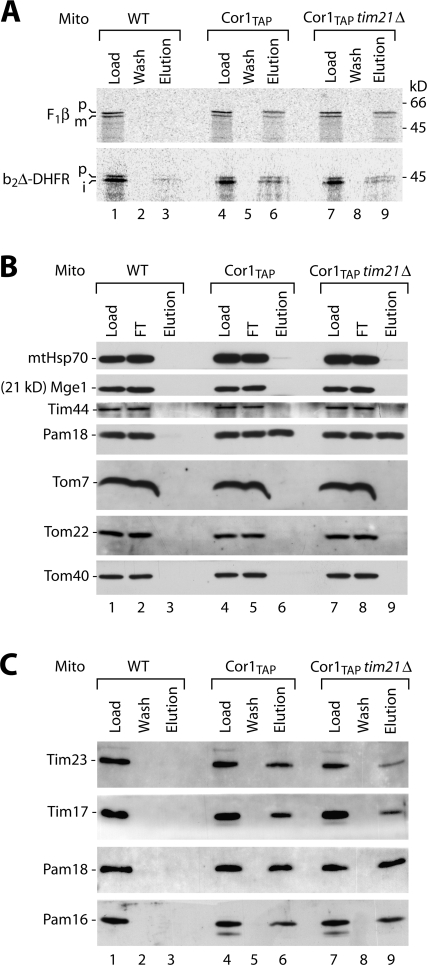
Pam16/18 but not other motor subunits interact with respiratory chain supercomplex
The experiments so far used b2-DHFR that contains the hydrophobic signal for lateral sorting into the inner membrane. We wondered whether matrix-targeted preproteins, which depend on TIM23Motor for import, could associate with the respiratory chain. The preprotein b2Δ-DHFR is identical to b2-DHFR except for deletion of the sorting signal, and, thus, it is imported into the matrix (Chacinska et al., 2005). Remarkably, b2Δ-DHFR, which was accumulated in mitochondrial import sites in the presence of methotrexate, was copurified with Cor1TAP (Fig. 5 A, lane 6). Moreover, the precursor of the matrix-located β subunit of the F1-ATPase (F1β), which was accumulated as transport intermediate in mitochondrial import sites at low temperature (Schleyer and Neupert, 1985), was copurified with Cor1TAP in a Tim21-independent manner (Fig. 5 A, lanes 6 and 9). The association of matrix-targeted preproteins with the respiratory chain did not fit the view of a strict separation of TIM23SORT and TIM23Motor and calls for a reconsideration of the relation between TIM23SORT, import motor, and respiratory chain.
Figure 5.
Pam16/18 interact with the respiratory chain. (A) [35S]b2(220)Δ-DHFR was imported into wild-type, Cor1TAP, and Cor1TAP tim21Δ mitochondria in the presence of methotrexate followed by IgG chromatography and SDS-PAGE. Load and wash, 2%; elution, 100%. [35S]F1β was imported at 2°C in the absence of added ATP. Load and wash, 1%; elution, 100%. p, precursor; i, intermediate; m, mature. (B) IgG chromatography was performed with wild-type, Cor1TAP, and Cor1TAP tim21Δ mitochondria followed by SDS-PAGE and immunodecoration. Load and flow through (FT), 2%; elution, 100%. (C) IgG chromatography was performed as described for B. Load and wash, 2.5%; elution, 100%.
So far, three subunits of the motor were analyzed and shown not to copurify with III/IV supercomplexes: mtHsp70, Tim44 (Fig. 5 B; van der Laan et al., 2006), and Pam17 (Figs. 2 and 4 A). Thus, we studied the three further motor subunits. Mge1 did not copurify with Cor1TAP like the control proteins of the TOM complex (Fig. 5 B, lane 6). Surprisingly, however, both Pam18 and Pam16 were found in the eluate after IgG affinity chromatography (Fig. 5, B and C; lane 6). We asked whether the association of Pam16/18 with the respiratory chain occurred indirectly via the Tim21-mediated interaction, but the deletion of Tim21 did not decrease the yield of copurification of these two motor subunits with Cor1TAP (Fig. 5, B and C; lane 9). Therefore, Pam16/18 are associated with III/IV supercomplexes independently of Tim21. We conclude that two regulatory subunits of the import motor associate with the respiratory chain.
Conclusions
We report that preproteins in transit through the presequence translocase of the mitochondrial inner membrane are associated with supercomplexes of the respiratory chain. Not only preproteins that are laterally sorted into the inner membrane but, unexpectedly, also preproteins directed to the matrix are found in the respiratory chain–bound translocase. This observation changes the current view of how different forms of the presequence translocase function in protein import. To date, two distinct forms, TIM23SORT and TIM23Motor, were thought to be selectively responsible for inner membrane–sorted and matrix-targeted preproteins, respectively, and the switch between both forms would occur en bloc. We report that not only Tim21 (a subunit of TIM23SORT) interacts with the respiratory chain, but the regulatory Pam16/18 motor module also associates with the respiratory chain in a Tim21-independent manner. As mtHsp70 and additional motor subunits are not associated with the respiratory chain, we propose that the switch between TIM23SORT and TIM23Motor does not occur in a one-step reaction, yet the motor is recruited to TIM23 in a modular fashion with the Pam16/18 module first.
The identification of a presequence translocase carrying a matrix-targeted preprotein despite the lack of mtHsp70 indicates that early steps of matrix import do not require a fully assembled motor. This provides an explanation for the puzzling observation that Tim21, which is located in mtHsp70-free TIM23SORT, functions in the early stage of preprotein transfer from TOM to TIM23 in cooperation with Tim50 for both inner membrane–sorted and matrix-targeted proteins (Geissler et al., 2002; Yamamoto et al., 2002; Chacinska et al., 2005; Mokranjac et al., 2005; Albrecht et al., 2006). Subsequently, TIM23SORT associates with the respiratory chain. Here, the import pathways diverge. Preproteins with hydrophobic sorting signal are inserted into the inner membrane by TIM23SORT in a Δp-driven process. In the case of matrix-targeted preproteins, we suggest that the Pam16/18 module is transferred to the translocase and replaces Tim21 at the stage of respiratory chain interaction, whereas the assembly of mtHsp70 and further motor subunits occurs only after release from the respiratory chain.
In summary, TIM23SORT and TIM23Motor represent two stages of a multistage import reaction. The switch between both forms occurs in a step-wise manner. Matrix-targeted preproteins use TIM23SORT in an early import stage followed by a respiratory chain–bound state involving a regulatory motor module. Finally, the fully assembled TIM23Motor complex drives transport into the matrix at the expense of ATP. We propose that respiratory chain supercomplexes associated with the presequence translocase serve two functions. They stimulate Δp-driven insertion of inner membrane proteins by TIM23SORT and may function as a scaffold for initiation of the sequential switch from TIM23SORT to TIM23Motor.
Materials and methods
Yeast strains and growth conditions
The Saccharomyces cerevisiae strains Cor1TAP and the corresponding wild-type BY4741 were acquired from EUROSCARF, and Cor1TAP tim21Δ was described previously (van der Laan et al., 2006). Deletions of COR1 and COR2 were performed in YPH499. Deletion of COR1 was achieved by replacing the open reading frame with a kanr cassette. COR2 was deleted by substitution with a HIS5 marker gene. For generation of the cor1Δ cor2Δ double deletion strain, a HIS5 marker was integrated into the COR2 locus in the cor1Δ strain. For isolation of mitochondria, cor1Δ, cor2Δ, and cor1Δ cor2Δ strains were grown at 30°C in YP medium supplemented with 2% sucrose, and Cor1TAP strains in YP medium were supplemented with 3% glycerol.
Import of preproteins into isolated mitochondria
Radiolabeled preproteins were synthesized in vitro by SP6 polymerase transcription of plasmid templates and translation in the presence of [35S]methionine in rabbit reticulocyte lysate (GE Healthcare; Stojanovski et al., 2007). The preproteins were imported into mitochondria isolated by differential centrifugation (Meisinger et al., 2006). Mitochondria were incubated with the precursor lysates in import buffer (3% [wt/vol] fatty acid–free BSA, 250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 5 mM KH2PO4, 5 mM methionine, and 10 mM MOPS-KOH, pH 7.2) in the presence of 2 mM ATP, an ATP-regenerating system (5 mM creatine phosphate and 0.1 mg/ml creatine kinase), and 2 mM NADH. Where indicated, 5 μM methotrexate was included. Δp was dissipated by incubation of mitochondria with 8 μM antimycin A, 1 μM valinomycin, and 10 μM oligomycin (Sigma-Aldrich).
Isolation of mitochondrial complexes and electrophoresis
Purified human IgG (MP Biomedicals) was coupled to 1 mM HCl-pretreated cyanogen bromide–activated Sepharose (GE Healthcare) for 2 h at room temperature in 0.1 M NaHCO3, pH 8.5. Cyanogen bromide–activated Sepharose was blocked overnight with 0.1 M Tris/HCl, pH 8.0, and kept at 4°C. Mitochondria were lysed in ice-cold 0.8% digitonin buffer (20 mM Tris-HCl, pH 7.4, 50 mM NaCl, 0.1 mM EDTA, and 10% [vol/vol] glycerol) and incubated with IgG Sepharose end over end at 4°C for between 1 and 2 h. The IgG Sepharose was washed and incubated with 25–50 U tobacco etch virus protease (Invitrogen) for 90 min at 16°C in digitonin buffer. Protein complexes were resolved by blue native electrophoresis, whereas denatured proteins were analyzed by SDS-PAGE. Immunodecorations were performed by semidry Western transfer onto polyvinylidene difluoride membranes (Millipore) and subsequent enhanced chemiluminescence (GE Healthcare).
Acknowledgments
We thank Claudia Prinz, Agnes Schulze-Specking, and Hanne Müller for expert technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 746, a European Molecular Biology Organization long-term fellowship (to M. van der Laan), the Gottfried Wilhelm Leibniz Program, a Max Planck Research Award, and Fonds der Chemischen Industrie.
N. Wiedemann and M. van der Laan contributed equally to this paper.
Abbreviations used in this paper: DHFR, dihydrofolate reductase; Hsp70, heat-shock protein 70; MPP, mitochondrial processing peptidase; mtHsp70, matrix Hsp70; PAM, presequence translocase-associated motor; TAP, tandem affinity purification; TIM, translocase of inner mitochondrial membrane; TOM, translocase of outer mitochondrial membrane.
References
- Albrecht, R., P. Rehling, A. Chacinska, J. Brix, S.A. Cadamuro, R. Volkmer, B. Guiard, N. Pfanner, and K. Zeth. 2006. The Tim21 binding domain connects the preprotein translocases of both mitochondrial membranes. EMBO Rep. 7:1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert, M., N. Pfanner, and M. van der Laan. 2007. A dynamic machinery for import of mitochondrial precursor proteins. FEBS Lett. 581:2802–2810. [DOI] [PubMed] [Google Scholar]
- Chacinska, A., M. Lind, A.E. Frazier, J. Dudek, C. Meisinger, A. Geissler, A. Sickmann, H.E. Meyer, K.N. Truscott, B. Guiard, et al. 2005. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell. 120:817–829. [DOI] [PubMed] [Google Scholar]
- Cruciat, C.M., S. Brunner, F. Baumann, W. Neupert, and R.A. Stuart. 2000. The cytochrome bc 1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem. 275:18093–18098. [DOI] [PubMed] [Google Scholar]
- Deng, K., S.K. Shenoy, S.C. Tso, L. Yu, and C.A. Yu. 2001. Reconstitution of mitochondrial processing peptidase from the core proteins (subunits I and II) of bovine heart mitochondrial cytochrome bc 1 complex. J. Biol. Chem. 276:6499–6505. [DOI] [PubMed] [Google Scholar]
- Dessi, P., C. Rudhe, and E. Glaser. 2000. Studies on the topology of the protein import channel in relation to the plant mitochondrial processing peptidase integrated into the cytochrome bc 1 complex. Plant J. 24:637–644. [DOI] [PubMed] [Google Scholar]
- Dolezal, P., V. Likic, J. Tachezy, and T. Lithgow. 2006. Evolution of the molecular machines for protein import into mitochondria. Science. 313:314–318. [DOI] [PubMed] [Google Scholar]
- D'Silva, P.R., B. Schilke, W. Walter, and E.A. Craig. 2005. Role of Pam16's degenerate J domain in protein import across the mitochondrial inner membrane. Proc. Natl. Acad. Sci. USA. 102:12419–12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler, A., A. Chacinska, K.N. Truscott, N. Wiedemann, K. Brander, A. Sickmann, H.E. Meyer, C. Meisinger, N. Pfanner, and P. Rehling. 2002. The mitochondrial presequence translocase: an essential role of Tim50 in directing preproteins to the import channel. Cell. 111:507–518. [DOI] [PubMed] [Google Scholar]
- Hoogenraad, N.J., L.A. Ward, and M.T. Ryan. 2002. Import and assembly of proteins into mitochondria of mammalian cells. Biochim. Biophys. Acta. 1592:97–105. [DOI] [PubMed] [Google Scholar]
- Huang, S., K.S. Ratliff, and A. Matouschek. 2002. Protein unfolding by the mitochondrial membrane potential. Nat. Struct. Biol. 9:301–307. [DOI] [PubMed] [Google Scholar]
- Jensen, R.E., and A.E. Johnson. 2001. Opening the door to mitochondrial protein import. Nat. Struct. Biol. 8:1008–1010. [DOI] [PubMed] [Google Scholar]
- Koehler, C.M. 2004. New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20:309–335. [DOI] [PubMed] [Google Scholar]
- Krayl, M., J.H. Lim, F. Martin, B. Guiard, and W. Voos. 2007. A cooperative action of the ATP-dependent import motor complex and the inner membrane potential drives mitochondrial preprotein import. Mol. Cell. Biol. 27:411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., J. Dudek, B. Guiard, N. Pfanner, P. Rehling, and W. Voos. 2004. The presequence translocase-associated protein import motor of mitochondria: Pam16 functions in an antagonistic manner to Pam18. J. Biol. Chem. 279:38047–38054. [DOI] [PubMed] [Google Scholar]
- Meinecke, M., R. Wagner, P. Kovermann, B. Guiard, D.U. Mick, D.P. Hutu, W. Voos, K.N. Truscott, A. Chacinska, N. Pfanner, and P. Rehling. 2006. Tim50 maintains the permeability barrier of the mitochondrial inner membrane. Science. 312:1523–1526. [DOI] [PubMed] [Google Scholar]
- Meisinger, C., N. Pfanner, and K.N. Truscott. 2006. Isolation of yeast mitochondria. Methods Mol. Biol. 313:33–39. [DOI] [PubMed] [Google Scholar]
- Mokranjac, D., D. Popov-Celeketic, K. Hell, and W. Neupert. 2005. Role of Tim21 in mitochondrial translocation contact sites. J. Biol. Chem. 280:23437–23440. [DOI] [PubMed] [Google Scholar]
- Mokranjac, D., G. Bourenkov, K. Hell, W. Neupert, and M. Groll. 2006. Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor. EMBO J. 25:4675–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokranjac, D., A. Berg, A. Adam, W. Neupert, and K. Hell. 2007. Association of the Tim14-Tim16 subcomplex with the TIM23 translocase is crucial for function of the mitochondrial protein import motor. J. Biol. Chem. 282:18037–18045. [DOI] [PubMed] [Google Scholar]
- Neupert, W., and J.M. Herrmann. 2007. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76:723–749. [DOI] [PubMed] [Google Scholar]
- Oka, T., and K. Mihara. 2005. A railroad switch in mitochondrial protein import. Mol. Cell. 18:145–146. [DOI] [PubMed] [Google Scholar]
- Schägger, H., and K. Pfeiffer. 2000. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 19:1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer, M., and W. Neupert. 1985. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 43:339–350. [DOI] [PubMed] [Google Scholar]
- Stojanovski, D., N. Pfanner, and N. Wiedemann. 2007. Import of proteins into mitochondria. Methods Cell Biol. 80:783–806. [DOI] [PubMed] [Google Scholar]
- Tamura, Y., Y. Harada, K. Yamano, K. Watanabe, D. Ishikawa, C. Ohshima, S. Nishikawa, H. Yamamoto, and T. Endo. 2006. Identification of Tam41 maintaining integrity of the TIM23 protein translocator complex in mitochondria. J. Cell Biol. 174:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan, M., A. Chacinska, M. Lind, I. Perschil, A. Sickmann, H.E. Meyer, B. Guiard, C. Meisinger, N. Pfanner, and P. Rehling. 2005. Pam17 is required for architecture and translocation activity of the mitochondrial protein import motor. Mol. Cell. Biol. 25:7449–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan, M., N. Wiedemann, D.U. Mick, B. Guiard, P. Rehling, and N. Pfanner. 2006. A role for Tim21 in membrane-potential-dependent preprotein sorting in mitochondria. Curr. Biol. 16:2271–2276. [DOI] [PubMed] [Google Scholar]
- van der Laan, M., M. Meinecke, J. Dudek, D.P. Hutu, M. Lind, I. Perschil, B. Guiard, R. Wagner, N. Pfanner, and P. Rehling. 2007. Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat. Cell Biol. 9:1152–1159. [DOI] [PubMed] [Google Scholar]
- Yamamoto, H., M. Esaki, T. Kanamori, Y. Tamura, S. Nishikawa, and T. Endo. 2002. Tim50 is a subunit of the TIM23 complex that links protein translocation across the outer and inner mitochondrial membranes. Cell. 111:519–528. [DOI] [PubMed] [Google Scholar]