Abstract
Rapid deactivation of the Drosophila light receptor rhodopsin, through a visual arrestin Arr2 and a pathway that involves a transcription factor dCAMTA, is required for timely termination of light responses in the photoreceptor neuron. Here we report that this process is also critical for maintenance of the photoreceptor sensitivity. In both dCAMTA- and arr2-mutant flies, the endocytosis of the major rhodopsin Rh1 was dramatically increased, which was mediated by a Gq protein that signals downstream of rhodopsin in the visual transduction pathway. Consequently, the Rh1 level was downregulated and the photoreceptor became less sensitive to light. Remarkably, the Gq-stimulated Rh1 endocytosis does not require phospholipase C, a known effector of Gq, but depends on a tetraspanin protein. Our work has identified an arrestin-independent endocytic pathway of G protein-coupled receptor in the fly. This pathway may also function in mammals and mediate an early feedback regulation of receptor signaling.
Keywords: CAMTA, endocytosis, GPCR, G protein, tetraspanin
Introduction
A variety of neurotransmitters, hormones, cytokines and sensory stimuli trigger intracellular signaling cascades through trimeric G protein-coupled receptors (GPCRs) on the cell membrane (Pierce et al, 2002; Kristiansen, 2004). To fine-tune the signaling event, cells express various regulatory molecules to control the activities of GPCR (Gainetdinov et al, 2004). Arrestins are a group of primary regulators of GPCR (Shenoy and Lefkowitz, 2003) that, upon binding to the activated receptor, uncouple the receptor from the G protein, a process termed deactivation or desensitization of GPCR. When a GPCR is continuously stimulated, the bound arrestin molecule will recruit clathrin proteins to internalize the receptor through dynamin-dependent endocytosis (Ferguson, 2001; Claing et al, 2002). Many internalized GPCRs undergo lysosomal degradation, leading to downregulation of the receptor level (Tsao et al, 2001; von Zastrow, 2001). Both internalization and downregulation of a GPCR reduce its density on the plasma membrane, and thus cause long-term desensitization of the cell to the extracellular stimulus. In addition to regulating the signaling, receptor downregulation may protect the cell from overexcitation, and has been implicated in neuronal tolerances to chronically applied drugs like morphine (Zuo, 2005; Marie et al, 2006).
Although arrestins are the primary regulators in the deactivation of GPCR, they are not the only molecules that mediate GPCR endocytosis and downregulation. Many GPCRs, such as the endothelin type B, muscarinic, vasoactive intestinal peptide type 1, bradykinin type 2 and cholecystokinin receptors, are internalized independent of arrestin (Claing et al, 2002; Prossnitz, 2004). The arrestin-independent pathway of GPCR endocytosis has yet to be characterized in vivo.
The Drosophila phototransduction cascade is a model pathway (Montell, 1999; Hardie and Raghu, 2001) for genetic dissection of GPCR signaling and regulation. This visual signaling cascade is localized in the rhabdomere (Hardie and Raghu, 2001), a highly packed microvillar structure that is analogous to the outer segment of mammalian photoreceptors. The light receptor rhodopsin activates a Gq protein to stimulate a norpA gene-encoded phospholipase C (PLC) (Bloomquist et al, 1988; Lee et al, 1994), which then opens TRP or TRPL Ca2+/cation channels (Montell, 1999) to depolarize the photoreceptor neuron. To ensure rapid termination of the visual response at the end of light stimulation, rhodopsin needs to be deactivated immediately after stimulating a single Gq molecule (Scott and Zuker, 1997, 1998). An arrestin protein Arr2 plays a pivotal role in the deactivation of rhodopsin (Dolph et al, 1993). Nonetheless, this visual arrestin does not appear to mediate rhodopsin endocytosis (Satoh and Ready, 2005) or downregulation in wild-type flies.
We have recently identified a new mechanism underlying the deactivation of rhodopsin. An F-box and leucine-rich repeat protein dFbxl4, which depends on a fly calmodulin-binding transcription activator dCAMTA for expression, facilitates rhodopsin deactivation in an unknown manner (Han et al, 2006). In the dCAMTA mutant flies tes1 and tes2 (for termination slow 1 and 2), because of the lack of dFbxl4-dependent rhodopsin deactivation, the Gq stimulation was prolonged, which leads to a slow termination of light response. Given that many F-box proteins act as substrate-recruiting subunits of SCF-type E3 ubiquitin ligases (Jin et al, 2004), and that several GPCRs undergo activity-dependent ubiquitination (Wojcikiewicz, 2004), it is possible that a reversible, dFbxl4-mediated ubiquitination of rhodopsin may abolish the rhodopsin–Gq interaction and thus deactivate the light receptor.
Another interesting phenotype caused by the loss of dCAMTA/dFbxl4 function is that the major rhodopsin protein Rh1, which is expressed in all six peripheral photoreceptors (O'Tousa et al, 1985), undergoes light-dependent downregulation (Han et al, 2006). Here we demonstrate that the Rh1 downregulation is due to increased endocytosis of Rh1, which is mediated by activity of the Gq protein. Due to the internalization of Rh1, the light sensitivity of the mutant photoreceptor decreased significantly. Thus, rapid deactivation of rhodopsin to prevent overstimulation of Gq is required for the maintenance of Rh1 level and photoreceptor sensitivity, as well as for the termination of light response. Our work has identified an arrestin-independent pathway of GPCR endocytosis in the fly eye, and suggests that G protein, the downstream signaling molecule of GPCR, may have a feedback control on the receptor signaling by triggering internalization and downregulation of the receptor.
Results
Light-dependent Rh1 endocytosis and downregulation in tes-mutant flies
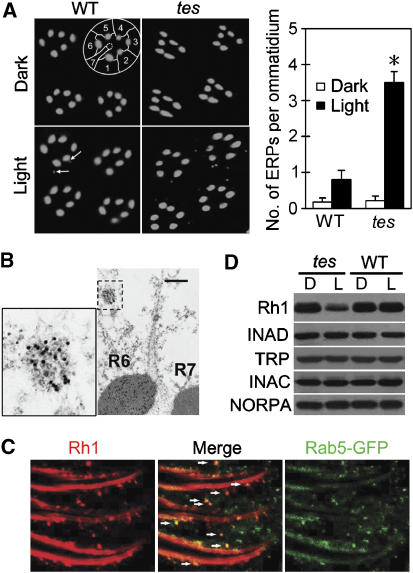
In dark-reared flies, all Rh1 proteins were localized in the rhabdomeres according to immunostaining results (Figure 1A). Upon exposure to ambient light (∼450 lux, from regular fluorescent tubes) for 3 h, a few potential endocytic Rh1 particles (ERPs) were observed in the cell bodies of wild-type photoreceptors. Interestingly, the same light treatment produced many more (∼4-fold) ERPs in photoreceptors of a tes-mutant fly (tes2) (Figure 1A). In electron microscopy images, each ERP appeared to be a collection of small vesicles (Figure 1B), a structure that has been reported to originate from endocytosis of Rh1 (Xu et al, 2004; Satoh and Ready, 2005). To provide further evidence that ERPs are derived from the endocytosis, we examined the distribution of Rab5, a small GTPase that mediates early endocytic pathways (Bucci et al, 1992), in a tes, ey-Gal4;p[UAS∷GFP-Rab5] fly. The staining result showed that all ERPs contained Rab5 (Figure 1C), suggesting that ERPs are made of small endocytic vesicles.
Figure 1.
Light-dependent Rh1 endocytosis and downregulation in tes-mutant flies. (A) Three-hour light stimulation induced more ERPs in tes flies than in wild type. The left panel shows cross-sections of eye that have been stained with a monoclonal Rh1 antibody. The cell bodies of photoreceptors in one wild-type ommatidium are outlined. Note that only the six peripheral rhabdomeres, not the central R7 one (dashed circle), contain Rh1. The arrows point to two ERPs in the light-stimulated wild-type section. The number of ERPs per ommatidium was calculated for each genotype and treatment. After averaging three sets of data, the means and s.e.ms (as error bars) are presented in the right panel. Except those in Figures 5 and 6A, all stained flies were exposed to light for 3 h. (B) Immunogold electron microscopy reveals that each ERP represents a collection of small endocytic vesicles. An ERP (boxed) in the R6 photoreceptor is enlarged in the left panel. Scale bar, 0.5 μm. (C) In a tes,ey-Gal4;p[UAS∷GFP-Rab5]fly, the ERPs (arrow heads) were colabeled with the Rab5 protein. Whole fly heads were stained with both Rh1 and GFP antibodies. The images show the side view of photoreceptors. (D) Western blots showing a reduced level of Rh1 in light exposed tes-mutant flies. All other visual signaling proteins had normal levels, indicating that no rhabdomeral degeneration occurred in the examined flies. D: dark-reared, never exposed to light, L: dark-reared but exposed to room light overnight before examination, WT: wild type. In all western blot analyses, each lane was loaded with one fly head. *Indicates that the sample is significantly different from others in the same group.
Probably due to the excessive endocytosis, the level of Rh1 protein in tes-mutant flies was significantly decreased (>2-fold) upon overnight exposure to the ambient light (Figure 1D). In contrast, the levels of all other visual signaling proteins, including TRP, NORPA, an eye protein kinase C INAC and a scaffold protein INAD (Montell, 1999), were not changed by the light stimulation.
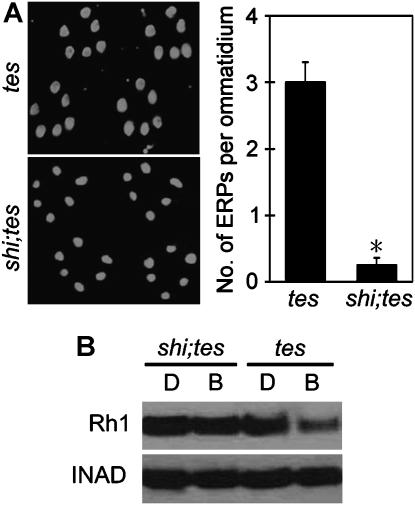
If Rh1 downregulation is really due to increased endocytosis and subsequent degradation of Rh1 in tes-mutant flies, it should be prevented when the endocytosis is blocked. Endocytosis of most GPCRs depends on the function of dynamin, a large GTPase that cleaves endocytic vesicles from the plasma membrane (Sever, 2002). We introduced a temperature-sensitive mutation of the dynamin gene shibire (shi) (Poodry and Edgar, 1979; Chen et al, 1991; van der Bliek and Meyerowitz, 1991) into the tes fly and found that this mutation virtually abolished the endocytosis of Rh1 at a restrictive temperature of 30.5°C (Figure 2A), indicating that Rh1 endocytosis is also dynamin-dependent.
Figure 2.
The Rh1 downregulation in tes-mutant flies is due to excessive endocytosis of Rh1. (A) The Rh1 endocytosis in tes flies depends on the shibire dynamin. During the 3-h light stimulation, both tes and the shi;tes double mutants were kept at 30.5°C, a temperature that prevents the mutant Shibire dynamin from function. (B) The shi mutation prevented light-induced Rh1 reduction in tes flies. The scaffold protein INAD was probed in parallel. For light stimulation in this particular experiment, the flies were exposed to pure blue light (750 lux) for three hours at 30.5°C. B, blue light. *Indicates that the sample is significantly different from others in the same group.
Next, we examined the effect of shi mutation on the downregulation of Rh1. We previously found that it took at least 10 h for ambient light to stimulate Rh1 reduction in the tes flies. Since such a long-time treatment of shi mutants at 30.5°C could cause damage to the general structure of rhabdomere, we instead stimulated the flies for 3 h with a pure blue light of 700 lux, which is a much stronger stimulation compared with the white ambient light. In tes-mutant flies, this treatment reduced the Rh1 level similar to that by overnight exposure to ambient light. In contrast, no significant downregulation of Rh1 was observed in the shi;tes double mutants (Figure 2B). These observations indicate that the Rh1 protein is indeed first internalized through dynamin-dependent endocytosis and then gets degraded later, likely in lysosomes.
Gq, but not Arr1, mediates endocytosis and downregulation of Rh1 in tes-mutant flies
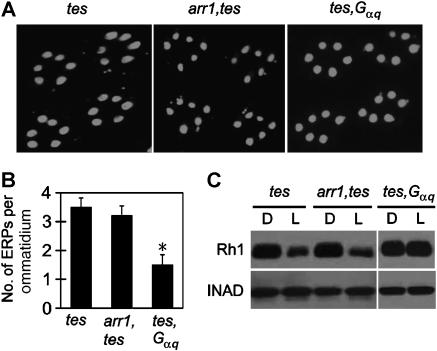
We further investigated which rhodopsin-interacting molecules are responsible for triggering the endocytosis of Rh1 in tes-mutant flies. Although Arr2 is dispensable for rhodopsin endocytosis, another visual arrestin Arr1 has been reported to mediate activity-dependent Rh1 endocytosis during late pupal development (Satoh and Ready, 2005). To examine whether Rh1 is internalized through Arr1 in the adult tes fly, we introduced an arr1 mutation (arr11) into the tes chromosome by recombination. Unexpectedly, the mutation of arr1 did not significantly inhibit the Rh1 endocytosis in the adult tes-mutant fly (Figure 3A and B) as it did in pupal photoreceptors (Satoh and Ready, 2005). The downregulation of Rh1 was also not suppressed in the arr1,tes flies (Figure 3C). As the Rh1 downregulation in a tes;arr2 double mutant was at least as severe as that in the tes single mutant (see Supplementary data), Rh1 appears to be internalized in the adult mutant flies through an arrestin-independent pathway.
Figure 3.
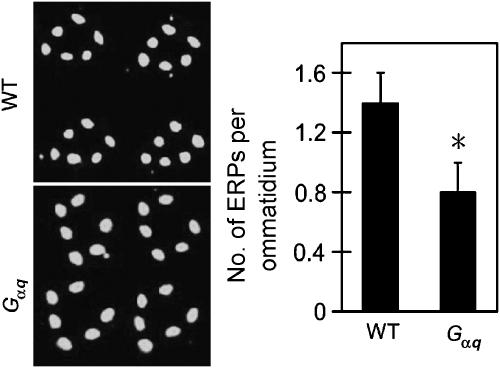
Gq, and not Arr1, is required for Rh1 endocytosis and downregulation in tes flies. (A) Mutation of Gαq, and not of arr1, inhibited endocytosis of Rh1 in tes flies. (B) Averaged ERP numbers per ommatidium. (C) Overnight light stimulation failed to trigger Rh1 downregulation in the tes, Gαq double mutant flies. *Indicates that the sample is significantly different from others in the same group.
In addition to arrestins, the Gq protein also interacts with Rh1 upon light stimulation. Considering that one consequence of tes mutation is the prolonged activation of Gq by Rh1, we hypothesized that the excessive Gq activity may somehow contribute to the increased endocytosis of Rh1. To test this, we recombined a mutant allele (Gαq1) of the Gq α-subunit gene (Scott et al, 1995) to the tes-mutant chromosome, and examined the Rh1 endocytosis and downregulation in the tes,Gαq double mutant flies. The results showed that the Gαq mutation greatly inhibited the endocytosis (Figure 3A and B), and blocked the downregulation of Rh1 (Figure 3C), suggesting that the Gq protein mediates Rh1 endocytosis in adult tes flies.
The Gq-dependent endocytosis and downregulation of Rh1 also occur in arr2-mutant flies
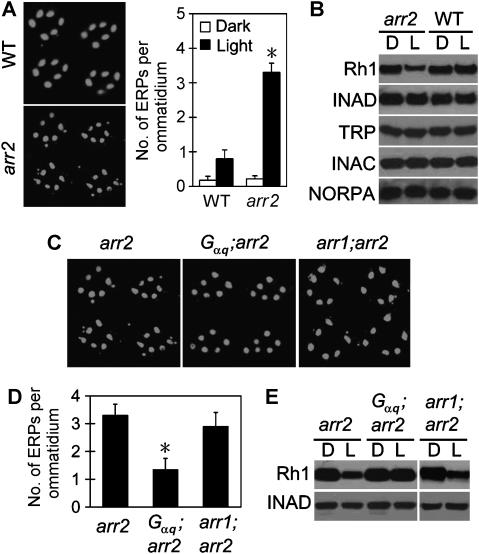
If the prolonged activation of Gq is indeed responsible for the endocytosis and downregulation of Rh1 in tes-mutant flies, we reasoned that a similar Rh1 downregulation should be observed in other mutants that have impaired deactivation of rhodopsin. Since Arr2 is the primary regulatory molecule required for rhodopsin deactivation (Dolph et al, 1993), we examined the internalization and the protein level of Rh1 in an arr2-null mutant that had been exposed to ambient light for 3 h or overnight, respectively.
Compared with wild type, the arr2-mutant photoreceptors contained many more ERPs (Figure 4A), indicating an increase of Rh1 endocytosis. Similar to tes flies, the light-exposed arr2 mutant had a reduced level of Rh1 protein (Figure 4B). The reduction in Rh1 level was not due to light-dependent retinal degeneration in this mutant background, since the overnight light stimulation did not cause any significant abnormality in rhabdomeral morphology according to electron microscopy. In addition, Rh1 downregulation, as well as the excessive endocytosis, was reversed in the dark (see Supplementary data). Moreover, all other examined rhabdomeral proteins displayed unchanged levels in the light-exposed arr2 flies (Figure 4B). It is thus highly likely that the excessive endocytosis has caused a specific downregulation of Rh1 in the arr2-mutant fly.
Figure 4.
Rh1 undergoes Gq-dependent endocytosis and downregulation in an arr2-null mutant fly. (A) Ambient light induced more ERPs in the arr2-mutant fly compared with wild type. (B) Overnight light stimulation caused Rh1 reduction in arr2 flies. All other rhabdomeral proteins had unchanged levels. (C) Mutation of Gαq, and not of arr1, inhibited the Rh1 endocytosis in arr2 flies. (D) Averaged ERP numbers per ommatidium. (E) Light exposure failed to induce Rh1 downregulation in the Gαq;arr2 double mutant. *Indicates that the sample is significantly different from others in the same group.
Remarkably, the endocytosis and the downregulation of Rh1 were greatly inhibited or suppressed, respectively, by the Gαq mutation in a Gαq;arr2 double mutant fly (Figure 4C–E). In contrast, the arr1 mutation had no significant effect on Rh1 endocytosis and downregulation in the arr2 background (Figure 4C–E). Thus, the excessive activity of Gq protein is required for Rh1 endocytosis and downregulation in both tes- and arr2-mutant flies.
Gq-dependent Rh1 endocytosis in the wild-type background
As a low level of Rh1 endocytosis was observed in wild-type flies, we tested whether this basal activity of endocytosis also involves Gq. Exposure of wild-type flies to ambient light for a longer time, 6 h, produced a few more ERPs (1.4±0.2 versus 0.8±0.3 per ommatidium) than the 3-h stimulation. In contrast, the same 6-h exposure only stimulated 0.8±0.2 ERPs per ommatidium in the Gαq mutant fly (Figure 5). These data suggest that Gq-mediated Rh1 endocytosis also occurs in wild-type flies, although the activity is too low to cause any detectable downregulation of Rh1.
Figure 5.
Mutation of Gαq partially inhibited Rh1 endocytosis in the wild-type background. Six-hour light exposure produced less ERPs in the Gαq mutant fly compared with wild type. *Indicates that the sample is significantly different from others in the same group.
The NORPA PLC does not mediate the Gq-dependent Rh1 endocytosis or downregulation
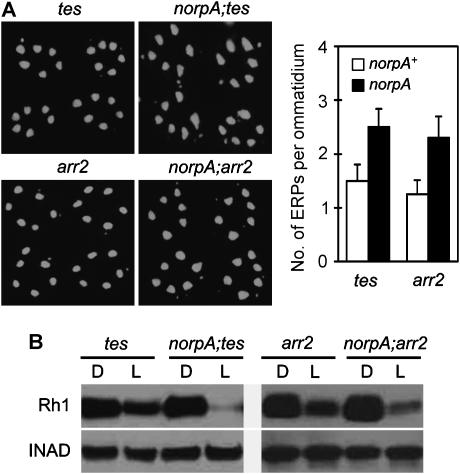
The primary function of Gq protein in fly visual signaling is to stimulate the norpA-encoded PLC, whose activity leads to the opening of TRP and TRPL ion channels (Hardie and Raghu, 2001). A possible explanation for the role of Gq in Rh1 endocytosis is that the Ca2+ influx through TRP channels might be required for the occurring of endocytosis. Nonetheless, the following observations argue against this hypothesis. A norpA mutation, which prevents Gq-dependent activation of TRP and TRPL channels, did not inhibit the endocytosis of Rh1 in either tes- or arr2-mutant background. Remarkably, in the norpA;tes and norpA;arr2 double mutant flies, a large amount of ERPs even appeared within 1.5 h after light exposure (Figure 6A), earlier than that in the tes and arr2 single-mutant background. As a consequence, the double mutants displayed even lower Rh1 levels upon overnight light exposure (Figure 6B). The norpA mutation enhances Rh1 endocytosis and downregulation probably because the NORPA protein is also required to deactivate Gq, and the mutation of norpA actually leads to the accumulation of more active Gq proteins (Cook et al, 2000). These data indicate that Gq may mediate Rh1 endocytosis through a mechanism that is independent of PLC.
Figure 6.
PLC does not mediate the Gαq-dependent endocytosis of Rh1. (A) The mutation of norpA caused more ERPs in tes- and arr2-mutant flies after 1.5 h of light exposure. (B) After overnight light stimulation, the Rh1 levels in the norpA;tes and the norpA;;arr2 flies were even lower than those in the tes and arr2 single mutants.
A tetraspanin Sunglass is involved in the Gq-dependent Rh1 endocytosis
It is reported that Sunglasses (Sun), a lysosomal tetraspanin protein, is required to transfer Rh1 to the lysosome for degradation upon blue light stimulation (Xu et al, 2004). In an attempt to block the Rh1 degradation and accumulate more ERPs in arr2-mutant photoreceptors, we introduced a sun-null mutation into the arr2 background. As expected, the sun mutation abolished the ambient light-induced Rh1 downregulation in the sun;arr2 flies (Figure 7A). Nonetheless, the number of ERPs did not increase but was dramatically reduced in these double mutant flies compared with that of the arr2 single mutant (Figure 7B). This observation suggests that Sun is required for the Gq-stimulated endocytosis, as well as for the lysosomal targeting, of Rh1.
Figure 7.
The tetraspanin Sun is involved in the Gαq-dependent Rh1 endocytosis. (A) A Sun-null mutation suppressed the downregulation of Rh1 in light-exposed arr2 flies. (B) The light stimulation failed to induce Rh1 endocytosis in the sun;arr2 double mutant flies. (C) Sun protein was detected in the peripheral rhabdomeres of p[rh1∷Myc-Sun] flies. The R7 one (arrow) had negative staining. The TRP protein was labeled to show the locations of all rhabdomeres. Three Sun-enriched areas in the cell bodies are indicated by arrowheads. (D) An MBP-Gq fusion protein, not the MBP alone, pulled down Myc-Sun from the head extracts. Lane one was loaded with 1/5 of input. The western blot was probed with an Myc antibody. *Indicates that the sample is significantly different from others in the same group.
It is difficult to explain why the lysosomal protein Sun has a role in the endocytosis of a plasma membrane protein. As the previous study used plastic sections to investigate the location of Sun (Xu et al, 2004) and a 24-h high-temperature step in the section preparation could have greatly reduced the immunoreactivity of the antigen protein, we reexamined the subcellular distribution of Sun by whole-head staining of intact eyes. In the same p[rh1∷Myc-Sun] transgenic fly used by Xu et al (2004), we detected a significant amount of Sun protein in the rhabdomeres of peripheral photoreceptors (Figure 7C). Thus, Sun is located on the plasma membrane of rhabdomere as well as in the lysosome. This observation has provided additional support for a role of Sun in the endocytosis of Rh1.
As both Sun and Gq are involved in the Rh1 endocytosis, it is possible that these two proteins may physically interact with each other. To test this, we coupled a maltose-binding protein (MBP)-tagged Gαq protein covalently to Sepharose 4B beads, and used these beads to pull down proteins from head extracts of the p[rh1∷Myc-Sun] fly. Using western blot, we successfully detected the Myc-Sun protein in the precipitants (Figure 7D). In control experiments, MBP alone, also coupled to Sepharose 4B, failed to pull down any Myc-Sun protein.
Excessive Rh1 endocytosis reduces the sensitivity of photoreceptor to light in tes- and arr2-mutant flies
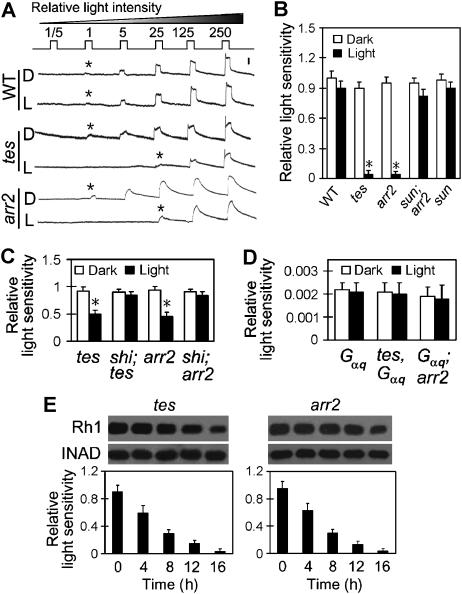
The light sensitivity of fly photoreceptor is determined by the density of rhodopsin on the rhabdomeral membrane (Johnson and Pak, 1986) as well as by the number of available Gq molecules (Scott et al, 1995). Both the endocytosis and the degradation of Rh1 in tes and arr2 flies will decrease the rhodopsin level on the membrane and thus could lead to reduced sensitivity of photoreceptor to light. By conducting intracellular recordings, we examined the sensitivities of photoreceptor after exposing the flies to light overnight. This overnight stimulation did not significantly change the light sensitivity of photoreceptor in wild-type flies (Figure 8A and B), probably because the amount of internalized Rh1 is too small. In contrast, light sensitivities in both tes- and arr2-mutant photoreceptors decreased approximately 25-fold after the light exposure (Figure 8A and B).
Figure 8.
Excessive endocytosis and downregulation of Rh1 leads to reduced light sensitivities of photoreceptor in tes- and arr2-mutant flies. (A) Intracellular recordings revealed decreased light sensitivities of photoreceptor in tes and arr2 flies that had been exposed to ambient light overnight. The fly eye was stimulated with a series of 1-s light pulses of increasing intensities as labeled on the top. The relative intensity 5 is approximately 1 lux. The first appearing response is marked with an asterisk. The scale bar next to the top trace is 10 mV. (B) Quantitation of light sensitivities of photoreceptor. The light groups had been exposed to light overnight. Note that mutation of sun prevented the reduction of sensitivity. The shown mean relative sensitivities were calculated as described in Materials and methods. The error bars represent s.e.m. (C) Mutation of shi prevented light-dependent sensitivity reduction in both tes- and arr2-mutant flies. The light group had been exposed to light for 4 h. All flies were kept at 30.5°C during the 4-h exposure. (D) Mutation of tes and arr2 failed to reduce the light sensitivity in the Gαq-mutant background after overnight light exposure. (E) Time courses of Rh1 downregulation (top) and photoreceptor sensitivity reduction (lower panels) in tes and arr2 flies during light exposure. *Indicates that the sample is significantly different from others in the same group.
Several observations suggest that the light-induced sensitivity reduction in tes and arr2 photoreceptors is due to the Gq-mediated Rh1 endocytosis and/or degradation. First, significant reduction of sensitivity was observed as early as 4 h after light exposure, and was abolished by the temperature-sensitive shi mutation in both tes and arr2 mutants at 30.5°C (Figure 8C). Second, in the Gαq-mutant fly, which already has a very low sensitivity to light due to the lack of Gq-mediated phototransduction (Scott et al, 1995), mutation of either tes or arr2 did not further reduce the photoreceptor sensitivity upon overnight light exposure (Figure 8D). Finally, the light-induced sensitivity reduction was not observed in the sun;arr2 double mutant fly (Figure 8B).
To investigate whether the degradation of Rh1 is the primary cause of sensitivity reduction, we examined the light sensitivity and the Rh1 protein level at a series of time points during the light exposure. In both tes and arr2 flies, the sensitivity started to decrease at about 4 h (Figure 8E), immediately after large amount of Rh1 had been internalized. Before the Rh1 level was significantly downregulated, the sensitivities had reduced at least fourfold (Figure 8E). Thus, the decrease of sensitivity in the mutant flies is due to the excessive endocytosis as well as the degradation of Rh1.
Discussion
Our data indicate that prolonged activation of the Gq protein stimulates Rh1 endocytosis and downregulation, which lead to decreased light sensitivity of fly photoreceptor. Interestingly, the Gq-mediated Rh1 endocytosis requires a tetraspanin protein Sun, but is independent of PLC and the visual arrestins.
Importance of rhodopsin deactivation for the maintenance of light sensitivity
The identification of Gq protein as a mediator of Rh1 endocytosis and downregulation has revealed a new role of rhodopsin deactivation. Since excessive activity of rhodopsin triggers the Gq-mediated endocytosis of Rh1, the deactivation of rhodopsin helps to concentrate Rh1 in the rhabdomere, and thus is critical for the maintenance of photoreceptor sensitivity. Therefore, molecules that promote rhodopsin deactivation, such as Arr2 and dCAMTA/dFbxl4, are important for both the sensitivity of photoreceptor and the termination of light response.
Gq/Sun-dependent Rh1 endocytosis and downregulation in adult flies
Although normal ambient light does not cause significant change in the overall level of Rh1, a low activity of rhodopsin degradation still occurs in wild-type flies. Stimulation with intense blue light enhances the degradation, leading to detectable downregulation of Rh1 (Xu et al, 2004). The degradation pathway, which involves the Sun protein, may help to remove photo-damaged rhodopsin molecules that cannot be deactivated otherwise, and thus protect the photoreceptor from overexcitation. In the sun-mutant fly, the photoreceptors undergo light-dependent degeneration (Xu et al, 2004) probably due to the existence of unregulated rhodopsin molecules. It is plausible that the Gq/Sun-mediated endocytosis may initiate the degradation of Rh1 in light-exposed wild-type flies.
Sun is a member of the tetraspanin family that have been implicated in cell adhesion, motility, invasion and proliferation (Hemler, 2005). Two human tetraspanins CD9 and CD81 have been shown to form dynamic complexes with a Gq protein and an orphan GPCR GPR56 on the membrane of cultured cells (Little et al, 2004). Although it has previously not been reported that a tetraspanin mediates signaling or endocytosis of any GPCR, tetraspanins including CD63, CD82 and CD151 have been found to promote internalization of other associated membrane proteins such as integrins, the EGF receptor and a H+–K+ ATPase, by mechanisms depending on either clathrin or protein kinase C (Berditchevski and Odintsova, 2007). It is possible that prolonged interaction between active Gq protein and Sun may trigger one of these mechanisms to internalize Rh1 together with Sun in the fly photoreceptor. In the future, it will be important to further characterize this Gq/Sun-dependent endocytosis.
The visual arrestin Arr2 is critical for the deactivation of rhodopsin, but does not mediate endocytosis of Rh1 in wild-type flies (Satoh and Ready, 2005). This could be due to a rhodopsin phosphatase RDGC (Steele et al, 1992) that keeps dephosphorylating Rh1 and the dephosphorylated Rh1 cannot form stable association with Arr2 (Kiselev et al, 2000). Although the other arrestin Arr1 is involved in Rh1 endocytosis during late pupal development (Satoh and Ready, 2005), it does not mediate the excessive endocytosis or the degradation of Rh1 in the adult mutant flies. It is likely that the Arr1-dependent endocytic activity is relatively lower in the adult and is not enhanced by the tes or arr2 mutation. In addition, given that many GPCRs internalized through arrestins are targeted for recycle instead of degradation (Yu et al, 1993; Pippig et al, 1995; Vines et al, 2003), the Arr1-mediated endocytosis of Rh1 may not lead to its downregulation.
A new role of Gq protein in GPCR signaling and regulation
A variety of mammalian GPCRs also undergo endocytosis independent of arrestin (Claing et al, 2002). Most of these, such as endothelin, muscarinic, bradykinin, cholecystokinin and thromboxane A2 receptors, are able to activate Gq proteins (Shenker et al, 1991; Hubbard and Hepler, 2006; Alexander et al, 2007). It has been shown in an in vitro system that the activity of Gq protein induces endocytosis of a thromboxane A2 β receptor (Rochdi and Parent, 2003). Our work here has provided in vivo evidence that a Gq protein mediates endocytosis of the fly light receptor Rh1. We propose that the Gq-dependent endocytosis could be a common mechanism that functions in all animal cells for the internalization of Gq-coupled receptors. As G protein is the molecule that signals immediately downstream of GPCR, this Gq-stimulated endocytosis of receptor may mediate an early feedback control of the receptor signaling on plasma membrane.
Remarkably, the Gq proteins internalize both Rh1 and the in vitro expressed thromboxane A2 β-receptor (Rochdi and Parent, 2003) independent of PLC, the known effector of Gq. Thus, the Gq stimulation of GPCR endocytosis represents a new branch of Gq signaling. In addition to controlling the receptor signaling, this new Gq pathway could also be involved in other cellular processes such as apoptotic neuronal degeneration (Iakhine et al, 2004) and deformation of membrane structures (Kosloff et al, 2003). A recent in vitro work on the thromboxane A2 receptor has shown that an ADP-ribosylation factor 6 could be included in this PLC-independent Gq-signaling pathway (Giguere et al, 2006). Our study of Rh1 endocytosis suggests that this Gq pathway also involves the tetraspanin protein Sun. It will be interesting to further characterize this endocytic Gq function in the future.
Materials and methods
Fly genetics and light treatment
All examined flies except the w;p[rh1∷Myc-Sun] transgenic fly had white eyes. Both wild-type flies and those containing the tes mutation were in a cn,bw background. All arr2 mutation-containing flies and the sun-mutant were in the white− background. The mutant alleles used for each gene in this work are arr11, arr25, Gαq1, norpA24, shi2, sun1 and tes2. To avoid age-dependent retinal degeneration in some mutant flies, all flies were examined at 1–2 days old.
All flies were reared in the dark at 22°C and transferred to new vials (with food) before light treatment. To induce Rh1 endocytosis, the flies in Figures 5 and 6A were exposed to room light (∼450 lux, from regular fluorescent tubes) for 6 and 1.5 h, respectively, and all the others for 3 h. To induce the downregulation of Rh1, except those in Figure 2B (as specified in the Results), all flies were exposed to room light for 16–18 h (overnight) before collecting the heads for western blot assay.
Immunostaining
For immunofluorescence staining of Rh1, fly heads were fixed with 4% paraformaldehyde in PBS, dehydrated with acetone and embedded in LR White resin as described (Porter and Montell, 1993). One-micrometer sections were cut across the top half of the eye and stained with a monoclonal Rh1 antibody (1:50, DSHB) and FITC-conjugated secondary antibodies. The activity of Rh1 endocytosis was quantitated as the number of ERPs per ommatidium. At least three sets of data were averaged for each genotype and light treatment.
Whole-head staining was performed to locate the GFP-Rab5 protein in the tes,ey-Gal4;p[UAS∷GFP-Rab5]fly, and the Myc-tagged Sun protein in the w;p[rh1∷Myc-Sun] fly. After fixation, the heads were double stained with the Rh1 and a GFP antibody (1:100), or with an Myc (1:200, Roche) and a TRP antibody (1:200), respectively, following a protocol described previously (Satoh and Ready, 2005). The stained eyes were examined under a LSM5 confocal microscope.
To examine ERPs with electron microscope (EM), fly heads were fixed and embedded in LR White as described (Li and Montell, 2000), except that ethanol was used for the dehydration. Thin sections of eye were cut and immunostained with the Rh1 antibody and anti-mouse IgGs conjugated with 15-nm gold particles. After staining with 1% aqueous uranyl acetate, the sections were examined using a transmission EM.
Gαq-binding assay
An MBP-Gαq fusion protein was expressed in BL-21 cells, purified with amylase resin (NEB) and coupled to CNBr-activated Sepharose 4B beads (Amersham). Approximately 50 μg protein on 50 μl beads were incubated with head extracts of 50 p[ninaE∷myc-sun];sun1 flies in PBS that contains 1% Triton X-100 and protease inhibitors. After three washes with PBS, proteins were eluted with 100 mM glycine in PBS and subjected to SDS–PAGE and western blotting.
Intracellular recording
Intracellular recording was performed as previously described (Wes et al, 1999), with minor modifications. In brief, flies were fixed with stripes of tape and a small opening was made on surface of the eye using fine tweezers. A glass microelectrode with resistance >30 MΩ (filled with 2 M KCl) was gradually inserted into the opening until light-induced membrane depolarization was observed. The reference electrode, filled with Ringer's solution, was put either on the thorax (for the data shown) or inside the eye at the retina layer, without causing difference in the measured sensitivities. Orange light of different intensities (through passing a series of neutral density filters) was used to trigger responses in the photoreceptor cells. Before recording, the flies were allowed to adapt for 3 min in the dark. The signal was amplified and recorded using a Warner IE210 Intracellular Electrometer.
The relative light sensitivity of a photoreceptor is defined as IWT/I, where IWT represents the mean light intensity required to stimulate a detectable response in wild-type photoreceptors that had previously not been exposed to light, and I is the lowest light intensity that is necessary for stimulation of the examined photoreceptor. For each genotype and condition, cells from >8 flies were examined and the relative sensitivities were averaged to obtain a mean. The standard error of means (s.e.m.) were calculated and presented as error bars in the figures.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary figure 3
Supplementary Figure Legends
Acknowledgments
We thank Craig Montell for INAC and INAD antibodies and sun mutant and transgenic flies; Charles S Zuker for Gαq1 and arr11 flies; Patrick J Dolph for the arr25 fly and Marcos González-Gaitán for the p[UAS∷GFP-Rab5] fly. We also thank Jianwu Bai and people in the Li laboratory for critical comments on the manuscript. This work was supported by an NIH grant R01-AG022508 awarded to HL.
References
- Alexander SP, Mathie A, Peters JA (2007) 7TM receptors. Br J Pharmacol 150 (Suppl 1): S4–S81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E (2007) Tetraspanins as regulators of protein trafficking. Traffic 8: 89–96 [DOI] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL (1988) Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 54: 723–733 [DOI] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M (1992) The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70: 715–728 [DOI] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB (1991) Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351: 583–586 [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ (2002) Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol 66: 61–79 [DOI] [PubMed] [Google Scholar]
- Cook B, Bar-Yaacov M, Cohen Ben-Ami H, Goldstein RE, Paroush Z, Selinger Z, Minke B (2000) Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat Cell Biol 2: 296–301 [DOI] [PubMed] [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS (1993) Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 260: 1910–1916 [DOI] [PubMed] [Google Scholar]
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24 [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27: 107–144 [DOI] [PubMed] [Google Scholar]
- Giguere P, Rochdi MD, Laroche G, Dupre E, Whorton MR, Sunahara RK, Claing A, Dupuis G, Parent JL (2006) ARF6 activation by Galpha q signaling: Galpha q forms molecular complexes with ARNO and ARF6. Cell Signal 18: 1988–1994 [DOI] [PubMed] [Google Scholar]
- Han J, Gong P, Reddig K, Mitra M, Guo P, Li HS (2006) The fly CAMTA transcription factor potentiates deactivation of rhodopsin, a G protein-coupled light receptor. Cell 127: 847–858 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P (2001) Visual transduction in Drosophila. Nature 413: 186–193 [DOI] [PubMed] [Google Scholar]
- Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6: 801–811 [DOI] [PubMed] [Google Scholar]
- Hubbard KB, Hepler JR (2006) Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 18: 135–150 [DOI] [PubMed] [Google Scholar]
- Iakhine R, Chorna-Ornan I, Zars T, Elia N, Cheng Y, Selinger Z, Minke B, Hyde DR (2004) Novel dominant rhodopsin mutation triggers two mechanisms of retinal degeneration and photoreceptor desensitization. J Neurosci 24: 2516–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW (2004) Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev 18: 2573–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Pak WL (1986) Electrophysiological study of Drosophila rhodopsin mutants. J Gen Physiol 88: 651–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R (2000) A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron 28: 139–152 [DOI] [PubMed] [Google Scholar]
- Kosloff M, Elia N, Joel-Almagor T, Timberg R, Zars TD, Hyde DR, Minke B, Selinger Z (2003) Regulation of light-dependent Gqalpha translocation and morphological changes in fly photoreceptors. EMBO J 22: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen K (2004) Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther 103: 21–80 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shah S, Suzuki E, Zars T, O'Day PM, Hyde DR (1994) The Drosophila dgq gene encodes a G alpha protein that mediates phototransduction. Neuron 13: 1143–1157 [DOI] [PubMed] [Google Scholar]
- Li HS, Montell C (2000) TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J Cell Biol 150: 1411–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KD, Hemler ME, Stipp CS (2004) Dynamic regulation of a GPCR–tetraspanin–G protein complex on intact cells: central role of CD81 in facilitating GPR56–Galpha q/11 association. Mol Biol Cell 15: 2375–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie N, Aguila B, Allouche S (2006) Tracking the opioid receptors on the way of desensitization. Cell Signal 18: 1815–1833 [DOI] [PubMed] [Google Scholar]
- Montell C (1999) Visual transduction in Drosophila. Annu Rev Cell Dev Biol 15: 231–268 [DOI] [PubMed] [Google Scholar]
- O'Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML (1985) The Drosophila ninaE gene encodes an opsin. Cell 40: 839–850 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002) Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650 [DOI] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ (1995) Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol 47: 666–676 [PubMed] [Google Scholar]
- Poodry CA, Edgar L (1979) Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J Cell Biol 81: 520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Montell C (1993) Distinct roles of the Drosophila ninaC kinase and myosin domains revealed by systematic mutagenesis. J Cell Biol 122: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER (2004) Novel roles for arrestins in the post-endocytic trafficking of G protein-coupled receptors. Life Sci 75: 893–899 [DOI] [PubMed] [Google Scholar]
- Rochdi MD, Parent JL (2003) Galphaq-coupled receptor internalization specifically induced by Galphaq signaling. Regulation by EBP50. J Biol Chem 278: 17827–17837 [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF (2005) Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol 15: 1722–1733 [DOI] [PubMed] [Google Scholar]
- Scott K, Becker A, Sun Y, Hardy R, Zuker C (1995) Gq alpha protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron 15: 919–927 [DOI] [PubMed] [Google Scholar]
- Scott K, Zuker C (1997) Lights out: deactivation of the phototransduction cascade. Trends Biochem Sci 22: 350–354 [DOI] [PubMed] [Google Scholar]
- Scott K, Zuker CS (1998) Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature 395: 805–808 [DOI] [PubMed] [Google Scholar]
- Sever S (2002) Dynamin and endocytosis. Curr Opin Cell Biol 14: 463–467 [DOI] [PubMed] [Google Scholar]
- Shenker A, Goldsmith P, Unson CG, Spiegel AM (1991) The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem 266: 9309–9313 [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ (2003) Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J 375: 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele FR, Washburn T, Rieger R, O'Tousa JE (1992) Drosophila retinal degeneration C (rdgC) encodes a novel serine/threonine protein phosphatase. Cell 69: 669–676 [DOI] [PubMed] [Google Scholar]
- Tsao P, Cao T, von Zastrow M (2001) Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci 22: 91–96 [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM (1991) Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351: 411–414 [DOI] [PubMed] [Google Scholar]
- Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, Kohout TA, Lefkowitz RJ, Prossnitz ER (2003) N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem 278: 41581–41584 [DOI] [PubMed] [Google Scholar]
- von Zastrow M (2001) Endocytosis and downregulation of G protein-coupled receptors. Parkinsonism Relat Disord 7: 265–271 [DOI] [PubMed] [Google Scholar]
- Wes PD, Xu XZ, Li HS, Chien F, Doberstein SK, Montell C (1999) Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat Neurosci 2: 447–453 [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ (2004) Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharmacol Sci 25: 35–41 [DOI] [PubMed] [Google Scholar]
- Xu H, Lee SJ, Suzuki E, Dugan KD, Stoddard A, Li HS, Chodosh LA, Montell C (2004) A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. EMBO J 23: 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Lefkowitz RJ, Hausdorff WP (1993) Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem 268: 337–341 [PubMed] [Google Scholar]
- Zuo Z (2005) The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth Analg 101: 728–734, table of contents [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary figure 3
Supplementary Figure Legends