Abstract
Pain is the primary reason that people seek medical care. At present chronic unremitting pain is the third greatest health problem after heart disease and cancer. Chronic pain is an economic burden in lost wages, lost productivity, medical expenses, legal fees and compensation. Chronic pain is defined as a pain of greater than two months duration and can be of an inflammatory or neuropathic origin that can arise following nerve injury or in the absence of any apparent injury. Chronic pain is characterized by an altered pain perception that includes allodynia (a response to a normally non-noxious stimuli), and hyperalgesia (an exaggerated response to a normally noxious stimuli). This type of pain is often insensitive to the traditional pain drugs or surgical intervention and thus the study of the cellular and molecular mechanisms that contribute to chronic pain are of the up-most importance for the development of a new generation of analgesic agents. Protein kinase C isozymes are under investigation as potential therapeutics for the treatment of chronic pain conditions. The anatomical localization of protein kinase C isozymes in both peripheral and central nervous system sites that process pain have made them the topic of basic science research for close to two decades. This review will outline the research to date on protein kinase C involvement in pain and analgesia. In addition, this review will try to synthesize these works to begin to develop a comprehensive mechanistic understanding of how protein kinase C may function as the master regulator of peripheral and central sensitization that underlies many chronic pain conditions.
Keywords: nociception, primary afferent, dorsal horn, spinal cord, opioid analgesia
I. Pain
Pain is defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” It is an evolutionarily conserved sensory experience that is physiologically necessary for an organism to detect and avoid injury. The importance of this sensory system is highlighted by conditions in which pain is absent such as congenital insensitivity to pain, leprosy, or diabetic neuropathy. The transmission of nociceptive input from the peripheral site of noxious stimuli to the central nervous system occurs through the activation of primary afferents (Figure 1). There are three main primary afferent fiber types; the large-diameter, heavily myelinated Aβ fibers that transmit non-noxious tactile sensations, the small-diameter, myelinated Aδ fibers that transmit “first pain” - the rapid shooting pain after you hit your thumb with a hammer, and the small-diameter, unmyelinated C fibers that transmit “second pain” – the throbbing in the thumb following the blow with the hammer. A noxious stimulus activates primary afferent nociceptors that synapse in the spinal cord on dorsal horn neurons. The dorsal horn of the spinal cord can be viewed as a gate at which millions of peripheral signals arrive and are sorted before being sent to supraspinal processing sites that determine the final response and add an emotional context to nociception (ie: remove your hand from the hot stove and “Ouch, that hurts”). Dorsal horn neurons not only convey pain sensation through ascending spinothalamic and spinoreticular projections, but also participate in segmental polysynaptic spinal reflexes (flexor, or withdrawal reflexes) to activate muscle groups that move a body part away from the stimulus and thus, protect the tissue from damage. These reflexes are commonly assessed as a measure of perceived pain in behavioral nociceptive tests in animal models of pain.
Figure 1. The pain pathway and its descending modulation.
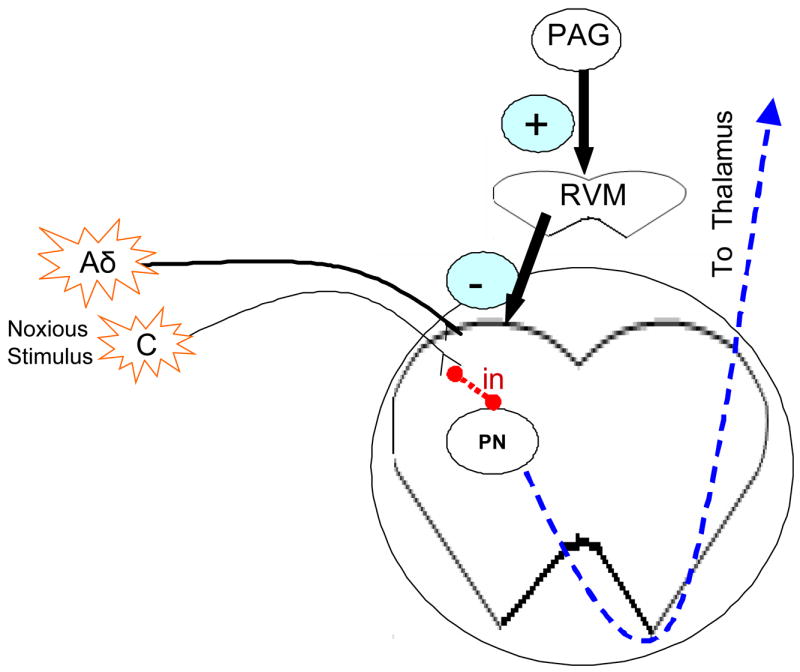
A noxious stimulus will excite peripheral nociceptors (Aδ and C fibers). These fibers synapse on second order dorsal horn neurons. Some of these dorsal horn neurons are excitatory or inhibitory interneurons (in). Others are ascending spinothalamic projection neurons (PN) that ascend via the contralateral ventrolateral funiculus to convey pain sensation to the brain. Dorsal horn neurons are also subject to descending modulation from the midbrain periaquaductal gray (PAG) through polysynaptic circuits through the medulla including through the rostral ventromedial medulla (RVM).
Pain pathways are modulated by both descending inhibitory and facilitatory control. In adult mammals, electrical stimulation of the periaqueductal gray (PAG) or microinjections of morphine into this midbrain site produces analgesia that can be robust enough to permit surgical procedures without the need for anesthetic agents(1). PAG neurons produce analgesia by activating descending inhibitory pathways to the spinal cord. These pathways include PAG axonal projections to rostral ventromedial medulla (RVM) neurons, which in turn project to the spinal cord via the dorsolateral funiculus to inhibit nociceptive primary afferents and spinal dorsal horn neurons(2–4). This effectively blocks pre-synaptic neurotransmitter release from primary afferent nociceptors and hyperpolarizes second order projection neurons in the spinothalamic or spinoreticular ascending pain pathways leading to a decrease in pain.
Pain can be categorized by a number of characteristics including physiological versus pathological, the duration of the painful stimulus (acute, subacute, tonic, chronic), the nature of the noxious insult (chemical/inflammatory, neuropathic, nociceptive), the type of response (spontaneous, evoked), and the location of the insult (cutaneous, subcutaneous, visceral, neuropathic). To this end, a multitude of animal models have been developed to examine nociceptive mechanisms and pharmacological selectivity. The functional role of protein kinase C (PKC) in pain and analgesia has been an area of intense research over the last two decades. In this review article we will summarize the current literature examining the role of PKC family members in pain and analgesia at each level of the neuro-axis involved in pain transmission.
II. Protein kinase C and Pain
PKC is a family of serine/threonine kinases that are divided into three groups based on calcium and diacylgycerol dependence. The α, βI, βII, and γ isozymes are calcium and diacylglycerol-dependent and are termed conventional (c) PKCs. The δ, ε, η, and θ isozymes are calcium-independent but diacylglycerol-dependent and are termed the novel (n) PKCs. Lastly, the ξ and λ/ι isozymes are calcium and diacylglycerol-independent and termed the atypical (a) PKCs. These different PKC isozymes function as key signal transducers in cells allowing them to regulate a number of cellular functions including differentiation, proliferation, cell migration, and apoptosis making them attractive therapeutic targets for a host of human diseases.
Over a decade ago expression of cPKCs (α, βI, βII, γ), nPKCs (ε, δ) and aPKC (ξ) was reported in the brain of rats (5–9). Since then PKC α, βI, βII, δ, ε, and ξ isozymes have been identified in primary afferents that transmit nociceptive signals from the peripheral site of injury to the superficial dorsal horn (10). Within the superficial laminae of the dorsal spinal cord, an area that has been implicated in pain processing, PKC α, βI, βII, and γ isoforms have been identified (11). With the evidence that PKC isozymes were in the anatomical regions that regulate pain the race was on to determine whether these isozymes were therapeutic targets for the treatment of pain. This review will consider the modulation of pain by PKC activity across the neuro-axis beginning in the primary afferent and ending in the brain.
III. Role of protein kinase C in pain and analgesia at different levels of the neuro-axis
III.a. The primary afferent peripheral terminal
Following tissue damage a variety of chemical mediators are released at the site of injury including, ATP, protons, bradykinin, prostaglandins, substance P, calcitonin gene related peptide (CGRP), and proinflammatory cytokines to name just a few. These chemicals can activate and sensitize primary afferent nociceptors leading to pain that can be characterized by hyperalgesia (ie: warm shower water is painful on sunburned skin), and allodynia (ie: the touch of clothing becomes painful). These substances have been used to model hyperalgesia and allodynia in animals and have highlighted the importance of specific PKC isozymes in nociceptive processing in the primary afferent.
The foundation suggesting a role of PKC in nociceptive processing in primary afferents came from work in isolated primary afferent neurons and isolated spinal cord preparations. These early studies showed that 1) PKC activation could depolarize unmyelinated afferent neurons (12, 13), 2) PKC activators could sensitize afferent neurons (14), 3) PKC activators could enhance currents activated by noxious thermal stimulus in afferent neurons (15), and 4) PKC inhibitors could block sensitization in afferent neurons (16, 17). Moving to in vivo models of cutaneous, inflammatory and neuropathic pain a variety of non-specific and isozyme specific PKC inhibitors have shown anti-nociceptive properties as outlined in Table 1.
Table 1. Inhibition of nociception by peripheral administration of PKC inhibitors.
A summary of the studies that implicate PKC in primary afferents in cutaneous, inflammatory, and neuropathic pain models.
| Pain Model | PKC treatment | Change elicited | Refs |
|---|---|---|---|
| CUTANEOUS PAIN | |||
| PKC activation | Phorbol ester
PKCε constituitively active |
Induces spontaneous nociception, TH, MA.
Enhanced heat activated membrane currents |
(19, 106, 107) |
| Bradykinin | PKCε inhibitor bisindolylmaleimide I | Decreased membrane currents MA decreased. | (19, 106) |
| Thermal stimulus | Chelerythrine | Decreased allodynia | (108) |
| Epinephrine | PKCε knock-out mice
εV1-2 inhibitory peptide |
Decreased TH and MH
Decreased MH |
(18) |
| Nerve Growth Factor | εV1-2 inhibitory peptide | Decreased MH | (18) |
| Capsaicin | PKC activator
Bisindolylmaleimide |
Increased capsaicin-induced currents.
Decreased capsaicin-induced currents. Contributes to C-fiber induced ERK activation |
(25)
(93) |
| PAR2 agonist & capsaicin | PKCε antagonist | Decreased TH | (109) |
| Endothelin-1 | GF109203X | Decreased MH | (32, 110) |
| INFLAMMATORY PAIN | |||
| Formalin | chelerythrine | Decreased second phase behaviors. | (106) |
| Carregeenan | εV1-2 inhibitory peptide | MH is attenuated | (18) |
| NEUROPATHIC PAIN | |||
| Sciatic nerve transection | No-treatment | Up-regulation of PKCα, β1, δ | (111) |
| Diabetic Neuropathy | PKC(19–36)
Staurosporine |
MH and C-fiber hyperexcitability blocked | (43) |
| Alcohol Neuropathy | non-specific inhibitors & εV1-2 inhibitory peptide | MH and C-fiber hyperexcitability blocked. | (112) |
MA – mechanical allodynia, MH – mechanical hyperalgesia, TH – thermal hyperalgesia
The development of the PKCε knockout mice (18) and PKC isozyme-selective small peptide inhibitors demonstrate that PKCε in primary afferents has a critical role in nociception (18, 19) (Figure 2). One of the most studied pathways by which PKCε modulates nociception is through activation of the TRPV1 receptor, a member of the transient receptor potential ion channel superfamily. TRPV1 (transient receptor potential vanilloid receptor 1) is a nonspecific cation channel that is activated by capsaicin, the pungent ingredient in hot chili peppers, as well as by noxious heat, extracellular acidification, and potentially the endogenous ligands anandamide (20), leukotriene B, and lipoxygenase products (21). TRPV1 knockout mouse studies have shown this receptor to be essential for thermal and inflammatory pain. TRPV1 receptors are expressed on unmyelinated C fibers that contain substance P and/or CGRP (22). Normally, TRPV1 is activated only at high temperature, but following tissue damage, TRPV1 conducts current at lower temperatures. This response to non-noxious temperatures is thought to underlie thermal hyperalgesia.
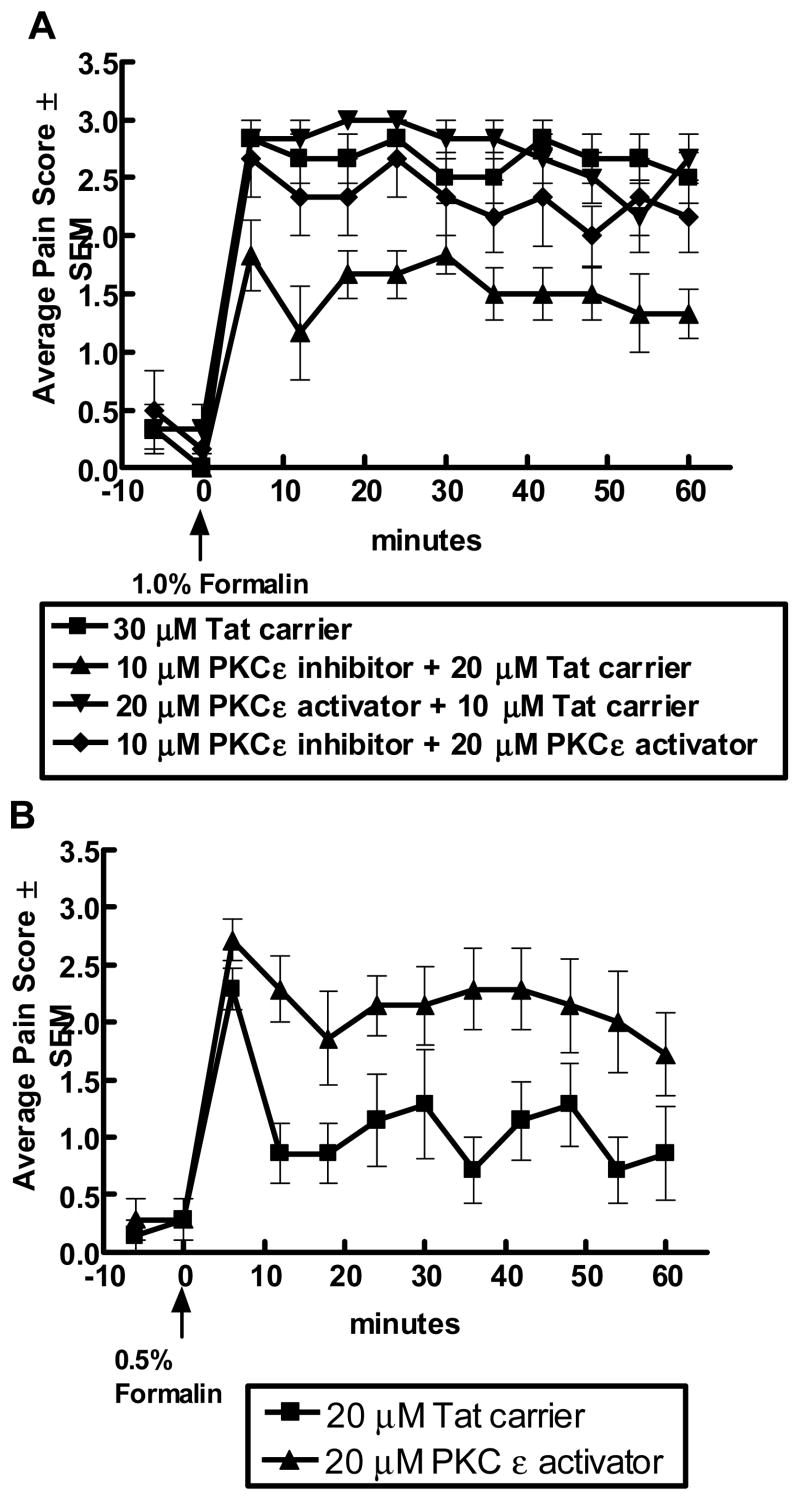
Figure 2. Protein kinase C ε modulates formalin-induced nociception.
Subcutaneous administration of dilute 1% formalin in the plantar hindpaw of a one week old rat produces spontaneous flinching and guarding of the hind paw that can be scored in 6 minute bins to provide a Pain Score. A score of 0 represents the absence of paw flinching and a score of 3 represents the presence of flinching or guarding of the hind paw. In panel A, intrathecal administration of an isozyme specific PKCε peptide inhibitor linked to a Tat carrier peptide reduced the average pain score. Attenuation by the PKCε peptide inhibitor could be reversed by co-adminstration with a PKCε peptide activator. The Tat carrier peptide did not alter behaviors as compared to saline or no treatment (148). PKCε peptide activator alone did not increase behaviors with the recognition that behaviors were already maximally stimulated with 1% formalin. In panel B, intrathecal administration of the PKCε peptide activator potentiated pain-associated behaviors stimulated with a sub-maximal concentration of formalin (0.5%). These findings indicate a role for PKCε in nociception.
Capsaicin applied to the skin produces thermal hyperalgesia and has proven a useful agent to study pain mechanisms in primary afferent neurons. It has been suggested that there are two independent pathways for TRPV1 activation: 1) direct ligand binding (endogenous ligands) which can be modulated by allosteric binding of protons 2) extracellular ligands coupled to PKC by intracellular signaling (23). In rat afferent neurons, PKC activation promotes exocytosis of TRPV1 to the cell surface (24), and increases capsaicin-induced TRPV1 currents while PKC inhibition decreases currents (25). Activation of PKC increases depolarization of TRPV1 allowing the receptor to conduct at lower temperatures (non-noxious temperatures) and in the presence of more physiologically relevant pH (26). As shown in Figure 3, application of capsaicin to the hind paw of a rat increases PKCε immunoreactivity in afferent fibers entering the dorsal horn of the spinal cord. PKCε has been implicated in phosphorylation of the TRPV1 receptor on Ser502 and Ser800 responsible for the potentiation of capsaicin-evoked currents (27, 28). Alternatively, it has been reported that PKCα may also activate TRPV1 independent from the binding of TRPV1 ligand. Down-regulation of PKCα does not alter conductance following TRPV1 ligand suggesting that phosphorylation of TRPV1 is not necessary for normal activity only for augmented activity. Further study needs to be done to clarify whether PKC ε and α may have isozyme specific roles in regard to the necessity of extracellular ligand-binding for activity.
Figure 3. Protein kinase C ε and γ immunoreactivity in the spinal cord after topical capsaicin.
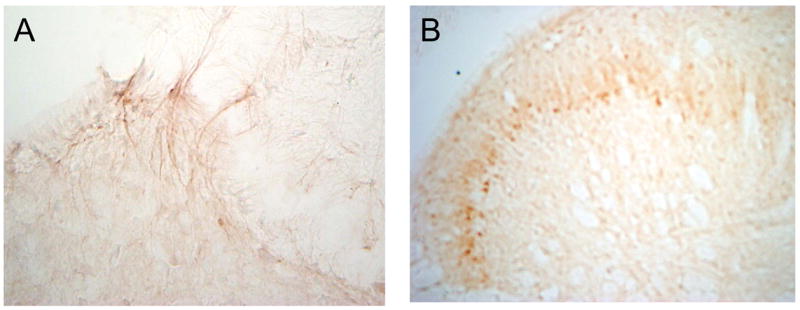
Topical capsaicin was applied to the rat hind paw resulting in thermal hyperalgesia that lasted at least one hour. At the end of the hour rats underwent transcardiac perfusion and lumbar spinal cords were collected for immunohistochemical analysis of protein kinase C ε and γ. In panel A, PKC ε immunoreactivity is seen in the dorsal horn of the spinal cord. The PKC ε appears to be on primary afferent fibers entering into the superficial lamina of the dorsal horn. Using serial spinal cord sections in panel B, PKC γ immunoreactivity is seen in lamina II of the dorsal horn of the spinal cord. In contrast with PKCε, PKCγ is located in dorsal horn neurons.
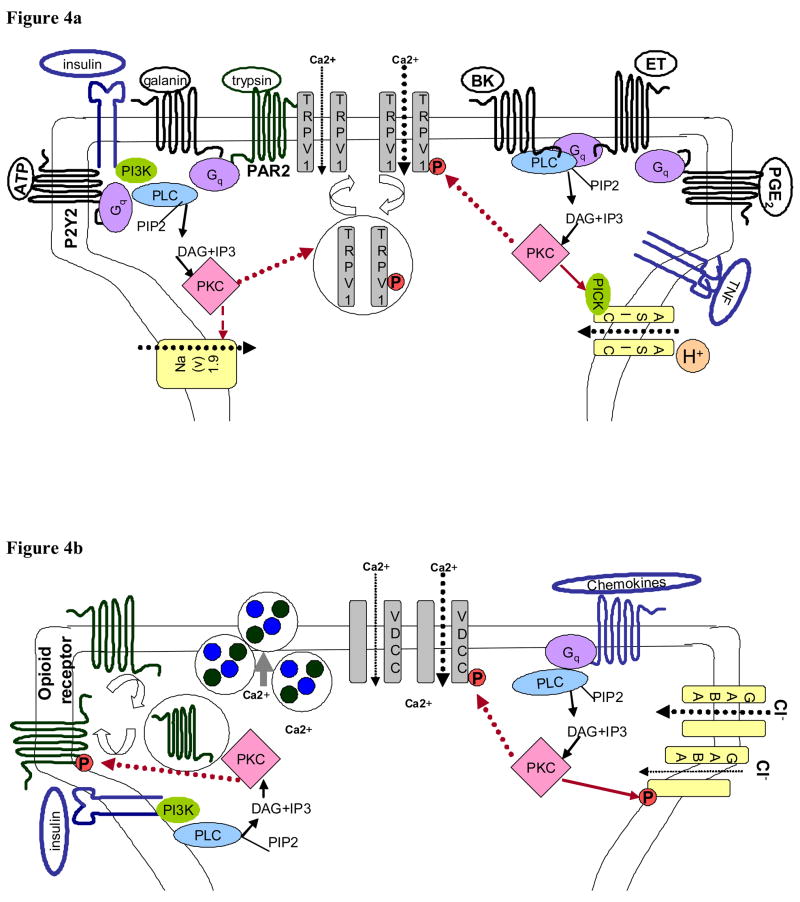
Furthermore, substances released locally following tissue damage such as protons, bradykinin, ATP, and prostaglandins may potentiate the activation of TRPV1 by thermal stimulus. PKC may serve as a master switch by which TRPV1 integrates heat and tissue damage to produce hyperalgesia. There is a large literature that suggests numerous inflammatory mediators may enhance the activity of TRPV1 via PKC-dependent pathways. The integration of these various pathways is briefly outlined below and shown schematically in Figure 4A. Bradykinin is one potential extracellular ligand that has been shown to activate PKCε (29), and induce translocation of PKCε (19) in DRG neurons, thus, enhancing TRPV1 activity in a PKC-dependent manner (30–32). Similarly, galanin (33), ATP via P2Y2 receptors (34, 35), trypsin/tryptase released during inflammation via proteinase-activated receptor (PAR) 2 (36), prostaglandin E2 and I2 acting at EP1 and IP (37), acid sensing ion channels (ASIC) via association with PICK-1 (protein interacting with C-kinase) (38),(39), have been implicated in potentiating TRPV1 currents and shifting TRPV1 activation to a more physiological pH. Further evidence for the role of PKC in primary afferents in pathological pain, have come from studies looking at the role of PKC in hyperalgesic priming. These studies suggest that TNF acting through the TNFR1 during acute carrageenan-induced inflammation induces hyperalgesic priming by activating neuronal PKCε (10, 40, 41).
Figure 4. PKC is positioned to play a central role in peripheral and central sensitization.
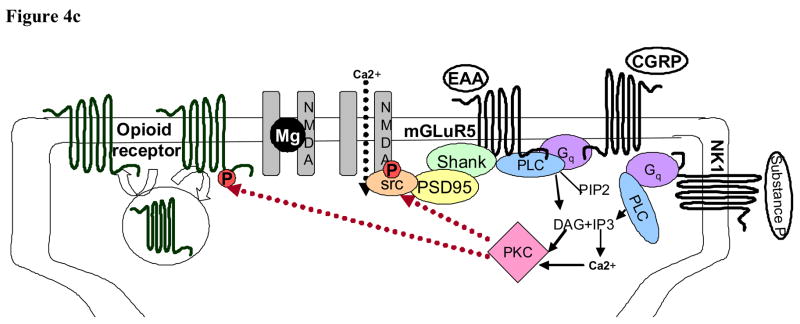
Panel A, PKC in peripheral sensitization in the primary afferent peripheral terminal. PKC is activated in the primary afferent by a large number of substances that are released in response to injury. These include bradykinin (BK), endothelin-1 (ET), prostaglandins (PGE2), cytokines (tumor necrosis factor, TNF), ATP, trypsin, galanin, and insulin. The receptors for these proteins activate phospholipase C (PLC) and stimulate the production of diacylgycerol (DAG) which activates PKCs. Activated PKC subsequently can increase cation flow into the peripheral terminal via actions on the capsaicin receptor (TRPV1), the acid sensing ion channel (ASIC), and tetrodotoxin-insensitive sodium channels (Nav 1.9). Panel B, PKC is positioned to influence neurotransmitter release from the central primary afferent terminals in the spinal cord. Binding of G-protein coupled pre-synaptic receptors and receptor tyrosine kinases signal through phospholipase C (PLC) to activate PKC. This activated PKC can then enhance calcium influx into the pre-synaptic terminal by phosphorylation of voltage gated calcium channels (VDCC). Simultaneously, PKC can decrease inhibitory tone in the pre-synaptic terminal by phosphorylation of opioid receptors and inhibitory GABAA receptors effectively decreasing pre-synaptic inhibition. This increase in excitatory tone and decrease in inhibitory tone can potentiate the release of neurotransmitter from the pre-synaptic terminal. Panel C, PKC in the post-synaptic dorsal horn neuron regulates neuronal activity. PKC is activated in dorsal horn neurons subsequent to G protein coupled signaling through phospholipase C (PLC). A variety of G proteins are involved including, but not limited to, neurokinin receptor (NK1), metabotrophic glutamate receptors (mGluR) that bind excitatory amino acids (EAA), and receptors for calcitonin gene-related peptide (CGRP). Activted PKC then phosphorylates Src thus increasing NMDA receptor activity. As in pre-synaptic primary afferent terminals, activated PKC can also modulate post-synaptic opioid receptor activity.
Similarly, insulin and insulin-like growth factors activate receptor tyrosine kinases that activate PKC leading to both increased TRPV1 receptor translocation to the cell membrane and enhanced receptor sensitivity (42). In a rodent model of painful diabetic neuropathy, PKC inhibitors decrease thermal hyperalgesia and the hyperresponsiveness of C afferent neurons (43). Diabetic neuropathy is accompanied by increased phosphorylation (activation) of PKCα, no change in PKCβII, and a down-regulation of PKCδ and TRPV1 protein (44, 45). Functionally, increased capsaicin activated inward currents were observed and PKC activation further potentiated the currents(45). This apparent contradication in expression and activity of TRPV1 can be explained by the observation that although there were fewer TRPV1 receptors, those that remained were more likely to be phosphorylated (via PKC) and present on the plasma membrane. Furthermore, not only does PKC potentiate pain signaling but it may also decrease analgesia. For instance, opioid-mediated activation of inhibitory G protein function is associated with increased PKC-dependent phosphorylation of the Goα subunit (44). In conclusion, PKC appears to be critical to the development of peripheral sensitization in primary afferent fibers resulting in the development of thermal hyperalgesia following injury.
III. b. The primary afferent-spinal cord synapse
In addition to activity in the peripheral nerve terminal, PKC is likely to modulate the neurotransmission at the central terminal in the dorsal horn of the spinal cord. PKC has been shown to increase excitatory neurotransmission and decrease inhibitory tone at the primary afferent-spinal cord synapse (Figure 4B).
Peripheral application of capsaicin releases neuropeptides and excitatory amino acids into the dorsal horn of the spinal cord (46, 47). In addition, to the sensitization of the TRPV1 receptor discussed above, PKC activation also enhances tetrodotoxin-resistant Na+ currents (48), thus increasing action potential propagation to the central terminals of afferent neurons. Electrophysiological studies have demonstrated that PKC activation potentiates capsaicin-induced depolarization in afferent neurons (25), a finding that correlates with enhanced capsaicin-induced release of substance P from spinal cord slices (49). While enhanced release of neurotransmitters may be secondary to PKC mediated events at the peripheral terminal there is evidence that local PKC activity at the pre-synaptic terminal also contributes. In afferent neurons, PKC activation alone increases substance P and CGRP release as well as potentiates potassium- and capsaicin-stimulated release of these neuropeptides (49–51). More specifically, inhibition of PKCε decreases capsaicin-induced release of glutamate and CGRP in isolated spinal cords (52). In addition to topical capsaicin, chemokines stimulate the release of CGRP from afferent neurons in a PKC dependent manner (53). One potential mechanism by which pre-synaptic PKC activity can augment neurotransmitter release is via PKC-dependent sensitization of voltage-dependent L-type Ca2+ channels (54). An alternative mechanism may be a PKC-mediated decrease in pre-synaptic inhibition.
In vitro electrophysiology suggests that PKC is involved in modulating opioid and GABAA receptor function. PKC activators have been shown to inhibit μ, δ, and κ opioid receptor agonist-stimulated analgesia (55–58). Chronic use of opioid analgesics increases activity and expression of PKC that correlates with a decrease in analgesia. In addition, PKC inhibitors attenuate the development of opioid tolerance (59, 60). Whether tolerance is due to a de-sensitization of opioid receptors or to the concomitant development of an opioid-induced hyperalgesia remains un-resolved. PKC may be involved in both the desensitization of opioid receptors as well as in the development of opioid-induced hyperalgesia. De-sensitization can occur in an agonist-dependent (homologous) and agonist-independent (heterologous) manner (For review see (61). While homologous de-sensitization is thought to involve the G protein coupled receptor kinases (GRK) (62–65), heterologous de-sensitization involves PKC mediated phosphorylation of the opioid receptor (62, 64, 66, 67). Both NMDA receptor (68, 69) and insulin-induced tyrosine kinase receptor activity (70) have been reported to activate PKC resulting in heterologous de-sensitization of the μ opioid receptor. PKC mediated phosphorylation of the μ opioid receptor inhibits internalization thus, preventing re-sensitization of the receptor (71). In contrast, in δ opioid receptors, PKC mediated phosphorylation of serine 344 produces internalization (72). This suggests that PKC may differentially modulate the opioid receptor sub-types.
GABA functions as an inhibitory neurotransmitter in the spinal cord and can act pre-synaptically to reduce the release of neurotransmitters from primary afferent terminals. Similar to opioid receptors, the inhibitory GABAA receptor is modulated by phosphorylation status (73–75). Both cholecystokinin and substance P decrease inhibitory GABAA currents via PKC-dependent phosphorylation of the receptor (74–76). These findings suggest that PKC acts on numerous receptor types in primary afferents to both enhance excitatory neurotransmission and to attenuate inhibitory tone at the synapse.
III. c. Spinal cord
Increased translocation and activation of PKC in dorsal horn neurons has been shown in a number of pain models(77–79) including following topical administration of capsaicin (Figure 3B). Spinal administration of non-specific inhibitors of PKC has highlighted the importance of spinally located PKC in pain (Table 2). Findings in PKC γ knockout mice suggest that PKCγ is a critical regulator of central sensitization while leaving acute pain processing intact (80).
Table 2. Inhibition of nociception by spinal (intrathecal) administration of PKC inhibitors.
A summary of the studies that implicate PKC in spinal cord in cutaneous, inflammatory, and neuropathic pain models.
| Pain Model | PKC treatment | Change Elicited | Refs |
|---|---|---|---|
| CUTANEOUS PAIN | |||
| Acute pain | PKC γ KO mice | No change | (80) |
| PKC activator | Phorbol esters | Induced pain-like behaviors (mice)
Increased activity in spinothalamic tract neurons (primate) |
(113, 114)
(115) |
| Tail flick | calphostin C | Enhanced [D-Ala2]deltorphin II-induced antinociception | (57) |
| Capsaicin | NPC15437 | Reversed MA | (116) |
| Thermal injury | GF109203X chelerythrine | decreased MH in the contralateral paw | (117) |
| INFLAMMATORY PAIN | |||
| Formalin | GF109203X chelerythrine
εV1-2 inhibitor γV3-5 inhibitor |
decreased nociception – 2nd phase
decreased c-fos in lumbar dorsal horn Decreased nociception – 1st & 2nd Decreased nociception – 2nd phase |
(118–120)
(121) |
| Bee Venom | Chelerythrine | Decreased primary TH
No effect on MH Decreased spontaneous nociception Decreased mirror image TH |
(122, 123) |
| Complete Freud’s Adjuvant | RO-320432 | No effect | (124) |
| Mustard Oil | PKC inhibitors | attenuation of neuronal activity mustard oil-induced | (125) |
| NEUROPATHIC PAIN | |||
| sciatic nerve ligation | PKC γ KO mice
Calphostin C RO-320432 |
Decreased MA & TA
Decreased TH Decreased TH Increased cPKC activity in dorsal horn |
(80)
(126) (124) |
| Chung Model | chelerythrine | Anti-allodynic | (127) |
| Chronic Constriction Injury | PKCγ knock-out mice
Chelerythrine MK-801 |
Decreased neuropathic pain
Decreased TH Decreased PKCγ immunoreactivity |
(78, 97, 128) |
| Pertussis toxin | Chelerythrine | Decreased TH | (129) |
| Dihydroxyphenylglycine | GF109203X | Decreased spontaneous nociceptive behaviors & TH | (130) |
| Diabetic Neuropathy | calphostin | Increased tail flick latencies | (131) |
| Alcohol Neuropathy | ODN to PKCε | inhibits hyperalgesia | (132) |
| OTHER PAIN | |||
| Substance P | GF109203X
H7 |
Inhibits TH | (119, 133) |
| Brain Derived Neurotrophic Factor | RO-320432 | Inhibits TH, MA | (134) |
| Muscle Pain | GF109203X
NPC15437 Chelerythrine |
No change in mechanical thresholds | (135) |
| Acute Ethanol withdrawal | εV1-2 inhibitor
γV3-5 inhibitor |
Prevents MA & TH | (136) |
| MORPHINE HYPERALGESIA & TOLERANCE | |||
| Opioid analgesia | PKC activator | Suppression of analgesia
Decreased opioid reward |
(55, 57)
(114) |
| Opioid analgesia | PKCγ knock-out mice | Enhanced μ opioid receptor analgesia & G protein signaling | (137) |
| Acute morphine withdrawal | εV1-2 inhibitor
γV3-5 inhibitor |
Decreases MA & TH | (138) |
MA – mechanical allodynia, MH – mechanical hyperalgesia, TH – thermal hyperalgesia, ODN - antisense oligodeoxynucleotide
Sensitization of dorsal horn neurons is characterized by lower thresholds for neuronal activation and increased spontaneous and evoked discharges. As in peripheral nociceptive neurons, in central spinal cord neurons PKC is likely to serve as a point of convergence for the development of sensitization (Figure 4C). In dorsal horn neurons activation of PKC increases excitability, shortens the latency to first spike, increases spike frequency, and increases action potential amplitude (81). Findings in PKCγ knockout mice coupled with the localization of PKCγ in a sub-set of excitatory interneurons in lamina II of the spinal cord (80, 82) have implicated an important role for spinal PKCγ both in inflammatory and neuropathic pain. PKCγ containing glutamatergic interneurons receive input from a subset of TPRV1 positive primary afferents (22), and can modulate lamina V transmission neurons (83). This provides a bridge from peripheral stimuli to ascending transmission neurons. In addition, to an ideal anatomical localization for the transmission of nociceptive stimuli, PKCγ has been found to potentiate NMDA-induced currents in the spinal cord via alleviation of its voltage-dependent Mg2+ block (84) providing an ideal mechanism for PKC- dependent central sensitization.
As in the peripheral nervous system in which PKC activation was a point of convergence for the numerous peripheral effectors to ultimately alter current flow through the TPRV1 receptor resulting in thermal hyperalgesia, PKCγ activation in lamina II interneurons and the resulting change in NMDA currents serves as a point of convergence for multiple excitatory neurotransmitter and neuromodulatory peptides released at the primary afferent-spinal cord synapse. For instance, group I metabotropic glutamate receptors and neurokinin 1 receptors (binds Substance P) are G protein coupled receptors that couple to Gαq activating PLCβ leading to the production of IP3, DAG, and liberation of intracellular Ca2+ facilitating the activation of PKC. Group I metabotropic glutamate receptor activation has been shown to enhance NMDA receptor activity via PKC (84–88) and inhibition of these metabotropic receptors or PKC alleviate pain (78, 89). Phosphorylation of the NMDA receptor appears to occur indirectly via the formation of a signalling complex that includes metabotropic glutamate receptor 5, Shank (postsynaptic density protein associated with metabotropic glutamate receptors), postsynaptic density-95, and the tyrosine kinase Src (which is activated up-stream by PKC). Phosphorylation of the NMDA receptor NR2B subunit by Src increases channel kinetics and time spent open (90–93). Similarly, substance P can bind spinal neurokinin 1 receptors and activate PKC (94, 95). This evidence supports a role of PKC in directly mediating neuronal excitability within the spinal cord.
As in afferent neurons, PKC in dorsal horn neurons also plays a significant role in opioid tolerance. Daily intrathecal administration of morphine increases membrane bound PKC in lamina I and II (96). In addition, PKC α and γ are up-regulated in the spinal cord of morphine tolerant rats (97, 98). The NMDA NR1 sub-unit is also up-regulated in morphine tolerant animals (99) and blockade of the NMDA receptor can diminish the development of tolerance (100, 101). These findings suggest overlap in nociception and opioid tolerance highlighting the potential dual utility of PKC isozyme specific inhibitors in the treatment of chronic pain and the prevention of opioid tolerance.
III. d. Descending modulation from the brain
Much less is known about PKC in the brain as it relates to pain and analgesia. Descending pathways from the PAG through the RVM to the dorsal horn of the spinal cord is an important mechanism for modulating ascending spinal nociceptive transmission and another potential level for modulation of pain and analgesia by PKC (Table 3). Electrical stimulation of the PAG results in a potent analgesia at the spinal level. PKC activation in the spinal cord can antagonize this descending inhibition (102). In addition, PKC activation in a model of inflammatory pain can enhance descending facilitation from the RVM via phosphorylation of the AMPA receptor (103), and the NR2a subunit of the NMDA receptor (104). So, similar to at the level of the spinal cord, activation of PKC in the brain can enhance nociception. In addition, as at the other levels of the neuro-axis supraspinal morphine exposure activates PKC and enhances excitatory glutamate signaling (105). While much work remains in understanding how PKC in the brain modulates pain and analgesia the data to date suggests similarity to mechanisms described in afferent and spinal cord neurons.
Table 3. Inhibition of nociception by brain (intracerebrobentricular) administration of PKC inhibitors.
A summary of the studies that implicate PKC in the brain in cutaneous, inflammatory, and neuropathic pain models.
| Pain Model | PKC treatment | Change Elicited | Refs |
|---|---|---|---|
| CUTANEOUS PAIN | |||
| Patch clamp PAG neurons | PMA GF109203X | modulated Cl- current | (139) |
| Abdominal constriction | calphostin C chelerythrine | Acute tolerance to N2O-induced antinociception | (140) |
| Acetyl-L-carnitine antinociception | Calphostin C and chelerytrine | Potentiated the antinociceptive effect of ALCARe | (141) |
| INFLAMMATORY PAIN | |||
| Brain Derived Neurotrophic Factor | chelerythrine | Decreased NR2A receptor phosphorylation in RVM | (104) |
| Complete Freud’s Adjuvant | chelerytrine | Attenuated upregulation of phosphoserine 831 GluR1 RVM | (103) |
| Histamine agonist | Calphostin C chelerytrine | Reverse hyperalgesia | (142) |
| MORPHINE HYPERALGESIA & TOLERANCE | |||
| Acute Morphine | NPC 15437 (PKC antagonist) | Decrease c-Fos expression in striatum and cingulate cortex
Translocation of PKC βII to plasma membrane in cortical and striatum neurons |
(143) |
| Low dose morphine hyperalgesia | Calphostin C
ODN to PKCγ |
Prevent morphine hyperalgesia
Mild analgesic Downregulated PKCγ in PAG |
(105) |
| Opioid analgesia | ODN to PKCγ
PKC α, γ, ε inhibitors |
Enhanced analgesia | (105, 144) |
| Chronic morphine tolerance | GF109203X
Bisindolylmaleimide I, Go-7874 |
Decreased glutamatergic synaptic transmission in NRM
Reversed tolerance |
(145–147) |
MA – mechanical allodynia, MH – mechanical hyperalgesia, TH – thermal hyperalgesia, ODN - antisense oligodeoxynucleotide, PAG – periaqueductal gray, RVM – rostral ventromedial medulla, NRM – nucleus raphe magnus
IV. Conclusions
In summary, this review has highlighted the importance of different PKC isozymes at different levels of the neuro-axis in both pain and analgesia such that PKC α and ε appear to be involved in peripheral nociception while PKCγ is important to central nociception. This does not exclude a role for other PKC isozymes in pain and analgesia but highlights the limited research on these other isozymes. This review also highlights the importance of PKC isozymes in mediating a switch from a protective and evolutionarily conserved physiological pain (those necessary to avoid tissue damage) to a more prolonged and pathological pain (pain of a higher intensity or longer duration than required to avoid or resolve tissue damage) as highlighted by the intact acute pain but absent inflammatory or neuropathic pain in PKC ε and γ knockout mice. This bodes well for the pursuit of isozyme specific inhibitors for the treatment of pain since the end goal in the development of novel analgesic agents is to develop therapeutics that leave intact normal physiological pain systems while preventing or attenuating pathological pain. New therepeutics that target specific isozymes of PKC may be on the horizon. In fact, preclinical development of small peptide PKC ε and γ inhibitors to treat inflammatory and neuropathic pain is underway (Kai Pharmaceuticals, South San Francisco, CA).
In conclusion, this review highlights the role of PKCε in peripheral sensitization and PKCγ in central sensitization. In both cases of sensitization it appears that PKC functions to integrate numerous receptor pathways into final effectors that increase excitatory signaling and decrease inhibitory signaling, thus promoting pain. These results highlight the idea that adequate analgesia will require either a cocktail approach at the level of the individual receptor or alternatively inhibition of second messenger pathways in an isozyme specific manner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–5. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–62. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Fields HL. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol. 1979;187:513–31. doi: 10.1002/cne.901870304. [DOI] [PubMed] [Google Scholar]
- 4.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–45. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- 5.Mochly-Rosen D, Basbaum AI, Koshl DE., Jr Distinct cellular and regional localization of immunoreactive protein kinase C in rat brain. Proc Natl Acad Sci U S A. 1987;84:4660–4. doi: 10.1073/pnas.84.13.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang FL, Young WS, 3rd, Yoshida Y, Huang KP. Developmental expression of protein kinase C isozymes in rat cerebellum. Brain Res Dev Brain Res. 1990;52:121–30. doi: 10.1016/0165-3806(90)90227-p. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Ase K, Sawamura S, Kikkawa U, Saito N, Tanaka C, Nishizuka Y. Postnatal development of brain-specific subspecies of protein kinase C in rat. Journal Neuroscience. 1988;8:1678–1683. doi: 10.1523/JNEUROSCI.08-05-01678.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–33. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito Y, Teshima R, Takagi K, Ikebuchi H, Yamazaki T, Sawada J. Activation of protein kinase C alpha enhances human growth hormone-binding protein release. Mol Cell Endocrinol. 1998;146:197–205. doi: 10.1016/s0303-7207(98)00151-8. [DOI] [PubMed] [Google Scholar]
- 10.Aley K, Messing R, Mochly-Rosen D, Levine J. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. Journal Neuroscience. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igwe O, Chronwall B. Hyperalgesia induced by peripheral inflammation is mediated by protein kinase C beta II isozyme in the rat spinal cord. Neuroscience. 2001;104:875–890. doi: 10.1016/s0306-4522(01)00107-5. [DOI] [PubMed] [Google Scholar]
- 12.Dray A, Bettaney J, Forster P, Perkins MN. Bradykinin-induced stimulation of afferent fibres is mediated through protein kinase C. Neurosci Lett. 1988;91:301–7. doi: 10.1016/0304-3940(88)90697-0. [DOI] [PubMed] [Google Scholar]
- 13.Rang HP, Ritchie JM. Depolarization of nonmyelinated fibers of the rat vagus nerve produced by activation of protein kinase C. J Neurosci. 1988;8:2606–17. doi: 10.1523/JNEUROSCI.08-07-02606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schepelmann K, Messlinger K, Schmidt RF. The effects of phorbol ester on slowly conducting afferents of the cat’s knee joint. Exp Brain Res. 1993;92:391–8. doi: 10.1007/BF00229027. [DOI] [PubMed] [Google Scholar]
- 15.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–9. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuirk SM, Dolphin AC. G-protein mediation in nociceptive signal transduction: an investigation into the excitatory action of bradykinin in a subpopulation of cultured rat sensory neurons. Neuroscience. 1992;49:117–28. doi: 10.1016/0306-4522(92)90079-h. [DOI] [PubMed] [Google Scholar]
- 17.Burgess GM, Mullaney I, McNeill M, Dunn PM, Rang HP. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989;9:3314–25. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–60. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–24. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 20.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–60. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 23.Olah Z, Karai L, Iadarola MJ. Protein kinase C(alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem. 2002;277:35752–9. doi: 10.1074/jbc.M201551200. [DOI] [PubMed] [Google Scholar]
- 24.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–72. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhou ZS, Zhao ZQ. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacology. 2001;41:601–608. doi: 10.1016/s0028-3908(01)00106-x. [DOI] [PubMed] [Google Scholar]
- 26.Crandall M, Kwash J, Yu W, White G. Activation of protein kinase C sensitizes human VR1 to capsaicin and to moderate decreases in pH at physiological temperatures in Xenopus oocytes. Pain. 2002;98:109–17. doi: 10.1016/s0304-3959(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 27.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–8. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 28.Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–16. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 30.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–90. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 31.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–25. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Cunha JM, Rae GA, Ferreira SH, Cunha Fde Q. Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase C. Eur J Pharmacol. 2004;501:87–94. doi: 10.1016/j.ejphar.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Jimenez-Andrade JM, Zhou S, Yamani A, Valencia de Ita S, Castaneda-Hernandez G, Carlton SM. Mechanism by which peripheral galanin increases acute inflammatory pain. Brain Res. 2005;1056:113–7. doi: 10.1016/j.brainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–62. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–9. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem. 2002;277:50463–8. doi: 10.1074/jbc.M208848200. [DOI] [PubMed] [Google Scholar]
- 39.Deval E, Salinas M, Baron A, Lingueglia E, Lazdunski M. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J Biol Chem. 2004;279:19531–9. doi: 10.1074/jbc.M313078200. [DOI] [PubMed] [Google Scholar]
- 40.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–52. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 41.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–90. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahlgren SC, Levine JD. Protein kinase C inhibitors decrease hyperalgesia and C-fiber hyperexcitability in the streptozotocin-diabetic rat. J Neurophysiol. 1994;72:684–92. doi: 10.1152/jn.1994.72.2.684. [DOI] [PubMed] [Google Scholar]
- 44.Shangguan Y, Hall KE, Neubig RR, Wiley JW. Diabetic neuropathy: inhibitory G protein dysfunction involves PKC-dependent phosphorylation of Goalpha. J Neurochem. 2003;86:1006–14. doi: 10.1046/j.1471-4159.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 45.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–27. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- 46.Gamse R, Molnar A, Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979;25:629–36. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- 47.Sorkin LS, McAdoo DJ. Amino acids and serotonin are released into the lumbar spinal cord of the anesthetized cat following intradermal capsaicin injections. Brain Res. 1993;607:89–98. doi: 10.1016/0006-8993(93)91492-b. [DOI] [PubMed] [Google Scholar]
- 48.Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–67. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frayer SM, Barber LA, Vasko MR. Activation of protein kinase C enhances peptide release from rat spinal cord slices. Neurosci Lett. 1999;265:17–20. doi: 10.1016/s0304-3940(99)00090-7. [DOI] [PubMed] [Google Scholar]
- 50.Barber LA, Vasko MR. Activation of protein kinase C augments peptide release from rat sensory neurons. J Neurochem. 1996;67:72–80. doi: 10.1046/j.1471-4159.1996.67010072.x. [DOI] [PubMed] [Google Scholar]
- 51.Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br J Pharmacol. 1998;125:1625–6. doi: 10.1038/sj.bjp.0702260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sweitzer S, Allen C, Zissen M, Kendig JJ. Mechanical alloydnia and thermal hyperalgesia upon acute opioid withdrawal in the neonatal rat. Pain. doi: 10.1016/j.pain.2004.04.003. (companion paper) [DOI] [PubMed] [Google Scholar]
- 53.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- 54.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–14. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 55.Zhang LJ, Wang XJ, Han JS. Phorbol ester suppression of opioid analgesia in rats. Life Sci. 1990;47:1775–82. doi: 10.1016/0024-3205(90)90352-r. [DOI] [PubMed] [Google Scholar]
- 56.Kavaliers M, Ossenkopp KP, Tysdale DM. Evidence for the involvement of protein kinase C in the modulation of morphine-induced ‘analgesia’ and the inhibitory effects of exposure to 60-Hz magnetic fields in the snail, Cepaea nemoralis. Brain Res. 1991;554:65–71. doi: 10.1016/0006-8993(91)90172-r. [DOI] [PubMed] [Google Scholar]
- 57.Narita M, Mizoguchi H, Kampine JP, Tseng LF. Role of protein kinase C in desensitization of spinal delta-opioid-mediated antinociception in the mouse. Br J Pharmacol. 1996;118:1829–35. doi: 10.1111/j.1476-5381.1996.tb15610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narita M, Ohsawa M, Mizoguchi H, Kamei J, Tseng LF. Pretreatment with protein kinase C activator phorbol 12,13-dibutyrate attenuates the antinociception induced by mu- but not epsilon-opioid receptor agonist in the mouse. Neuroscience. 1997;76:291–8. doi: 10.1016/s0306-4522(96)00354-5. [DOI] [PubMed] [Google Scholar]
- 59.Narita M, Tseng LF. Stimulation of spinal delta-opioid receptors in mice selectively enhances the attenuation of delta-opioid receptor-mediated antinociception by antisense oligodeoxynucleotide. Eur J Pharmacol. 1995;284:185–9. doi: 10.1016/0014-2999(95)00414-g. [DOI] [PubMed] [Google Scholar]
- 60.Aley K, Levine J. Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in rat. Journal Neuroscience. 1997;17:8018–8023. doi: 10.1523/JNEUROSCI.17-20-08018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci. 2006;27:558–65. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Agonist-dependent phosphorylation of the mouse delta-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase C. Mol Pharmacol. 1995;48:173–7. [PubMed] [Google Scholar]
- 63.Kovoor A, Nappey V, Kieffer BL, Chavkin C. Mu and delta opioid receptors are differentially desensitized by the coexpression of beta-adrenergic receptor kinase 2 and beta-arrestin 2 in xenopus oocytes. J Biol Chem. 1997;272:27605–11. doi: 10.1074/jbc.272.44.27605. [DOI] [PubMed] [Google Scholar]
- 64.Zhao J, Pei G, Huang YL, Zhong FM, Ma L. Carboxyl terminus of delta opioid receptor is required for agonist-dependent receptor phosphorylation. Biochem Biophys Res Commun. 1997;238:71–6. doi: 10.1006/bbrc.1997.7242. [DOI] [PubMed] [Google Scholar]
- 65.Appleyard SM, Celver J, Pineda V, Kovoor A, Wayman GA, Chavkin C. Agonist-dependent desensitization of the kappa opioid receptor by G protein receptor kinase and beta-arrestin. J Biol Chem. 1999;274:23802–7. doi: 10.1074/jbc.274.34.23802. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. Differential mu opiate receptor phosphorylation and desensitization induced by agonists and phorbol esters. J Biol Chem. 1996;271:11449–54. doi: 10.1074/jbc.271.19.11449. [DOI] [PubMed] [Google Scholar]
- 67.El Kouhen R, Kouhen OM, Law PY, Loh HH. The absence of a direct correlation between the loss of [D-Ala2, MePhe4,Gly5-ol]Enkephalin inhibition of adenylyl cyclase activity and agonist-induced mu-opioid receptor phosphorylation. J Biol Chem. 1999;274:9207–15. doi: 10.1074/jbc.274.14.9207. [DOI] [PubMed] [Google Scholar]
- 68.Fan GH, Zhao J, Wu YL, Lou LG, Zhang Z, Jing Q, Ma L, Pei G. N-Methyl-D-aspartate attenuates opioid receptor-mediated G protein activation and this process involves protein kinase C. Mol Pharmacol. 1998;53:684–90. doi: 10.1124/mol.53.4.684. [DOI] [PubMed] [Google Scholar]
- 69.Cai YC, Ma L, Fan GH, Zhao J, Jiang LZ, Pei G. Activation of N-methyl-D-aspartate receptor attenuates acute responsiveness of delta-opioid receptors. Mol Pharmacol. 1997;51:583–7. doi: 10.1124/mol.51.4.583. [DOI] [PubMed] [Google Scholar]
- 70.Ohsawa M, Tanaka S, Kamei J. Possible mechanisms for insulin-induced attenuation of the antinociceptive effect of [D-Ala2, N-MePhe4, Gly-ol5]enkephalin. Eur J Pharmacol. 1999;373:181–6. doi: 10.1016/s0014-2999(99)00273-3. [DOI] [PubMed] [Google Scholar]
- 71.Ueda H, Inoue M, Matsumoto T. Protein kinase C-mediated inhibition of mu-opioid receptor internalization and its involvement in the development of acute tolerance to peripheral mu-agonist analgesia. J Neurosci. 2001;21:2967–73. doi: 10.1523/JNEUROSCI.21-09-02967.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiang B, Yu GH, Guo J, Chen L, Hu W, Pei G, Ma L. Heterologous activation of protein kinase C stimulates phosphorylation of delta-opioid receptor at serine 344, resulting in beta-arrestin- and clathrin-mediated receptor internalization. J Biol Chem. 2001;276:4709–16. doi: 10.1074/jbc.M006187200. [DOI] [PubMed] [Google Scholar]
- 73.McMahon S, Koltzenburg M. The changing role of primary afferent neurones in pain. Pain. 1990;43:269–72. doi: 10.1016/0304-3959(90)90024-8. [DOI] [PubMed] [Google Scholar]
- 74.Si JQ, Zhang ZQ, Li CX, Wang LF, Yang YL, Li ZW. Modulatory effect of substance P on GABA-activated currents from rat dorsal root ganglion. Acta Pharmacol Sin. 2004;25:623–9. [PubMed] [Google Scholar]
- 75.Yamada K, Akasu T. Substance P suppresses GABAA receptor function via protein kinase C in primary sensory neurones of bullfrogs. J Physiol. 1996;496(Pt 2):439–49. doi: 10.1113/jphysiol.1996.sp021697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma KT, Si JQ, Zhang ZQ, Zhao L, Fan P, Jin JL, Li XZ, Zhu L. Modulatory effect of CCK-8S on GABA-induced depolarization from rat dorsal root ganglion. Brain Res. 2006;1121:66–75. doi: 10.1016/j.brainres.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 77.Yashpal K, Pitcher G, Parent A, Quirion R, Coderre T. Noxious thermal and chemical stimulation induce increases in 3H-phorbol 12,13-dibutyrate binding in spinal cord dorsal horn as well as persistent pain and hyperalgesia, which is reduced by inhibition of protein kinase C. Journal Neuroscience. 1995;15:3263–3272. doi: 10.1523/JNEUROSCI.15-05-03263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yashpal K, Fisher K, Chabot JG, Coderre TJ. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain. 2001;94:17–29. doi: 10.1016/S0304-3959(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 79.Mao J, Mayer DJ, Hayes RL, Price DD. Spatial patterns of increased spinal cord membrane-bound protein kinase C and their relation to increases in 14C-2-deoxyglucose metabolic activity in rats with painful peripheral mononeuropathy. J Neurophysiol. 1993;70:470–81. doi: 10.1152/jn.1993.70.2.470. [DOI] [PubMed] [Google Scholar]
- 80.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–83. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 81.Hu HJ, Gereau RWt. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–8. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- 82.Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- 83.Martin WJ, Malmberg AB, Basbaum AI. PKCgamma contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci. 2001;21:5321–7. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen L, Huang L. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 85.Harvey J, Collingridge GL. Signal transduction pathways involved in the acute potentiation of NMDA responses by 1S,3R-ACPD in rat hippocampal slices. Br J Pharmacol. 1993;109:1085–90. doi: 10.1111/j.1476-5381.1993.tb13733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelso SR, Nelson TE, Leonard JP. Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J Physiol. 1992;449:705–18. doi: 10.1113/jphysiol.1992.sp019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kitamura Y, Miyazaki A, Yamanaka Y, Nomura Y. Stimulatory effects of protein kinase C and calmodulin kinase II on N-methyl-D-aspartate receptor/channels in the postsynaptic density of rat brain. J Neurochem. 1993;61:100–9. doi: 10.1111/j.1471-4159.1993.tb03542.x. [DOI] [PubMed] [Google Scholar]
- 88.Raymond LA, Tingley WG, Blackstone CD, Roche KW, Huganir RL. Glutamate receptor modulation by protein phosphorylation. J Physiol Paris. 1994;88:181–92. doi: 10.1016/0928-4257(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 89.Fundytus ME, Yashpal K, Chabot JG, Osborne MG, Lefebvre CD, Dray A, Henry JL, Coderre TJ. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–67. doi: 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–73. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 92.Yu XM, Askalan R, Keil GJ, 2nd, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–8. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 93.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–21. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coderre TJ, Yashpal K. Intracellular messengers contributing to persistent nociception and hyperalgesia induced by L-glutamate and substance P in the rat formalin pain model. Eur J Neurosci. 1994;6:1328–34. doi: 10.1111/j.1460-9568.1994.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 95.Monastyrskaya K, Hostettler A, Buergi S, Draeger A. The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J Biol Chem. 2005;280:7135–46. doi: 10.1074/jbc.M405806200. [DOI] [PubMed] [Google Scholar]
- 96.Mayer DJ, Mao J, Price DD. The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain. 1995;61:365–74. doi: 10.1016/0304-3959(95)00023-L. [DOI] [PubMed] [Google Scholar]
- 97.Mao J, Price DD, Phillips LL, Lu J, Mayer DJ. Increases in protein kinase C gamma immunoreactivity in the spinal cord of rats associated with tolerance to the analgesic effects of morphine. Brain Res. 1995;677:257–67. doi: 10.1016/0006-8993(95)00161-i. [DOI] [PubMed] [Google Scholar]
- 98.Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh T. Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain. 2000;85:395–404. doi: 10.1016/S0304-3959(99)00281-X. [DOI] [PubMed] [Google Scholar]
- 99.Lim G, Wang S, Zeng Q, Sung B, Yang L, Mao J. Expression of spinal NMDA receptor and PKCgamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–54. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–12. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dunbar S, Yaksh TL. Concurrent spinal infusion of MK801 blocks spinal tolerance and dependence induced by chronic intrathecal morphine in the rat. Anesthesiology. 1996;84:1177–88. doi: 10.1097/00000542-199605000-00020. [DOI] [PubMed] [Google Scholar]
- 102.Peng YB, Lin Q, Willis WD. Involvement of protein kinase C in responses of rat dorsal horn neurons to mechanical stimuli and periaqueductal gray descending inhibition. Exp Brain Res. 1997;114:561–70. doi: 10.1007/pl00005664. [DOI] [PubMed] [Google Scholar]
- 103.Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366:201–5. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 104.Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–37. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galeotti N, Stefano GB, Guarna M, Bianchi E, Ghelardini C. Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain. 2006;123:294–305. doi: 10.1016/j.pain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Souza AL, Moreira FA, Almeida KR, Bertollo CM, Costa KA, Coelho MM. In vivo evidence for a role of protein kinase C in peripheral nociceptive processing. Br J Pharmacol. 2002;135:239–47. doi: 10.1038/sj.bjp.0704434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferreira J, Triches KM, Medeiros R, Calixto JB. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain. 2005;117:171–81. doi: 10.1016/j.pain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Jones TL, Sorkin LS. Activated PKA and PKC, but not CaMKIIalpha, are required for AMPA/Kainate-mediated pain behavior in the thermal stimulus model. Pain. 2005;117:259–70. doi: 10.1016/j.pain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 109.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–71. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Motta EM, Calixto JB, Rae GA. Mechanical hyperalgesia induced by endothelin-1 in rats is mediated via phospholipase C, protein kinase C, and MAP kinases. Exp Biol Med (Maywood) 2006;231:1141–5. [PubMed] [Google Scholar]
- 111.Yang L, Zhang FX, Huang F, Lu YJ, Li GD, Bao L, Xiao HS, Zhang X. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur J Neurosci. 2004;19:871–83. doi: 10.1111/j.0953-816x.2004.03121.x. [DOI] [PubMed] [Google Scholar]
- 112.Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000;20:8614–9. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Narita M, Imai S, Oe K, Narita M, Kubota C, Yajima Y, Yamazaki M, Suzuki T. Induction of c-fos expression in the mouse brain associated with hyperalgesia induced by intrathecal injection of protein kinase C activator. Brain Res. 2004;1015:189–93. doi: 10.1016/j.brainres.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 114.Oe K, Narita M, Imai S, Shibasaki M, Kubota C, Kasukawa A, Hamaguchi M, Yajima Y, Yamazaki M, Suzuki T. Inhibition of the morphine-induced rewarding effect by direct activation of spinal protein kinase C in mice. Psychopharmacology (Berl) 2004;177:55–60. doi: 10.1007/s00213-004-1929-0. [DOI] [PubMed] [Google Scholar]
- 115.Palecek J, Paleckova V, Dougherty PM, Willis WD. The effect of phorbol esters on the responses of primate spinothalamic neurons to mechanical and thermal stimuli. J Neurophysiol. 1994;71:529–37. doi: 10.1152/jn.1994.71.2.529. [DOI] [PubMed] [Google Scholar]
- 116.Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Capsaicin-induced sensitization of primate spinothalamic tract cells is prevented by a protein kinase C inhibitor. Brain Res. 1997;772:82–6. doi: 10.1016/s0006-8993(97)00876-7. [DOI] [PubMed] [Google Scholar]
- 117.Coderre TJ. Contribution of protein kinase C to central sensitization and persistent pain following tissue injury. Neurosci Lett. 1992;140:181–4. doi: 10.1016/0304-3940(92)90097-q. [DOI] [PubMed] [Google Scholar]
- 118.Coderre TJ, Yashpal K, Henry JL. Specific contribution of lumbar spinal mechanisms to persistent nociceptive responses in the formalin test. Neuroreport. 1994;5:1337–40. [PubMed] [Google Scholar]
- 119.Wajima Z, Hua XY, Yaksh TL. Inhibition of spinal protein kinase C blocks substance P-mediated hyperalgesia. Brain Res. 2000;877:314–21. doi: 10.1016/s0006-8993(00)02714-1. [DOI] [PubMed] [Google Scholar]
- 120.Nie H, Wang H, Zhang RX, Gao WC, Qiao JT, Dafny N. Is protein kinase C (PKC) involved in nociception? Int J Neurosci. 2006;116:1115–24. doi: 10.1080/00207450600575466. [DOI] [PubMed] [Google Scholar]
- 121.Sweitzer SM, Wong SM, Peters MC, Mochly-Rosen D, Yeomans DC, Kendig JJ. Protein kinase C {epsilon} and {gamma}: Involvement in formalin-induced nociception in neonatal rats. J Pharmacol Exp Ther. 2004:616–625. doi: 10.1124/jpet.103.060350. [DOI] [PubMed] [Google Scholar]
- 122.Li KC, Chen J. Differential roles of spinal protein kinases C and a in development of primary heat and mechanical hypersensitivity induced by subcutaneous bee venom chemical injury in the rat. Neurosignals. 2003;12:292–301. doi: 10.1159/000075311. [DOI] [PubMed] [Google Scholar]
- 123.Li KC, Zheng JH, Chen J. Involvement of spinal protein kinase C in induction and maintenance of both persistent spontaneous flinching reflex and contralateral heat hyperalgesia induced by subcutaneous bee venom in the conscious rat. Neurosci Lett. 2000;285:103–6. doi: 10.1016/s0304-3940(00)01039-9. [DOI] [PubMed] [Google Scholar]
- 124.Yajima Y, Narita M, Shimamura M, Narita M, Kubota C, Suzuki T. Differential involvement of spinal protein kinase C and protein kinase A in neuropathic and inflammatory pain in mice. Brain Res. 2003;992:288–93. doi: 10.1016/j.brainres.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 125.Munro FE, Fleetwood-Walker SM, Mitchell R. Evidence for a role of protein kinase C in the sustained activation of rat dorsal horn neurons evoked by cutaneous mustard oil application. Neurosci Lett. 1994;170:199–202. doi: 10.1016/0304-3940(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 126.Ohsawa M, Narita M, Mizoguchi H, Cheng E, Tseng LF. Reduced hyperalgesia induced by nerve injury, but not by inflammation in mice lacking protein kinase C gamma isoform. Eur J Pharmacol. 2001;429:157–60. doi: 10.1016/s0014-2999(01)01317-6. [DOI] [PubMed] [Google Scholar]
- 127.Hua XY, Chen P, Yaksh TL. Inhibition of spinal protein kinase C reduces nerve injury-induced tactile allodynia in neuropathic rats. Neurosci Lett. 1999;276:99–102. doi: 10.1016/s0304-3940(99)00818-6. [DOI] [PubMed] [Google Scholar]
- 128.Gardell LR, Ibrahim M, Wang R, Wang Z, Ossipov MH, Malan TP, Jr, Porreca F, Lai J. Mouse strains that lack spinal dynorphin upregulation after peripheral nerve injury do not develop neuropathic pain. Neuroscience. 2004;123:43–52. doi: 10.1016/j.neuroscience.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 129.Wen ZH, Guo YW, Chang YC, Wong CS. D-2-amino-5-phosphonopentanoic acid inhibits intrathecal pertussis toxin-induced thermal hyperalgesia and protein kinase Cgamma up-regulation. Brain Res. 2003;963:1–7. doi: 10.1016/s0006-8993(02)03751-4. [DOI] [PubMed] [Google Scholar]
- 130.Ambrosini SS, Coderre TJ. Intracellular messengers involved in spontaneous pain, heat hyperalgesia, and mechanical allodynia induced by intrathecal dihydroxyphenylglycine. Neurosci Lett. 2006;409:224–9. doi: 10.1016/j.neulet.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 131.Ohsawa M, Kamei J. Modification of the expression of naloxone-precipitated withdrawal signs in morphine-dependent mice by diabetes: possible involvement of protein kinase C. Jpn J Pharmacol. 1999;79:303–11. doi: 10.1254/jjp.79.303. [DOI] [PubMed] [Google Scholar]
- 132.Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. Eur J Neurosci. 2006;24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- 133.Zhang WJ, Shi ZX, Wang BB, Cui YJ, Guo JZ, Li B. Allitridum mimics effect of ischemic preconditioning by activation of protein kinase C. Acta Pharmacol Sin. 2001;22:132–6. [PubMed] [Google Scholar]
- 134.Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–94. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 135.Sluka KA, Audette KM. Activation of protein kinase C in the spinal cord produces mechanical hyperalgesia by activating glutamate receptors, but does not mediate chronic muscle-induced hyperalgesia. Mol Pain. 2006;2:13. doi: 10.1186/1744-8069-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shumilla JA, Liron T, Kendig M-RDJ, SM S. Ethanol withdrawal-associated allodynia and hyperalgesia: age-dependent regulation by protein kinase C epsilon and gamma isozymes. J of Pain. 2005;6:539–45. doi: 10.1016/j.jpain.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 137.Narita M, Mizoguchi H, Suzuki T, Narita M, Dun NJ, Imai S, Yajima Y, Nagase H, Suzuki T, Tseng LF. Enhanced mu-opioid responses in the spinal cord of mice lacking protein kinase Cgamma isoform. J Biol Chem. 2001;276:15409–14. doi: 10.1074/jbc.M009716200. [DOI] [PubMed] [Google Scholar]
- 138.Sweitzer SM, Allen CP, Zissen MH, Kendig JJ. Mechanical allodynia and thermal hyperalgesia upon acute opioid withdrawal in the neonatal rat. Pain. 2004;110:269–80. doi: 10.1016/j.pain.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Lee JJ, Hahm ET, Min BI, Han SH, Cho JJ, Cho YW. Roles of protein kinase A and C in the opioid potentiation of the GABAA response in rat periaqueductal gray neuron. Neuropharmacology. 2003;44:573–83. doi: 10.1016/s0028-3908(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 140.Ishikawa M, Matsushita Y, Abe K, Utsunomiya I, Hoshi K, Quock RM, Taguchi K. Involvement of brain protein kinase C in nitrous oxide-induced antinociception in mice. Neuroscience. 2006;140:227–33. doi: 10.1016/j.neuroscience.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Galeotti N, Bartolini A, Calvani M, Nicolai R, Ghelardini C. Acetyl-L-carnitine requires phospholipase C-IP3 pathway activation to induce antinociception. Neuropharmacology. 2004;47:286–94. doi: 10.1016/j.neuropharm.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 142.Galeotti N, Malmberg-Aiello P, Bartolini A, Schunack W, Ghelardini C. H1-receptor stimulation induces hyperalgesia through activation of the phospholipase C-PKC pathway. Neuropharmacology. 2004;47:295–303. doi: 10.1016/j.neuropharm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 143.Harlan RE, Kailas SR, Tagoe CE, Garcia MM. Morphine actions in the rat forebrain: role of protein kinase C. Brain Res Bull. 2004;62:285–95. doi: 10.1016/j.brainresbull.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 144.Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain. 2006 doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 145.Bie B, Pan ZZ. Increased glutamate synaptic transmission in the nucleus raphe magnus neurons from morphine-tolerant rats. Mol Pain. 2005;1:7. doi: 10.1186/1744-8069-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Smith FL, Javed RR, Elzey MJ, Dewey WL. The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res. 2003;985:78–88. doi: 10.1016/s0006-8993(03)03170-6. [DOI] [PubMed] [Google Scholar]
- 147.Smith FL, Javed RR, Smith PA, Dewey WL, Gabra BH. PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol Res. 2006;54:474–80. doi: 10.1016/j.phrs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 148.Sweitzer S, Kheifets V, Jones T, Mochly-Rosen D, Kendig J, Yeomans D. Developmental regulation of formalin-induced nociception by protein kinase C epsilon and gamma. 10th World Congress On Pain; San Diego, CA. 2002. [Google Scholar]