Abstract
OBJECTIVES
We sought to prospectively evaluate evidence of myocardial ischemia following surgical repair of anomalous aortic origin of a coronary artery with an interarterial course (AAOCA).
BACKGROUND
AAOCA is a rare anomaly associated with increased myocardial ischemia and sudden death risk in children. Data evaluating ischemia after AAOCA repair are limited.
METHODS
We included children who underwent AAOCA surgery between 10/01–12/06. They were prospectively assessed using exercise stress test (EST), stress echocardiogram (SE), and stress myocardial perfusion scan (MPS).
RESULTS
Of 24/26 eligible children, 15 (63%) were male, 16 (67%) had anomalous right coronary (ARCA), and 7 (29%) were asymptomatic. Median age was 12 (5–18) years; follow-up was 15 (2–48) months. All had unobstructed neo-coronary ostia by echocardiogram and were asymptomatic. One ALCA and 8 ARCA patients had post-operative evaluations suggestive of ischemia. The ALCA patient had reversible apical septal and mid-anteroseptal hypokinesis on SE. Of the ARCA patients, 2 had inferior ST depression on EST; subsequently, one had normal tests but the other developed anterolateral Q waves. Two had blunted blood pressure response with EST, one had fixed apical inferior hypokinesis on SE, two had reversible perfusion defects on MPS, and one had a fixed perfusion defect on MPS.
CONCLUSIONS
Subclinical changes suggestive of ischemia may occur despite patent neo-coronary ostia, notably after ARCA repair. The implication of these results on indication for surgery and subsequent sudden death risk is unknown. Serial EST, SE, and MPS are essential in evaluating ongoing ischemia risk after AAOCA repair.
Anomalous aortic origin of a coronary artery that courses between the great vessels (AAOCA) is a rare anomaly associated with increased myocardial ischemia and sudden death risk in children. We prospectively assessed children using exercise stress test, stress echocardiogram, and stress myocardial perfusion scan. One ALCA and 8 ARCA patients had positive ischemia evaluations. Subclinical changes suggestive of ischemia may occur despite patent neo-coronary ostia, notably after ARCA repair.
Keywords: coronary artery anomalies, pediatric cardiac surgery, ischemia
Anomalous aortic origin of a coronary artery that courses between the two great vessels (AAOCA) is a rare anomaly that carries an increased risk for myocardial ischemia and sudden cardiac death (1–4) during or just after exercise, notably among otherwise healthy children and young adults (2,5–7). Anomalous left main coronary artery from the right sinus of Valsalva (ALCA) and right coronary from the left sinus (ARCA) are both associated with sudden death but the former carries a higher risk (1–4). The true prevalence of AAOCA is unknown but estimates range from 0.1–0.3% (1,7–9). Patients are often asymptomatic (7,8) but when symptoms are present, they usually include: chest pain, pre-syncope, or syncope with exertion (2,10–13). Because the risk of sudden death is unknown, patient management remains controversial: some children are followed clinically with exercise restrictions while others are referred for surgery with exercise limitations until surgical repair is completed (14).
Data is limited evaluating children for myocardial ischemia after AAOCA repair (10–12). No studies to date have evaluated children for post-operative ischemia utilizing multiple imaging modalities, including exercise test (EST), stress echocardiogram (SE), and myocardial perfusion scan (MPS). The purpose of our study was to prospectively evaluate evidence of rest and stress myocardial ischemia in children with AAOCA following surgical repair.
METHODS
Patients
The study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Patients with AAOCA who underwent surgical repair at our institution between October 2001 and December 2006 were eligible for study inclusion. We excluded patients with other coronary artery anomalies or significant structural heart disease.
After obtaining parental consent and child assent we reviewed each patient’s medical record for presenting signs and symptoms, pre-operative and post-operative testing, and surgical repair data. We defined cardiovascular symptoms as chest pain, pre-syncope, or syncope with or just after exertion and cardiovascular signs as ventricular arrhythmia, exercise-induced ischemia, myocardial infarction, or aborted sudden death. Enrolled patients underwent prospective testing as described below.
Exercise Stress Test Protocol
Subjects exercised to maximal ability using a ramp bicycle protocol (SensorMedics, Yorba Linda, CA). Cardiac rhythm was monitored continuously. Four subjects who did not meet the height requirements for cycle ergometry utilized an incremental treadmill (Marquette Series 2000, Milwaukee, WI) protocol. Four subjects tested at an outside hospital exercised using a similar treadmill protocol.
Stress Echocardiogram Protocol
Two-dimensional images were obtained using a Sonos 5500 or iE 33 ultrasound system (Phillips, Andover, MA). To assess regional wall motion, images were obtained in standard parasternal long- and short-axis views and apical 4-chamber and 2-chamber views. The left ventricle was divided into 17 segments to analyze regional myocardial function (15).
Myocardial Perfusion Scan Protocol
Intravenous 99mTc-sestamibi was administered at rest and peak exercise and images were obtained at rest and within 30 minutes of peak exercise. Locations of perfusion defects were noted using the same 17-segment model utilized for SE (15).
Statistical Analysis
Baseline clinical characteristics and positive post-operative tests for ischemia were described. Descriptive statistics were expressed as mean (±SD) and median (range) for normally and non-normally distributed data, respectively. Two-tailed Fisher’s exact test was used to compare gender and cardiovascular symptoms with coronary anatomy (i.e., ARCA or ALCA).
RESULTS
Baseline Demographics
Twenty-four of 26 consecutive patients who met inclusion criteria consented to participate. Fifteen were male and 16 had ARCA. Seven of eight ALCA patients compared to 8 of 16 ARCA patients were male (P=0.18). Table 1 describes presenting signs and symptoms and associated coronary anatomy of the cohort. Of the 12 patients with cardiovascular complaints, five had ALCA compared to seven with ARCA (P=0.67). Six of seven ARCA patients who presented with cardiovascular symptoms had chest pain with exercise.
Table 1.
Presenting Signs and Symptoms (N=24)
| Symptom | N (%) | Coronary Anomaly |
|---|---|---|
| Chest Pain | ||
| Rest | 3(13) | ARCA (n=2), ALCA (n=1) |
| Exercise | 6 (25) | ARCA |
| Pre-Syncope | ||
| Rest | 1 (4) | ARCA |
| Exercise | 1 (4) | ALCA |
| Syncope | ||
| Rest | 1 (4) | ARCA |
| Exercise | 3 (13) | ALCA (n=2), ARCA (n=1) |
| Aborted Sudden Death | ||
| Exercise | 1 (4) | ALCA |
| Palpitations/PVCs | 1 (4) | ALCA |
| Asymptomatic | ||
| Murmur Evaluation | 6 (25) | ARCA (n=4), ALCA (n=2) |
| Family History | 1 (4) | ARCA |
ALCA = anomalous left main coronary artery; ARCA = anomalous right coronary artery.
Pre-operative Ischemia Evaluations
Sixteen patients had pre-operative EST; of these, nine had presenting symptoms suggestive of ischemia. One ARCA patient had a blunted blood pressure response on EST with no ECG abnormalities. One ALCA patient with palpitations had frequent premature ventricular contractions on Holter monitor; a second had aborted sudden cardiac death after non-competitive exercise. All other pre-operative ischemia evaluations were normal.
Surgical Repair
The median age at surgery was 12 (5–18) years. Surgical repair was performed in 23 patients using the unroofing procedure (16). Coronary reimplantation (17) was utilized in one ARCA patient due to the short intramural course of the anomalous vessel. In 12 patients, the aortic commissure was detached prior to unroofing. All survived the operation. One ALCA patient required emergent surgery on the first post-operative night for an aortic incision disruption that was successfully repaired. Another ALCA patient needed surgical drainage of a loculated pericardial effusion two weeks postoperatively. No patients developed significant aortic insufficiency during the follow-up period.
Post-operative Ischemia Evaluations
Prospective evaluations for myocardial ischemia were performed a median of 15 (2–48) months after surgery. All patients had unobstructed neo-coronary ostia by routine echocardiogram. One ALCA and eight ARCA patients had evaluations suggestive of myocardial ischemia (see Table 2). As an example of a positive ischemia evaluation, one ARCA patient underwent EST six weeks post-operatively that demonstrated inferior lead ST segment depression (Figure 1A) with a normal MPS. At 20 months post-operatively, baseline Q waves were noted without ischemic changes during exercise (Figure 1B) with a normal SE and MPS.
Table 2.
Positive Post-Operative Ischemia Tests (N=9)
| ID | Presenting Symptom | Anatomy | F/U time, mo | Exercise Stress Test | Stress Echocardiogram | Myocardial Perfusion Scan |
|---|---|---|---|---|---|---|
| 1* | Chest Pain, Exercise | ARCA | 4.0 | Inferior ST depression | — | Normal |
| 1† | 48.0 | Normal | Normal | Normal | ||
| 2* | Chest Pain, Exercise | ARCA | 1.5 | Inferior ST depression | — | Normal |
| 2† | 20.0 | Anterolateral Q waves | Normal | Normal | ||
| 3* | Chest Pain, Rest | ARCA | 15.0 | Normal | — | — |
| 3† | 27.0 | Blunted BP response | Normal | Normal | ||
| 4† | Murmur Evaluation | ARCA | 22.0 | Blunted BP response | Normal | — |
| 5† | Pre-Syncope, Rest | ARCA | 41.5 | Normal | Fixed apical- inferior hypokinesis | Normal |
| 6† | Family History ARCA | ARCA | 15.5 | Normal | Normal | Inferoseptal defect Reversible |
| 7† | Chest Pain, Exercise | ARCA | 3.5 | Normal | — | Anterior wall defect Reversible |
| 8† | Syncope, Exercise | ARCA | 3.5 | Normal | Normal | Anteroseptal defect Fixed |
| 9† | Aborted Sudden Death | ALCA | 8.5 | Normal | Apical septal & mid antero- septal hypokinesis with exercise | Normal |
First post-operative test
Prospective post-operative test for study
patient did not have test
ARCA = anomalous right coronary artery; ALCA = anomalous left coronary artery; BP = blood pressure; F/U = follow-up; ID = patient identification number; mo = months.
Figure 1.

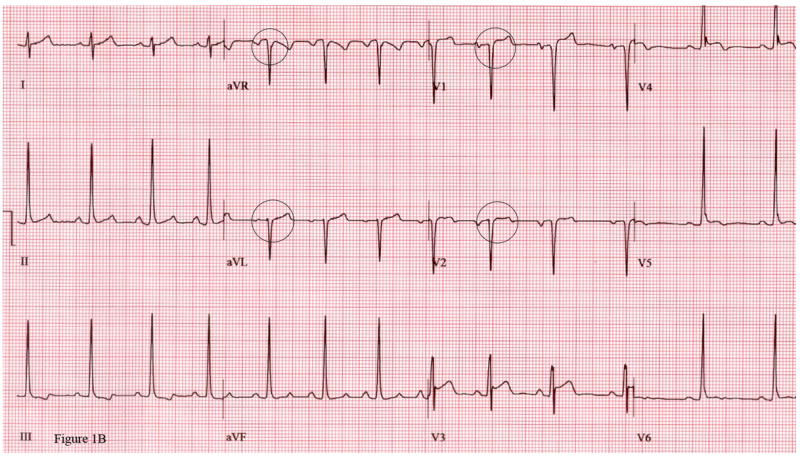
Figure 1A. ECG from maximal exercise stress test 6 weeks after unroofing procedure for anomalous right coronary artery (ARCA) shows inferior lead ST segment depression.
Figure 1B. Same patient 20 months after ARCA surgery shows baseline Q waves in leads V1, V2, aVR, aVL at rest with no ischemic changes during maximal exercise stress test.
DISCUSSION
AAOCA is a rare congenital anomaly associated with increased risk of myocardial ischemia and sudden death in children and young adults (1–6). We present the most comprehensive prospective ischemia evaluation following AAOCA repair on the largest number of pediatric patients to date. We found evidence suggestive of ischemia in one ALCA and eight ARCA patients; these may not have been identified had we used one or even two testing modalities. All patients remained asymptomatic during testing, calling into question using patient complaints in risk assessment for ischemia. While most patients had symptoms at diagnosis, we classified one-half as cardiovascular in origin. Those with ALCA presented more frequently with cardiovascular complaints than those with ARCA.
What remains controversial is the management of asymptomatic children who appear to have a higher risk of sudden death than those identified later in adulthood (1,6). Serial evaluation with EST may be useful. However, basing one’s management on a single test is worrisome because EST can be falsely negative, especially since ischemia is intermittent with AAOCA (2). Indeed, in our study, nine of 16 patients who had a pre-operative EST presented with cardiovascular symptoms and only one had an abnormal EST.
A limitation of our study was the inability to determine risk of surgery versus observation on myocardial ischemia and sudden death because this was not a randomized study. Further, as this was a prospective study initiated after surgical repair, we lacked pre-operative ischemia evaluations on eight patients; this would have made for a more complete assessment of pre- and post-operative ischemia. Last, because the unroofing procedure has only been utilized fairly recently, we were only able to obtain mid-but not long-term follow-up evaluations.
In conclusion, subclinical changes suggestive of ischemia may occur after AAOCA surgery despite patent neo-coronary ostia, notably after ARCA repair. The implication of these results on indication for surgery and subsequent sudden death risk is unclear. This study highlights that while surgery is often performed on children in whom the pre-operative risk of myocardial ischemia is unknown, we currently have suboptimal ways to follow surgical results. Until more accurate testing modalities are found, serial EST, SE, and MPS are essential in evaluating ongoing ischemia risk after AAOCA repair.
Indeed, larger numbers of children need to be followed over time to determine if the risk of myocardial ischemia after surgery is truly diminished. Angelini et al. (18) and Pellicia et al. (19) have suggested that a multi-center, multi-national database of patients with AAOCA be established. We concur with this and are developing such a database of children and young adults in order to obtain the natural history of those who do not undergo surgery and the “unnatural” history of those who do to assess the utility of surgical intervention in decreasing long-term risk of myocardial ischemia and sudden cardiac death.
Acknowledgments
Dr. Davis was supported by an NIH Institutional National Research Service Award Training Grant (T32HL007915).
Abbreviations and Acronyms
- AAOCA
anomalous aortic origin of a coronary artery with an interarterial course
- ALCA
anomalous origin of left main coronary artery
- ARCA
anomalous origin of right coronary artery
- ECG
electrocardiogram
- EST
exercise stress test
- SE
stress echocardiogram
- MPS
myocardial perfusion scan
Footnotes
There are no Conflicts of Interest
References
- 1.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20:640–7. doi: 10.1016/0735-1097(92)90019-j. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 3.Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998;29:689–95. doi: 10.1016/s0046-8177(98)90277-5. [DOI] [PubMed] [Google Scholar]
- 4.Kragel AH, Roberts WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. Am J Cardiol. 1988;62:771–7. doi: 10.1016/0002-9149(88)91220-9. [DOI] [PubMed] [Google Scholar]
- 5.Corrado D, Thiene G, Nava A, Rossi L, Pennelli N. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am J Med. 1990;89:588–96. doi: 10.1016/0002-9343(90)90176-e. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: “high-risk” abnormalities in the initial coronary artery course and heterogeneous clinical outcomes. Am Heart J. 1997;133:428–35. doi: 10.1016/s0002-8703(97)70184-4. [DOI] [PubMed] [Google Scholar]
- 7.Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37:593–7. doi: 10.1016/s0735-1097(00)01136-0. [DOI] [PubMed] [Google Scholar]
- 8.Zeppilli P, dello Russo A, Santini C, et al. In vivo detection of coronary artery anomalies in asymptomatic athletes by echocardiographic screening. Chest. 1998;114:89–93. doi: 10.1378/chest.114.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 10.Frommelt PC, Frommelt MA, Tweddell JS, Jaquiss RDB. Prospective echocardiographic diagnosis and surgical repair of anomalous origin of a coronary artery from the opposite sinus with an interarterial course. J Am Coll Cardiol. 2003;42:148–54. doi: 10.1016/s0735-1097(03)00503-5. [DOI] [PubMed] [Google Scholar]
- 11.Romp RL, Herlong JR, Landolfo CK, et al. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg. 2003;76:589–96. doi: 10.1016/s0003-4975(03)00436-3. [DOI] [PubMed] [Google Scholar]
- 12.Erez E, Tam VKH, Doublin NA, Stakes J. Anomalous coronary artery with aortic origin and course between the great arteries: improved diagnosis, anatomic findings, and surgical treatment. Ann Thorac Surg. 2006;82:973–7. doi: 10.1016/j.athoracsur.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 13.Frommelt PC, Berger S, Pelech AN, Bergstrom S, Williamson JG. Prospective identification of anomalous origin of left coronary artery from the right sinus of Valsalva using transthoracic echocardiography: importance of color Doppler flow mapping. Pediatr Cardiol. 2001;22:327–332. doi: 10.1007/s002460010239. [DOI] [PubMed] [Google Scholar]
- 14.Graham TP, Jr, Driscoll DJ, Gersony WM, Newburger JW, Rocchini A, Towbin JA. 36th Bethesda conference: eligibility recommendations for competitive athletes with cardiovascular abnormalities. Task Force 2: Congenital Heart Disease. J Am Coll Cardiol. 2005;45:1326–33. doi: 10.1016/j.jacc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa I, Gula G, Radley-Smith R, Durrer S, Yacoub M. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death.Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg. 1981;82:297–300. [PubMed] [Google Scholar]
- 17.DiLello F, Munk JF, Flemma RJ, Mullen DC. Successful coronary reimplantation for anomalous origin of the right coronary artery from the left sinus of Valsalva. J Thorac Cardiovasc Surg. 1991;102:455–6. [PubMed] [Google Scholar]
- 18.Angelini P, Velasco JA, Flamm S. Coronary Anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–54. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 19.Pelliccia A. Congenital coronary artery anomalies in young patients. New perspectives for timely identification. J Am Coll Cardiol. 2001;37:598–600. doi: 10.1016/s0735-1097(00)01122-0. [DOI] [PubMed] [Google Scholar]


