Abstract
Genome stability relies on faithful DNA repair both in mitosis and in meiosis. Here, we report on a Caenorhabditis elegans protein that we found to be homologous to the mammalian repair-related protein CtIP and to the budding yeast Com1/Sae2 recombination protein. A com-1 mutant displays normal meiotic chromosome pairing but forms irregular chromatin aggregates instead of diakinesis bivalents. While meiotic DNA double-strand breaks (DSBs) are formed, they appear to persist or undergo improper repair. Despite the presence of DSBs, the recombination protein RAD-51, which is known to associate with single-stranded DNA (ssDNA) flanking DSBs, does not localize to meiotic chromosomes in the com-1 mutant. Exposure of the mutant to γ-radiation, however, induces RAD-51 foci, which suggests that the failure of RAD-51 to load is specific to meiotic (SPO-11-generated) DSBs. These results suggest that C. elegans COM-1 plays a role in the generation of ssDNA tails that can load RAD-51, invade homologous DNA tracts and thereby initiate recombination. Extrapolating from the worm homolog, we expect similar phenotypes for mutations in the mammalian tumor suppressor CtIP.
Keywords: Caenorhabditis elegans, crossing over, DNA repair, meiosis, recombination
Introduction
To compensate for genome duplication upon fertilization, sexually reproducing organisms rely on the haploidization of their chromosome complement during meiosis. This reduction is achieved by a sequence of two divisions without intervening DNA replication. During the first of these divisions (meiosis I), homologous (corresponding paternal and maternal) chromosomes separate, leaving nuclei with a single copy of each chromosome. To ensure the orderly segregation of homologous chromosomes in meiosis I, they form pairs (bivalents), first by being connected by the synaptonemal complex (SC) and after its disassembly, by chiasmata (for a review, see Zickler and Kleckner, 1999). Chiasmata are the consequence of a physical exchange (recombination) between DNA molecules of the homologous chromosomes (crossing over).
Crossing over and other forms of meiotic recombination are initiated by double-strand breaks (DSBs) in DNA, which are generated by the meiosis-specific protein SPO-11. SPO-11 cuts DNA by a topoisomerase II-like transesterase reaction by which SPO-11 is covalently bound to the DNA ends at DSBs (Bergerat et al, 1997; Keeney et al, 1997). In the next step, the 5′–3′ half strands of the flanking DNA are resected, creating 3′ single-stranded overhangs on both sides. The loading of the single strands with RAD-51 (and in most organisms also Dmc1) protomers (for a review, see Shinohara and Shinohara, 2004) helps these strands to invade double-stranded homologous DNA regions (with a preference for DNA on the homologous chromosome) and to initiate strand exchange. At this point, a decision is made as to whether the strand exchange will result in a crossover or a non-reciprocal exchange, a conversion (for recent reviews, see Bishop and Zickler, 2004; Hollingsworth and Brill, 2004; Whitby, 2005). In either case, the DSB will be repaired by using the homologous sequence as a template for the synthesis of DNA spanning the DSB.
The removal of SPO-11 and single-stranded DNA (ssDNA) resection is essential for the recombinational repair of DSBs. There are several mutations known in budding yeast in which these steps are compromised. Mre11 and Rad50, both of which also have mitotic functions, are required for the formation of DSBs, but certain rad50 and mre11 separation-of-function mutants exist, which cannot remove Spo11 from DSBs (Alani et al, 1990; Baudat and Nicolas, 1997; Nairz and Klein, 1997; Keeney, 2001). In addition, in com1Δ/sae2Δ mutants, Spo11 remains covalently attached to DSB ends, which are left unresected (McKee and Kleckner, 1997; Prinz et al, 1997; Neale et al, 2005; Prieler et al, 2005).
COM1/SAE2 was originally discovered independently by two screens in budding yeast for mutants that can only sporulate if meiotic DSBs are not induced due to the absence of Spo11p. Null mutants featured unresected DSBs, absence of recombination, reduced homologous synapsis and a very weak sensitivity to methyl methanesulfonate (MMS) (McKee and Kleckner, 1997; Prinz et al, 1997). Here, we report the discovery of a putative Caenorhabditis elegans homolog of Com1p/Sae2p and the study of its meiotic function by mutant analysis. As recombination is not required for SC formation in C. elegans, pairing functions are not compromised by defects in the recombination pathway in this organism, which therefore facilitates the study of recombination-specific phenotypes elicited by the com-1 mutation.
In addition to the homology among the Com1/Sae2 proteins with meiotic roles in C. elegans, Saccharomyces cerevisiae and Arabidopsis (accompanying paper by Uanschou et al), we note their relationship to the mammalian CtIP tumor suppressor protein. Our results, therefore, also predict a potential function of CtIP in meiotic recombination and genome stability in metazoans.
Results
Identification of the C. elegans com-1 gene and of com-1 mutants
Alleles of C. elegans com-1 were first obtained from a mutagenesis screen in the laboratory of RS and characterized as causing maternal-effect embryonic lethality (Gönczy et al, 1999). The screen was designed to recover mutations that are linked to the unc-32 locus and map to the right portion of chromosome III from tra-1 to dpy-1 (see Materials and methods).
Two mutants from this screen, carrying alleles let(t1626) and let(t1489) showed hallmarks of meiotic recombination defects (see below). Crosses of animals carrying the two alleles demonstrated that let(t1626) and let(t1489) are non-complementing and represent a single locus. To identify candidate genes within the interval between tra-1 and dpy-1, which could correspond to the mutated locus, we assessed the presence of homologs of known DNA-repair and/or meiotic proteins in this region. With this aim, we extracted all protein sequences from the worm and human genomic databases associated with the gene ontology terms ‘DNA repair' or ‘meiosis' (http://wormbase.org; Hammond and Birney, 2004). Approximately 60 genes within the tra-1 to dpy-1 interval could be directly linked to a known meiotic or repair protein based on sequence similarity (Altschul et al, 1997). Among those, the protein encoded by open reading frame (ORF) C44B9.5 stood out as the potential ortholog of the mammalian BRCA1-associating protein CtIP (NP_976036) (Yu et al, 2006 and lit. cit. therein). Similarity searches against the human and worm proteomes using C44B9.5 and CtIP show that these proteins are each other's best and only reciprocal BLAST hits (using full-length sequences with low complexity regions and coiled coils masked off; E-value cut-off 0.001) (Figure 1A). Strikingly, C44B9.5p could be identified as a homolog of S. cerevisiae Com1p/Sae2p (McKee and Kleckner, 1997; Prinz et al, 1997) when subjecting the very C-terminal 100 amino acids (aa) of C44B9.5p (NP_499398) to a PSI-BLAST search against the NCBI non-redundant (nr) database (nr version 02/2007; standard settings; NP_011340 hit in round 7 with an Expect of 3e-05) (Altschul et al, 1997; Marchler-Bauer et al, 2002). The search converged in round 8 with one representative per species, the conserved segment always being situated in the very C-terminus of the gathered proteins (Figure 1A). The collected set of Com1p/Sae2p—C44B9.5p—CtIP homologs (Figure 1B) is in agreement with the protein family identified in rigorous reciprocal searches starting with yeast Com1p/Sae2p (accompanying paper by Uanschou et al).
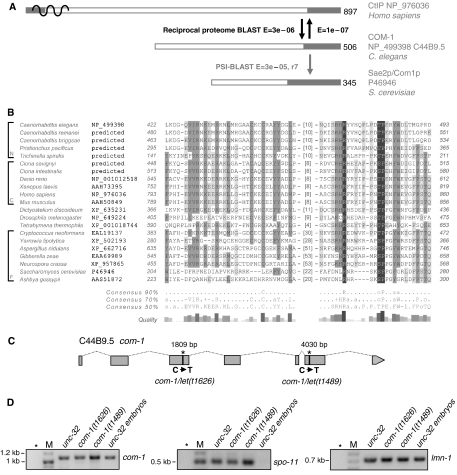
Figure 1.
Homologous relationships between C. elegans C44B9.5p and other family members and a map of the C44B9.5 locus. (A) Protein architecture of the homologous sequences CtIP (RBBP8), C44B9.5p (COM-1) and Com1p/Sae2p. The organization of proteins in this family is dominated by intrinsically unstructured regions. Pronounced ordered segments, indicated by gray boxes, are commonly found in the very C-terminus. Reciprocal proteome BLAST searches performed using coils and low-complexity filtered full-length proteins are indicated by black arrows. The gray arrow indicates PSI-BLAST searches started with the C-terminal 100 amino acids (aa) of C. elegans C44B9.5p and leading to S. cerevisiae Com1p/Sae2p in round 7. (B) Multiple sequence alignment of the C-terminal conserved region in Com1/Sae2/C44B9.5/CtIP homologs. Note that the sequenced cDNA reveals a longer C. elegans COM-1 protein sequence due to an additional exon, than predicted by the current WormBase Release WS178. Alignment visualization and gray-scale conservation shading are performed using GeneDoc (Nicholas et al, 1997; http://www.psc.edu/biomed/genedoc/gdfeedb.htm). A sequence consensus is shown beneath the alignment if a relative frequency cut-off (50, 70 or 90%) is met by individual aa or one of the following aa categories: alcoholic (denoted by ‘o' and including S and T), aliphatic (‘l' for I, L and V), aromatic (‘a' for F, H, W and Y), positive (‘+' for H, K and R), negative (‘−' for D and E). An indication of selected taxonomic groups is included in front of the species names for nematodes (N), chordates (C) and fungi (F). Genomic sequences as the basis for ‘predicted' protein sequences were derived from www.genome.wustl.edu for nematodes and www.ensembl.org for chordates. (C) Map of the C44B9.5 (com-1) locus on chromosome III with the positions of the mutations in alleles t1626 and t1489. (D) Amplification of com-1, spo-11 and ubiquitously expressed control lmn-1 cDNAs in wild-type and mutant backgrounds (asterisks: null-template control, M: size marker). Stable com-1 transcripts are recovered from com-1(t1626) and com-1(t1489) mutant hermaphrodites. com-1 but not the meiosis-specific recombination gene spo-11 is mitotically expressed in wild-type embryos.
Sequencing of ORF C44B9.5 in the let(t1626) and let(t1489) mutants showed that indeed they carry mutations in C44B9.5. let(t1626) is a C to T transition at base pair (bp) 1809 in the third exon of the ORF, and let(t1489) is a C to T transition at bp 4030 in the sixth exon of the 5357 bp 7-exon ORF. (Sequencing of C44B9.5 cDNA of adult wild-type hermaphrodites revealed a deviation from the annotated mRNA sequence in the current release WS178 of the C. elegans WormBase. It comprises an additional small (57 bp) fifth exon.) Both mutations define a premature stop codon (Figure 1C) and therefore produce proteins that are missing the most conserved and, hence, presumably the functionally most significant C-terminal portion. According to the germline gene expression profile, the expression of C44B9.5 is not enhanced during meiosis (Reinke et al, 2000, 2004; see http://workhorse.stanford.edu/germline/). We confirmed the mitotic expression of ORF C44B9.5 by RT–PCR (Figure 1D).
Owing to the protein sequence homology and the similar phenotypes of S. cerevisiae com1/sae2 and C. elegans let(t1626) mutants, we renamed the mutants com-1(t1626) and com-1(t1489) following the designation in budding yeast for Completion Of Meiotic recombination (Prinz et al, 1997), a function the gene is likely to exert in C. elegans as well (see below). com-1(t1626) is considered the strongest allele (phenotypically resembling the knockout by cosuppression, see below) and we used it to further explore the function of the gene.
com-1 mutant animals display a normal somatic phenotype but are infertile
The constitutive expression of com-1 mRNA (see above) suggests that this gene has a role during somatic growth. However, we found that the development of homozygous com-1 mutant worms originating from heterozygous mothers was not notably affected or retarded compared to unc-32 control animals. On the other hand, anaphase bridges were observed in at least 12% of mitoses (n=47) in embryos produced by these worms (inset in Figure 2). Defective anaphases may be a consequence of the irregular disjunction of chromosomes in the wake of an aberrant meiosis (see below) or could indicate a slight effect of the com-1 mutation on mitosis.
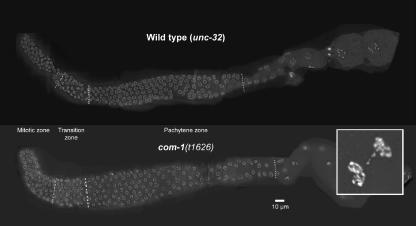
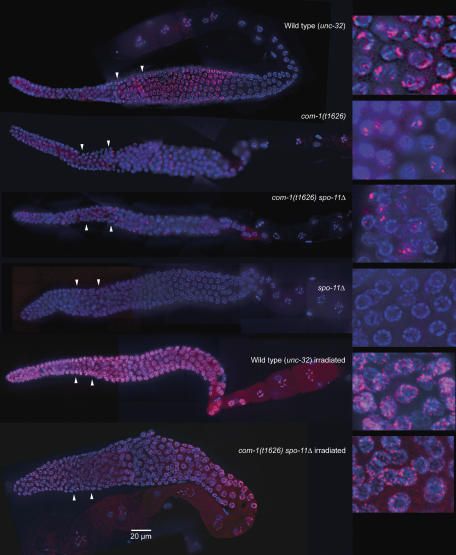
Figure 2.
Comparison of wild-type (unc-32) and com-1 unc-32 mutant gonads (composite images). The morphology of com-1(t1626) mutant gonads does not notably differ from that of wild-type gonads. The mitotic zone is followed by a distinct transition zone displaying the typical polarized arrangement of meiotic chromosomes. In the pachytene zone, nuclei with DAPI-positive parallel tracks of chromatin are found. The inset shows nuclei of a com-1 embryo with a DNA bridge. DNA is stained with DAPI.
As yeast com1Δ/sae2Δ cells are reported to be sensitive to genotoxic stress (see Discussion), we tested whether C. elegans com-1 mutant homozygotes display a somatic growth defect in the presence of MMS. We did not observe an effect of MMS on somatic growth (data not shown), but it is possible that it is prevented by a pool of COM-1 received from the heterozygous mothers. The low fertility of the com-1 mutant (see below) precluded the investigation of a somatic requirement for COM-1 in following generations.
The brood size of homozygous com-1 mutants was reduced and the viability of the offspring strongly reduced. Of a total of 1813 fertilized eggs laid by 12 com-1 unc-32 mutant worms (average brood size: 151 fertilized eggs), 12 hatched but died at the L1 or L2 stage, which is a hatch rate of 0.7%. By comparison, of the 1183 eggs laid by 6 unc-32 control animals (average brood size: 197 fertilized eggs), 1177 (99.5%) produced adult progeny. To find out the cause for the sterility of the mutants, we studied gonadal development and meiosis under the microscope.
Gonad morphology and meiotic pairing are normal in the com-1 mutant
DAPI staining revealed morphologically inconspicuous com-1(t1626) mutant gonads (Figure 2). Like wild-type gonads, they were subdivided into a mitotic plus meiotic entry zone, a transition zone and a pachytene zone (Albertson et al, 1997; Schedl, 1997; Figure 2).
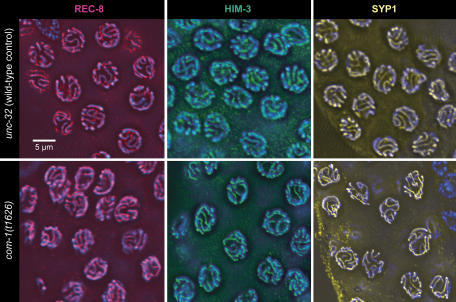
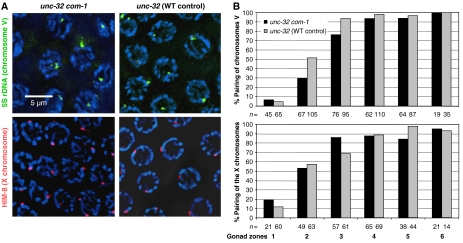
To determine whether a normal SC was formed in the pachytene zone, nuclei were immunostained for REC-8 and HIM-3, two components of the lateral elements of the SC. Moreover, the presence and localization of the SC transversal protein SYP-1 was tested by immunostaining. All three proteins showed a normal abundance and distribution in pachytene (Figure 3). Having thus confirmed that extensive SC was formed between chromosomes in the mutant, it was determined whether it connected homologous chromosome regions. For this, homologous chromosome loci were highlighted by HIM-8 staining and FISH. HIM-8 specifically binds to the pairing center of the X chromosome and can be used to mark this locus near the left end of this chromosome (Phillips et al, 2005). Similarly, FISH was used to delineate the 5S rDNA locus on the right arm of chromosome V. Both HIM-8 staining and FISH produced a single signal or two closely associated signals in most pachytene nuclei (Figure 4A), revealing that the corresponding chromosomal regions were homologously paired in com-1(t1626) (Figure 4B).
Figure 3.
Immunostaining of SC components in the pachytene zone of wild-type and com-1 mutant gonads. Axial element components REC-8 (red) and HIM-3 (green) and the transversal filament component SYP-1 (yellow) appear normal in the mutant indicating normal SC assembly. Chromatin is counterstained with DAPI (blue).
Figure 4.
Homologous pairing is not affected in the com-1 mutant. (A) FISH of the 5S rDNA locus on chromosome V and immunostaining of the X chromosomal protein HIM-8 both produce a single spot (or two closely adjacent spots) in pachytene nuclei of unc-32 com-1 and of unc-32 control gonads, indicating that the corresponding homologous chromosome regions are paired. Chromatin is counterstained by DAPI (blue). (B) Frequencies of paired chromosomes V and X (as monitored by the association of FISH signals or HIM-8 foci, respectively) along the gonad. Two signals touching each other or merged into a single signal were scored as paired. Gonads were divided into six intervals of equal lengths between the distal tip and the end of the pachytene zone. The lines labelled ‘n=' indicate the number of nuclei evaluated for the corresponding data point. For the FISH experiment, they were sampled from four and for the HIM-8 experiment from three gonads.
No distinct diakinesis bivalents are formed in the com-1 mutant
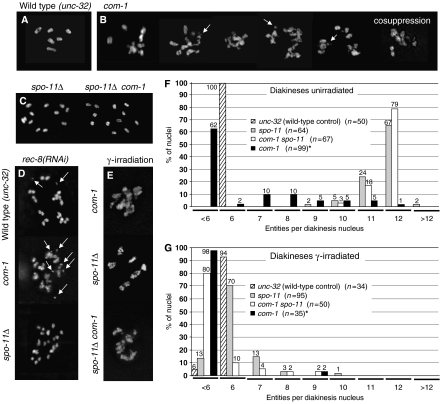
Unlike the wild type where six distinct bivalents can be observed per diakinesis cell, a variable number of diffuse DAPI-stained entities were present in the com-1 mutant (Figure 5A and B). Scoring of 68 late diakineses from 44 gonads revealed a range from 12 chromatin masses to a single large clump. Chromatin masses appeared less condensed than wild-type bivalents at the corresponding stage and they were sometimes connected by thin DAPI-positive threads. Judging from their sizes, some of the free chromatin structures may be bivalents and others, individual chromosomes, whereas yet some others could represent chromosome fragments. A similar phenotype was observed after depletion of COM-1 by cosuppression (Figure 5B). The clumping, stickiness and fragmentation of diakinesis chromosomes possibly indicate inefficient repair of DSBs, as it is frequently observed in mutants in which DSBs are not normally repaired (Rinaldo et al, 2002; Alpi et al, 2003; Takanami et al, 2003; Martin et al, 2005; see Discussion).
Figure 5.
Appearance and quantification of chromosomal structures in diakinesis nuclei; DAPI staining. (A–E) Diakinesis in the wild type and in mutants (all of the unc-32 background) without and after γ-irradiation. (A) Six bivalents are present in the wild type. (B) Examples of chromatin masses in the com-1 mutant. The mutant displays a variable number of sometimes interconnected chromatin masses ranging from more than six to a single clump plus a few small fragments (arrows). The rightmost image shows a diakinesis from a gonad in a com-1 cosuppression line. (C) Univalent formation by the spo-11Δ mutant (left), and the com-1 spo-11Δ double mutant (right). (D) Depletion of REC-8 by RNAi causes the formation of chromosome fragments (arrows) as an indication of DSBs in the wild type (top) and in the com-1 mutant (middle), but not in the spo-11Δ mutant (bottom). (E) Diakineses after γ-irradiation. Top: dispersed chromatin masses in the com-1 mutant; they do not notably differ from those in unirradiated cells. Middle: irradiation restores bivalent formation in the spo-11Δ mutant. Bivalents are irregularly shaped presumably because the frequency and distribution of crossovers is not wild type. Bottom: irradiation restores chromatin clumping in com-1 spo-11Δ mutant diakineses. (F, G) Frequency distribution of DAPI-positive entities in diakinesis nuclei without (F) and after (G) γ-irradiation. *The frequencies of DAPI-positive entities in the com-1 mutant are based on estimates, as the dispersed and partially interconnected chromatin masses precluded exact counting.
To test whether meiotic DSBs are formed in the first place and whether they contribute to the abnormal chromosomal morphology in the com-1 mutant, we constructed a spo-11Δ com-1 double mutant. In the spo-11Δ background, DSBs do not form in the first place (Dernburg et al, 1998) and DSB-dependent clumping and undercondensation should not occur (Figure 5C). Indeed, the com-1 mutant phenotype was largely alleviated by the double mutation (Figure 5C). Of 67 cells in diakinesis, 53 (79%) showed 12 distinct DAPI-positive structures, that is, univalents and another 18% showed 11 entities (Figure 5F). Similarly, the mre-11Δ mutation (which also prevents DSB formation; Chin and Villeneuve, 2001; see below) abolished the clumping phenotype of com-1 and restored normally shaped univalents in a double mutant (data not shown). The absence of clumping in the com-1 spo-11Δ and the com-1 mre-11Δ double mutants suggests that it may be caused by unrepaired or inappropriately processed DSBs in the com-1 mutant.
To directly visualize meiotic DSBs in the com-1 mutant, we depleted REC-8 by RNAi in the mutant. In the absence of REC-8, homologous chromosomes are unpaired and sister chromatids are separated which impedes the repair of DSBs by homologous and sister chromatid recombination. Moreover, unrepaired DSBs are exposed as free chromosome fragments which would not be seen if they were attached to their corresponding (intact) sisters in the presence of REC-8 (Pasierbek et al, 2001; Colaiácovo et al, 2003; Figure 5D). Indeed, numerous chromosome fragments were present in diakineses of com-1 mutant animals deprived of REC-8 (Figure 5D), whereas in the spo-11Δ rec-8(RNAi) control no fragments were found (Figure 5D). This observation confirms the occurrence of SPO-11-dependent DSBs in the com-1 mutant.
Viability of the com-1 mutant progeny is improved by the absence of meiotic DSB
To test whether the high embryonic lethality of the com-1 mutant depends on the induction of meiotic DSBs, we decided to analyze the viability of the progeny in the DSB-less com-1 mre-11Δ double mutant. In this background, viability should be improved to the level permitted by the production of chromosomally balanced gametes and embryos due to the random segregation of univalents. Embryo viability of mre-11Δ hermaphrodites had been found to be 3.1% (Chin and Villeneuve, 2001). (Embryo viability of the spo-11Δ mutant is much lower, which precluded its use for this experiment.) In our hands, the mre-11Δ unc-32 control produced 4.1% viable embryos (n=1763 fertilized eggs laid by 10 animals). The hatch rate of eggs of a com-1 mre-11Δ double mutant was 4.3% (n=1885 eggs from 12 hermaphrodites). Thus, while the hatch rate of com-1 mutant progeny is only 0.7% (see above), the viability of embryos of a com-1 mre-11Δ double mutant is restored to the level of the mre-11Δ single mutant, which is consistent with the interpretation that unrepaired or improperly repaired DSBs are the cause of embryonic lethality in the com-1 mutant.
RAD-51 protein foci do not form normally on chromatin in the com-1 mutant
The recombination protein RAD-51 associates with 3′ ssDNA overhangs flanking DSBs to form long nucleoprotein filaments (Shinohara et al, 1992). In the wild type, RAD-51 localizes to meiotic chromosomes from late zygotene to mid-pachytene (Alpi et al, 2003; Colaiácovo et al, 2003; Figure 6). This corresponds to the region where recombination is believed to take place. In contrast, in the com-1 mutant, despite the formation and persistence of DSBs, RAD-51 foci were virtually completely missing in the corresponding zone of the gonad (Figure 6; Supplementary Figure 1). As western analysis demonstrated that the RAD-51 protein was expressed in the com-1 mutant (data not shown), the absence of RAD-51 foci must be due to the inability of RAD-51 to associate with DNA flanking the DSBs.
Figure 6.
Immunostaining of the recombination protein RAD-51 (red) in whole gonads (composite images—left). Enlarged details from the corresponding gonads are shown to the right. While in the wild type abundant RAD-51 foci indicate ongoing recombination in late transition zone to early pachytene, there is no such RAD-51-rich zone in the com-1 mutant gonad. Throughout the gonad, however, stray nuclei show few strong RAD-51 foci. These foci are missing in the spo-11Δ mutant strain but are present in the com-1 spo-11Δ double mutant. (For the frequencies of RAD-51 foci per nucleus and the distribution of RAD-51 foci-bearing nuclei within the gonads see Supplementary Figure 1.) γ-irradiation induces RAD-51 foci formation in all strains, including the com-1 mutant (data not shown) and the com-1 spo-11Δ double mutant. In each gonad, the left arrowhead denotes the border between the mitotic zone and the transition zone and the right arrowhead the border between the transition zone and the pachytene zone.
Remarkably, however, immunostaining with RAD-51 antibody produced strong foci in a few nuclei scattered all along the gonad (Figure 6). In the six gonads where these foci were counted, the average number of foci/gonad was 24.5 and the maximum was 41. They occurred frequently in pairs within a nucleus. These sporadic foci did not depend on (untimely expressed) SPO-11, as they also occurred in a spo-11Δ com-1 double mutant (Figure 6; average number 27.3 foci/gonad with a maximum of 37; n=6 gonads). In com-1+/+ controls, such extra foci were virtually missing (unc-32 spo-11Δ strain: ∅ 2.1 foci/gonad, n=8 gonads; unc-32 strain: ∅ 1.9 foci outside the zygotene-mid-pachytene zone, n=11 gonads).
Notably, RAD-51 foci, similar in number and distribution to those in the com-1 mutant, were found in an mre-11Δ strain (data not shown). We assume that these foci represent RAD-51-binding repair intermediates at certain DNA lesions that, in the absence of COM-1 and MRE-11, cannot undergo efficient repair. The occurrence of RAD-51 foci in the com-1 mutant also suggests that, while COM-1 is necessary for the loading of RAD-51 at meiotic DSBs, it could play a role subsequent to RAD-51 loading in other repair processes. Recently, spontaneous (SPO-11-independent) RAD-51 foci were also detected in rad-50 mutant germ lines and interpreted as spontaneous DNA breaks that arose during the course of DNA replication (Hayashi et al, 2007).
γ-irradiation induces RAD-51 foci in the absence of COM-1
To further test whether the inability to load RAD-51 is specific to masked DNA ends (such as those at meiotic DSBs), we induced DSBs by γ-irradiation. We observed massive RAD-51 foci formation in nuclei along the entire gonad not only in young embryos in the wild type and in the spo-11 mutant but also in the com-1 mutant and the spo-11Δ com-1 double mutant (Figure 6). This demonstrates that RAD-51 is in principle able to load onto DSB-flanking ssDNA in the absence of COM-1. In a similar experiment, we induced DNA interstrand-crosslinks (ICL) by mitomycin treatment. In this case, COM-1 was also not required for the formation of RAD-51 foci which likely indicate the loading of RAD-51 at DSB intermediates during ICL formation (Dronkert and Kanaar, 2001; data not shown). These observations suggest that loading of RAD-51 foci is prevented only in the case of SPO-11-generated (meiotic) DSBs.
Irradiation-induced meiotic DSBs do not restore normal bivalent formation in the absence of COM-1
As the above observations suggest that COM-1 plays a role in the processing of meiotic SPO-11-induced DSBs, DSBs formed independently of SPO-11 should be processed normally in the com-1 mutant. In C. elegans, it has been shown that a high frequency of bivalent formation can be restored in spo-11Δ mutants by γ-irradiation (Dernburg et al, 1998). We used this approach to induce DSBs in a spo-11Δ com-1 double mutant to see whether SPO-11-independent DSBs can be converted into chiasmata in the absence of COM-1.
Irradiation of the com-1 mutant (for experimental conditions see the Materials and methods section) did not notably change the appearance of the dispersed and clumped chromatin masses (Figure 5E and G). When we irradiated the spo-11 null mutant strain, 92% of cells in diakinesis (n=75) showed six bivalents (Figure 5E); only 8% displayed more than six entities, indicating the presence of univalents (Figure 5G). As was mentioned above, spo-11 is epistatic to com-1 and distinct univalents were prevalent in the double mutant (Figure 5C). Irradiation of the spo-11Δ com-1 double mutant did not induce normal bivalent formation during diakinesis, but it restored dispersed chromatin aggregates resembling those of the untreated com-1 mutant (Figure 5E). This suggests that, while radiation-induced DSBs occur and load RAD-51 in the absence of COM-1 and SPO-11, this is not sufficient to rescue normal meiosis. It is possible that COM-1 function continues to be required after RAD-51 loading or that RAD-51 loading onto radiation-induced lesions is only partial.
Apoptotic cell death is not increased in the com-1 mutant
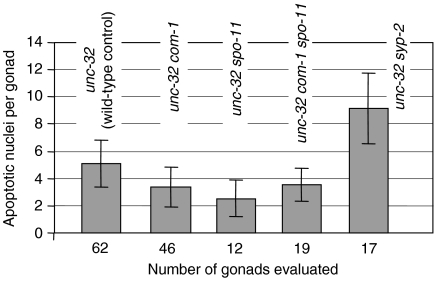
Under normal growth conditions, approximately 50% of female germ cells are fated to die by programmed cell death, which is genetically distinct to checkpoint-induced cell death, and results in a steady-state level of 0–4 morphologically apoptotic cells per gonad (Gumienny et al, 1999). As the com-1 mutant arrests with unrepaired DSBs, we wanted to determine whether the com-1 mutation elicits elevated germ cell apoptosis due to the activation of the C. elegans DNA damage checkpoint response (Gartner et al, 2000). By staining cell corpses with acridine orange, we found that the frequency of apoptotic nuclei was not higher in the mutant as compared to the wild type (Figure 7). It was only nonsignificantly elevated over an spo-11Δ control, which shows only background levels of dead cells due to DSB-independent programmed cell death (Colaiácovo et al, 2003). This suggests that either the DSB intermediates in the com-1 mutant do not trigger a checkpoint response or alternatively, that COM-1 is required for the signalling of apoptosis.
Figure 7.
The frequency of apoptotic nuclei is not increased in the mutant as compared to the wild type and to the spo-11Δ mutant where DSB-induced apoptosis does not occur. The syp-2Δ mutant where apoptotic cell death was reported to be increased (Colaiácovo et al, 2003) served as a positive control.
Discussion
COM-1 has a function in DNA repair and recombination
Here, we studied by mutational analysis the meiotic function of COM-1, a newly identified C. elegans homolog of the S. cerevisiae Com1/Sae2 protein. In accordance with the reported role of the protein in budding yeast (McKee and Kleckner, 1997; Prinz et al, 1997; Usui et al, 2001; Bilic, 2003; Lisby et al, 2004; Clerici et al, 2005), we found that it is not only required for the processing of meiotic DSBs but may also have a repair function in non-meiotic cells.
In com-1 rec-8(RNAi) animals, we detected SPO-11-dependent chromosome fragments diagnostic of DSBs (Pasierbek et al, 2001; Colaiácovo et al, 2003), which indicates that meiotic DSBs are formed by the com-1 mutant. Moreover, in diakinesis of the com-1 mutant, we occasionally observed small chromatin entities, which indicates that these DSBs remain unrepaired. The mutant also featured clumped and poorly condensed chromatin masses resembling those which occur in the absence of RAD-51 (Rinaldo et al, 2002; Alpi et al, 2003; Takanami et al, 2003; Martin et al, 2005) or of BRC-2 (Martin et al, 2005). The diffuse appearance of diakinesis chromatin has been interpreted to be a consequence of massive DNA fragmentation that occurs due to the generation of multiple DSBs that cannot be repaired in rad-51 mutants, as Rinaldo et al (2002) found that it was suppressed in rad51(RNAi) spo-11 and rad51(RNAi) mre-11 mutant strains, which fail to generate meiotic DSBs. Furthermore, irradiated diakinesis nuclei of mre-11 mutants look similar to those lacking RAD-51, possibly because breaks that are generated upon irradiation cannot be repaired (Chin and Villeneuve, 2001).
Our interpretation that chromatin undercondensation and clumping at diakinesis indicate the persistence of DSBs is supported by the alleviation of this phenotype in spo-11 and mre-11 mutant backgrounds, where DSBs are not generated in the first place. Moreover, the absence of RAD-51 foci (which mark the strand invasion step in the recombinational repair of DSBs) suggests that the repair of meiotic DSBs is canceled before homologous strand invasion in the absence of COM-1.
We detected neither a somatic growth defect in com-1 mutant animals nor a higher sensitivity to MMS treatment, but both might possibly be prevented by the action of maternally inherited COM-1 of F2 animals. Later generations could not be tested because of the low fertility of com-1 mutants. However, we obtained circumstantial evidence for the involvement of COM-1 in the non-meiotic DNA repair activities, as in its absence RAD-51 foci were formed on chromatin in nuclei throughout the germ line. These foci, which were also detected in worms lacking MRE-11, could signal stalled repair or attempted alternative repair pathways in the absence of these two proteins. It should also be mentioned that a double mutant of com-1 with lig-4 (which is defective in non-homologous end joining (NHEJ)—Martin et al, 2005) is very likely synthetically lethal as no offspring in later generations could be recovered after RNAi depletion of LIG-4 in com-1 animals (data not shown). This suggests that in the absence of COM-1 certain lesions cannot undergo recombinational repair and must rely on NHEJ.
COM-1 may act preferentially on meiotic DSBs
With COM-1's role in mitotic repair being insignificant or redundant, it is possible that the mutant's repair defect exclusively or primarily concerns meiotic (SPO-11-generated) DSBs. Our observation that RAD-51 is unable to associate with meiotic DSBs but able to associate with γ-irradiation-generated (and rare spontaneous) DNA lesions is consistent with the reported role of Com1p/Sae2p in budding yeast, namely to help generate ssDNA, which is capable of loading RAD-51.
At the same time, we noticed that the presence of (radiation-induced) RAD-51-decorated DNA lesions is not sufficient to support normal bivalent formation in the com-1 mutant. One possible explanation could be that COM-1 may also exert a function following the formation of the RAD-51 nucleofilament. Another hint at such an additional function comes from the observation of putative repair intermediates, which can load but not remove RAD-51 in the absence of COM-1 (Figure 6).
Considering the fact that there is only a limited requirement of Com1p/Sae2p for mitotic DSB repair also in budding yeast (see below), Bilic (2003) and Franz Klein (personal communication) suggested that a specific requirement for Com1p/Sae2p might exist under meiotic conditions (e.g., the axial elements) in yeast by which crossover-incompetent modes of repair are restrained. This conjecture is corroborated by the observation that the com1Δ mutant phenotype is partially suppressed by mutations in RED1 and HOP1, which cause reduced formation of axial elements (Woltering et al, 2000). A requirement of C. elegans COM-1 for DSB repair specifically in the meiotic context would also explain our observation that irradiation-induced DSBs in meiosis, although they can load RAD-51, do not promote the formation of normal bivalents in the absence of SPO-11.
Recombination intermediates that accumulate in the com-1 mutant do not signal a checkpoint
The com-1 mutation did not trigger apoptotic cell death above wild-type level. It has previously been reported that unrepaired DSBs per se do not trigger cell death in cases where DSB repair was defective due to the unavailability of the homolog as the template for repair in rec-8(RNAi) and him-3 mutants (Alpi et al, 2003). On the other hand, apoptosis was increased in a rad-51 mutant (Alpi et al, 2003; Martin et al, 2005) where ssDNA at DSBs is not associated with a RAD-51 array. Colaiácovo et al (2003) attributed the disposition of recombination intermediates to trigger the pachytene checkpoint to different stages in their processing, such as featuring unengaged 3′ ssDNA ends or nascent strand exchange intermediates. Hence, it is conceivable that unmasked ssDNA, such as in the rad-51 mutant, is efficient in eliciting a DNA damage checkpoint response, whereas if ssDNA ends are concealed or absent, the lesion is not visible to the checkpoint machinery. This would be consistent with the interpretation that DNA flanking meiotic DSBs is not resected in the absence of COM-1. An alternative explanation would be that com-1 may act in the regulation of checkpoint signalling of unrepaired DSBs (compare Clerici et al, 2005).
The Com1/Sae2 protein is moderately conserved and related to the mammalian tumor suppressor CtIP
A moderate sequence conservation between the COM-1 group proteins over a wide spectrum of organisms was demonstrated by sequence analytical methods (Figure 1). These proteins also possess a similar general architecture. They are mostly disordered and low-complex (Pdisorder; www.softberry.com) except for the C-terminal 70–100 aa corresponding to the region of highest sequence conservation. The conserved C-terminal segment is always preceded by a predominantly low-complex region of variable length.
In addition to the similarities at the sequence level, studies of C. elegans com-1 (this paper) and Arabidopsis Atgr1/com1 mutants (accompanying paper by Uanschou et al) show functional similarities of the respective proteins to S. cerevisiae Com1p/Sae2p.
In budding yeast com1/sae2 mutants, meiotic recombination is defective as Spo11p remains covalently attached to DNA ends, which are left unresected (McKee and Kleckner, 1997; Prinz et al, 1997; Neale et al, 2005; Prieler et al, 2005). Yeast Com1p/Sae2p also plays a role in vegetative growth. com1Δ/sae2Δ mutants exhibit slight sensitivity to MMS, hydroxyurea or ionizing radiation and subtle repair defects of spontaneous lesions (Usui et al, 2001; Lisby et al, 2004; Clerici et al, 2005). In addition, the phosphorylation of Com1p/Sae2p in vegetative cells following DNA damage suggests that it is active in DNA repair (Baroni et al, 2004). All these observations are consistent with a role of Com1p/Sae2p in the processing of DSBs.
The evolutionarily conserved MRX protein complex (a.k.a MRN complex) has multiple roles not only in DNA repair processes (Assenmacher and Hopfner, 2004), but also in the formation of meiotic DSBs by generating 3′ ssDNA tails at sites of DSB lesion (Neale et al, 2005). It was proposed that Com1p/Sae2p interacts with the MRX complex (Clerici et al, 2005) and is involved in the removal of Spo11p, possibly by regulating the nuclease activity of the MRX complex (Rattray et al, 2001) and thus allowing loading of Rad51p, homologous strand invasion and recombinational repair to proceed.
In mitosis, Com1p/Sae2p may be dispensable because of partially redundant functions such as that of exonuclease Exo1, which contributes to DSB resection independently of the MRX complex (Llorente and Symington, 2004; Nakada et al, 2004). The existence of redundant pathways is also suggested by the co-lethality of com1Δ and rad27Δ (Debrauwère et al, 2001; Tong et al, 2001) and com1Δ and top3Δ (Franz Klein, personal communication).
While the somatic roles of Com1p are less well defined, the importance of the mammalian Com1p homolog, CtIP (a.k.a. RBBP8) for genomic integrity was demonstrated by heterozygous CtIP+/− mice, which suffer from a higher incidence of tumors than wild-type mice (Chen et al, 2005). CtIP is a multifunctional protein with roles in checkpoint control, transcriptional regulation of genes that are important for DNA-damage response and cell-cycle progression, in DNA replication and developmental control (Barber and Boulton, 2006; Wu and Lee, 2006). Human CtIP is required for the DNA damage checkpoint response and/or repair, most likely by its interaction with BRCA1 (Yu and Chen, 2004; Greenberg et al, 2006). BRCA1 E3 ligase specifically binds to phosphorylated forms of CtIP and promotes CtIP ubiquitylation. Ubiquitylated CtIP binds to chromatin (possibly at the sites of DNA damage) (Yu et al, 2006). It is conceivable that CtIP's checkpoint activity is due to its involvement in the formation of a ssDNA repair intermediate that can be sensed by the checkpoint machinery. Such a role would be consistent with the proposed roles of its homologs of the Com1 family. While we could not confirm a COM-1–BRC-1 interaction in a yeast two-hybrid assay despite the documented interaction of human CtIP and BRCA1, the assay suggested COM-1 homodimerization (data not shown), which is consistent with the documented homodimerization for CtIP (Dubin et al, 2004).
In support of a potential role of CtIP in meiotic recombination, northern analysis had shown that CtIP mRNA was most abundant in testis as compared to other healthy tissues (Wong et al, 1998). Furthermore, we noted that worms carrying the com-1(t1489) allele lacking only the C-terminal conserved part (Figure 1C) displayed the full-fledged mutant phenotype (data not shown), although mRNA is stably expressed (Figure 1D). Thus, allele com-1(t1489) demonstrates that the region which is conserved between COM-1 and CtIP, is important for meiosis. The meiotic role of this region is confirmed by mutagenesis studies in yeast by Uanschou et al (accompanying paper). Substitution of proline 268 and other conserved amino acids in the C-terminal part of yeast Com1 caused a substantial reduction of sporulation efficiency and spore viability. As ctip−/− mice are embryonic lethal (Chen et al, 2005), the confirmation of whether CtIP shares a meiotic function with its homologs has to await a conditional germline knockout.
Materials and methods
Worm strains and culture conditions
Strains N2 (wild-type), AV106 spo-11(ok79) (Dernburg et al, 1998) and AV112 mre-11(ok179) (Chin and Villeneuve, 2001) were obtained from the Caenorhabditis Genetics Center (University of Minnesota, St Paul, MN). Strains GE2258 unc-32(e189) com-1 (t1626)/qC1 III him-3(e1147) IV and GE2212 unc-32(e189) com-1 (t1489)/qC1 III him-3(e1147) IV were generated in the laboratory of RS. They were produced by mutagenesis of an unc-32(e189)/qC1III balancer strain, and candidate mutations were preselected for their linkage to the unc-32 locus within the tra-1 to dpy-1 interval (Gönczy et al, 1999), which is spanned by the qC1 balancer (Edgley et al, 1995). They carry the weak e1147 allele of him-3, which was used to raise the percentage of male progeny to ∼3.5% (Zetka et al, 1999). him-3 was outcrossed by crossing GE2258 and GE2212 males to N2 hermaphrodites and selecting for F1 progeny segregating Unc F2. In addition, unc-32(e189) homozygotes were produced to serve as com-1+ wild-type controls. com-1 spo-11 and com-1 mre-11 double mutants were obtained by crossings of heterozygotes of com-1(t1626) and spo-11(ok79) or mre-11(ok179). Worms were grown on NGM plates seeded with Escherichia coli OP50 (Brenner, 1974) at 20°C.
cDNA preparation and analysis
Total RNA was isolated from homogenized adult hermaphrodites or eggs by TRIzol Reagent (Invitrogen), following the manufacturer's instructions. mRNA purification was performed with Dynabeads Oligo (dT)25 (Invitrogen), following the manufacturer's instructions. cDNA libraries were made with the RETROscript kit (Ambion). To test the expression of C44B9.5 (com-1), the first 1092 bp of the corresponding cDNA were amplified. spo-11 transcripts were detected by the amplification of 502 bp of the cDNA comprising exon 3 to exon 5. Amplification of 723 bp (exon 1 to exon 5) of the ubiquitously expressed lmn-1 cDNA served as a control of the quality and quantity of the cDNA.
rec-8 RNA interference
Production of double-stranded RNA (dsRNA) for rec-8RNAi was carried out as in Pasierbek et al (2001). rec-8RNAi was performed by injection according to Colaiácovo et al (2002). In short, 1–5 μg/μl dsRNA was injected into the body cavity of young adult hermaphrodites. Injected worms were kept at 20°C. Diakinesis nuclei of the germ line of young adult F1 worms were evaluated by DAPI staining (see below).
com-1 cosuppression
Germline-specific depletion of com-1 mRNA was performed by cosuppression (Dernburg et al, 2000). A PCR product comprising 1.5 kb of 5′ regulatory sequence and the first three exons was coinjected with the rol-6(su1006) marker. The PCR product was generated with primers MJ1486 (5′-cgattaccgcataaaccactacg-3′) and MJ1487 (5′-gccagtgtgaaatcgagttgctc-3′). Gonads of young adult F3 hermaphrodites expressing the roller phenotype were dissected, DAPI-stained (see below) and inspected for their diakinesis phenotype.
γ-irradiation assay
Irradiation experiments were performed according to Dernburg et al (1998) to score irradiation-induced bivalent formation at diakinesis. In short, young adult hermaphrodites were exposed to 5000 rads of γ-radiation from a 60Co-source. To determine the time during which pachytene cells with γ-ray-induced DSBs matured into diakineses, spo-11(ok79) unc-32(e189) control worms were examined at various times after irradiation. Despite the fact that the gonads of unc-32(e189) worms are somewhat shorter than those of N2 worms, the maximum frequency of bivalents was found to be in accordance with Dernburg et al (1998) 18 h after exposure. For the analysis of irradiation-induced RAD-51 loading, worms were cut open 90 min after irradiation. Eggs of irradiated com-1 spo-11 animals, which were used for scoring diakineses were tested by PCR for homozygosity of the com-1(t1626) allele, as it tended to segregate away at a low frequency from the unc-32 marker (separation: 5.5 cM) by which it was selected for.
Cytological techniques
Hermaphrodites were cut open to release the gonads in 6 μl of 1 × PBS on a microscope slide and fixed by the addition of an equal volume of 2% formaldehyde. The material was immediately covered with a coverslip and kept at room temperature for 5 min. The coverslip was removed after freezing the preparations in liquid nitrogen and the slides were then transferred to ice-cold methanol for 5 min. After washing the slides three times for 5 min in 1 × PBS supplemented with 0.1% Tween 20 (PBS-T), they were mounted in Vectashield antifading medium (Vector Laboratories Inc., Burlingame, CA) containing 2 μg/ml 4′ 6-diamidino-2-phenylindole (DAPI) for the staining of chromatin and chromosomes.
For immunostaining, worms were cut on a slide, covered with a coverslip, which was removed after freezing in liquid nitrogen. The preparations were fixed for 1 min in ice-cold methanol and were immediately transferred to PBS-T without drying. Preparations were transferred to fresh PBS-T twice for 5 min each and were blocked with 3% BSA in 1 × PBS for 20 min at room temperature in a humidity chamber. The primary antibody was applied and the specimen was incubated overnight at 4°C in a humidity chamber. Antibodies were diluted in 1 × PBS containing 0.01% NaN3 as follows: 1:100 anti-REC-8, 1:100 anti-RAD-51, 1:50 anti-SYP-1, 1:400 anti-HIM-3, 1:500 anti-HIM-8. After washing three times in 1 × PBS plus 0.1% Tween 20 secondary antibodies were applied at the following dilutions: anti-Guinea pig Alexa 568 (1:400), anti-rabbit Cy3 (1:250), anti-rabbit FITC (1:300) or anti-rat FITC (1:300). After 90 min incubation at room temperature, slides were washed and mounted in Vectashield supplemented with DAPI.
For fluorescence in situ hybridization (FISH), gonads were dissected in 6 μl of 1 × PBS and fixed by the addition of an equal volume of 7.4% formaldehyde. The specimens were immediately covered with a coverslip and transferred to liquid nitrogen. After removal of the coverslip, the tissue was dehydrated by incubation in increasing ethanol concentrations, dried on air and kept at room temperature until FISH was performed. PCR-amplified 5S rDNA was used as a probe for the right arm of chromosome V (Pasierbek et al, 2001). The probe was PCR-labelled with digoxigenin-11-dUTP. The probe was applied to cytological preparations, and chromosomal and probe DNA were heat-denatured and allowed to hybridize. Hybridized DNA on the slides was detected with FITC-conjugated anti-digoxigenin antibodies.
After immunostainings and FISH, fluorescence-labelled antibodies and DAPI were visualized by UV excitation and detection at appropriate wavelengths. Optical sectioning was performed on the preparations and images were recorded at separate wavelengths, deconvolved, projected and color-merged.
Monitoring of apoptotic germline nuclei
Apoptotic cells were detected by their selective intake of acridine orange (see Gartner et al, 2004). In short, 0.5 ml of staining solution (50 μg acridine orange/ml M9 buffer) was added to young adult worms on a 35 mm worm plate. The plates were stored in the dark for 1 h before the worms were washed in M9 buffer and transferred to a new plate. After a further 1 h incubation at room temperature in the dark, the worms were mounted in a drop of M9 buffer supplemented with 0.1% NaN3 on agarose beds and scored under a fluorescence microscope.
Note added in proof
After this paper went to press, we became aware of a recent study on a fission yeast CtlP homologue with roles both in vegetative and meiotic DSB repair (Limbo et al, 2007, Mol Cell 28: 134–146). This dual function substantiates our notion on the possible orthology of C. elegans COM-1 and mammalian CtlP.
Supplementary Material
Supplementary Figure 1
Acknowledgments
We are grateful to Peter Schlögelhofer and Franz Klein for communicating results prior to publication and their insightful comments on the manuscript. Many thanks go to Simon Boulton for constructs and to Maria Siomos (Gregor Mendel Institute, Vienna) for improving the manuscript. Technical assistance by Christian Pflügl, Lucia Riedmann, Markus Ladurner and Jiradet Gloggnitzer is gratefully acknowledged. This work was supported by project P17329 and an Elise Richter grant of the Austrian Research Fund (FWF), and a WWTF grant (LS0905).
References
- Alani E, Padmore R, Kleckner N (1990) Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell 61: 419–436 [DOI] [PubMed] [Google Scholar]
- Albertson DG, Rose AM, Villeneuve AM (1997) Chromosome organization, mitosis, and meiosis. In C. elegans II, Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds), pp 47–78. Plainview, NY: Cold Spring Harbor Laboratory Press [PubMed] [Google Scholar]
- Alpi A, Pasierbek P, Gartner A, Loidl J (2003) Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6–16 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenmacher N, Hopfner K-P (2004) MRE11/RAD50/NBS1: complex activities. Chromosoma 113: 157–166 [DOI] [PubMed] [Google Scholar]
- Barber LJ, Boulton SJ (2006) BRCA1 ubiquitylation of CtIP: just the tIP of the iceberg? DNA Repair 9: 1499–1504 [DOI] [PubMed] [Google Scholar]
- Baroni E, Viscardi V, Cartagena-Lirola H, Lucchini G, Longhese MP (2004) The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol Cell Biol 24: 4151–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, Nicolas A (1997) Clustering of meiotic double-strand breaks on yeast chromosome III. Proc Natl Acad Sci USA 94: 5213–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerat A, de Massy B, Gadelle D, Varoutas P-C, Nicolas A, Forterre P (1997) An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature 386: 414–417 [DOI] [PubMed] [Google Scholar]
- Bilic I (2003) The role of Com1 in double strand break repair in Saccharomyces cerevisiae. PhD Thesis, University of Vienna
- Bishop DK, Zickler D (2004) Early decision: meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117: 9–15 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-L, Liu F, Cai S, Lin X, Li A, Chen Y, Gu B, Lee E-P, Lee W-H (2005) Inactivation of CtIP leads to early embryonic lethality mediated by G1 restraint and to tumorigenesis by haploid insufficiency. Mol Cell Biol 25: 3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin GM, Villeneuve AM (2001) C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G2 DNA damage checkpoint. Genes Dev 15: 522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Mantiero D, Lucchini G, Longhese MP (2005) The Saccharomyces cerevisiae Sae2 protein promotes resection and bridging of double strand break ends. J Biol Chem 280: 38631–38638 [DOI] [PubMed] [Google Scholar]
- Colaiácovo MP, MacQueen AJ, Martinez-Perez E, McDonald K, Adamo A, La Volpe A, Villeneuve AM (2003) Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell 5: 463–474 [DOI] [PubMed] [Google Scholar]
- Colaiácovo MP, Stanfield GM, Reddy KC, Reinke V, Kim SK, Villeneuve AM (2002) A targeted RNAi screen for genes involved in chromosome morphogenesis and nuclear organization in the Caenorhabditis elegans germline. Genetics 162: 113–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrauwère H, Loeillet S, Lin W, Lopes J, Nicolas A (2001) Links between replication and recombination in Saccharomyces cerevisiae: a hypersensitive requirement for homologous recombination in the absence of Rad27 activity. Proc Natl Acad Sci USA 98: 8263–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg AF, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve AM (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387–398 [DOI] [PubMed] [Google Scholar]
- Dernburg AF, Zalevsky J, Colaiácovo MP, Villeneuve AM (2000) Transgene-mediated cosuppression in the C. elegans germ line. Genes Dev 14: 1578–1583 [PMC free article] [PubMed] [Google Scholar]
- Dronkert ML, Kanaar R (2001) Repair of DNA interstrand cross-links. Mutat Res 486: 217–247 [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Stokes PH, Sum EYM, Williams RS, Valova VA, Robinson PJ, Lindeman GJ, Glover JNM, Visvader JE, Matthews JM (2004) Dimerization of CtIP, a BRCA1-and CtBP-interacting protein, is mediated by an N-terminal coiled-coil motif. J Biol Chem 279: 26932–26938 [DOI] [PubMed] [Google Scholar]
- Edgley ML, Baillie DL, Riddle DL, Rose AM (1995) Genetic balancers. In Caenorhabditis Elegans. Modern Biological Analysis of an Organism, Epstein HF, Shakes DC (eds), pp 147–184. San Diego: Academic Press [Google Scholar]
- Gartner A, MacQueen AJ, Villeneuve AM (2004) Methods for analyzing checkpoint responses in Caenorhabditis elegans. Meth Mol Biol 280: 257–274 [DOI] [PubMed] [Google Scholar]
- Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO (2000) A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol Cell 5: 435–443 [DOI] [PubMed] [Google Scholar]
- Gönczy P, Schnabel H, Kaletta T, Amores AD, Hyman T, Schnabel R (1999) Dissection of cell division processes in the one cell stage Caenorhabditis elegans embryo by mutational analysis. J Cell Biol 144: 927–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg RA, Sobhian B, Pathania S, Cantor SB, Nakatani Y, Livingston DM (2006) Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1-containing complexes. Genes Dev 20: 34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO (1999) Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126: 1011–1022 [DOI] [PubMed] [Google Scholar]
- Hammond MP, Birney E (2004) Genome information resources—developments at Ensembl. Trends Genet 20: 268–272 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Chin M, Villeneuve AM (2007) C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression. PLoS Genet 3: e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth NM, Brill SJ (2004) The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions. Genes Dev 18: 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S (2001) Mechanism and control of meiotic recombination initiation. Curr Topics Dev Biol 52: 1–53 [DOI] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384 [DOI] [PubMed] [Google Scholar]
- Lisby M, Barlow JH, Burgess RC, Rothstein R (2004) Choreography of the DNA damage response. Spatiotemporal relationships among checkpoint and repair proteins. Cell 118: 699–713 [DOI] [PubMed] [Google Scholar]
- Llorente B, Symington LS (2004) The Mre11 nuclease is not required for 5′–3′ resection at multiple HO-induced double-strand breaks. Mol Cell Biol 24: 9682–9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Panchenko AR, Ariel N, Bryant SH (2002) Comparison of sequence and structure alignments for protein domains. Proteins 48: 439–446 [DOI] [PubMed] [Google Scholar]
- Martin JS, Winkelmann N, Petalcorin MIR, McIlwraith MJ, Boulton SJ (2005) RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25: 3127–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AHZ, Kleckner N (1997) A general method for identifying recessive diploid- specific mutations in Saccharomyces cerevisiae, its application to the isolation of mutants blocked at intermediate stages of meiotic prophase and characterization of a new gene SAE2. Genetics 146: 797–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz K, Klein F (1997) mre11S—a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev 11: 2272–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Hirano Y, Sugimoto K (2004) Requirement of the Mre11 complex and exonuclease 1 for activation of the Mec1 signaling pathway. Mol Cell Biol 24: 10016–10025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MJ, Pan J, Keeney S (2005) Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature 436: 1053–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB Jr, Deerfield DWII (1997) GeneDoc: analysis and visualization of genetic variation. EMBNEW News 4: 14 [Google Scholar]
- Pasierbek P, Jantsch M, Melcher M, Schleiffer A, Schweizer D, Loidl J (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15: 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CM, Wong C, Bhalla N, Carlton PM, Weiser P, Meneely PM, Dernburg AF (2005) HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123: 1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieler S, Penkner A, Borde V, Klein F (2005) The control of Spo11's interaction with meiotic recombination hotspots. Genes Dev 19: 255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz S, Amon A, Klein F (1997) Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics 146: 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray AJ, McGill CB, Shafer BK, Strathern JN (2001) Fidelity of mitotic double-strand-break repair in Saccharomyces cerevisiae: a role for SAE2/COM1. Genetics 158: 109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V, Gil IS, Ward S, Kazmer K (2004) Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131: 311–323 [DOI] [PubMed] [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJM, Davis EB, Scherer S, Ward S, Kim SK (2000) A global profile of germline gene expression in C. elegans. Mol Cell 6: 605–616 [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Bazzicalupo P, Hilliard M, La Volpe A (2002) Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics 160: 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl T (1997) Developmental genetics of the germ line. In C. elegans II, Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds), pp 241–269. Plainview, NY: Cold Spring Harbor Laboratory Press [PubMed] [Google Scholar]
- Shinohara A, Ogawa H, Ogawa T (1992) Rad51 protein involved in repair and recombination in Saccharomyces cerevisiae is a RecA-like protein. Cell 69: 457–470 [DOI] [PubMed] [Google Scholar]
- Shinohara A, Shinohara M (2004) Roles of RecA homologues Rad51 and Dmc1 during meiotic recombination. Cytogenet Genome Res 107: 201–207 [DOI] [PubMed] [Google Scholar]
- Takanami T, Mori A, Takahashi H, Horiuchi S, Higashitani A (2003) Caenorhabditis elegans Ce-rdh-1/rad-51 functions after double- strand break formation of meiotic recombination. Chromosome Res 11: 125–135 [DOI] [PubMed] [Google Scholar]
- Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, Andrews B, Tyers M, Boone C (2001) Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294: 2364–2368 [DOI] [PubMed] [Google Scholar]
- Usui T, Ogawa H, Petrini JHJ (2001) A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol Cell 7: 1255–1266 [DOI] [PubMed] [Google Scholar]
- Whitby MC (2005) Making crossovers during meiosis. Biochem Soc Transact 33: 1451–1455 [DOI] [PubMed] [Google Scholar]
- Woltering D, Baumgartner B, Bagchi S, Larkin B, Loidl J, de los Santos T, Hollingsworth NM (2000) Meiotic segregation, synapsis and recombination checkpoint functions require physical interaction between the chromosomal proteins Red1p and Hop1p. Mol Cell Biol 20: 6646–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AKC, Ormonde PA, Pero R, Chen Y, Lian L, Salada G, Berry S, Lawrence Q, Dayananth P, Ha P, Tavtigian SV, Teng DHF, Bartel PL (1998) Characterization of a carboxy-terminal BRCA1 interacting protein. Oncogene 17: 2279–2285 [DOI] [PubMed] [Google Scholar]
- Wu G, Lee WH (2006) CtIP, a multivalent adaptor connecting transcriptional regulation, checkpoint control and tumor suppression. Cell Cycle 5: 1592–1596 [DOI] [PubMed] [Google Scholar]
- Yu X, Chen J (2004) DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol Cell Biol 24: 9478–9486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Fu S, Lai M, Baer R, Chen J (2006) BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev 20: 1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetka MC, Kawasaki I, Strome S, Muller F (1999) Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev 13: 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33: 603–754 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1