Abstract
Cell adhesion molecules (CAMs) are thought to mediate interactions between innervating axons and their targets. However, such interactions have not been directly observed in vivo. In this paper, we study the function and dynamics of Fasciclin2 (Fas2), a homophilic CAM expressed both pre- and postsynaptically during neuromuscular synapse formation in Drosophila melanogaster. We apply live imaging of functional fluorescent fusion proteins expressed in muscles and find that Fas2 and Discs-Large (Dlg; a scaffolding protein known to bind Fas2) accumulate at the synaptic contact site soon after the arrival of the nerve. Genetic, deletion, and photobleaching analyses suggest that Fas2-mediated trans-synaptic adhesion is important for the postsynaptic accumulation of both Fas2 itself and Dlg. In fas2 mutants, many aspects of synapse formation appear normal; however, we see a reduction in the synaptic accumulation of Scribble (another scaffolding protein) and glutamate receptor subunits GluRIIA and GluRIIB. We propose that Fas2 mediates trans-synaptic adhesion, which contributes to postsynaptic molecular assembly at the onset of synaptogenesis.
Introduction
How the innervating axons induce the assembly of postsynaptic components at the site of synaptic contact is a fundamental issue in neuroscience. Synaptic induction is thought to be mediated by membrane-bound and/or diffusible molecules (Goda and Davis, 2003; Li and Sheng, 2003; Scheiffele, 2003; Yamagata et al., 2003; Waites et al., 2005). Cell adhesion molecules (CAMs) in particular have long been proposed to have instrumental roles in triggering synaptic assembly for the following two reasons. First, CAMs may provide an adhesive link between pre- and postsynaptic cells by holding the synaptic contacts together. Second, by binding to other synaptic components such as synaptic scaffolding proteins, CAMs may induce the intracellular signaling necessary for synaptic differentiation. Therefore, CAMs may provide the earliest trans-synaptic scaffold upon which other synaptic components are later recruited. Consistent with this idea, several CAMs have previously been implicated in synapse formation in vitro. For example, neurexin in the presynaptic axons can induce glutamate and GABA postsynaptic specializations via neuroligin (Graf et al., 2004; Chih et al., 2005). Conversely, postsynaptic neuroligin can induce presynaptic specialization by binding to presynaptic neurexin (Scheiffele et al., 2000). Similarly, Syn-CAM, a homophilic CAM with Ig domains, can induce presynaptic specialization (Biederer et al., 2002). These molecules not only link pre- and postsynaptic cells through extracellular interaction but also bind to and recruit synaptic PDZ-domain scaffold proteins, such as PSD-95 and CASK, through intracellular interaction. Therefore, these CAMs are thought to drive assembly of the synaptic structures by coupling intercellular adhesion with intracellular signaling. However, little is known about the roles of these and other trans-synaptic CAMs, such as Syn-CAM, cadherins, and EphrinB/EphB2, in synapse formation in vivo (Missler et al., 2003; Yamagata et al., 2003; Waites et al., 2005; Varoqueaux et al., 2006).
Little is also known about the dynamics of CAMs or other synaptic molecules when the synapses are initially formed in the embryo (Jontes et al., 2004; Kasthuri and Lichtman, 2004; Niell and Smith, 2004). If cell adhesion is indeed a mechanism of postsynaptic induction, recruitment of postsynaptic CAMs at the site of axon-target contact would be the earliest step of the postsynaptic assembly. However, to our knowledge, no study has yet directly examined this process in the intact organism. This is likely because it is usually difficult to identify newly forming synapses in the embryo and, furthermore, to image the process of molecular assembly. In this study, we characterized the function and dynamics of a CAM, Fasciclin2 (Fas2), during synapse formation de novo by applying live imaging, photobleaching, and genetic analyses to the highly accessible neuromuscular synapses in Drosophila melanogaster embryos (Keshishian et al., 1996).
Fas2 is a homophilic CAM of an Ig superfamily and is expressed both pre- and postsynaptically during the formation of neuromuscular synapses in the embryo and at the mature neuromuscular junction (NMJ) in the larvae (Schuster et al., 1996). Fas2 binds to Discs-Large (Dlg), a homologue of the vertebrate PSD-95, via a PDZ-binding motif in its intracellular domain. At the mature NMJ in the larvae, Fas2 and Dlg form a molecular complex that is essential for synaptic stabilization and growth (Guan et al., 1996; Thomas et al., 1997; Zito et al., 1997). During this process, Dlg regulates the synaptic localization of Fas2 through intracellular interaction (Thomas et al., 1997; Zito et al., 1997). Because Fas2 and Dlg are also expressed in all motor neurons and muscles in the embryo (Schuster et al., 1996), they may well play important roles in the initial assembly of the synapse. However, little is known about their function and dynamics during initial synapse formation in the embryo. Previous studies showed that Fas2 is expressed at a low level across the entire surface of the muscles before the arrival of the motor axons but becomes concentrated at the postsynaptic site at the mature NMJ in the larvae (Schuster et al., 1996). From these and other observations, it has been suggested that Fas2 is actively clustered at the synaptic site. However, how this process is achieved in vivo has remained unknown.
In this study, we show that Fas2 mediates the initial pre- and postsynaptic interaction that is important for the postsynaptic accumulation of Fas2 itself and Dlg at the nascent synapse. We expressed a functional Fas2-GFP (or a color derivative, YFP) or Dlg-GFP fusion protein in the postsynaptic muscle and followed their dynamics during synapse formation. We found that both Fas2- and Dlg-GFP became concentrated at the presumptive postsynaptic site immediately after the formation of the synaptic contact site. The accumulation of Fas2- and Dlg-GFP failed to occur in fas2 mutants, suggesting that unlike in the larvae, Fas2-mediated trans-synaptic adhesion regulates the synaptic localization of postsynaptic Fas2 and Dlg in the embryo. This notion is further supported by deletion and photobleaching analyses of Fas2. In fas2 mutants, synaptic accumulation of another PDZ-containing scaffolding protein Scribble (Scrib) and glutamate receptor subunits GluRIIA and GluRIIB were also reduced, although many other aspects of synapse formation seemed to proceed normally. These results provide in vivo evidence for the role of trans-synaptic adhesion in triggering molecular assembly at nascent postsynaptic sites.
Results
Accumulation of Fas2-YFP at nascent synaptic sites
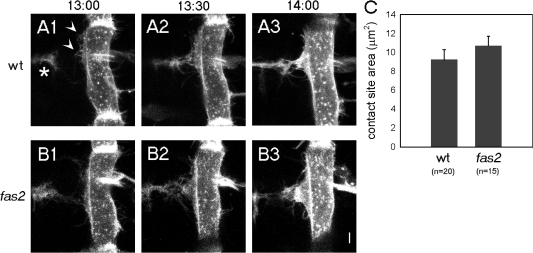
Before tracing molecular dynamics during synapse formation, we first examined the morphological change of pre- and postsynaptic cells during this process. It has been reported that the initial pre- and postsynaptic interaction during the formation of neuromuscular synapses in the Drosophila embryo occurs between filopodia extending from motor neurons and myopodia extending from muscles (Ritzenthaler et al., 2000). Previous imaging analysis of the postsynaptic myopodia showed that they progressively cluster at the prospective synaptic site as they contact and intermingle with the presynaptic filopodia to form the nascent synaptic site. In this study, we conducted simultaneous imaging of both presynaptic filopodia and postsynaptic myopodia by genetically expressing myristylated GFP (mGFP) in motor neurons and a target muscle (M12) and by capturing time-lapse images that span the period of initial neuromuscular interaction (Fig. 1 A). This dual imaging confirmed and extended previous studies (Ritzenthaler et al., 2000) by showing that (1) the synapse is formed through stabilization of certain contacts between neuronal filopodia and myopodia and their subsequent clustering, and (2) myopodia clustering occurs shortly after the initial nerve contact (<40 min; Fig. 1 A and unpublished data). These observations are consistent with the idea that myopodia clustering and subsequent postsynaptic differentiation is induced by contact with the presynaptic cells.
Figure 1.
Cellular interactions between pre- and postsynaptic cells during initial synapse formation. (A) Time-lapse sequence of myopodia and presynaptic filopodia showing their dynamic interaction in a wild-type (wt) embryo 13–14 h after egg laying. Motor neuronal growth cones (asterisk) and myopodia (arrowheads) of ventral muscle 12 are labeled with membrane-bound GFP. (B) The same imaging analysis as in A in a fas2 mutant embryo at 13 h, 13 h 30 min, and 14 h. (C) Quantification of the area of the neuromuscular contact site at 14 h in wild-type (n = 20) and fas2 mutant (n = 15) embryos. Error bars represent SEM. Bar, 5 μm.
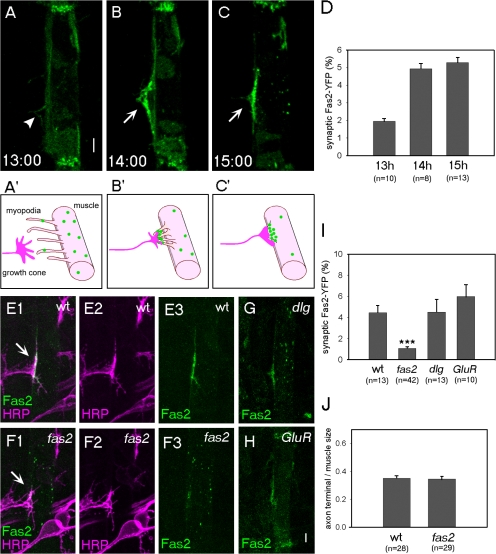
We next studied the dynamics and function of Fas2 as a candidate protein that could mediate the initial pre- and postsynaptic interaction. Fas2 is known to be expressed by motor neurons and muscles in the embryo (see Introduction). However, because possible postsynaptic accumulation of these molecules is obscured by the presence of presynaptic expression, whether and how Fas2 is assembled at the nascent postsynaptic sites was unknown. Therefore, we expressed YFP-tagged Fas2 in the ventral muscle M12 and examined its behavior during the period of filopodia–myopodia interaction. The Fas2-YFP used in this study was functional by genetic and cell biological criteria (see Materials and methods). We found that Fas2-YFP distribution was initially diffuse but became concentrated at the postsynaptic sites upon the arrival of the nerve (Fig. 2, A–D). Before the arrival of the motor axons, Fas2-YFP was uniformly distributed on the entire surface of the muscle with the exception of enriched localization at the muscle attachment sites (Fig. 2 A). In particular, no Fas2 accumulation was observed in myopodia, which extend in random directions at this stage (Fig. 2 A, arrowhead). When the myopodia clustered in response to innervation at 14 h, however, Fas2-YFP accumulated in the clustered myopodia (Fig. 2 B). A high concentration of Fas2-YFP was also observed at the nascent postsynaptic site at 15 h (Fig. 2 C). These results suggest that postsynaptic Fas2 is rapidly rcruited to the nerve contact site. To further characterize the Fas2 accumulation, we performed dual-color live imaging analysis of presynaptic filopodia and postsynaptic Fas2 by expressing myristylated YFP (mYFP) in neurons and Fas2-CFP in muscles (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200705154/DC1). Time-lapse analysis with 4-min intervals revealed that Fas2-CFP accumulation does occur concurrently with the formation of the initial nerve contact site. We also performed dual-color live imaging analysis of myopodia and postsynaptic Fas2 by simultaneously expressing mYFP and Fas2-CFP in muscles. This analysis showed that Fas2-CFP accumulation occurs focally in clustered myopodia but not in transient myopodia outside the synaptic contact site (Fig. S2). Thus, accumulation of Fas2 appears to be one of the earliest events of postsynaptic differentiation immediately after the period of mutual exploration by axonal filopodia and myopodia.
Figure 2.
Accumulation of Fas2-YFP to the nascent synaptic site and its dependence on endogenous Fas2. (A–C) Accumulation of Fas2-YFP on muscle 12 in wild-type (wt) embryos. (A) At 13 h, Fas2-YFP was distributed evenly, including myopodia (arrowhead). (B and C) At 14 (B) and 15 h (C), strong Fas2-YFP concentration was seen in the myopodia cluster (B; arrow) or nascent synaptic site (C; arrow). (A′–C′) Schematic diagrams of the morphology of filopodia and myopodia and accumulation of Fas2 during the stages shown in A–C. (D) Time course of Fas2-YFP accumulation. (E and F) Confocal images of dissected wild-type (E) and fas2 mutant (F) embryos at 15 h expressing Fas2-YFP on muscle 12 (green) and immunostained for the axonal marker anti-HRP antigen (magenta). The presynaptic arborization at the prospective synaptic site (arrows) was normal in fas2 mutants. Nonetheless, Fas2-YFP accumulation at the synaptic site was greatly diminished. (G and H) Fas2-YFP localization in dlgX1-2 (G) and GluRIISP22/Df(2L)clh4 (H). (I) Quantification of the synaptic accumulation of Fas2-YFP. ***, P < 0.001 by t test. (J) Quantification of the terminal size of the axon on muscle 12. Error bars represent SEM. Bar, 5 μm.
Postsynaptic accumulation of Fas2-YFP is reduced in the absence of presynaptic Fas2
To explore the mechanisms underlying the postsynaptic accumulation of Fas2, we searched for candidate genes responsible for the accumulation. As described in the Introduction, Fas2 localization at the larval NMJ depends on the function of Dlg (Thomas et al., 1997; Zito et al., 1997). Because endogenous Dlg is expressed at the nascent synapse (Guan et al., 1996), the initial accumulation of Fas2 in the embryo could similarly depend on intracellular interaction with Dlg. Alternatively, it could be induced by the presynaptic cells through the action of secreted or cell surface molecules, just like acetylcholine receptor clustering is induced by agrin at the vertebrate NMJ (Kummer et al., 2006). Because Fas2 is a homophilic CAM and is present on motor neuron growth cones, direct binding with presynaptic Fas2 could be a mechanism of such nerve-dependent recruitment. We were also interested in whether neural activity is involved in the Fas2 accumulation.
To investigate these possibilities, we analyzed postsynaptic Fas2-YFP distribution in various mutants. We first examined pros mutants in which motor neuronal axon outgrowth fails or is delayed (Broadie and Bate, 1993). Fas2-YFP accumulation was largely lost in pros mutants, suggesting that it is dependent on presynaptic innervation (unpublished data). We next studied fas2, dlg, and GluRIISP22 mutants. GluRIISP22 mutants completely lack synaptic transmission because they are deficient for both the GluRIIA and GluRIIB genes, which encode glutamate receptor subunits (DiAntonio et al., 1999). Fas2-YFP accumulation at the postsynaptic site was greatly reduced in fas2 but not in dlg or GluRIISP22 mutants (Fig. 2, E–I). Thus, Fas2-mediated adhesion is important for the initial Fas2 accumulation. Because postsynaptic Fas2-YFP is functional, the results also support the requirement of presynaptic Fas2 in the postsynaptic accumulation of Fas2.
Normally sized presynaptic terminals form in fas2 mutants
The reduction of Fas2-YFP accumulation in fas2 mutants suggests the possibility that presynaptic Fas2 recruits postsynaptic Fas2 to the synaptic contact site through direct binding. However, because presynaptic Fas2 may also be required for formation of the synaptic contact site itself, the reduced postsynaptic Fas2 accumulation could be an indirect consequence of an absence or reduction in the synaptic contact sites. Thus, we studied early synaptic interaction in fas2 mutants. As has previously been reported, differentiation of motor neurons and muscles and initial outgrowth of motor axons to the vicinity of target muscles were normal in fas2 mutants (Schuster et al., 1996). We also used the aforementioned pre- and postsynaptic dual imaging to confirm that motor neuronal axons reached the target region and touched the target muscles as in wild type (Fig. 1 B). No obvious abnormalities were seen in the timing and dynamics of the myopodia–filopodia interactions to form the synaptic contact site (the size of the clustered myopodia was 9.2 ± 1.1 μm2 in wild type [n = 20] compared with 10.7 ± 1.0 μm2 in fas2 [n = 15]; P > 0.3 by t test; Fig. 1 B). T-shaped presynaptic terminals of normal size also formed in the prospective synaptic site along the proximal edge of target muscle 12 (Fig. 2, E, F, and J; Schuster et al., 1996). Thus, Fas2 is not required for the formation of synaptic contact sites. Therefore, the results suggest a specific function of presynaptic Fas2 in the accumulation of postsynaptic Fas2.
Fas2 is required for the synaptic accumulation of Dlg
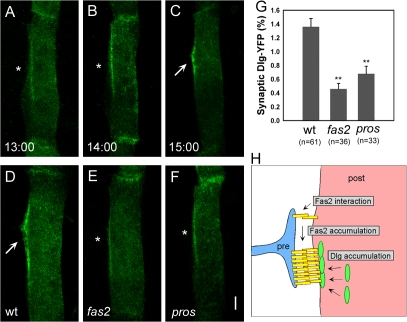
Next, we asked whether Fas2 is required for the assembly of other postsynaptic components. We first studied the localization of Dlg and its dependence on Fas2. Because Dlg, like Fas2, is expressed both pre- and postsynaptically during the formation of neuromuscular synapses, we used Dlg-YFP expressed in the target muscle to monitor Dlg dynamics in the postsynaptic cell. Just like Fas2-YFP, Dlg-YFP was only weakly localized at the prospective synaptic site before nerve innervation but became concentrated at the nascent postsynaptic site upon the arrival of the motor neurons (Fig. 3, A–C). This Dlg-YFP accumulation was greatly reduced in fas2 mutants, indicating that delivery of Dlg to the postsynaptic site is dependent on Fas2 (Fig. 3, D and E). Only ∼30% of the normal level of accumulation was observed in fas2 mutants (Fig. 3 G). A similar level of Dlg-YFP accumulation was seen before the arrival of the nerve (Fig. 3 A) and in pros mutants that lacked presynaptic innervation (Fig. 3, F and G), suggesting that the residual Dlg-YFP accumulation occurs by nerve-independent mechanisms. These results indicate that Fas2 plays a major role in the nerve-dependent induction of Dlg assembly at the postsynaptic site. Endogenous Dlg also accumulated at the nascent synaptic site (Guan et al., 1996), and the accumulation was reduced in fas2 mutants (Fig. S4, A and B; available at http://www.jcb.org/cgi/content/full/jcb.200705154/DC1). However, because both motor neurons and muscles express Dlg, the pre- and postsynaptic contribution could not be distinguished in these experiments.
Figure 3.
Fas2 is necessary for the synaptic accumulation of Dlg. (A–C) Accumulation of Dlg-YFP on muscle 12 in wild-type embryos. At 13 h (A; asterisk), Dlg-YFP was weakly localized. From 14 (B; asterisk) to 15 h (C; arrow), Dlg-YFP accumulated strongly at the postsynaptic site. (D–F) Distribution of Dlg-YFP on muscle 12 in wild-type (D), fas2eB112 (E), and prosm4 (F) embryos at 15 h shows strong (D; arrow) and weak (E and F; asterisks) accumulation. (G) Quantification of the synaptic accumulation of Dlg-YFP in wild-type (wt), fas2eB112, and prosm4 embryos. **, P < 0.005 by t test. Error bars represent SEM. (H) Schematic diagram describing a model of the postsynaptic assembly of Fas2 and Dlg. Bar, 5 μm.
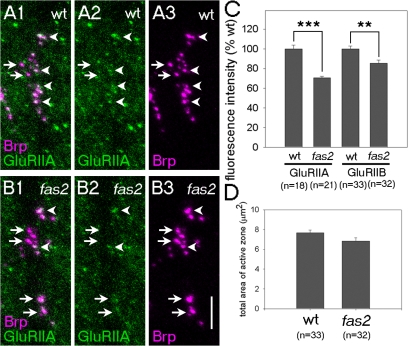
To study whether fas2 is required for the accumulation of other postsynaptic molecules than Dlg, we tested the embryonic expression of 14 proteins that are known to be present at the larval NMJ (Fig. S4; and see Materials and methods). Three of them—Scrib, α-Spectrin, and β-Spectrin—were found to be localized at the nascent NMJ at 15 h. Because all three proteins, like Dlg, were present in the presynaptic axons, their possible localization in the postsynaptic sites was obscured by presynaptic staining. Nonetheless, we found that the expression of Scrib but not α- or β-Spectrin is significantly reduced in fas2 mutants. Scrib is a PDZ-containing scaffolding protein and is known to colocalize and indirectly interact with Dlg at the larval NMJ (Roche et al., 2002). We also studied localization of the glutamate receptor subunits GluRIIA and GluRIIB, which are known to be recruited to the synapse much later. Synaptic accumulation of these molecules, as assessed by antibody staining, does not occur until 18 h, and it apposes the presynaptic active zone marker Bruchpilot (Brp; Marrus et al., 2004). The accumulation of GluRIIA and GluRIIB was significantly reduced in fas2 mutants, as assessed by the total fluorescence intensity for GluRIIA and GluRIIB staining apposing the Brp-positive area (Fig. 4). Note that clustering of Brp at the presynaptic site was normal in fas2 mutants (Fig. 4 D). These results are consistent with the idea that the Fas2/Dlg scaffold is required for the proper assembly of subsets of postsynaptic components in the embryo.
Figure 4.
Fas2 is necessary for the proper synaptic accumulation of GluRIIA and GluRIIB. (A and B) Distribution of GluRIIA (green) and presynaptic active zone marker Brp (magenta) in wild-type (A) and fas2 (B) embryos at 18 h. Brp-positive active zones with (arrowheads) or without (arrows) the apposing GluRIIA cluster in the postsynaptic site are indicated. (C) Quantification of the synaptic accumulation of GluRIIA and GluRIIB in wild-type (wt) and fas2 embryos. **, P < 0.005; ***, P < 0.001 by t test. (D) Total area of the Brp-positive active zones on ventral muscles 6, 7, 12, 13, and 30 is normal in fas2 mutants. Statistical difference was calculated using the t test. Error bars represent SEM. Bar, 5 μm.
Extracellular interaction is sufficient for the postsynaptic assembly of Fas2
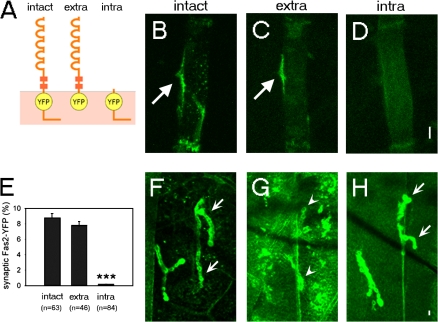
The aforementioned results suggest that formation of the Fas2 adhesion complex is a critical early event in postsynaptic assembly. To further study how the initial postsynaptic Fas2 accumulation is induced during development, we performed deletion and photobleaching experiments. We first performed deletion analysis of Fas2 to test whether trans-synaptic extracellular interaction is indeed responsible for the Fas2 accumulation. We constructed YFP fusion proteins containing only the extracellular (extra–Fas2-YFP) or intracellular (intra–Fas2-YFP) domains of Fas2 (Fig. 5 A) and studied their localization in wild-type embryos. If the initial accumulation of Fas2 at the postsynaptic site is solely dependent on the interaction with presynaptic Fas2, the extracellular domain should be sufficient for the localization. On the other hand, if Fas2 accumulation is regulated by interaction with Dlg as in the larvae (Thomas et al., 1997; Zito et al., 1997), the intracellular domain should be necessary and sufficient. We found that extra–Fas2-YFP accumulated at the nascent postsynaptic site, just as intact Fas2-YFP does (Fig. 5, B and C; arrows; and quantitative data shown in E). This suggests that extracellular interaction alone is sufficient for the synaptic localization and that interaction with intracellular proteins such as Dlg is not necessary. In contrast, intra–Fas2-YFP did not accumulate at the nascent synaptic site (Fig. 5, D and E). The same intra–Fas2-YFP construct but not extra–Fas2-YFP strongly accumulated at the larval NMJ as previously reported (Fig. 5, F–H; Thomas et al., 1997; Zito et al., 1997). These results indicate that unlike in the larvae, Dlg interaction is not sufficient for the initial localization of Fas2 at the postsynaptic site. Together with the normal distribution of Fas2-YFP in dlg mutants described in the previous paragraph, these results indicate that Dlg interaction does not play a major role in the initial accumulation of Fas2 at the postsynaptic site. Instead, our results support the idea of recruitment by direct interaction with presynaptic Fas2.
Figure 5.
Extracellular domain is sufficient for the synaptic localization of Fas2-YFP. (A) Schematic diagram of Fas2-YFP constructs. (B–D) Distribution of intact Fas2-YFP (B), extra–Fas2-YFP (C), and intra–Fas2-YFP (D) expressed on muscle 12 in wild-type embryos. Fas2-YFP strongly accumulated at the nascent postsynaptic sites of muscle 12 (arrows). (E) Quantification of the synaptic accumulation of different Fas2-YFP constructs in wild-type embryos at 15 h. ***, P < 0.001 by t test. Error bars represent SEM. (F–H) Distribution of intact Fas2-YFP (F), extra–Fas2-YFP (G), and intra–Fas2-YFP (H) expressed on all muscles in wild-type larvae. Strong (arrows) and weak (arrowheads) accumulation of Fas2-YFP constructs at the synaptic sites of muscle 12 are shown. Bars, 5 μm.
Mobility of Fas2 at the postsynaptic site is regulated by extracellular interaction
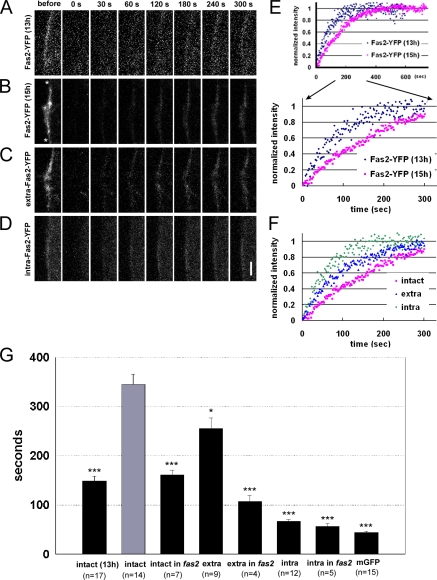
We next performed FRAP analysis to gain further insights on how Fas2 accumulates at the nascent synapse. In general, when a group of molecules is immobile at a specific region, they are replaced with a lower turnover rate and with a slower kinetics. In FRAP experiments, after bleaching fluorescence in a restricted region and time window, fluorescence recovery within the region, which takes place by exchange between bleached and unbleached molecules, is traced (Sprague and McNally, 2005). We used this method to evaluate the change in mobility of postsynaptic Fas2 during the process of synapse formation. We photobleached the fluorescence of Fas2-YFP at the prospective synaptic site and monitored its recovery, which indicates the exchange of bleached molecules at the synaptic site with unbleached molecules from the rest of the muscle. By normalizing these recovery data with fluorescence at the extrasynaptic region, we evaluated turnover fractions and turnover time constants of Fas2-YFP (Fig. 6; and see Materials and methods). First, we asked whether there was a change in the motility of the postsynaptic Fas2 during synapse formation by performing FRAP of Fas2-YFP before (13 h) and after (15 h) nerve innervation. At both stages, >80% of Fas2-YFP showed turnover after 10 min, suggesting that a majority of Fas2-YFP are mobile at both of these stages (unpublished data). However, we observed a large increase in the turnover time constant of these mobile fractions (turnover time constant of 148 ± 10 s before innervation compared with 345 ± 20 s after innervation; Fig. 6, A, B, E, and G). Furthermore, the turnover time constant in a proximal region within the synaptic site (i.e., near the axon entry point) was longer than that in a distal region (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200705154/DC1). These results suggest that the mobility of Fas2 is reduced by the influence of the innervating nerve.
Figure 6.
FRAP analysis of postsynaptic Fas2-YFP. (A–D) FRAP for postsynaptic Fas2. The prospective postsynaptic sites (between the asterisks in B) of M12 expressing Fas2-YFP (A and B), extra–Fas2-YFP (C), and intra–Fas2-YFP (D), before (A; 13 h) and after (B–D; 15 h), were selectively photobleached, and fluorescence recovery at the site was monitored by time-lapse imaging. The elapsed time after the end of the bleaching is shown. (E and F) Recovery time course of postsynaptic YFP fluorescence shown in A–D. (E) Comparison before and after innervation. (F) Comparison between the intact and deleted forms of Fas2-YFP. (G) Turnover time constants obtained by fitting the recovery curve with the single exponential. Error bars represent SEM. *, P < 0.01; ***, P < 0.001 by t test (see Materials and methods). Bar, 5 μm.
To ask whether presynaptic Fas2 is involved in the reduction of postsynaptic Fas2 motility, we next performed FRAP of Fas2-YFP in fas2 mutant embryos. The recovery time constant of postsynaptic Fas2-YFP in fas2 mutants was much shorter than that in the wild type (turnover time constant of 161 ± 10 s in fas2 mutants compared with 345 ± 20 s in wild type; Fig. 6 G), suggesting that binding with presynaptic Fas2 reduces the motility of postsynaptic Fas2. To further address this issue, we performed FRAP of deleted forms of Fas2. The time constant of intra–Fas2-YFP was much shorter than that of the intact Fas2-YFP and was similar to that of mGFP (Fig. 6, B–D, F, and G). In contrast, the time constant of extra–Fas2-YFP was much longer, although it was significantly shorter than that of intact Fas2-YFP. Furthermore, the time constant of extra-Fas2 was reduced in fas2 mutants, whereas that of intra-Fas2 was not (Fig. 6 G). These results collectively suggest that association with the presynaptic Fas2 is responsible for the reduction in the mobility of postsynaptic Fas2.
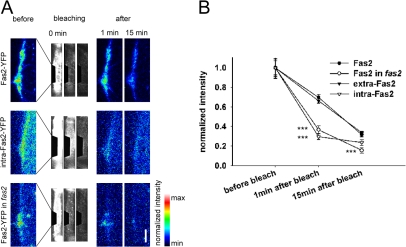
We next performed inverse FRAP (iFRAP) experiments of Fas2-YFP (Dundr et al., 2002; Rabut et al., 2004). The entire cellular fluorescence except for the synaptic site was photobleached, and loss of the fluorescence from the synaptic site over time was monitored. Because iFRAP directly traces the dissociation of the fluorescent molecules from the region of interest (in this case, the presumptive synaptic site), it is suitable to analyze the mobility of bound molecules (Rabut et al., 2004). We applied iFRAP to muscles expressing intact Fas2-YFP, extra–Fas2-YFP, or intra–Fas2-YFP and measured the reduction in synaptic fluorescent signal present at the presumptive synaptic site 1 and 15 min after the bleaching. The reduction in synaptic fluorescence was much smaller for Fas2-YFP and extra–Fas2-YFP compared with intra–Fas2-YFP (Fig. 7), suggesting that Fas2-YFP and extra–Fas2-YFP are more stably bound to the synaptic site than intra–Fas2-YFP. The results are consistent with the FRAP results and suggest that Fas2 mobility is regulated by extracellular interaction. Furthermore, the dissociation rate of Fas2-YFP as assessed by iFRAP was considerably increased in fas2 mutants to a level comparable with that of intra–Fas2-YFP in wild type, suggesting that binding with presynaptic Fas2 is involved in the regulation of Fas2 motility. These FRAP and iFRAP results further support the notion that Fas2 is targeted to the synaptic contact site by binding to the presynaptic Fas2.
Figure 7.
iFRAP analysis of postsynaptic Fas2-YFP. (A) iFRAP for postsynaptic Fas2-YFP (top) and intra–Fas2-YFP (middle) in wild type and Fas2-YFP in fas2 mutant (bottom). The fluorescence intensity at the prospective postsynaptic site before and 1 and 15 min after photobleaching the entire extrasynaptic region are shown in pseudocolor in the left panel and in the right two panels. The middle three panels show the process of photobleaching. (B) Synaptic fluorescence intensities 1 and 15 min after photobleaching normalized by those before photobleaching are shown (Fas2, n = 8; Fas2 in fas2 mutant, n = 8; extra-Fas2, n = 13; intra-Fas2, n = 7). ***, P < 0.001 by t test. Error bars indicate SEM. Bar, 5 μm.
Discussion
In this study, we took advantage of the highly specific and stereotyped development of the Drosophila NMJ to observe and molecularly dissect distinct steps of early pre- and postsynaptic interaction. By looking at individual processes of synapse formation in real time in the intact organism, we demonstrated the specific function of Fas2 during initial pre- and postsynaptic interaction. The results of our combined genetic and live imaging analyses are consistent with the idea that Fas2 functions as a trans-synaptic inducer of early postsynaptic assembly at this synapse.
Recruitment of postsynaptic Fas2 by trans-synaptic adhesion
The accumulation of Fas2 at the synaptic site appears to be one of the earliest events of postsynaptic differentiation. We observed that synaptic accumulation of Fas2-YFP immediately follows the period of filopodial exploration and occurs much earlier than the accumulation of glutamate receptors. Our genetic and deletion analyses suggest that interaction with presynaptic Fas2 plays a dominant role in the postsynaptic accumulation of Fas2. First, we found that postsynaptic Fas2-YFP failed to accumulate properly in fas2 mutants. Because Fas2 is normally expressed on the growth cones of motor neurons and on muscles during the period of synapse formation and because Fas2-YFP expressed on muscles is functional, the results strongly suggest that presynaptic Fas2 is required for the proper accumulation of postsynaptic Fas2. Because of technical difficulties, we did not perform nerve-specific rescue of the phenotype, so the involvement of Fas2 in other surrounding tissues cannot be excluded. However, specificity in the location of the observed abnormality at the neuromuscular contact site argues against this possibility. Second, we showed that the extracellular domain but not the intracellular domain of Fas2 is sufficient for its localization to the postsynaptic site. The results indicate that extracellular interaction, most likely with presynaptic Fas2, is sufficient for the Fas2 localization, and, unlike in the larvae, intracellular interaction with other molecules such as Dlg is not necessary.
Photobleaching experiments demonstrated that the motility of Fas2 is also regulated by extracellular interaction. We found that the turnover time constant for FRAP of Fas2-YFP at the prospective postsynaptic site is greatly increased by innervation. Furthermore, the increase was dependent on the presence of presynaptic Fas2 and on extracellular interaction. iFRAP experiments also showed that the rate of dissociation of Fas2-YFP from the synaptic site is dependent on its extracellular domain and on the presence of presynaptic Fas2. These results indicate that mobility of Fas2 at the postsynaptic site is limited by interaction with presynaptic Fas2 and support the notion that postsynaptic Fas2 is entrapped and accumulated at the synaptic contact site by binding with presynaptic Fas2. Collectively, our genetic, imaging, and photobleaching experiments provide in vivo support for the role of trans-synaptic adhesion in the initial assembly of postsynaptic CAMs.
The role of Fas2 in early synapse formation
We found that Fas2 is also required for the synaptic accumulation of Dlg, which, like that of Fas2, takes place immediately after the formation of the synaptic site. Accumulation of Dlg-YFP at the nascent synapse is greatly reduced in fas2 mutants. In contrast, accumulation of Fas2-YFP at the synapse was normal in dlg mutants. Collectively, our molecular genetic analysis of Fas2 and Dlg support a model in which (1) presynaptic Fas2 first induces the assembly of postsynaptic Fas2 to the nerve innervation sites through homophilic adhesion, and (2) postsynaptic Fas2, in turn, recruits Dlg through intracellular interaction (Fig. 3 H). This is in contrast to the situation in more mature synapses in the larvae in which Dlg plays a major role in the synaptic localization of Fas2 (Thomas et al., 1997; Zito et al., 1997) and reveals a novel mechanism by which the initial targeting of Fas2 and Dlg to synapses is accomplished.
Although our results suggest that Fas2 plays a prominent role in the initial assembly of the Fas2–Dlg complex at the synapse, we do not exclude the possibility that the reverse signaling (i.e., recruitment of Fas2 by Dlg) also contributes to this process. Because Dlg is maternally supplied, weak Dlg expression was present in the mutant embryos analyzed in this study (see Materials and methods; Guan et al., 1996). Therefore, Dlg could contribute in a redundant manner to the postsynaptic accumulation of Fas2. Such bidirectional control has been suggested for mammalian PSD-95 and neuroligin: neuroligin promotes clustering of PSD-95 at the synapse and vice versa (Prange et al., 2004; Chih et al., 2005). We also found that Dlg can partially accumulate at the postsynaptic site independent of the innervating nerve. Similar nerve-independent aggregates of acetylcholine receptors were observed at the NMJ in vertebrates (Kummer et al., 2006). These results suggest that multiple mechanisms contribute to the postsynaptic assembly of Dlg. However, the large reduction in the assembly of Dlg-YFP observed in fas2 mutants suggests that Fas2-mediated trans-synaptic signaling is critical for the nerve-induced accumulation of Dlg.
We also found that fas2 is required for the proper recruitment of Scrib, GluRIIA, and GluRIIB to the synaptic sites. Scrib regulates synaptic plasticity and synaptic vesicle dynamics at the larval NMJ. A previous study showed that Scrib indirectly interacts with Dlg and that Dlg is critical for the synaptic localization of Scrib (Roche et al., 2002). There is no evidence for molecular interaction between GluRIIA/B and Dlg or Fas2, although it has previously been reported that synaptic localization of GluRIIB but not GluRIIA is reduced in dlg mutants (Chen and Featherstone, 2005). The reduction in the accumulation of Scrib, GluRIIA, and GluRIIB in fas2 mutants was not as drastic as that of Dlg-YFP. Therefore, it is likely that Fas2 partly and indirectly regulates the synaptic localization of these molecules.
Our live imaging analysis showed that Fas2 is not required for the initial formation of the synaptic contact sites. No abnormality was seen in the dynamics or stabilization of the filopodia in fas2 mutants. This is consistent with the observation that Fas2 accumulates in the myopodia only after their clustering. Presynaptic terminals with normal size also formed at the prospective synaptic sites. Thus, Fas2 is not required for the formation of the synaptic contact site itself but rather for assembly of the postsynaptic molecular complex, including Fas2 and Dlg at the nascent postsynaptic site. It should also be noted that many other aspects of synaptic differentiation are normal in fas2 mutants. Clustering of the active zone marker Brp to the presynaptic sites is normal in fas2 mutants. Previous EM analysis of late embryos also showed synapses with normal morphology, and bouton numbers form in fas2 mutants (Schuster et al., 1996). Therefore, Fas2-mediated adhesion is likely a component of the mechanisms that regulate the assembly of synapses. This is consistent with the generally accepted view that synapse formation likely uses multiple redundant mechanisms (Goda and Davis, 2003; Waites et al., 2005).
Induction of postsynaptic molecular assembly by trans-synaptic adhesion
The mechanism of early postsynaptic assembly in vivo is not well understood. At the NMJ in vertebrates, Agrin secreted by motor neurons can induce acetylcholine receptor clustering on the surface of the postsynaptic muscle (Kummer et al., 2006). In agrin mutants, the NMJ failed to develop properly. However, a recent study suggests that the function of Agrin is required for the maintenance rather than the initial formation of synapses (Kummer et al., 2006). On the other hand, trans-synaptic cell adhesion has long been implicated in synaptic induction because it can provide a simple and direct way by which the innervating axon induces molecular assembly at the apposing postsynaptic sites (Chow and Poo, 1985; Fannon and Colman, 1996; Uchida et al., 1996). However, the role of cell adhesion at the onset of synaptogenesis has never been directly demonstrated in vivo, possibly as a result of the difficulty in examining distinct steps of synaptic development in intact organisms and redundancy in the synaptogenic program. We showed by high-resolution in vivo imaging of the early course of synaptic assembly that a CAM, Fas2, regulates specific aspects of synaptic differentiation. In particular, our results suggest that presynaptic Fas2 induces the accumulation of postsynaptic Fas2 and Dlg at the synaptic contact site through trans-synaptic adhesion. Much evidence suggests that Dlg and its mammalian homologue PSD-95 are, in turn, critical for the clustering of several neurotransmitter receptors and adaptor proteins (Funke et al., 2005). Our results provide in vivo evidence for the role of trans-synaptic adhesion in triggering postsynaptic molecular assembly.
Materials and methods
Fly strains
The GAL4-UAS system (Brand and Perrimon, 1993) was used to express mGFP and YFP-tagged Fas2 and Dlg in muscles and/or motor neurons. Gene expression in muscle 12 and motor neurons was driven by the Gal4 lines GAL4-5053A (Ritzenthaler et al., 2000) and elav-GAL4-3E1 (Davis et al., 1997), respectively. For vital visualization of cell morphology, we used UAS-mGFP (Ritzenthaler et al., 2000). The genotypes of mutants were prosm4 (Broadie and Bate, 1993), fas2eB112/Y (Schuster et al., 1996), dlgX1-2/Y (Guan et al., 1996), and GluRIISP22/Df(2L)clh4 (DiAntonio et al., 1999). Although Dlg is known to be provided maternally (Guan et al., 1996), immunostaining showed that the amount of Dlg present in dlgX1-2/Y embryos is only 20% of that in wild-type embryos at 15 h. The developmental stage of embryos was expressed in terms of hours after egg laying. To narrow the time window of the staging, the eggs were laid for 30 min. The half-period of the egg laying (15 min) was assigned as hour 0. The developmental stage of embryos was also verified by morphological criteria of the midgut.
Plasmid construction and generation of transgenic lines
Construction of the pUAST vectors and germline transformation were performed according to standard protocols (Brand and Perrimon, 1993). The Fas2-YFP expression construct contains the ORF of YFP inserted via a five–amino acid linker into the C-terminal end of the transmembrane domain of Fas2. Fas2-YFP was verified to be functional according to its normal synaptic localization, adhesive activity when expressed on larval muscles (Nose et al., 1997) and S2 cells (Grenningloh et al., 1990), and ability to rescue the lethality of fas2eB112 when expressed in subsets of muscles and neurons (Schuster et al., 1996). The Fas2 deletion constructs, extra–Fas2-YFP, and intra–Fas2-YFP contained amino acids 1–794, 1–45, and 736–844, respectively, of the PEST(−) form of Fas2 and the ORF of YFP at the same position as in the intact Fas2-YFP. The Dlg-YFP construct contained the ORF of YFP connected to the N terminus of Dlg, as previously reported for Dlg-GFP (Koh et al., 1999). UAS-mYFP/CFP were designed as described for UAS-mGFP (Ritzenthaler et al., 2000). The elav-mYFP construct contained the elav promoter (Sone et al., 1997) at the 5′ end of mYFP/CFP.
Live visualization and quantification
The dynamics of Fas2- and Dlg-YFP was imaged in dissected embryos immersed in insect saline using a confocal system (LSM510; Carl Zeiss, Inc.) with a 100× NA 1.0 water immersion objective lens (Carl Zeiss, Inc.) at 25°C. The accumulation of Fas2- and Dlg-YFP at the nascent synapse was quantified using IPLab software (BD Biosciences). First, particle analysis was used to objectively assign fluorescent signals to the synaptic site. Fluorescence intensity in each pixel in the image of the muscle was measured, and the mean (m) and SD (σ) was calculated. Particles (continuous clusters of pixels) at the nerve innervation site with fluorescence intensity higher than m + 2σ was defined as the postsynaptic assembly. The ratio of the total amount of fluorescence in the postsynaptic assembly (thus defined) to that in the entire muscle was then used as a measure of synaptic accumulation. Z-stack images are used in these analyses; thus, total amounts of proteins present in the synaptic sites are quantified. The formation of the synaptic contact site was assessed by the area of the clustered myopodia/filopodia, which was approximated as a tetragonum and measured using IPLab software.
Dual imaging of Fas2-CFP in muscles and mYFP in axons or muscles was conducted with a confocal microscope (FV1000; Olympus) with 440- and 514-nm laser lines and a 100× NA 1.0 water immersion objective lens (Olympus) at 25°C. For time-lapse dual imaging of presynaptic filopodia and Fas2-CFP (Fig. S1), we used moderate power laser illumination with faster scanning speed (10 μs/pixel). Because the high power laser beam necessary for the visualization of myopodia (Fig. S2) photobleached fluorescent molecules, only one image could be obtained in each imaging.
Photobleaching experiments
FRAP and iFRAP experiments were performed using the FV1000 confocal microscope with 488- and 514-nm argon laser lines and a 100× NA 1.0 water immersion objective lens at 25°C. The sequential image acquisition and photobleaching procedures were performed using a macro program in Fluoview software (Olympus). For FRAP, the photobleaching of the region of interest (typically 3 × 15 μm) with 100% laser intensity was controlled by acousto-optic tunable filters and took ∼5 s. Time-lapse images before and after photobleaching were captured at 0.5–2 Hz with low-power illumination, which caused only 1% per min photobleaching. For subsynaptic analysis, we analyzed a proximal (adjacent to the nerve entry point) and distal region in the postsynaptic site separated by a mean distance of 7.2 μm (n = 13). For iFRAP, the entire cellular fluorescence in the muscle except for the nascent postsynaptic site was photobleached. This bleaching process took ∼45 s. Change in the fluorescence intensity at the postsynaptic site was monitored 1 and 15 min after the start of the photobleaching.
FRAP and iFRAP image analyses were performed using Fluoview software. For each image in a FRAP or iFRAP experiment, the mean fluorescent intensity of a region of interest was measured, and background intensity of an off-cell region was subtracted. For iFRAP experiments, the fluorescence signals detected at different confocal planes were integrated. All values reported are mean ± SEM; datasets were compared by t test.
To obtain the time constant of FRAP, individual recovery data were fit using KaleidaGraph (Hulinks) with the following single exponential equation:
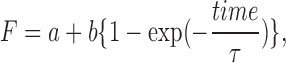 |
where F is the background-subtracted fluorescence intensity, a is an intensity value just after bleaching (time = 0), the second term is fluorescence from recovered molecules, and τ is the time constant for recovery. The apparent time constant and turnover fraction obtained from these analyses were further corrected as described below (see Consideration for the effect of nonnegligible amounts…) to alleviate the effect of nonnegligible amounts of bleached Fas2-YFP.
Immunocytochemistry
To vitally label innervating motor neuronal axons, we incubated dissected embryos with anti-HRP antibodies conjugated with Cy5 (Invitrogen) for 4 h at 4°C. Rabbit polyclonal antibodies against GluRIIA and GluRIIB (Saitoe et al., 1997; Marrus et al., 2004), mouse monoclonal antibodies against an active zone marker, Brp (Nc82; Wagh et al., 2006), and Dlg (Parnas et al., 2001) were used at dilutions of 1:1,000, 1:2,500, 1:100, and 1:50, respectively. We used goat anti-HRP antibody as an axonal marker at a dilution of 1:4,000. As putative embryonic synaptic markers, we tested the expression of the following antigens, which were previously reported to be localized at larval neuromuscular synapses: Scrib (Mathew et al., 2002), Coracle (Chen et al., 2005), GUK-holder (Mathew et al., 2002), dLin7 (Bachmann et al., 2004), Bazooka (Ruiz-Canada et al., 2004), PAK (Sone et al., 2000), GRIP (Ataman et al., 2006), dystrophins (van der Plas et al., 2006), α-Spectrin (Featherstone et al., 2001), β-Spectrin (Featherstone et al., 2001), ankyrin (Pielage et al., 2006), αPS2-integrin (Beumer et al., 1999), and αPS1-integrin (Beumer et al., 1999). Secondary antibodies used were goat anti–rabbit IgG AlexaFluor488, goat anti–mouse IgG Cy3, donkey anti–rabbit IgG AlexaFluor488, donkey anti–mouse IgG AlexaFluor488, donkey anti–guinea pig IgG AlexaFluor488, and donkey anti–goat IgG Cy3 (Invitrogen). Confocal images were acquired with an FV1000 microscope with a 100× NA 1.0 objective lens. Fluorescence intensities of the synaptic markers apposing the motoneuronal terminal or active zone were quantified using IPLab software.
Consideration for the effect of nonnegligible amounts of bleached Fas2-YFP in obtaining the FRAP turnover fraction and time constant
In the FRAP experiments, molecular turnover of Fas2-YFP within the prospective synaptic site was monitored as the recruitment of unbleached, fluorescent Fas2-YFP from the extrasynaptic region. Because a large amount of Fas2-YFP present in the synaptic site was bleached and gradually moved to the extrasynaptic region, nonnegligible amounts of bleached Fas2-YFP can reoccupy the synaptic site during FRAP. This affects the FRAP recovery curve. We considered and corrected this effect in obtaining the turnover fraction (ρ) and turnover time constant (τ) from the FRAP curve. We assumed that at the end of photobleaching, the synaptic site contained N bleached Fas2-YFP molecules, of which φN molecules are mobile, and that the extrasynaptic region contained M fluorescent Fas2-YFP. We also assumed that at time t, the synaptic site contained x fluorescent Fas2-YFP in place of the bleached molecules (this also means that the extrasynaptic region contained x bleached molecules). We assumed that the rate of exchange between Fas2-YFP in the synaptic site and that in the extrasynaptic region is mainly determined by the unbinding of Fas2-YFP from the synaptic site. The number of molecules exchanged per unit of time within the synaptic site can then be defined as kφN, where k is a time-independent constant. The change in fluorescence, Δx, from time t to time t + Δt is determined by the following two turnover events: (1) exchange between bleached Fas2-YFP at the synaptic site (φN − x molecules out of φN molecules at time t) and unbleached Fas2-YFP in the extrasynaptic region (M − x out of M molecules) and (2) exchange between unbleached Fas2-YFP at the synaptic region (x molecules out of φN) and bleached Fas2-YFP in the extrasynaptic region (x molecules out of M). Thus, the following equation is obtained:
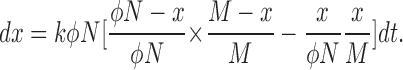 |
This equation gives
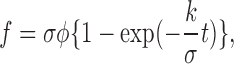 |
where
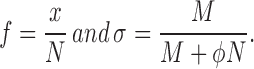 |
Accordingly, the apparent turnover time constant, τ′ = σ/k, is σ times smaller (σ < 1) than the actual τ. Similarly, the apparent turnover fraction, ρ′ = f(t → ∞), is σ times smaller than the actual ρ. As the estimated value of σ, we used the ratio of fluorescence intensity in the extrasynaptic region at the end of the FRAP experiment to that before bleaching, σ0. The apparent turnover fraction and turnover time constant obtained from the FRAP curve were therefore divided by σ0 to obtain ρ and τ.
Online supplemental material
Fig. S1 shows that Fas2 accumulates at postsynaptic sites immediately after motoneuronal innervation. Fig. S2 shows that Fas2 accumulates in myopodia that contact presynaptic filopodia. Fig. S3 shows FRAP analysis of Fas2-YFP at a subsynaptic level. Fig. S4 shows that synaptic accumulation of endogenous Dlg and Scrib are reduced in fas2 mutants. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200705154/DC1.
Supplementary Material
Acknowledgments
We thank Drs. A. Chiba, Y. Kidokoro, A. DiAntonio, V. Budnik, C. Hama, D. Bilder, R.G. Fehon, U. Thomas, A. Wodarz, L.G. Fradkin, R.R. Dubreuil, the Bloomington Stock Center, the Drosophila Genetic Resource Center at the Kyoto Institute of Technology, and the Developmental Studies Hybridoma Bank for fly stocks and reagents. We also thank E. Suzuki and members of the Nose laboratory for discussion; Y. Fujioka, H. Kato, K. Ishibashi, and Y. Suzuki for technical assistance; A. Chiba and H. Taniguchi for critical reading of the manuscript; and A. Chiba for communicating unpublished results.
This study was supported by a grant-in-aid to A. Nose for Scientific Research B and for Scientific Research on Priority Areas (Molecular Brain Science) from the Ministry of Education, Culture, Sports, Science and Technology. H. Kohsaka was supported by research fellowships from the Japan Society for the Promotion of Science for Young Scientists.
Abbreviations used in this paper: Brp, Bruchpilot; CAM, cell adhesion molecule; Dlg, Discs-Large; Fas2, Fasciclin2; iFRAP, inverse FRAP; mGFP, myristylated GFP; mYFP, myristylated YFP; NMJ, neuromuscular junction; Scrib, Scribble.
References
- Ataman, B., J. Ashley, D. Gorczyca, M. Gorczyca, D. Mathew, C. Wichmann, S.J. Sigrist, and V. Budnik. 2006. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc. Natl. Acad. Sci. USA. 103:7841–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann, A., M. Timmer, J. Sierralta, G. Pietrini, E.D. Gundelfinger, E. Knust, and U. Thomas. 2004. Cell type-specific recruitment of Drosophila Lin-7 to distinct MAGUK-based protein complexes defines novel roles for Sdt and Dlg-S97. J. Cell Sci. 117:1899–1909. [DOI] [PubMed] [Google Scholar]
- Beumer, K.J., J. Rohrbough, A. Prokop, and K. Broadie. 1999. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 126:5833–5846. [DOI] [PubMed] [Google Scholar]
- Biederer, T., Y. Sara, M. Mozhayeva, D. Atasoy, X. Liu, E.T. Kavalali, and T.C. Südhof. 2002. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 297:1525–1531. [DOI] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Broadie, K., and M. Bate. 1993. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 361:350–353. [DOI] [PubMed] [Google Scholar]
- Chen, K., and D.E. Featherstone. 2005. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K., C. Merino, S.J. Sigrist, and D.E. Featherstone. 2005. The 4.1 protein Coracle mediates subunit-selective anchoring of Drosophila glutamate receptors to the postsynaptic actin cytoskeleton. J. Neurosci. 25:6667–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih, B., H. Engelman, and P. Scheiffele. 2005. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 307:1324–1328. [DOI] [PubMed] [Google Scholar]
- Chow, I., and M.M. Poo. 1985. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J. Neurosci. 5:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, G.W., C.M. Schuster, and C.S. Goodman. 1997. Genetic analysis of the mechanisms controlling target selection: target-derived Fasciclin II regulates the pattern of synapse formation. Neuron. 19:561–573. [DOI] [PubMed] [Google Scholar]
- DiAntonio, A., S.A. Petersen, M. Heckmann, and C.S. Goodman. 1999. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J. Neurosci. 19:3023–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr, M., U. Hoffmann-Rohrer, Q. Hu, I. Grummt, L.I. Rothblum, R.D. Phair, and T. Misteli. 2002. A kinetic framework for a mammalian RNA polymerase in vivo. Science. 298:1623–1626. [DOI] [PubMed] [Google Scholar]
- Fannon, A.M., and D.R. Colman. 1996. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 17:423–434. [DOI] [PubMed] [Google Scholar]
- Featherstone, D.E., W.S. Davis, R.R. Dubreuil, and K. Broadie. 2001. Drosophila α-and β-Spectrin mutations disrupt presynaptic neurotransmitter release. J. Neurosci. 21:4215–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke, L., S. Dakoji, and D.S. Bredt. 2005. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu. Rev. Biochem. 74:219–245. [DOI] [PubMed] [Google Scholar]
- Goda, Y., and G.W. Davis. 2003. Mechanisms of synapse assembly and disassembly. Neuron. 40:243–264. [DOI] [PubMed] [Google Scholar]
- Graf, E.R., X. Zhang, S.X. Jin, M.W. Linhoff, and A.M. Craig. 2004. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 119:1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh, G., A.J. Bieber, E.J. Rehm, P.M. Snow, Z.R. Traquina, M. Hortsch, N.H. Patel, and C.S. Goodman. 1990. Molecular genetics of neuronal recognition in Drosophila: evolution and function of immunoglobulin superfamily cell adhesion molecules. Cold Spring Harb. Symp. Quant. Biol. 55:327–340. [DOI] [PubMed] [Google Scholar]
- Guan, B., B. Hartmann, Y.H. Kho, M. Gorczyca, and V. Budnik. 1996. The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr. Biol. 6:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes, J.D., M.R. Emond, and S.J. Smith. 2004. In vivo trafficking and targeting of N-cadherin to nascent presynaptic terminals. J. Neurosci. 24:9027–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasthuri, N., and J.W. Lichtman. 2004. Structural dynamics of synapses in living animals. Curr. Opin. Neurobiol. 14:105–111. [DOI] [PubMed] [Google Scholar]
- Keshishian, H., K. Broadie, A. Chiba, and M. Bate. 1996. The Drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu. Rev. Neurosci. 19:545–575. [DOI] [PubMed] [Google Scholar]
- Koh, Y.H., E. Popova, U. Thomas, L.C. Griffith, and V. Budnik. 1999. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 98:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer, T.T., T. Misgeld, and J.R. Sanes. 2006. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr. Opin. Neurobiol. 16:74–82. [DOI] [PubMed] [Google Scholar]
- Li, Z., and M. Sheng. 2003. Some assembly required: the development of neuronal synapses. Nat. Rev. Mol. Cell Biol. 4:833–841. [DOI] [PubMed] [Google Scholar]
- Marrus, S.B., S.L. Portman, M.J. Allen, K.G. Moffat, and A. DiAntonio. 2004. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 24:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew, D., L.S. Gramates, M. Packard, U. Thomas, D. Bilder, N. Perrimon, M. Gorczyca, and V. Budnik. 2002. Recruitment of Scribble to the synaptic scaffolding complex requires GUK-holder, a novel DLG binding protein. Curr. Biol. 12:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler, M., W. Zhang, A. Rohlmann, G. Kattenstroth, R.E. Hammer, K. Gottmann, and T.C. Südhof. 2003. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 423:939–948. [DOI] [PubMed] [Google Scholar]
- Niell, C.M., and S.J. Smith. 2004. Live optical imaging of nervous system development. Annu. Rev. Physiol. 66:771–798. [DOI] [PubMed] [Google Scholar]
- Nose, A., T. Umeda, and M. Takeichi. 1997. Neuromuscular target recognition by a homophilic interaction of connectin cell adhesion molecules in Drosophila. Development. 124:1433–1441. [DOI] [PubMed] [Google Scholar]
- Parnas, D., A.P. Haghighi, R.D. Fetter, S.W. Kim, and C.S. Goodman. 2001. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 32:415–424. [DOI] [PubMed] [Google Scholar]
- Pielage, J., R.D. Fetter, and G.W. Davis. 2006. A postsynaptic Spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J. Cell Biol. 175:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange, O., T.P. Wong, K. Gerrow, Y.T. Wang, and A. El-Husseini. 2004. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA. 101:13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut, G., V. Doye, and J. Ellenberg. 2004. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol. 6:1114–1121. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler, S., E. Suzuki, and A. Chiba. 2000. Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat. Neurosci. 3:1012–1017. [DOI] [PubMed] [Google Scholar]
- Roche, J.P., M.C. Packard, S. Moeckel-Cole, and V. Budnik. 2002. Regulation of synaptic plasticity and synaptic vesicle dynamics by the PDZ protein Scribble. J. Neurosci. 22:6471–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Canada, C., J. Ashley, S. Moeckel-Cole, E. Drier, J. Yin, and V. Budnik. 2004. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron. 42:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoe, M., S. Tanaka, K. Takata, and Y. Kidokoro. 1997. Neural activity affects distribution of glutamate receptors during neuromuscular junction formation in Drosophila embryos. Dev. Biol. 184:48–60. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P. 2003. Cell-cell signaling during synapse formation in the CNS. Annu. Rev. Neurosci. 26:485–508. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., J. Fan, J. Choih, R. Fetter, and T. Serafini. 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 101:657–669. [DOI] [PubMed] [Google Scholar]
- Schuster, C.M., G.W. Davis, R.D. Fetter, and C.S. Goodman. 1996. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 17:641–654. [DOI] [PubMed] [Google Scholar]
- Sone, M., M. Hoshino, E. Suzuki, S. Kuroda, K. Kaibuchi, H. Nakagoshi, K. Saigo, Y. Nabeshima, and C. Hama. 1997. Still life, a protein in synaptic terminals of Drosophila homologous to GDP-GTP exchangers. Science. 275:543–547. [DOI] [PubMed] [Google Scholar]
- Sone, M., E. Suzuki, M. Hoshino, D. Hou, H. Kuromi, M. Fukata, S. Kuroda, K. Kaibuchi, Y. Nabeshima, and C. Hama. 2000. Synaptic development is controlled in the periactive zones of Drosophila synapse. Development. 127:4157–4168. [DOI] [PubMed] [Google Scholar]
- Sprague, B.L., and J.G. McNally. 2005. FRAP analysis of binding: proper and fitting. Trends Cell Biol. 15:84–91. [DOI] [PubMed] [Google Scholar]
- Thomas, U., E. Kim, S. Kuhlendahl, Y.H. Koh, E.D. Gundelfinger, M. Sheng, C.C. Garner, and V. Budnik. 1997. Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 19:787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, N., Y. Honjo, K.R. Johnson, M.J. Wheelock, and M. Takeichi. 1996. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol. 135:767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas, M.C., G.S. Pilgram, J.J. Plomp, A. de Jong, L.G. Fradkin, and J.N. Noordermeer. 2006. Dystrophin is required for appropriate retrograde control of neurotransmitter release at the Drosophila neuromuscular junction. J. Neurosci. 26:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux, F., G. Aramuni, R.L. Rawson, R. Mohrmann, M. Missler, K. Gottmann, W. Zhang, T.C. Südhof, and N. Brose. 2006. Neuroligins determine synapse maturation and function. Neuron. 51:741–754. [DOI] [PubMed] [Google Scholar]
- Wagh, D.A., T.M. Rasse, E. Asan, A. Hofbauer, I. Schwenkert, H. Dürrbeck, S. Buchner, M.C. Dabauvalle, M. Schmidt, G. Qin, et al. 2006. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 49:833–844. [DOI] [PubMed] [Google Scholar]
- Waites, C.L., A.M. Craig, and C.C. Garner. 2005. Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci. 28:251–274. [DOI] [PubMed] [Google Scholar]
- Yamagata, M., J.R. Sanes, and J.A. Weiner. 2003. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 15:621–632. [DOI] [PubMed] [Google Scholar]
- Zito, K., R.D. Fetter, C.S. Goodman, and E.Y. Isacoff. 1997. Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron. 19:1007–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.