Abstract
We investigated the function of cyclin-dependent kinase 2 (Cdk2) in neural progenitor cells during postnatal development. Chondroitin sulfate proteoglycan (NG2)–expressing progenitor cells of the subventricular zone (SVZ) show no significant difference in density and proliferation between Cdk2−/− and wild-type mice at perinatal ages and are reduced only in adult Cdk2−/− mice. Adult Cdk2−/− SVZ cells in culture display decreased self-renewal capacity and enhanced differentiation. Compensatory mechanisms in perinatal Cdk2−/− SVZ cells, which persist until postnatal day 15, involve increased Cdk4 expression that results in retinoblastoma protein inactivation. A subsequent decline in Cdk4 activity to wild-type levels in postnatal day 28 Cdk2−/− cells coincides with lower NG2+ proliferation and self-renewal capacity similar to adult levels. Cdk4 silencing in perinatal Cdk2−/− SVZ cells abolishes Cdk4 up-regulation and reduces cell proliferation and self- renewal to adult levels. Conversely, Cdk4 overexpression in adult SVZ cells restores proliferative capacity to wild-type levels. Thus, although Cdk2 is functionally redundant in perinatal SVZ, it is important for adult progenitor cell proliferation and self-renewal through age-dependent regulation of Cdk4.
Introduction
Two main neurogenic regions are present in the postnatal mouse forebrain: the rostral subependymal zone of the lateral ventricle, the so-called subventricular zone (SVZ), and the subgranular layer of the hippocampal dentate gyrus (Luskin, 1993; Lois and Alvarez-Buylla, 1994; Morshead et al., 1998). In these regions, cell divisions continue to take place throughout life, as complex interactions between intrinsic molecular programs and extrinsic determinants induce neural stem cells and progenitors to proliferate, differentiate, and undergo apoptotic cell death. Significant progress has been recently made in the identification of extracellular factors that regulate neural stem cells and progenitor proliferation and differentiation in the SVZ (Conover et al., 2000; Coskun and Luskin, 2002; Doetsch et al., 2002; Lai et al., 2003; Molofsky et al., 2003; Anton et al., 2004; Leone et al., 2005; Ghashghaei et al., 2006; Lledo et al., 2006; Meng et al., 2006; Ricard et al., 2006). However, several lines of evidence also indicate that neural progenitor proliferation is intrinsically controlled by a molecular network of kinases and accessory proteins.
Cell cycle progression from G1 to S phase is positively regulated by (a) Cdks (Cdk2, 4, and 6), which are activated upon binding of the specific regulatory subunits (cyclin E, D, and A, respectively; Morgan, 1997), and (b) phosphorylation of their target retinoblastoma protein (Rb), which causes the release of E2F1-4 transcription factors (Mittnacht, 1998; Knudsen and Knudsen, 2006). Additionally, the Cdk inhibitors p19INK4d, p21Cip1, and p27Kip1 function as negative regulators of proliferation in the SVZ (Durand et al., 1998; Casaccia-Bonnefil et al., 1999; Ghiani et al., 1999; Coskun and Luskin, 2002; Doetsch et al., 2002). Possible changes in the role and function of these intrinsic regulators during postnatal development of neural progenitors are still largely unexplored but are likely to play a major role in modifying the proliferation rate of SVZ progenitors between birth and adulthood.
In the anterior SVZ (ASVZ), neural progenitors expressing the chondroitin sulfate proteoglycan NG2 (NG2+ progenitors) represent a principal population of proliferating cells (Dawson et al., 2000; Aguirre et al., 2004). NG2+ cells are present in the SVZ from birth until adulthood and display cellular properties of transient-amplifying (type C) cells, generating γ-aminobutyric acid–ergic interneurons and oligodendrocytes in the olfactory bulb (Aguirre and Gallo, 2004; Menn et al., 2006). Previous studies have demonstrated that the Cdk2–cyclin E complex plays an important role in regulating NG2+ progenitor cell cycle in culture and that Cdk2 expression is down-regulated in adult progenitors (Ghiani and Gallo, 2001; Belachew et al., 2002). However, it is still unknown whether Cdk2 is functionally required for NG2+ progenitor proliferation in vivo and whether this requirement changes during postnatal development of the SVZ. Furthermore, a previous analysis also revealed higher levels of the Cdk4/6–cyclin D complex in dividing versus quiescent NG2+ progenitors, which suggests a possible involvement of this kinase in maintaining cell proliferation (Ghiani and Gallo, 2001).
To assess the role of intrinsic cell cycle regulators in progenitor proliferation during developmental and adult neuro- and gliogenesis, we analyzed the NG2+ progenitor cell population in Cdk2−/− mice. Our results demonstrate that the lack of Cdk2 significantly affects progenitor cell proliferation in neurogenic regions only after reaching a juvenile stage of postnatal development, which results in a severe reduction of NG2+ cell quantity and proliferation. We also provide direct evidence that the defect in NG2+ progenitor cell proliferation observed in the adult SVZ of the Cdk2−/− mouse is compensated by Cdk4 at earlier developmental stages. Finally, we show that Cdk2 also plays an important role in controlling oligodendrocyte and neuron lineage commitment in the adult brain.
Results
Loss of Cdk2 causes a decrease in cell proliferation in neurogenic regions of the adult brain
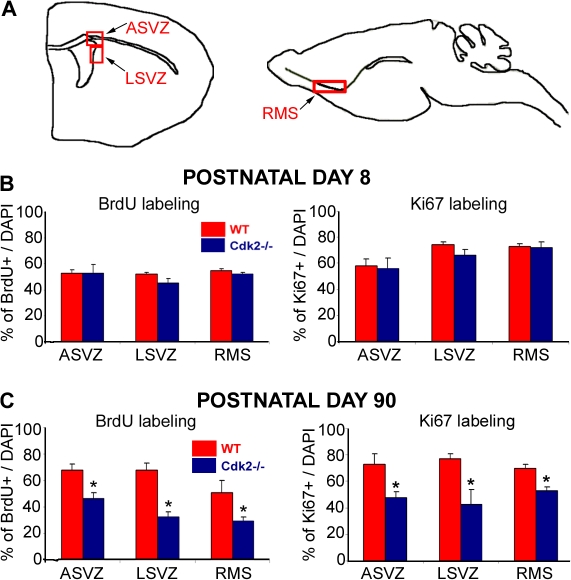
We investigated the role of Cdk2 on SVZ neural progenitor cell proliferation by comparing Cdk2−/− and wild-type mice. We first analyzed two developmental stages, one corresponding to the peak of NG2+ progenitor proliferation (postnatal day 8 [P8]) and the other corresponding to a proliferative plateau (P90; Belachew et al., 2002; Aguirre et al., 2004). We used anti-BrdU staining to identify cells progressing through S phase and anti-Ki67 staining for cells in G1, S, M, or G2 phase. We analyzed neurogenic areas of the brain where stem cells and neural progenitors proliferate and differentiate, including the ASVZ, the lateral SVZ (LSVZ; Fig. 1 A, left), and the path of cell migration along the rostral migratory stream (RMS) toward the olfactory bulb (Fig. 1 A, right). At P8, the loss of Cdk2 did not affect the percentage of BrdU+ and Ki67+ cells in all brain regions examined (Fig. 1 B). Conversely, in P90 brains, the percentage of cells positive for these cell cycle markers was significantly decreased in the ASVZ, LSVZ, and RMS of the Cdk2−/− mouse as compared with the wild type (Figs. 1 C and S1, available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1).
Figure 1.
Loss of Cdk2 causes a reduction of cell proliferation in the SVZ and RMS of the adult brain. (A) Schematic drawings of coronal (left) and sagittal (right) planes of sections showing neurogenic areas of the brain. Proliferating cells were labeled with anti-BrdU and -Ki67 in the ASVZ (left), LSVZ (left), and RMS (right). (B and C) Percentages of proliferating cells were calculated based on the total number of DAPI cells. At P8, the percentages of BrdU+ (left) or Ki67+ (right) cells in the ASVZ, LSVZ, and RMS of the Cdk2−/− mouse were similar to the wild type. At P90, a significant decrease in BrdU+ (left) and Ki67+ (right) cells was observed in all regions of the Cdk2−/− mouse. Results are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t test (six to eight hemispheres were used for analysis for each age and marker).
Loss of Cdk2 selectively impairs NG2-expressing progenitor cell proliferation in the adult SVZ
In the adult SVZ, slowly dividing (type B) stem cells expressing glial fibrillary acidic protein (GFAP) and vimentin give rise to highly proliferative transient-amplifying (type C) cells, which express Mash1 and Dlx2. These cells differentiate into neuroblast (type A) cells characterized by class III β-tubulin (Tuj1), a polysialylated form of neural cell adhesion molecule, or doublecortin (Dcx) expression (Doetsch et al., 2002). To identify cell populations of the neurogenic niche directly affected by loss of the Cdk2 gene, we performed immunohistochemical analysis using antibodies against GFAP and the proteoglycan NG2 in combination with a variety of other cellular markers. The percentage of GFAP-expressing cells was higher in the LSVZ than in the ASVZ in both P8 and 90 brains of wild-type and Cdk2−/− mice (Fig. S2, J1 and 3, available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1). This finding was consistent with previous analyses demonstrating that most stem cells are born in the LSVZ, where their self-renewal is supported by endothelial cells (Shen et al., 2004). Loss of Cdk2 had no effect on the proliferative potential of GFAP+ cells in the ASVZ and LSVZ of P8 or 90 mice (Fig. S2, J2 and 4), which suggests that Cdk2 activity is either nonessential for slowly dividing type B cells or can be compensated by other Cdks at both ages.
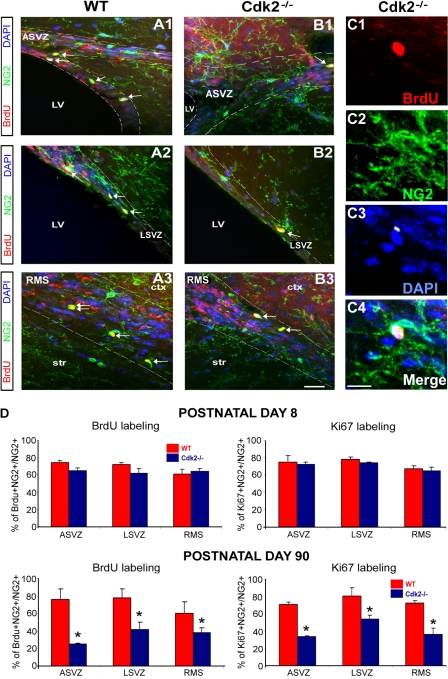
Because anti-GFAP antibodies also label astrocytes, we used nestin as a marker of undifferentiated cells to identify the adult stem cell population. The percentage of GFAP+–nestin+ cells was higher in P90 than in P8 SVZ, confirming that this is a major cell population in the adult SVZ (Fig. S3, I and J, available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1). Interestingly, in both P8 and 90 brains, no significant changes were found in the percentage of GFAP+–nestin+ neural stem cells in all structures (Fig. S3). Conversely, a significant effect on NG2+ progenitor cell proliferation was observed in the absence of Cdk2 (Fig. 2). At P90, NG2+ proliferation was significantly reduced in neurogenic regions of the Cdk2−/− brain (Fig. 2 D), whereas no changes were observed at P8 (Fig. 2 D).
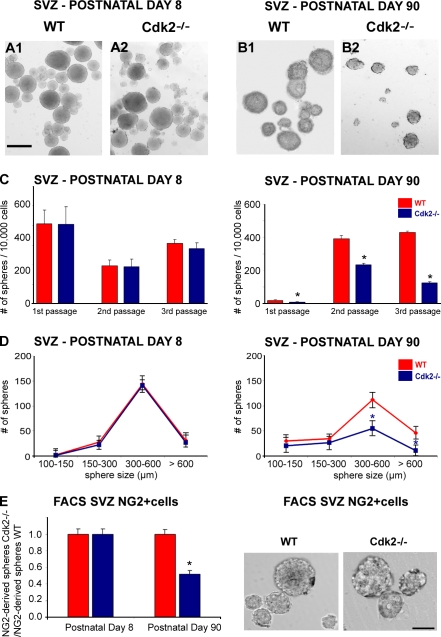
Figure 2.
Decreased proliferation of NG2-expressing progenitors in neurogenic areas of the adult Cdk2−/− mouse. (A–C) Tricolored images show NG2+ progenitor cells (green) in the ASVZ, LSVZ, and RMS (LV, lateral ventricle; ctx, cortex; str, striatum) on sagittal sections obtained from wild-type (A1–3) and Cdk2−/− (B1–3) mice stained with anti-BrdU (red) and DAPI (blue). Arrows indicate proliferating NG2+ progenitors. White dotted lines delineate the ASVZ (A1 and B1), LSVZ (A2 and B2), and RMS (A3 and B3). (C1–4) Cdk2−/− mouse. Magnified view of NG2+ progenitors (C2, anti-NG2) labeled with anti-BrdU (C1) and DAPI (C3). Merged image is shown in C4. (D) Percentages of double-labeled cells. At P8, no differences in the percentage of NG2+–BrdU+ or NG2+–Ki67+ cells were observed in the ASVZ, LSVZ, and RMS between wild-type and Cdk2−/− mice (D, top left and right), whereas at P90 there was a significant reduction in the percentage of proliferating NG2+ cells in the Cdk2−/− brain (D, bottom left and right). Results are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t test (six to eight hemispheres were taken for analysis for each age and marker). Bars: (A and B) 50 μm; (C) 12 μm.
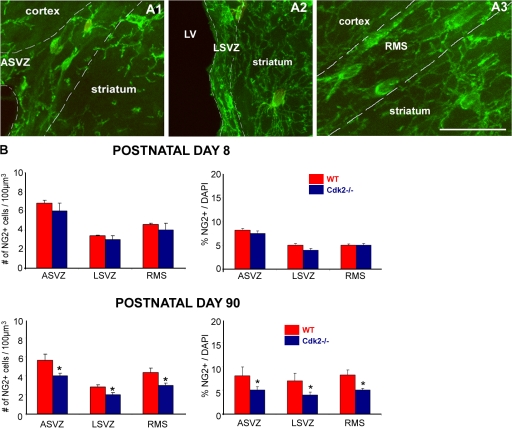
Consistent with these findings, we also observed a significant decrease of NG2+ cell density in the SVZ and RMS of the Cdk2−/− mouse. NG2+ cells were counted in the ASVZ, LSVZ, and RMS using confocal z stack images. In the SVZ and RMS, the typical morphology of migratory NG2+ cells was characterized by an oval cell body and one or two long leading processes (Fig. 3, A1–3). However, in the striatum, NG2+ cells displayed a postmigratory morphology with a larger and more rounded cell body and many branched processes (Fig. 3, A1–3). Two independent counting methods (volume density and cell number relative to the total number of cells) demonstrated that in the adult Cdk2−/− neurogenic brain areas, there was a significant reduction in the density of NG2+ progenitors (Fig. 3 B, bottom). Consistent with the cell proliferation results, no differences in cell density were observed between Cdk2−/− and wild-type brains at P8 (Fig. 3 B, top). Consistent with the cell proliferation and density data, counts of the total number of NG2+ cells in the SVZ also showed a significant decrease at P90 (wild type, 425 ± 55 cells; Cdk2−/−, 290 ± 36 cells; n = 4 brains; P < 0.04, results were analyzed with a t test) but not at P8 (wild type, 409 ± 50 cells; Cdk2−/−, 400 ± 51 cells; n = 3 brains).
Figure 3.
Decreased density of NG2-expressing progenitors in neurogenic areas of the adult Cdk2−/− mouse. (A) NG2+ progenitors present in the ASVZ, LSVZ, and RMS display a migratory cellular morphology as opposed to those in the striatum. White dotted lines delineate the ASVZ (A1), LSVZ (A2), and RMS (A3). Bar, 100 μm. (B) NG2+ progenitor number was estimated as total number of cells per 100 μm3 throughout the entire depth of the sections (left) and as a percentage of total DAPI-labeled cells (right) using confocal z-stack analysis. At P8, no changes in the density and proportion of NG2+ progenitors between the wild type and Cdk2−/− were observed, whereas a significant reduction of NG2+ cell density and proportion was observed at P90 in the ASVZ, LSVZ, and RMS of the Cdk2−/− mouse. Results are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t test (six to eight hemispheres were taken for analysis for each age and marker).
Finally, we investigated the contribution of apoptosis to the reduction of progenitor cell proliferation in Cdk2−/− mouse brains. Caspase-3+ apoptotic cells were quantified and no differences were observed in cell death between Cdk2−/− and wild-type mice at P8 or 90 (Fig. S4, A–F, available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1). Similarly, no differences were observed in the percentage of TUNEL+ cells at both ages (Fig. S4, G and H).
Collectively, these data indicate that the lack of Cdk2 has important cell- and developmental stage–specific effects in the adult SVZ because (a) it selectively decreases NG2+ cell proliferation without altering GFAP+ stem cell production and (b) it only affects NG2+ cells in the adult brain.
Loss of Cdk2 promotes lineage commitment of NG2-expressing progenitors in the adult brain
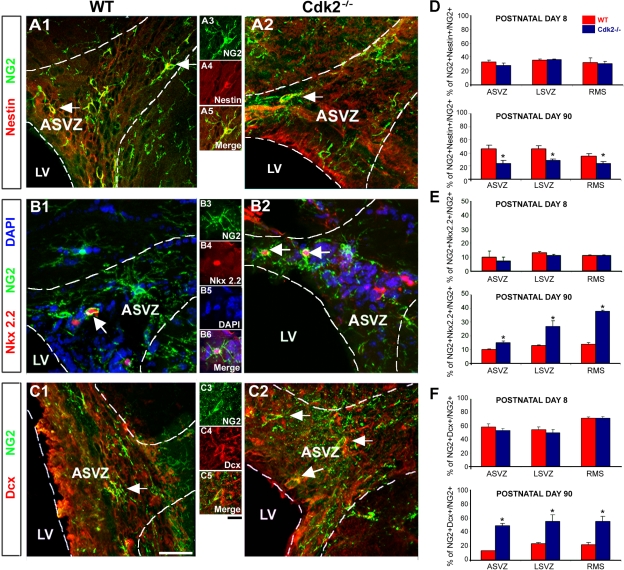
Double immunostaining of NG2+ cells with anti-nestin antibodies indicated that the population of undifferentiated NG2+–nestin+ progenitors decreased by ∼48, 37, and 32%, respectively, in the ASVZ, LSVZ, and RMS of adult Cdk2−/− mice (Fig. 4 D, bottom). In contrast, no changes in the percentage of NG2+–nestin+ cells were observed at P8 (Fig. 4 D, top).
Figure 4.
Cdk2 loss promotes lineage specification of adult NG2+ progenitor cells. (A–C) All micrographs were taken from the ASVZ in wild-type and Cdk2−/− mice at P90. Immunostaining shows undifferentiated NG2+–nestin+ progenitors (A1 and 2 and higher magnification in A3–5), Nkx2.2+ oligodendrocyte progenitors (B1 and 2 and higher magnification in B3–6), and Dcx+ neuroblasts (C1 and 2 and higher magnification in C3–5). White dotted lines delineate the ASVZ. (D–F) Histograms represent percentages of NG2+–nestin+, NG2+–Nkx2.2+, and NG2+–Dcx+ cells calculated from the number of total NG2+ cells. At P8, no changes between wild-type and Cdk2−/− mice were observed in the number of NG2+ undifferentiated cells (D, top) as well as oligodendrocyte progenitors (E, top) and neuroblasts (F, top) within the ASVZ, LSVZ, and RMS. In the P90 Cdk2−/− brains, the pool of undifferentiated NG2+ progenitors was partially depleted in the ASVZ, LSVZ, and RMS (D, bottom), whereas a significant increase was observed in the percentage of committed Nkx2.2+ oligodendrocyte progenitors (E, bottom) and Dcx+ postmitotic neuroblasts (F, bottom). Data are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t test (six to eight hemispheres were taken for analysis for each age and marker). Bars: (A1 and 2, B1 and 2, and C1 and 2) 50 μm; (A3–5, B3–6, and C3–5) 15 μm.
We have previously demonstrated that postnatal SVZ NG2+ progenitors acquire expression of the committed oligodendrocyte and neuronal progenitor markers Nkx2.2 and Dcx, respectively (Aguirre and Gallo, 2004). At P90, we observed an increase in the percentage of NG2+–Nkx2.2+ and NG2+–Dcx+ cells in the ASVZ, LSVZ, and RMS of the Cdk2−/− mouse (P < 0.05, t test; Fig. 4, E and F, bottom). In contrast, NG2+ cell differentiation was not modified by Cdk2 loss at P8 (Fig. 4, E and F, top). No NG2+–GFAP+ cells were detected in the brain areas analyzed at P8 or 90 (unpublished data).
In conclusion, these findings indicate that Cdk2 does not uniformly regulate NG2+ cell differentiation throughout ontogenic development because Cdk2 loss promotes oligodendrocytic and neuronal commitment at P90 but not P8. Consistent with these data, Cdk2 loss causes a significant decrease in the pool of undifferentiated NG2+–nestin+ cells only in the adult SVZ.
Loss of Cdk2 function causes a decrease in self-renewal potential and an increase in the differentiation of adult neural progenitor cells
SVZ NG2+ cells are able to form neurospheres (Aguirre et al., 2004; Aguirre and Gallo, 2004) and differentiate into neurons, oligodendrocytes, and astrocytes in culture (Belachew et al., 2002). To correlate the selective decrease of adult NG2+ cell proliferation in Cdk2−/− SVZ in vivo with a functional in vitro assessment of their self-renewal potential, we performed neurosphere formation assays using Cdk2−/− and wild-type SVZ cells at P8 and 90. In these experiments and in the experiments shown in Figs. 5–10 , we used total SVZ cells or tissue for our biochemical and cellular analysis because of the limitations imposed by the system (i.e., number of cells in the SVZ) and the use of Cdk2−/− mice (the limited number of Cdk2−/− mice per litter). Furthermore, rapidly dividing NG2+ progenitor cells represent the largest progenitor cell population in the postnatal and adult SVZ (Aguirre et al., 2004; Aguirre and Gallo, 2004), and GFAP+ type B cells are not affected by Cdk2 loss; therefore, our findings in total SVZ cells relate to the NG2+ cell population.
Figure 5.
The absence of Cdk2 selectively impairs neurosphere formation in adult Cdk2−/−cultures. (A and B) SVZ neurospheres were obtained from single cell suspensions from wild-type and Cdk2−/− mice at P8 (A1 and 2) and P90 (B1 and 2). Bar, 500 μm. (C) To assess self-renewal potential, neurosphere numbers were counted after the first, second, and third passage, respectively. No changes in the number of growing neurospheres were observed in P8 cultures (C, left), whereas a significant decrease of neurosphere growth was observed in P90 Cdk2−/− cultures after each passage (C, right). Data were obtained from three independent experiments and are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t test. (D) For size analysis, only neurospheres within the range of 100–600 μm were counted. The number of neurospheres bigger than 300 μm was significantly reduced in P90 Cdk2−/− cultures (D, right) but was unaffected in P8 Cdk2−/− cultures (D, left). *, P < 0.05, results were analyzed by a t test. (E) Neurosphere formation from FACS-purified NG2+ SVZ cells (wild type and Cdk2−/−) at P8 and 90. (E, left) At P8, no differences were found in the number of NG2+ cell–derived neurospheres between Cdk2−/− and wild-type cells. Conversely, a significant decrease in the number of neurospheres was found in FACS-sorted NG2+ Cdk2−/− cells at P90 as compared with the wild type. Data were obtained from three independent experiments and are expressed as a ratio between Cdk2−/− and wild-type NG2+ cell–derived neurospheres. All data are expressed as means ± the SEM. *, P < 0.005; results were analyzed by a t test. (E, right) Images of wild-type and Cdk2−/− neurospheres from FACS-sorted P90 NG2+ cells. Bar, 250 μm.
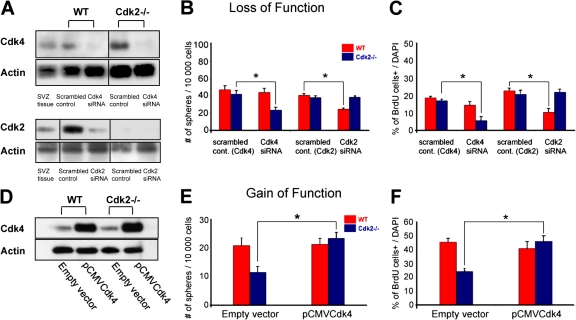
Figure 10.
siRNA-induced knockdown of Cdk4 and 2 inhibits proliferation in P8 Cdk2−/− and wild-type SVZ cell cultures, respectively. (A–C) Loss- of-function experiments in perinatal SVZ cells. Cells from P8 brains were transfected with scrambled (control), Cdk4-silencing, or Cdk2-silencing siRNA and harvested 24 h after transfection. (A) Cell and SVZ tissue lysates were immunoblotted with anti-Cdk4 and -Cdk2 antibodies and band intensities from siRNA-treated cells were measured and normalized to actin. In both wild-type and Cdk2−/− cells, a reduction of 50–70% in Cdk4 levels was obtained after siRNA transfection. Wild-type P8 SVZ tissue lysate was also used as a positive control for Cdk4 expression. Transfection with Cdk2 siRNA decreased Cdk2 expression in wild-type SVZ cells by ∼50% but did not modify Cdk2 expression in Cdk2−/− P8 SVZ cells. Wild-type P8 SVZ tissue lysate was also used as a positive control for Cdk2 expression. Black lines indicate that the intervening lanes have been spliced out. (B) Transfection of Cdk2−/− cells with scrambled siRNA control did not modify neurosphere formation but Cdk4 siRNA caused a significant decrease in the number of neurospheres. No effect was observed in wild-type cultures. Transfection with Cdk2 siRNA reduced the number of neurospheres in wild-type cultures as compared with treatment with scrambled control. No effect was observed in Cdk2−/− cells. Data were obtained from second passage neurospheres of three independent experiments. (C) BrdU immunolabeling showed that neural progenitor cell proliferation was impaired in Cdk2−/− cultures after Cdk4 siRNA treatment but not in wild-type cultures. After Cdk2 siRNA transfection, proliferation was impaired in wild-type but not Cdk2−/− cultures. Data represent BrdU+ cells as percentages of total DAPI-labeled cells and were obtained from three separate experiments (three independent cell cultures). The total number of cells counted was 454 (scrambled control Cdk4), 782 (scrambled control Cdk2), 458 (Cdk4 siRNA), and 636 (Cdk2 siRNA) for the wild type; and 647 (scrambled control Cdk4), 765 (scrambled control Cdk2), 624 (Cdk4 siRNA), and 643 (Cdk2 siRNA) for Cdk2−/−. (D–F) Gain-of-function experiments in adult SVZ cells. Plasmid pCMV-Cdk4 and empty vector were transfected to P90 wild-type and Cdk2−/− cells. (D) Western blot analysis shows higher Cdk4 expression in both wild-type and Cdk2−/− SVZ cells after transfection with pCMV-Cdk4 as compared with transfection with an empty vector. (E) Transfection of Cdk2−/− cells with pCMV-Cdk4 caused a significant increase in the number of neurospheres compared with wild-type cells. Data were obtained from second passage neurospheres of three independent experiments. (F) BrdU incorporation assays. Cdk4 overexpression did not modify cell proliferation in wild-type cells but greatly increased cell proliferation in Cdk2−/− cells to levels similar to the wild type. Data were obtained from three independent experiments. The total number of cells counted was 511 and 451 for the empty vector (wild type and Cdk2−/−, respectively) and 425 and 608 for pCMV-Cdk4 (wild type and Cdk2−/−, respectively). Data are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t-test.
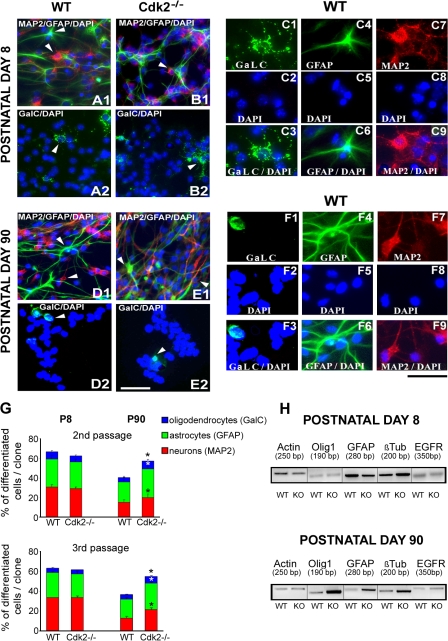
Figure 6.
Cell differentiation of SVZ progenitors is enhanced by loss of Cdk2. (A–F) Cells plated from SVZ neurospheres obtained from wild-type (A1 and 2 and D1 and 2) and Cdk2−/− (B1 and 2 and E1 and 2) mice differentiated into oligodendrocytes, astrocytes, and neurons as stained with antibodies against GalC, GFAP, and MAP2, respectively. Higher magnification images show the individual cell types at P8 (C1–9) and P90 (F1–9). (G) Comparable percentages of neurons, astrocytes, and oligodendrocytes were obtained from Cdk2−/− and wild-type neurospheres at P8 after second and third passages, but significantly higher percentages of all differentiated cell phenotypes were found in Cdk2−/− cells at P90. Data are expressed as means ± the SEM. *, P < 0.05; results were analyzed using a t test. (H) Semiquantitative RT-PCR analysis confirmed immunolabeling of cells and showed increased levels of oligodendrocyte transcription factor 1 (Olig1), GFAP, and Tubb3 gene expression in P90 Cdk2−/− cells. At P8, no differences in gene expression were observed. Actin and EGFR were used as internal positive controls. Bars: (A, B, D, and E) 50 μm; (C and F) 25 μm.
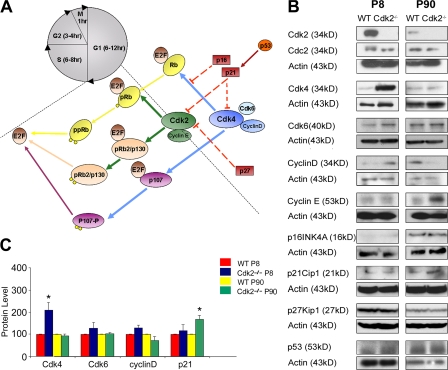
Figure 7.
Expression of cell cycle–related proteins is modified in the Cdk2−/− SVZ. (A) Schematic drawing of the major components of the Cdk2 and Cdk4/6 pathways. (B) Western blot analysis shows that at P8, Cdk4, Cdk6, and cyclin D protein levels were elevated in Cdk2−/− SVZ as compared with the wild type. At P90, only p21Cip1 and cyclin E expression were higher in Cdk2−/− than in wild-type SVZ. Actin was used as a loading control. (C) At P8, significant differences were found only for Cdk4 expression, whereas differences in Cdk6 and cyclin D were not significant. At P90, the increase in p21Cip1 expression was significant. Data are expressed as means ± the SEM. *, P < 0.001; results were analyzed by a t test. Each histogram was obtained from the independent Western blot analysis of three to four SVZs.
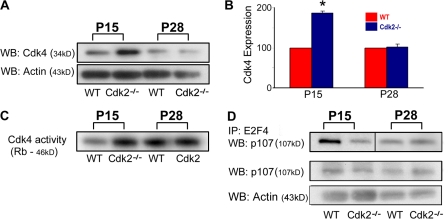
Figure 8.
Up-regulation of Cdk4 expression and activity in the Cdk2−/− SVZ decline to adult levels between P15 and 28. (A and B) SVZ tissue lysates were immunoblotted with anti-Cdk4 antibodies and band intensities were measured and normalized to actin. Note that the increase in Cdk4 expression in Cdk2−/− tissue is developmentally regulated and was lost at P28. Each histogram was obtained from an independent Western blot analysis of three to four SVZs. *, P < 0.05; results were analyzed by a t test. (C) Cdk4 activity was up-regulated in Cdk2−/− SVZ at P15 but not P28. Cdk4 activity was measured using glutathione S-transferase Rb protein as a substrate. (D) At P15, E2F4-bound p107 was lower in Cdk2−/− than in wild-type SVZ, which indicates enhanced activation of the Cdk4 pathway. Conversely, at P28, E2F4-bound p107 was similar in Cdk2−/− and wild-type SVZ. E2F4-bound p107 was coimmunoprecipitated with anti-E2F4 antibodies and probed on Western blot with anti-p107 antibodies. Importantly, p107 expression levels did not change at either age in Cdk2−/− versus wild-type SVZ as shown by Western blot with anti-p107 antibodies.
Figure 9.
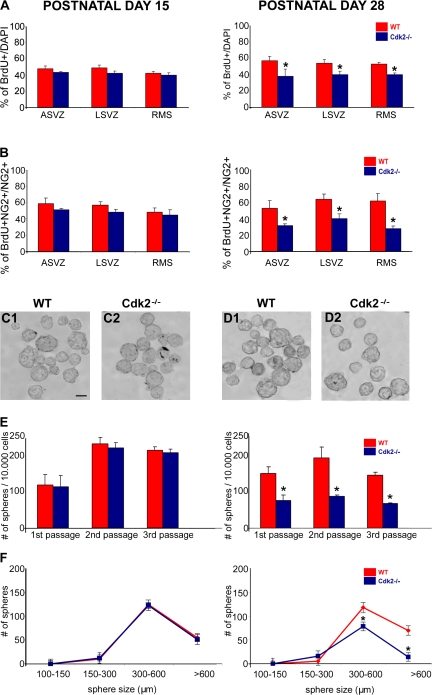
Reduction of Cdk4 levels in Cdk2−/− SVZ during postnatal development correlates with a decrease in neural progenitor cell proliferation and self-renewal. (A) BrdU immunolabeling shows that proliferation of total SVZ cells is similar in Cdk2−/− and wild-type brains at P15 (A, left), whereas cell proliferation is significantly decreased in the Cdk2−/− SVZ at P28 (A, right). Similar results were obtained by analyzing NG2+ progenitors at P15 (B, left) and P28 (B, right). Data represent BrdU+ cells as percentages of total DAPI-labeled cells (A1 and 2) or total NG2+ cells (B1 and 2). Data were obtained from at least three separate brains. *, P < 0.001; results were analyzed by a t test. (C–E) Neurosphere numbers were counted after the first, second, and third passage, respectively. No changes in the number of neurospheres were observed in P15 cultures (C1 and 2 and E, left), whereas a significant decreased was observed in P28 Cdk2−/− cultures (D1 and 2 and E, right). Bar, 500 μm. Data were obtained from three independent experiments. (F) For size analysis, only neurospheres within the range of 100–600 μm were counted. The number of neurospheres bigger than 300 μm was significantly reduced in P28 Cdk2−/− cultures but was unaffected in P15 Cdk2−/− cultures. All data are expressed as means ± the SEM. *, P < 0.05; results were analyzed by a t test.
The number of neurospheres obtained from Cdk2−/− and wild-type SVZ cells was determined after three successive passages during a total time window of 21 d in vitro. At P8, the number of growing neurospheres per 10,000 seeded cells was similar in wild-type and Cdk2−/− mice during all passages (mean number of cells ± the SEM; Fig. 5 C, left), which indicates that SVZ cell self-renewal potential was not modified by Cdk2 loss. The first passage of P90 cultures showed a much lower number of neurospheres as compared with P8. This was likely caused by the lower percentage of neurosphere-forming cells in the adult SVZ as compared with earlier developmental stages. In P90 cells, the number of neurospheres greatly increased between the first and second passages (Fig. 5 C, right), most likely because of the selective survival of highly proliferative cells after the first passage.
Consistent with the data obtained in situ, loss of Cdk2 caused a significant decrease in neurosphere formation in P90 cultures (mean number of cells ± the SEM; Fig. 5 C, right), which demonstrates that adult SVZ cell self-renewal potential was significantly impaired by the loss of Cdk2. Neurosphere size analysis indicated a slight reduction in the number of adult Cdk2−/− spheres within the range of 100–300 μm in diameter and a significant decrease in the number of spheres within the 300–600-μm range and >600 μm (percentage of decrease: 0–100 μm, 6%; 100–150 μm, 23%; 150–300 μm, 95%; 300–600 μm, 97%; Fig. 5 D, right). Conversely, in P8 cultures, the size of neurospheres was not affected by the loss of Cdk2 (Fig. 5 D, left). These results were confirmed in neurospheres obtained from NG2+ cells that were FACS purified from P8 and 90 SVZ tissues (Fig. 5 E).
We investigated whether the absence of Cdk2 also regulates the multilineage potential of neurospheres and performed clonal analysis on neurospheres differentiated in culture for 7 d (Fig. 6). Among the progeny of Cdk2−/− neurospheres obtained from P90 mice, we observed a significant overall increase of differentiated cell phenotypes, including microtubule-associated protein 2+ (MAP2+) neurons, galactocerebroside+ (GalC+) oligodendrocytes, and GFAP+ astrocytes (Fig. 6 G). Conversely, similar percentages of all differentiated cell types were found in the second and third passages of P8 Cdk2−/− and wild-type cultures (Fig. 6 G). Semiquantitative RT-PCR analysis demonstrated an increase in oligodendrocyte transcription factor 1 (Olig1), GFAP, and Tubb3 gene expression in P90 but not P8 Cdk2−/− neurospheres as compared with wild-type cells (Fig. 6 H). Altogether, these in vitro data confirmed our results in situ and strongly support the idea that, in the adult brain, Cdk2 plays a critical role not only in SVZ cell proliferation but also in neural progenitor self-renewal potential and differentiation.
Cdk4 transiently compensates for Cdk2 loss during early postnatal development of the SVZ
Cell cycle progression is associated with the activity of four Cdks (Cdc2/Cdk1 and Cdk2, 4, and 6). Knockout mouse models have provided important insight into the functional interplay between these Cdks (Berthet et al., 2006). Most of these single knockout models do not exhibit major cell cycle defects, which reveals redundancies and suggests that a single Cdk might be sufficient to drive the cell cycle at least during embryonic development.
To gain further insight into the molecular mechanisms underlying the involvement of Cdk2 in SVZ cell proliferation, we first analyzed expression of (a) Cdc2/Cdk1, Cdk4, and Cdk6; (b) their activating partners cyclin D and E; and (c) the Cdk inhibitors p16Ink4D, p53, p21Cip1, and p27Kip1 in wild-type and Cdk2−/− SVZ protein extracts from P8 and 90 mice. At P8, Western blot analysis showed strong up-regulation of Cdk4 protein levels and kinase activity and a nonsignificant increase of cyclin D and Cdk6 expression in Cdk2−/− SVZ as compared with the wild type (Fig. 7, B and C; and Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1). At P8, a 2.2-fold increase in Cdk4 activity was observed (Fig. S5).
Conversely, at P90, the levels of all Cdks analyzed and Cdk4 activity were similar in Cdk2−/− and wild-type SVZ tissue extracts but an elevation of p21Cip1 expression was detected in Cdk2−/− SVZ (Fig. 7, B and C; and Fig. S5). At this age, a nonsignificant decrease in cyclin D and a nonsignificant increase in cyclin E were also observed (Fig. 7 B). These findings suggest that the elevation of Cdk4 levels and activity observed at P8 might compensate for the loss of Cdk2 and contribute to the maintenance of similar proliferation in Cdk2−/− and wild-type SVZ cells.
A developmental time course revealed that the loss of Cdk4 overexpression in the Cdk2−/− SVZ occurred between P15 and 28 (Fig. 8, A and B) in parallel with a decline of Cdk4 kinase activity (Fig. 8 C). Finally, we also assessed by immunoprecipitation the ability of E2F-4 transcription factors to associate with the Cdk4-activated pRb family member p107 (Fig. 8 D; Beijersbergen et al., 1995). Only the unphosphorylated form of p107 can bind E2F-4 (Beijersbergen et al., 1995); therefore, the analysis of p107–E2F-4 interaction represents an indirect assessment of a Cdk4-activated pathway (Fig. 8 D). A direct comparison between Cdk2−/− and the wild type at P8 and 15 indicated that the levels of p107 associated with E2F-4 were lower in Cdk2−/− than in wild-type SVZ cells, suggesting that Cdk4-mediated phosphorylation of p107 in the Cdk2−/− SVZ at this developmental stage was increased (Figs. 8 D and S5). Conversely, p107 was found to be associated with E2F-4 to a similar extent in Cdk2−/− and wild-type SVZ cells at P28 (Figs. 8 D and S5).
We also wanted to define whether the developmental decline of Cdk4 overexpression that occurred between P15 and 28 in the Cdk2−/− SVZ (Fig. 8) correlated with a decrease in NG2+ cell proliferation and neurosphere formation. At P15, the total number of BrdU+ cells (Fig. 9 A, left) and NG2+ cell proliferation (Fig. 9 B, left) in the ASVZ, LSVZ, and RMS, as well as neurosphere formation (number and size; Fig. 9, C1 and 2, E [left], and F [left]) were undistinguishable in Cdk2−/− and wild-type cells. Conversely, at P28, when Cdk4 expression and activity were similar in the Cdk2−/− and wild-type SVZ (Fig. 8), total cell and NG2+ cell proliferation and neurosphere formation were significantly decreased in Cdk2−/− cells (Fig. 9, A and B and D–F, right).
Altogether, these results point to a possible compensatory role of the Cdk4 pathway, which might be involved in maintaining the appropriate pool of SVZ NG2+ progenitors in the absence of Cdk2 during earlier stages of postnatal development.
Cdk4 loss- and gain-of-function modifies perinatal and adult Cdk2−/− SVZ progenitor cell proliferation and self-renewal, respectively
We predicted that, if Cdk4 compensated for the loss of Cdk2 in P8 SVZ cells, down-regulation of its expression in Cdk2−/− SVZ cells at this developmental stage would cause a decrease in their proliferation. Conversely, Cdk4 overexpression in P90 Cdk2−/− SVZ cells would enhance their proliferative potential.
Loss of function.
We performed siRNA-mediated Cdk4 knockdown in P8 SVZ cultures from wild-type and Cdk2−/− mice. Transfection with Cdk4 siRNA significantly decreased Cdk4 expression levels in both cell types (53% in wild-type and 47% in Cdk2−/− cells; Fig. 10 A). In the same set of experiments, the levels of Cdk4 proteins were unaffected by the scrambled siRNA (Fig. 10 A). Furthermore, as expected, cell transfection with Cdk2 siRNA decreased Cdk2 expression in wild-type cells but had no effect in Cdk2−/− cells (Fig. 10 A).
To study the functional effect of Cdk4 and 2 siRNA on neurosphere formation, we plated transfected SVZ cells from wild-type and Cdk2−/− brains and cultured them in a medium containing mitogenic factors. After 7 d, neurospheres were generated in both scrambled control and Cdk4 and 2 siRNA– transfected cells (Fig. 10 B). Treatment with scrambled Cdk4 and 2 siRNA did not significantly affect neurosphere formation in Cdk2−/− cultures as compared with the wild type (Fig. 10 B). Conversely, transfection of Cdk2−/− cultures with Cdk4 siRNA caused a 58% reduction in the number of neurospheres as compared with scrambled control (Fig. 10 B). Transfection of wild-type cultures with Cdk2 siRNA caused a 40% reduction in neurosphere quantity (Fig. 10 B). In wild-type and in Cdk2−/− cultures, the number of neurospheres was not affected by Cdk4 or 2 silencing, respectively (Fig. 10 B). This strongly suggests that Cdk4 mostly has a compensatory role for Cdk2 function in early postnatal development.
Cell proliferation analysis after Cdk4 siRNA transfection was consistent with the neurosphere formation assays. Percentages of BrdU+ cells in wild-type and Cdk2−/− neurospheres were unaffected after transfection with scrambled Cdk4 and 2 siRNA sequences, respectively (Fig. 10 C). Conversely, Cdk4 siRNA treatment significantly decreased cell proliferation in Cdk2−/− cultures, as shown by the 70% reduction in BrdU+ cells (Fig. 10 C). In wild-type neurospheres, cell proliferation was only slightly decreased by ∼15% (Fig. 10 C), which further supports the idea that SVZ cell proliferation also depends on Cdk4 activity. Finally, as expected, Cdk2 siRNA treatment significantly and selectively decreased cell proliferation in wild-type neurospheres but had no effect in Cdk2−/− cells (Fig. 10 C).
Gain of function.
We overexpressed Cdk4 in P90 Cdk2−/− cells to determine whether increased activity of this kinase would enhance cell proliferation and renewal. pCMV-Cdk4 transfection greatly increased Cdk4 expression in both wild-type and Cdk2−/− SVZ cells as compared with the empty vector (Fig. 10 D). Cdk4 overexpression completely reversed the phenotype in adult Cdk2−/− SVZ cells as demonstrated by neurosphere formation (Fig. 10 E) and cell proliferation (Fig. 10 F) assays. In both sets of experiments, the behavior of Cdk2−/− cells was indistinguishable from their wild-type counterparts, which indicates that restoring Cdk4 expression in P90 Cdk2−/− SVZ cells is sufficient to compensate for Cdk2 loss.
Altogether, these loss- and gain-of-function data confirm that Cdk4 is required for maintaining an appropriate pool of dividing SVZ cells in culture and that the endogenous up-regulation of Cdk4 that occurs in SVZ progenitors at the early postnatal stage can functionally compensate for the loss of Cdk2.
Discussion
The identification of extrinsic and intrinsic factors governing progenitor cell proliferation and differentiation in neurogenic regions of the postnatal brain is crucial to elucidate fundamental mechanisms of postnatal neuro- and gliogenesis. Although our knowledge of extracellular signals that regulate neural progenitor cell division is quite extensive, the specific role of intrinsic regulators is less well defined. In this paper, we took advantage of a viable Cdk2−/− mouse mutant to investigate the functional role of cell cycle regulators in neural progenitor proliferation and differentiation in neurogenic regions of the postnatal brain. Our findings point to Cdk2 as a cell-intrinsic determinant of progenitor cell proliferation and differentiation and indicate that Cdk2 plays a nonredundant role in maintaining the pool of dividing and undifferentiated progenitor cells in the SVZ of the adult brain.
Age- and cell type–specific function of Cdk2 in the SVZ
Previous studies identified NG2-expressing progenitors of the postnatal SVZ as the transient-amplifying type C cells based on their antigenic and cellular properties as well as their lineage potential (Aguirre et al., 2004; Parras et al., 2004; Menn et al., 2006). The NG2-expressing cell population is highly proliferative, can form neurospheres in culture, and can generate both neurons and oligodendrocytes (Aguirre et al., 2004; Aguirre and Gallo, 2004). In this paper, we show that NG2+ cell proliferation is selectively impaired in the adult SVZ of mice lacking Cdk2. Importantly, loss of Cdk2 did not affect proliferation of slowly dividing GFAP+–nestin+ stem cells, indicating that intrinsic cell cycle regulators play distinct roles in different cell populations of the adult SVZ. Analysis in cultures obtained from adult SVZ cells is consistent with the in vivo findings, as a significant decrease in the formation of neurospheres was also observed in Cdk2−/− cells. Our results place Cdk2 among previously described cell type–specific modulators that regulate SVZ progenitor proliferation, such as p27Kip1 or p19INK4d (Casaccia-Bonnefil et al., 1999; Coskun and Luskin, 2001; Doetsch et al., 2002; Li et al., 2006). Moreover, it appears that Cdk2 might play a prominent role in adult neural progenitors because loss of this kinase did not affect proliferation of embryonic fibroblasts or human colon cancer cell lines in culture (Berthet et al., 2003; Ortega et al., 2003; Tetsu and McCormick, 2003).
The specific mechanism by which Cdk2 participates in the regulation of NG2+ progenitor cell proliferation is still undefined. Deregulation of Cdk2 activity is often associated with changes in the rate of entry into S phase (Duronio et al., 1996; Hua et al., 1997; Krude et al., 1997). Previous analysis demonstrated that the absence of Cdk2 significantly delays the entry of synchronized fibroblasts into S phase (Berthet et al., 2003); therefore, it is likely that Cdk2 might also be involved in the regulation of S phase entry in NG2+ progenitors. Although our data do not directly rule out other possible mechanisms, it is likely that loss of Cdk2 might be linked with changes in the length of G1 phase and therefore in the overall cell cycle duration.
At variance with our findings in the adult SVZ, NG2+ cell proliferation was not modified by the loss of the Cdk2 gene during the first two postnatal weeks. In vivo and in vitro analysis demonstrated that both NG2+ cell proliferation and self-renewal capacities were not affected in the Cdk2−/− mouse up to P15. These findings not only revealed a developmentally regulated requirement for Cdk2 function in progenitor cells but also raised the important question of possible molecular differences in the expression and function of other cell cycle regulatory proteins in NG2+ progenitors of the juvenile and adult brain.
Compensation of Cdk2 loss by Cdk4 in SVZ progenitors is developmentally regulated
Western blot analysis showed that within a specific set of cell cycle proteins, only Cdk4 was significantly up-regulated in the SVZ of the Cdk2−/− mouse at P8 as compared with the wild type. No differences in Cdk4 expression and kinase activity could be detected at P90. The time course of Cdk4 up-regulation observed during postnatal development of the Cdk2−/− mouse correlated with the cell proliferation and self-renewal rate of SVZ progenitors. Elevated levels of Cdk4 protein expression and activity in Cdk2−/− tissue were observed until P15 and then subsequently declined at P28 and 90, when no differences in Cdk4 expression were observed. In the P8 and 15 Cdk2−/− SVZ, a decreased level of p107 associated with E2F-4 also reflected higher Cdk4 kinase activity but no differences in the p107–E2F-4 complex were observed at P28 and 90. Consistent with this, NG2+ cell proliferation and neurosphere formation were indistinguishable in Cdk2−/− and wild-type mice up to P15 but were drastically reduced at P28 and 90.
To ascertain that the Cdk4 pathway could be engaged in compensating for the loss of Cdk2 in SVZ NG2+ cells during the first two postnatal weeks (Zezula et al., 2001), we used a loss- and gain-of-function approach. First, we directly prevented Cdk4 up-regulation in cells isolated from the SVZ of Cdk2−/− early postnatal brains. siRNA-mediated knockdown of Cdk4 in neurospheres from P8 Cdk2−/− mice drastically decreased Cdk4 expression levels and reduced both SVZ progenitor cell proliferation and neurosphere formation. Second, we overexpressed Cdk4 in adult SVZ cells to compensate for its developmental loss. Under these conditions, both neurosphere numbers and cell proliferation were completely restored to wild-type levels.
In conclusion, these results indicate that Cdk4 plays an important role as a regulatory protein in compensatory mechanisms active during the first two postnatal weeks of the Cdk2−/− mouse lifespan. We propose that these compensatory mechanisms sustain NG2+ cell proliferation and self-renewal in the SVZ during the first two postnatal weeks and preserve the pool of these progenitors in neurogenic areas of the early postnatal brain.
Both Cdk2 and 4 are activated by their regulatory proteins cyclin E and D to sequentially phosphorylate Rb (Mittnacht, 1998; Knudsen and Knudsen, 2006). Rb hyperphosphorylation causes dissociation of E2F transcription factors, which in turn activate S phase gene transcription, leading to G1/S cell cycle progression. Impaired neurogenesis was observed in adult mice lacking E2F-1 (Cooper-Kuhn et al., 2002). Because both Cdk2 and 4 are needed for Rb activation, the question of the sequence of the molecular events that maintain NG2+ cell proliferation in the Cdk2 null mice during the first two postnatal weeks arises. The Rb-like protein p107 also binds the E2F family of transcription factors and is phosphorylated by Cdk4 but not by Cdk2 (Beijersbergen et al., 1995). Therefore, we propose that an alternative cell cycle pathway active in Cdk2 null mice involves both Cdk4 and p107. Future experiments in cells isolated from the Cdk2−/− mouse SVZ will define the specific involvement of p107 phosphorylation in this alternative pathway.
Cdk2 involvement in neural lineage commitment
The decrease in NG2+ cell proliferation observed in the adult SVZ may result from a shift in balance between cell proliferation and differentiation and/or cell death. Previous studies demonstrated that Cdks as well as their inhibitors belonging to the Cip1/Kip1 and INK families play an essential role in regulating cell cycle and survival in neural tissue (Coqueret, 2003). It was shown that the loss of p27Kip1 up-regulates proliferation of transient-amplifying progenitors in the SVZ concomitantly with a reduction in the number of neuroblasts caused by extensive apoptosis (Doetsch et al., 2002). Our results indicate that the loss of Cdk2 did not affect the number of c-Caspase3+ cells during development; therefore, we exclude the possibility that apoptosis may have a significant impact on the regulation of the NG2+ cell number in Cdk2−/− mice.
We have previously demonstrated that NG2+ progenitors are multipotential and generate oligodendrocytes and γ-aminobutric acid–ergic interneurons (Belachew et al., 2003; Aguirre et al., 2004; Aguirre and Gallo, 2004; Menn et al., 2006). Consistent with these findings, we observed newly generated NG2+–Nkx2.2+ committed oligodendrocytes and NG2+–Dcx+ committed neuroblasts originating from the ASVZ and migrating along the RMS in juvenile and adult brains. Cellular and molecular analyses in vivo and in vitro demonstrate that the loss of Cdk2 promotes NG2+ cell lineage commitment and differentiation to oligodendrocytes and neurons in the adult SVZ. In the adult Cdk2−/− but not early postnatal brain, we found an increased number of fate-determined NG2+ cells. Consistently, only in Cdk2−/− neurospheres isolated from the adult SVZ did we observe elevated expression of mRNA encoding for cell type–specific markers as well as an increased percentage of differentiated neurons, oligodendrocyes, and astrocytes. These findings strongly support a correlation between the reduction in the number of uncommitted proliferating NG2+ cells and the increase in differentiated phenotypes observed in the SVZ and RMS of the Cdk2−/− brain.
It is known that neural differentiation is controlled by Cdk inhibitors, which block the activity of cyclin–Cdk complexes (Zindy et al., 1997; Levine et al., 2000; Coskun and Luskin, 2001; Ohnuma et al., 2001; Zezula et al., 2001). A previous study indicated that p21Cip1 acts as a positive regulator of S phase progression by supporting the formation of the Cdk4–cyclin D1 complex (LaBaer et al., 1997), whereas other studies have shown that p21Cip1 is required for oligodendrocyte differentiation (Zezula et al., 2001). p21Cip1 also promotes cell differentiation in the immune system by interaction with multiple regulatory proteins, including the transcription factors Stat3 and CCAAT/enhancer binding protein (Steinman, 2002). Our data demonstrate an elevated expression of p21Cip1 in adult Cdk2−/− SVZ tissue in parallel with enhanced NG2+ cell differentiation at this age, which suggests that p21Cip1 might be involved in this effect.
It is known that p21Cip1 can cooperate with other Cdk inhibitors, including p27Kip1, involved in neuronal differentiation and fate specification of glial cells (Durand et al., 1998; Zezula et al., 2001). p27Kip1 promotes neuronal differentiation of cortical progenitors by stabilizing neurogenin 2 protein (Nguyen et al., 2006). However, p27Kip1 expression was not modified in adult Cdk2−/− SVZ, which indicates that this protein is unlikely to be implicated in SVZ cell differentiation in Cdk2−/− mice.
Conclusions
Our paper identifies Cdk2 as an important gene for neural progenitor cell proliferation and self-renewal in a major neurogenic region of the adult brain, the SVZ. Together with other intrinsic regulators, including p16INK4a (Molofsky et al., 2006), both down-regulation of Cdk2 in adult tissue and the decline of a Cdk4-dependent compensatory mechanism appear to participate in the progressive decrease of multipotent progenitor cell proliferation and self-renewal potential with aging. It is likely that the reduced regenerative capacities that characterize aging SVZ progenitors may at least in part be caused by the progressive Cdk2-dependent depletion of the NG2+ progenitor pool. The elucidation of the critical molecular cues that control proliferation, self-renewal, and differentiation of endogenous adult neural progenitor cells may open new avenues for the development of neuronal replacement therapies for neurodegenerative diseases and other central nervous system injuries that require manipulation of endogenous progenitors or transplantation of exogenous cells.
Materials and methods
Animals
The colony of Cdk2−/− null mice (Berthet et al., 2003) was maintained in the animal facility of the Children's National Medical Center according to the Institutional Animal Care and Use Committee and National Institutes of Health guidelines. Cdk2−/− mice are viable and develop normally without any anatomical or histological malformations (Berthet et al., 2003). To breed Cdk2−/− mutants, we crossed two heterozygotes because previous data showed that Cdk2−/− male and female individuals are sterile. After birth, the newborns were genotyped according to the procedure established by Berthet et al. (2003) by PCR using three primers described in the supplemental Materials and methods (available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1). DNA products were loaded onto 2% agarose gels to resolve the specific bands: 150 bp for the wild type, 500 bp for Cdk2−/−, and both bands for heterozygotes. Heterozygote Cdk2+/− mice were not analyzed in this paper.
BrdU incorporation and labeling
Proliferating cells were labeled by intraperitoneal BrdU (Sigma-Aldrich) injections. P8 mice were injected with 100 μg/g BrdU once 2 h before being killed, whereas in adult mice (P90), 100 μg/g BrdU was given twice within 24 h before killing. These two different protocols of BrdU administration were based on previously described changes in the length of cell cycle of dividing cells throughout the lifespan (Takahashi et al., 1993; Smith and Luskin, 1998; Caviness et al., 2003). After BrdU injections, animals were anesthetized with isoflurane and transcardially perfused with 0.1 M PBS, pH 7.4, followed by 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde overnight. Serial coronal and sagittal sections (50 μm) were cut using a microtome (American Optical), collected in PBS, pH 7.4, and stored at 4°C until use. For BrdU labeling, the tissue was pretreated with 2 N HCl and neutralized in 0.1 M boric acid, pH 8.5. After washing, sections were incubated with primary antibody (1:50 anti-BrdU; BD Biosciences) overnight and then with the secondary antibody (1:200 TRITC-conjugated AffiniPure goat anti–mouse; Jackson ImmunoResearch Laboratories) for 1 h. After washing in PBS, pH 7.4, sections were mounted and analyzed by confocal microscopy (BX60; Olympus). Cells were scored as BrdU+ only when strong immunoreactivity was clearly detected specifically in the nucleus (Figs. 2 C1 and S2).
Immunocytochemistry and cell counting
Immunocytochemistry was performed on floating sections using antibodies against the following antigens: NG2, nestin, Dcx, GFAP (all obtained from Chemicon), BrdU (Becton Dickinson), Ki67 (NovoCastra), Nkx2.2 (provided by T. Jessell, Columbia University, New York, NY), and c-Caspase (Cell Signaling Technology). All antibodies dilutions were performed as described previously (Aguirre et al., 2005). Sections were incubated overnight at 4°C in primary antibodies diluted in 0.1 M PBS, pH 7.4, containing 0.1% Triton X-100 and 5% normal goat serum. Appropriate secondary antibodies were used as follows: TRITC-conjugated AffiniPure goat anti–mouse IgG (H + L), FITC-conjugated AffinitiPure goat anti–rabbit IgG, and TRITC-conjugated AffiniPure goat anti–mouse IgM (Jackson ImmunoResearch Laboratories). Sections were incubated with the secondary antibodies for 1 h at room temperature and then mounted. For TUNEL staining, an apoptosis detection kit (Roche) was used according to the manufacturer's instructions. Z stacks of 1-μm-thick single-plane images of the entire thickness of a slice were captured using a confocal microscope (MRC1024; Bio-Rad Laboratories) and collapsed before cell counting. Measurements were taken from at least 7–12 tissue sections obtained from three to four mice in each group. The results are presented as a mean ± the SEM and a t test was performed to establish statistical significance.
Analysis of FACS-purified NG2+ cells
To culture a pure NG2+ progenitor cell population, SVZs were dissected from wild-type and Cdk2−/− mice at P8 and 90. Isolated cell suspensions (see Cell cultures and clonal analysis) were incubated with anti-NG2 antibody (1:1,000; Chemicon) for 1 h at 4°C. Cells were washed twice with DME/F12 medium supplemented with 1% N2 and 1% B27 and incubated with R-phycoerythrin antibody (1:5,000; CALTAG Laboratories) for 20 min at 4°C. NG2-labeled cells were FACS purified as described previously (Influx; Cytopeia; Aguirre et al., 2005). After washing with DME/F12 medium, purified NG2+ cells were plated on 24-well dishes coated with poly-lysine at the density of 10 cells per microliter and cultured in stem cell medium (SCM; StemCell Technologies, Inc.) containing 20 ng/ml EGF and 10 ng/ml bFGF (Millipore). After 7 d, the numbers of growing neurospheres were counted in each dish.
Western blots, immunoprecipitation, and kinase activity assays
SVZs were homogenized in RIPA lysis buffer with proteinase inhibitors (Santa Cruz Biotechnology, Inc.). Protein extracts were boiled for 5 min before loading onto 4–20% gradient gels (20 μg of protein per each lane; GeneMate). Gels were electrotransferred to a 0.2-μm nitrocellulose membrane (Millipore). Blots were blocked in 5% milk in TBST for 1 h and incubated at 4°C overnight with one of the following antibodies: anti-Cdk2, -Cdk4, -Cdk6, -Cdc2, –cyclin D, –cyclin E, -p21CIP1, -p27KIP1 (Santa Cruz Biotechnology, Inc.), -p16INK4A, -p53 (Cell Signaling Technology), and -actin (Chemicon). Bands were detected with appropriate HRP-conjugated secondary antibodies, reacted with chemiluminescent ECL substrate (GE Healthcare), and visualized by exposure to x rays. Band intensity was measured using the ImageJ program (National Institutes of Health). Western blots were obtained from the SVZ of three to four different animals in each group and age. Data were averaged and were represented as means ± the SEM.
For immunoprecipitation, SVZ tissue extracts from wild-type and Cdk2−/− mice were prepared in RIPA buffer containing 2% Triton X-100 and 0.2% SDS. Aliquots (270 μg of tissue) were incubated overnight with antibodies against E2F4 (C-20; Santa Cruz Biotechnology, Inc.) and 15 μl of agarose A (Santa Cruz Biotechnology, Inc.). Immunocomplexes bound to agarose A were collected by centrifugation and washed twice in 500 μl RIPA buffer containing inhibitors. Precipitated proteins were analyzed by immunoblotting with anti-p107 antibody (Sigma-Aldrich). Bands were detected by using HRP-labeled polyclonal anti-mouse Ig (BD Biosciences) and developed with a chemiluminescent substrate (ECL; GE Healthcare). To measure Cdk4 activity, SVZ tissues were prepared as described in the previous paragraph. Protein extracts (350 μg) were immunoprecipitated with a rabbit polyclonal antibody against Cdk4 (Santa Cruz Biotechnology, Inc.) for 30 min at 37°C plus 18 μl of protein A–agarose (Santa Cruz Biotechnology, Inc.). The beads were washed twice with 500 μl of kinase buffer containing 1 mM DTT, 7.5 mM MgCl2, and 10 mM Tris, pH 7.4. 20-μl kinase reaction mixtures contained 5 μg of glutathione S-transferase–Rb (Santa Cruz Biotechnology, Inc.) as a substrate, 30 μM ATP, and 5 μCi [32P]ATP (PerkinElmer). Reactions were stopped by adding 20 μl of 2% SDS loading buffer and heating at 95°C for 3 min. Labeled proteins were resolved on 10% mini-SDS polyacrylamide gels. Phosphorylated pRb band was visualized and quantitated by PhosphorImager (Molecular Dynamics).
RT-PCR
cDNA was first synthesized from 1.5–2 μg of total RNA extracted from Cdk2−/− and wild-type differentiated neurospheres from P8 and 90 mice in a total volume of 11 μl, including 10 mM dNTPmix and 0.5 μg/μl Oligo dT (Invitrogen). Reaction mixtures were heated at 65°C for 5 min and then at 42°C for 50 min. From 2 μl of cDNA, sequences of interest were amplified in a thermocycler (Bio-Rad Laboratories) in a total volume of 25 μl of mixture with tag polymerase (Eppendorf). Primer pairs are described in the supplemental Materials and methods. PCR products were resolved by vertical electrophoresis on 2% agarose gels. The intensity of the bands was measured using ImageJ. For the analysis of each protein, three to four sets of second passage neurospheres were used.
Cell cultures and clonal analysis
SVZs were dissected from 300-μm-thick brain sections prepared from P8, 15, 28, and 90 mice and digested for 30 min at 37°C in Hanks' balanced salt solution (Invitrogen) containing 13 U/ml papain (Sigma-Aldrich), 5 U/ml DNase (Sigma-Aldrich), and trypsin (Sigma-Aldrich). SVZ cells were dissociated by trituration and resuspended in Hanks' buffer containing 1 M Hepes (Invitrogen), 15% sucrose, and penicillin/streptavidin. Cells were then plated onto poly-l-lysine–coated dishes at a density of 10 cells per microliter and cultured for 10 d in DME/F-12 medium (Invitrogen) supplemented with 1% N2, 1% B27 (Invitrogen), 20 ng/ml EGF, and 10 ng/ml bFGF (Millipore). Neurospheres obtained from wild-type and Cdk2−/− cells were counted and repassaged for secondary and tertiary neurosphere formation or, for differentiation assays, plated onto laminin-coated dishes (Invitrogen). Equal numbers of cells from Cdk2−/− and wild-type mice were used in all experiments and were cultured under the same conditions. For cell differentiation assays, neurospheres were cultured with 30 ng/ml NT3 and 10 ng/ml BDNF (Millipore). To immunolabel differentiated cells, standard protocols were used (Aguirre et al., 2005) with primary antibodies against GalC, MAP2, and GFAP (Chemicon).
siRNA-induced Cdk4 knockdown in P8 SVZ cells
Cell transfections were performed using the NeuroPORTER transfection reagent (Genlantis) according to the manufacturer's instructions. After SVZ dissection, cells were plated in 12-well cell culture dishes at a density of 50 cells per microliter for 24 h. At the time of transfection, cell cultures were ∼60% confluent. Three different, commercially available siRNA sequences directed toward murine Cdk4 (NM-009870) and Cdk2 (AM16704) were obtained from Ambion. A mixture of all three siRNAs (20 pM each) produced specific knockdown of Cdk4 at 7 h after transfection. In brief, 2 μl of 20 pM of each Cdk4 siRNA solution and 12 μl of the transfection reagent were incubated in 100 μl of OptiMEM medium (Invitrogen) for 20 min to facilitate complex formation. The siRNA transfection mix was added to the cells cultured in 10% FBS. Control consisted of nonspecific siRNA (Silencer negative; Ambion). Cells were transfected for 7 h at 37°C, washed with Hanks' buffer, and cultured in MEM with 10% FBS for an additional 24 h. The medium was then changed to SCM (20 ng/ml EGF and 10 ng/ml FGF). After 24 h, cells were lysed with 0.05% Trypsin-EDTA (Invitrogen) for 5 min and plated at a density of 10 cells per microliter in SCM with growth factors to generate neurospheres. 7 d later, formed spheres were counted in each dish and statistical analysis was performed using a t test. To assess the proliferative potential of cells, BrdU was added to the culture medium at a concentration of 10 μg/ml followed by a 60-min incubation at 37°C. Cells were then fixed in 4% paraformaldehyde and kept in PBS until use. BrdU incorporation was visualized by immunofluorescence using an anti–mouse BrdU antibody and TRITC-conjugated AffiniPure goat anti–mouse IgG. Percentages of BrdU+ cells were quantified in random fields captured under 10× magnification (total of >250 cells) from at least three different samples and subjected to statistical analysis. To demonstrate Cdk4 and 2 knockdown, Western blots analyses were performed on transfected wild-type and Cdk2−/− cells. After transfection, cells were lysed in 50 μl of ice-cold RIPA buffer. Protein samples were prepared and processed as described in the Western blots, immunoprecipitation… section. Membranes were incubated with anti-Cdk4 and -Cdk2 antibodies and results were normalized relatively to actin.
Overexpression of Cdk4 in SVZ cells
To overexpress Cdk4 in adult SVZ cells, we used a pCMV-Cdk4 plasmid (van den Heuvel and Harlow, 1993) and an empty vector as a control. Plasmid constructs were introduced into cultured cells by liposomal transfection (6 h) in 12-well dishes using 1.5 μg DNA and 12 μl of NeuroPORTER in SCM. After transfection, the cells were washed in SCM and plated on poly-lysine coated dishes for culturing (see Cell cultures and clonal analysis). After a 7-d analysis of neurospheres formation, BrdU staining and Western blotting were performed on wild-type and Cdk2−/− transfected cells (as described in the previous paragraph).
Online supplemental material
Fig. S1 shows proliferation of total SVZ and RMS cells and NG2+ progenitor cells after single and double BrdU injection at P90. Fig. S2 shows that loss of Cdk2 does not affect GFAP+ cell proliferation in the SVZ. Fig. S3 shows that loss of Cdk2 does not modify the GFAP+–nestin+ stem cell number in the SVZ. Fig. S4 shows that loss of Cdk2 does not influence cell apoptosis in the SVZ. Fig. S5 shows the developmental time course of Cdk4 activity in the SVZ of the Cdk2−/− mouse. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200702031/DC1.
Supplementary Material
Acknowledgments
We are particularly grateful to Drs. Li-Jin Chew, Josh Corbin, and Tarik Haydar for discussion and critical comments on this manuscript, and to Jodi Becker for help with some of the perfusion experiments. We thank Drs. Anne Baron-Van Evercooren and Brahim Nait-Oumesmar for stimulating discussions. We also thank Dr. Teresa Hawley and Ms. Bhragavi Rajan for their help with FACS purification of NG2-expressing cells.
This paper was supported by the US National Institutes of Health (grant R01NS045702 to V. Gallo), the European Leukodystrophy Association (grant to S. Belachew), and the National Institute of Child Health and Human Development Mental Retardation and Developmental Disabilities Research Center (grant P30HD40677).
Abbreviations used in this paper: ASVZ, anterior SVZ; Dcx, doublecortin; GalC, galactocerebroside; GFAP, glial fibrillary acidic protein; LSVZ, lateral SVZ; MAP2, microtubule-associated protein 2; P, postnatal day; Rb, retinoblastoma protein; RMS, rostral migratory stream; SCM, stem cell medium; SVZ, subventricular zone.
References
- Aguirre, A., and V. Gallo. 2004. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J. Neurosci. 24:10530–10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre, A.A., R. Chittajallu, S. Belachew, and V. Gallo. 2004. NG2-expressing cells in the subventricular zone are type C–like cells and contribute to interneuron generation in the postnatal hippocampus. J. Cell Biol. 165:575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre, A., T.A. Rizvi, N. Ratner, and V. Gallo. 2005. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J. Neurosci. 25:11092–11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton, E.S., H.T. Ghashghaei, J.L. Weber, C. McCann, T.M. Fischer, I.D. Cheung, M. Gassmann, A. Messing, R. Klein, M.H. Schwab, et al. 2004. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat. Neurosci. 7:1319–1328. [DOI] [PubMed] [Google Scholar]
- Beijersbergen, R.L., L. Carlee, R.M. Kerkhoven, and R. Bernards. 1995. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 9:1340–1353. [DOI] [PubMed] [Google Scholar]
- Belachew, S., A.A. Aguirre, H. Wang, F. Vautier, X. Yuan, S. Anderson, M. Kirby, and V. Gallo. 2002. Cyclin-dependent kinase-2 controls oligodendrocyte progenitor cell cycle progression and is downregulated in adult oligodendrocyte progenitors. J. Neurosci. 22:8553–8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew, S., R. Chittajallu, A.A. Aguirre, X. Yuan, M. Kirby, S. Anderson, and V. Gallo. 2003. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J. Cell Biol. 161:169–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 13:1775–1785. [DOI] [PubMed] [Google Scholar]
- Berthet, C., K.D. Klarmann, M.B. Hilton, H.C. Suh, J.R. Keller, H. Kiyokawa, and P. Kaldis. 2006. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev. Cell. 10:563–573. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil, P., R.J. Hardy, K.K. Teng, J.M. Levine, A. Koff, and M.V. Chao. 1999. Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development. 126:4027–4037. [DOI] [PubMed] [Google Scholar]
- Caviness, V.S., Jr., T. Goto, T. Tarui, T. Takahashi, P.G. Bhide, and R.S. Nowakowski. 2003. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb. Cortex. 13:592–598. [DOI] [PubMed] [Google Scholar]
- Conover, J.C., F. Doetsch, J.M. Garcia-Verdugo, N.W. Gale, G.D. Yancopoulos, and A. Alvarez-Buylla. 2000. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 3:1091–1097. [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn, C.M., M. Vroemen, J. Brown, H. Ye, M.A. Thompson, J. Winkler, and H.G. Kuhn. 2002. Impaired adult neurogenesis in mice lacking the transcription factor E2F1. Mol. Cell. Neurosci. 21:312–323. [DOI] [PubMed] [Google Scholar]
- Coqueret, O. 2003. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 13:65–70. [DOI] [PubMed] [Google Scholar]
- Coskun, V., and M.B. Luskin. 2001. The expression pattern of the cell cycle inhibitor p19(INK4d) by progenitor cells of the rat embryonic telencephalon and neonatal anterior subventricular zone. J. Neurosci. 21:3092–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun, V., and M.B. Luskin. 2002. Intrinsic and extrinsic regulation of the proliferation and differentiation of cells in the rodent rostral migratory stream. J. Neurosci. Res. 69:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, M.R., J.M. Levine, and R. Reynolds. 2000. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J. Neurosci. Res. 61:471–479. [DOI] [PubMed] [Google Scholar]
- Doetsch, F., J.M. Verdugo, I. Caille, A. Alvarez-Buylla, M.V. Chao, and P. Casaccia-Bonnefil. 2002. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J. Neurosci. 22:2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand, B., M.L. Fero, J.M. Roberts, and M.C. Raff. 1998. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 8:431–440. [DOI] [PubMed] [Google Scholar]
- Duronio, R.J., A. Brook, N. Dyson, and P.H. O'Farrell. 1996. E2F-induced S phase requires cyclin E. Genes Dev. 10:2505–2513. [DOI] [PubMed] [Google Scholar]
- Ghashghaei, H.T., J. Weber, L. Pevny, R. Schmid, M.H. Schwab, K.C. Lloyd, D.D. Eisenstat, C. Lai, and E.S. Anton. 2006. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc. Natl. Acad. Sci. USA. 103:1930–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani, C., and V. Gallo. 2001. Inhibition of cyclin E-cyclin-dependent kinase 2 complex formation and activity is associated with cell cycle arrest and withdrawal in oligodendrocyte progenitor cells. J. Neurosci. 21:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani, C.A., A.M. Eisen, X. Yuan, R.A. DePinho, C.J. McBain, and V. Gallo. 1999. Neurotransmitter receptor activation triggers p27(Kip1) and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors. Development. 126:1077–1090. [DOI] [PubMed] [Google Scholar]
- Hua, X.H., H. Yan, and J. Newport. 1997. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J. Cell Biol. 137:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, E.S., and K.E. Knudsen. 2006. Retinoblastoma tumor suppressor: where cancer meets the cell cycle. Exp. Biol. Med. (Maywood). 231:1271–1281. [DOI] [PubMed] [Google Scholar]
- Krude, T., M. Jackman, J. Pines, and R.A. Laskey. 1997. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 88:109–119. [DOI] [PubMed] [Google Scholar]
- LaBaer, J., M.D. Garrett, L.F. Stevenson, J.M. Slingerland, C. Sandhu, H.S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847–862. [DOI] [PubMed] [Google Scholar]
- Lai, K., B.K. Kaspar, F.H. Gage, and D.V. Schaffer. 2003. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6:21–27. [DOI] [PubMed] [Google Scholar]
- Leone, D.P., J.B. Relvas, L.S. Campos, S. Hemmi, C. Brakebusch, R. Fassler, C. Ffrench-Constant, and U. Suter. 2005. Regulation of neural progenitor proliferation and survival by beta1 integrins. J. Cell Sci. 118:2589–2599. [DOI] [PubMed] [Google Scholar]
- Levine, E.M., J. Close, M. Fero, A. Ostrovsky, and T.A. Reh. 2000. p27(Kip1) regulates cell cycle withdrawal of late multipotent progenitor cells in the mammalian retina. Dev. Biol. 219:299–314. [DOI] [PubMed] [Google Scholar]
- Li, L., Y. Iwamoto, A. Berezovskaya, and V.A. Boussiotis. 2006. A pathway regulated by cell cycle inhibitor p27(Kip1) and checkpoint inhibitor Smad3 is involved in the induction of T cell tolerance. Nat. Immunol. 7:1157–1165. [DOI] [PubMed] [Google Scholar]
- Lledo, P.M., M. Alonso, and M.S. Grubb. 2006. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7:179–193. [DOI] [PubMed] [Google Scholar]
- Lois, C., and A. Alvarez-Buylla. 1994. Long-distance neuronal migration in the adult mammalian brain. Science. 264:1145–1148. [DOI] [PubMed] [Google Scholar]
- Luskin, M.B. 1993. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 11:173–189. [DOI] [PubMed] [Google Scholar]
- Meng, H., Z. Zhang, R. Zhang, X. Liu, L. Wang, A.M. Robin, and M. Chopp. 2006. Biphasic effects of exogenous VEGF on VEGF expression of adult neural progenitors. Neurosci. Lett. 393:97–101. [DOI] [PubMed] [Google Scholar]
- Menn, B., J.M. Garcia-Verdugo, C. Yaschine, O. Gonzalez-Perez, D. Rowitch, and A. Alvarez-Buylla. 2006. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26:7907–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittnacht, S. 1998. Control of pRB phosphorylation. Curr. Opin. Genet. Dev. 8:21–27. [DOI] [PubMed] [Google Scholar]
- Molofsky, A.V., R. Pardal, T. Iwashita, I.K. Park, M.F. Clarke, and S.J. Morrison. 2003. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 425:962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky, A.V., S.G. Slutsky, N.M. Joseph, S. He, R. Pardal, J. Krishnamurthy, N.E. Sharpless, and S.J. Morrison. 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 443:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D.O. 1997. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13:261–291. [DOI] [PubMed] [Google Scholar]
- Morshead, C.M., C.G. Craig, and D. van der Kooy. 1998. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 125:2251–2261. [DOI] [PubMed] [Google Scholar]
- Nguyen, L., A. Besson, J.I. Heng, C. Schuurmans, L. Teboul, C. Parras, A. Philpott, J.M. Roberts, and F. Guillemot. 2006. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 20:1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma, S., A. Philpott, and W.A. Harris. 2001. Cell cycle and cell fate in the nervous system. Curr. Opin. Neurobiol. 11:66–73. [DOI] [PubMed] [Google Scholar]
- Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J.L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35:25–31. [DOI] [PubMed] [Google Scholar]
- Parras, C.M., R. Galli, O. Britz, S. Soares, C. Galichet, J. Battiste, J.E. Johnson, M. Nakafuku, A. Vescovi, and F. Guillemot. 2004. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 23:4495–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard, J., J. Salinas, L. Garcia, and D.J. Liebl. 2006. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol. Cell. Neurosci. 31:713–722. [DOI] [PubMed] [Google Scholar]
- Shen, Q., S.K. Goderie, L. Jin, N. Karanth, Y. Sun, N. Abramova, P. Vincent, K. Pumiglia, and S. Temple. 2004. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 304:1338–1340. [DOI] [PubMed] [Google Scholar]
- Smith, C.M., and M.B. Luskin. 1998. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev. Dyn. 213:220–227. [DOI] [PubMed] [Google Scholar]
- Steinman, R.A. 2002. Cell cycle regulators and hematopoiesis. Oncogene. 21:3403–3413. [DOI] [PubMed] [Google Scholar]
- Takahashi, T., R.S. Nowakowski, and V.S. Caviness Jr. 1993. Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J. Neurosci. 13:820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu, O., and F. McCormick. 2003. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 3:233–245. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 262:2050–2054. [DOI] [PubMed] [Google Scholar]
- Zezula, J., P. Casaccia-Bonnefil, S.A. Ezhevsky, D.J. Osterhout, J.M. Levine, S.F. Dowdy, M.V. Chao, and A. Koff. 2001. p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep. 2:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy, F., H. Soares, K.H. Herzog, J. Morgan, C.J. Sherr, and M.F. Roussel. 1997. Expression of INK4 inhibitors of cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 8:1139–1150. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.