Abstract
A prospective longitudinal inception cohort study of 33 patients undergoing surgery for cauda equina syndrome (CES) due to a herniated lumbar disc. To determine what factors influence spine and urinary outcome measures at 3 months and 1 year in CES specifically with regard to the timing of onset of symptoms and the timing of surgical decompression. CES consists of signs and symptoms caused by compression of lumbar and sacral nerve roots. Controversy exists regarding the relative importance of timing of surgery as a prognostic factor influencing outcome. Post-operative outcome was assessed at 3 months and 1 year using the Oswestry Disability Index (ODI), Visual Analogue Scale (VAS) scores for leg and back pain and an incontinence questionnaire. Statistical analysis was used to determine the association between pre-operative variables and these post-operative outcomes with a specific emphasis on the timing of surgery. Surgery was performed on 12 (36%) patients within 48 h of the onset of symptoms including seven patients (21%) who underwent surgery within 24 h. Follow up was achieved in 27 (82%) and 25 (76%) patients at 3 and 12 months, respectively. There was no statistically significant difference in outcome between three groups of patients with respect to length of time from symptom onset to surgery- <24, 24–48 and >48 h. A significantly better outcome was found in patients who were continent of urine at presentation compared with those who were incontinent. The duration of symptoms prior to surgery does not appear to influence the outcome. This finding has significant implications for the medico-legal sequelae of this condition. The data suggests that the severity of bladder dysfunction at the time of surgery is the dominant factor in recovery of bladder function.
Keywords: Cauda equina syndrome, Surgical outcome, Timing, Herniated nucleus pulposus
Introduction
Cauda equina syndrome (CES) is a complex of clinical symptoms and signs most commonly secondary to a massive prolapsed intervertebral disc [21]. Although the clinical presentation varies according to the involved nerve roots, the salient clinical features include altered perineal sensation and disturbance of bladder and bowel function [18].
Problems arise in perceived delay in management and there are a variety of opinions regarding the optimum timing for surgery [1, 4, 5, 10, 16, 20, 22]. Most publications have been retrospective with a limited number of patients and inconsistent follow up intervals. A meta-analysis of surgically treated CES cases suggested benefit if decompression was undertaken within 48 h from symptom onset [1] in pooled data from retrospective studies. Several studies argue that a continuum exists with respect to progressive lengthening in the time to surgery yielding increasing poor outcomes [2, 10, 16, 22]. However, not all studies support this argument which has raised the notion that the principal determinant of outcome may not be timing but the extent of the neurological deficit prior to surgery [4, 5]. Despite conflicting evidence regarding the relationship between the timing of surgery and the functional outcome, there is an overwhelming consensus amongst spine surgeons in favour of urgent decompression surgery in CES. Although CES is uncommon it is common enough to cause difficulties in recognition and management. Perceived delays in diagnosis and treatment may result in litigation due to the devastating impact of disordered bowel, bladder and sexual function on quality of life [11].
We have prospectively evaluated the outcomes from patients undergoing surgery for CES secondary to lumbar disc herniation with a particular emphasis on the timing of onset of symptoms to surgery. The study was undertaken through National recruitment with early and late outcomes assessed through validated questionnaires relating to spine, bowel and bladder function [3, 13]. The relationship between such outcomes and the timing of surgery has never been studied prospectively before.
Patients and methods
Thirty-three patients undergoing surgery for CES were prospectively identified from April 2002 to August 2003 through the return of a standard questionnaire including signed patient consent which had been circulated through the British Association of Spine Surgeons. Basic demographics including symptoms and signs at presentation and the duration of symptoms prior to referral were prospectively gathered on all patients. All patients underwent MRI scanning prior to surgery. The time from referral to scan to surgery was noted. Follow up, at a mean of 3 months and 1 year, involved completing the Oswestry Disability Index (ODI), Visual Analogue Scales (VAS) (0–10 scale) for back and leg pain and a Short Form Incontinence Questionnaire (SFIQ) based on the Leicester MRC Incontinence Study [13] looking at the severity of urological and bowel dysfunction (see Table 1). The responses for Questions 2, 3 and 4 in the Incontinence Questionnaire generated numerical scores to aid statistical analysis with increasing values reflecting increasing severity of symptoms. Statistical analysis was performed using SPSS Version 14 for Windows and statistical significance was set at P < 0.05.
Table 1.
Short form incontinence questionnaire (SFIC)
| Question 1. Do you ever leak urine when you do not mean to? | ||||
| Yes | ||||
| No | ||||
| Question 2. Do your urinary symptoms | ||||
| A lot | A little | Not at all | Number urinary symptoms | |
| (a) Bother you? | 3 | 2 | 1 | 0 |
| (b) Cause physical discomfort? | 3 | 2 | 1 | 0 |
| (c) Interfere with daily activities? | 3 | 2 | 1 | 0 |
| (d) Interfere with social life? | 3 | 2 | 1 | 0 |
| (e) Affect your relationships? | 3 | 2 | 1 | 0 |
| (f) Upset or distress you? | 3 | 2 | 1 | 0 |
| (g) Affect your sleep? | 3 | 2 | 1 | 0 |
| (h) Affect your overall quality of life | 3 | 2 | 1 | 0 |
| Total score | /24 | |||
| Question 3. If you were to spend the rest of your life with your urinary pattern just the way it is now, how would you feel about that? | ||||
| Delighted | 1 | |||
| Pleased | 2 | |||
| Mostly satisfied | 3 | |||
| Mixed—equally dissatisfied and satisfied | 4 | |||
| Dissatisfied | 5 | |||
| Mostly dissatisfied | 6 | |||
| Unhappy | 7 | |||
| Terrible | 8 | |||
| Question 4. Do you ever leak from your bowels when you do not mean to? | ||||
| Never/rarely | 1 | |||
| Several times a year | 2 | |||
| Several times a month | 3 | |||
| Several times a week | 4 | |||
| Several times a day | 5 | |||
| Continuously | 6 | |||
| Question 5. Do you have to use a catheter? | ||||
| Yes | ||||
| No | ||||
Results
Eighteen surgeons provided 33 patients with complete data. There were 19 men and 14 women ranging in age from 30 to 79 years (mean age 43). The presenting signs and symptoms, ranked in order of prevalence are shown in Table 2. In 15 of the patients (45%), MRI revealed a massive disc herniation occupying more than 75% of the spinal canal. Seventeen patients had smaller disc herniations and the level of disc herniation is shown in Table 3.
Table 2.
Presenting symptoms and signs in 33 patients with cauda equina syndrome
| Symptom/sign | Number of patients | Per cent |
|---|---|---|
| Back pain | 31 | 94 |
| Urological dysfunction | 30 | 91 |
| Loss of urinary sensation | 21 | 64 |
| Urinary incontinence | 16 | 48 |
| Painful retention | 6 | 18 |
| Sciatica | 28 | 85 |
| Unilateral | 18 | 55 |
| Bilateral | 10 | 30 |
| Perineal numbness | 27 | 82 |
| Faecal symptoms | 10 | 30 |
| Soiling | 6 | 18 |
| Incontinence | 4 | 12 |
| Loss of perineal pinprick sensation | 25 | 76 |
| Bilateral loss | 20 | 61 |
| Unilateral loss | 5 | 15 |
| Absent anal tone | 18 | 55 |
| Absent ankle reflexes | 18 | 55 |
| Bilateral | 10 | 30 |
| Unilateral | 8 | 25 |
| Foot drop | 8 | 24 |
| Bilateral | 3 | 9 |
| Unilateral | 5 | 15 |
| Absent bulbocavernosus reflex | 1 | 3 |
Table 3.
Level of herniated disc and greatest canal compromise on axial MRI
| Number of patients | ||
|---|---|---|
| Level of herniated disc | L2/3 | 2 |
| L3/4 | 1 | |
| L4/5 | 18 | |
| L5/S1 | 12 | |
| Greatest canal compromise | 0–25% | 1 |
| 26–50% | 3 | |
| 51–75% | 13 | |
| 76–100% | 15 | |
Time to surgery
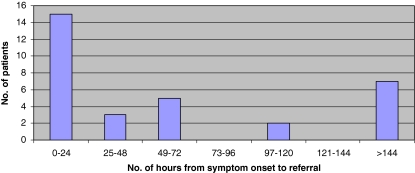
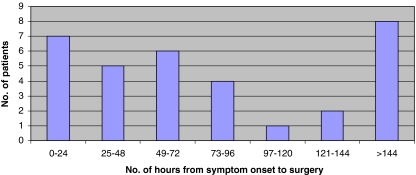
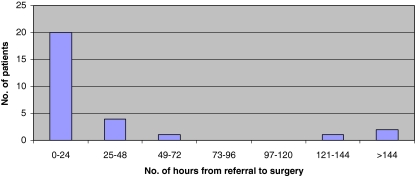
Fifteen (45%) of the 33 patients were seen within 24 h of developing symptoms, six patients presented more than 1 week after the onset of symptoms (Fig. 1). Time from presentation to diagnostic imaging was available for 28 patients. MRI was performed within 24 h of referral for 26 of these cases. The mean time from onset of symptoms to surgery was 131 h (range 6–627 h) (Fig. 2). Surgery was performed on 12 (36%) patients within 48 h of symptom onset and seven patients (21%) were within 24 h (Group 1). Five patients underwent decompression between 24 and 48 h (Group 2). In the 21 patients (64%) where time from onset of symptoms to surgery exceeded 48 h (Group 3), 13 patients had surgery within 24 h of referral (Fig. 3). Eight patients experienced delays between referral and surgery of 45–408 h (mean 110.9 h). In two patients the MRI was performed more than 24 h after referral and in one case it was the principal delay in time from onset of symptoms to surgery.
Fig. 1.
Time from onset of symptoms to referral. Number of patients in each 24 h interval from onset of symptoms to time of referral
Fig. 2.
Time from onset of symptoms to surgery. Number of patients in each 24 h interval from onset of symptoms to time of surgery
Fig. 3.
Time from referral to surgery. Number of patients in each 24 h interval from time of referral to time of surgery
Surgical approach and complications
Surgical decompression of the cauda equina was documented as interlaminar discectomy in 15 patients, six of these were a microdiscectomy approach. Discectomy and laminectomy was performed in the other 18 patients with six patients having a hemilaminectomy. Intra-operative difficulties were reported in seven cases. There was one approach to the wrong level which was recognized with X-ray. Three incidental durotomies occurred and there was one instance of significant epidural bleeding which was controlled. Morbid obesity, noted in three patients and spinal stenosis also caused intra-operative difficulty. One post-operative ileus resolved.
Results at follow up
At early follow up (mean 3 months), 27 patients (82%), consisting of 16 men and 11 women, returned completed questionnaires. At late follow up (mean 12 months) there were 25 respondents (76%). One patient committed suicide between follow ups.
The Oswestry disability index (ODI) and visual analogue scale (VAS) scores for leg and back pain
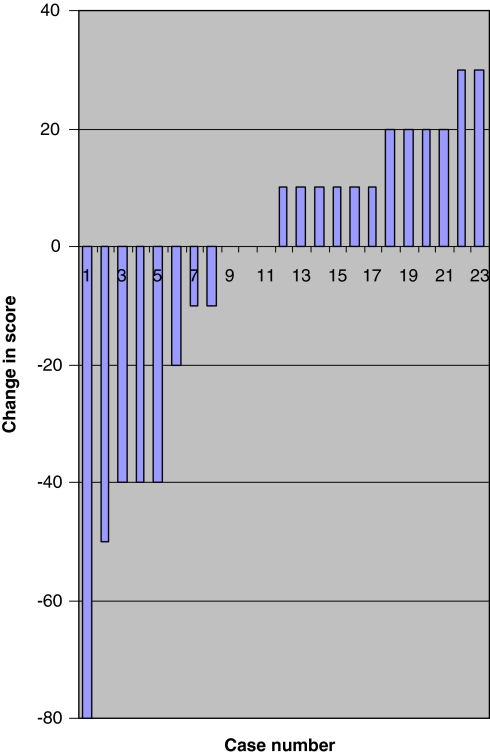
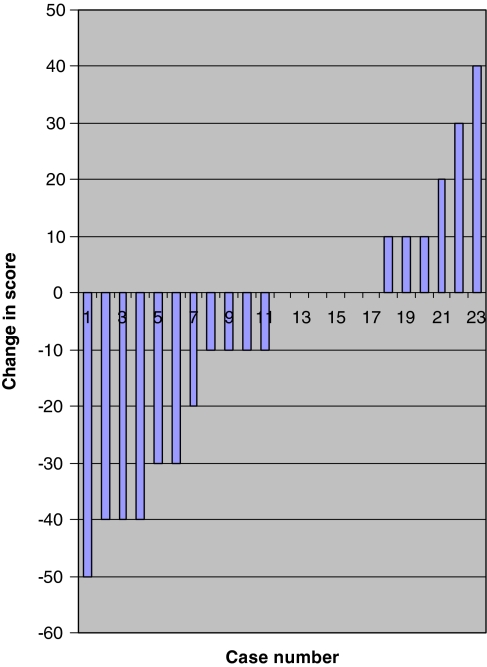
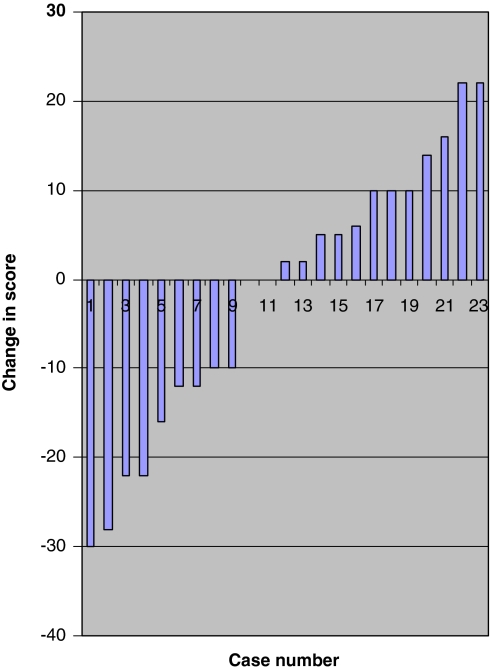
There was no statistically significant difference between these outcomes at early and late follow up. A greater number of patients experienced improvement in their VAS back pain and ODI scores compared to VAS leg pain scores (Figs. 4, 5, 6). The median scores for the three groups based on length of time from symptom onset to surgery at early and late follow up are shown in Table 4.
Fig. 4.
Change in visual analogue score for back pain between follow ups. Difference in scores between early and late follow up for each patient plotted in ascending order
Fig. 5.
Change in visual analogue score for leg pain between follow ups. Difference in scores between early and late follow up for each patient plotted in ascending order
Fig. 6.
Change in oswestry disability index between follow ups. Difference in scores between early and late follow up for each patient plotted in ascending order
Table 4.
Median outcome scores for VAS leg and back pain and ODI for patients undergoing surgery within 24, 24–48 and after 48 h
| Length of time to surgery | |||
|---|---|---|---|
| <24 h | 24–48 h | >48 h | |
| Number of patients | 7 | 5 | 21 |
| Early follow up | |||
| Number of patients responding | 6 | 5 | 16 |
| Median VAS back pain | 35 | 20 | 30 |
| Median VAS leg pain | 5 | 30 | 35 |
| Median ODI | 22.5 | 38 | 26.5 |
| Late follow up | |||
| Number of patients responding | 4 | 5 | 16 |
| Median VAS back pain | 30 | 40 | 40 |
| Median VAS leg pain | 15 | 70 | 35 |
| Median ODI | 18 | 42 | 27 |
Urological and bowel outcome
Twelve (48%) out of 25 patients inadvertently leaked urine at early follow up (SFIQ Q1) with three of 26 patients reporting the use of a urinary catheter. At late follow up, three patients were no longer leaking urine, nine patients had persistent leakage and two patients had developed this symptom. Two of the three patients with urinary catheters at early follow up had them at final follow up.
At early follow up, the median score for impact of urological dysfunction on quality of life (SFIQ Q2) was 12.6 (range 0–24), this was unchanged at late follow up. Eleven of 25 patients (44%) felt either “mostly dissatisfied” (n = 2), “unhappy”(n = 1) or “terrible” (n = 8) about having to spend the rest of their life with their current urinary pattern. At final follow up this reduced to eight of 23 patients (34%) with six patients feeling “terrible.”
Out of 25 patients responding at early follow up, 17 never or rarely leaked from their bowels. Seven of the remaining nine patients experienced inadvertent bowel leakage at least several times a month and this number was unchanged at late follow up.
The results for the SFIQ grouped according to length of time from symptom onset to surgery are shown in Table 5.
Table 5.
Results of short form incontinence questionnaire for patients undergoing surgery within 24, 24–48 and after 48 h (numbers in brackets indicate number of patients who responded to this question)
| Length of time to surgery | |||
|---|---|---|---|
| <24 h | 24–48 h | >48 h | |
| Number of patients | 7 | 5 | 21 |
| Early follow up | |||
| Number of patients leaking urine | 1(5) | 5(5) | 6(15) |
| Median score for impact of urological | |||
| Dysfunction on quality of life | 5(5) | 21(5) | 9(15) |
| Satisfaction with current urinary symptoms | |||
| Delighted/pleased/mostly satisfied | 3 | 1 | 10 |
| Mixed feelings | 1 | 0 | 0 |
| Terrible/unhappy/mostly dissatisfied | 2 | 4 | 5 |
| Frequency of bowel leakage | |||
| Never/several times a year | 5 | 2 | 11 |
| Several times a month/week/day | 1 | 3 | 5 |
| Urinary catheter usage | 1(6) | 1(5) | 1(15) |
| Late follow up | |||
| Number of patients leaking urine | 2(4) | 3(5) | 7(16) |
| Median score for impact of urological | |||
| Dysfunction on quality of life | 5.5(4) | 20(4) | 10.5(10) |
| Satisfaction with current urinary symptoms | |||
| Delighted/pleased/mostly satisfied | 3 | 1 | 8 |
| Mixed feelings | 0 | 1 | 1 |
| Terrible/unhappy/mostly dissatisfied | 1 | 3 | 4 |
| Frequency of bowel leakage | |||
| Never/several times a year | 3 | 1 | 14 |
| Several times a month/week/day | 1 | 4 | 2 |
| Urinary catheter usage | 1(4) | 1(5) | 0(16) |
Continent versus incontinent patients
At presentation, 16 patients had symptoms of urinary incontinence, 17 did not. All of the measured outcomes were compared between these two groups (Man-Whitney U-test) with the exception of urinary catheter usage due to the very small number of these patients. The results are shown in Table 6. There was a statistically significant improvement in the continent group compared to the incontinent group specifically in outcomes of VAS leg pain, frequency of bowel leakage at early follow up, VAS leg and back pain at late follow up and “the impact of urological dysfunction on the quality of life” and “the satisfaction with current urinary symptoms” at both early and late follow up.
Table 6.
Comparison of outcomes between patients who presented with urinary incontinence and those who were continent (numbers in brackets indicate number of patients who responded to this question)
| Incontinent | Continent | P-value | |
|---|---|---|---|
| Early follow up | |||
| Median VAS back | 20(12) | 35(14) | 0.374 |
| Median VAS leg | 40(12) | 10(14) | 0.046* |
| Median ODI | 27(12) | 26.5(14) | 0.667 |
| Number of patients leaking urine** | 8(11) | 4(13) | 0.199 |
| Median score for impact of urological | |||
| Dysfunction on quality of life | 21.5(11) | 8(13) | 0.002* |
| Satisfaction with current urinary symptoms | |||
| Delighted/pleased/mostly satisfied | 3 | 10 | 0.015* |
| Mixed feelings | 0 | 1 | |
| Terrible/unhappy/mostly dissatisfied | 8 | 3 | |
| Frequency of bowel leakage | |||
| Never/several times a year | 5 | 13 | 0.038* |
| Several times a month/week/day | 6 | 1 | |
| Urinary catheter usage | 3(11) | 0(14) | |
| Late follow up | |||
| Median VAS back | 40(12) | 20(12) | 0.020* |
| Median VAS leg | 70(12) | 10(12) | 0.003* |
| Median ODI | 52(12) | 18(12) | 0.060 |
| Number of patients leaking urine** | 9(12) | 2(12) | 0.199 |
| Median score for impact of urological | |||
| Dysfunction on quality of life | 20(8) | 8(9) | 0.006* |
| Satisfaction with current urinary symptoms | |||
| Delighted/pleased/mostly satisfied | 3 | 10 | 0.014* |
| Mixed feelings | 0 | 0 | |
| Terrible/unhappy/mostly dissatisfied | 8 | 0 | |
| Frequency of bowel leakage | |||
| Never/several times a year | 7 | 10 | 0.347 |
| Several times a month/week/day | 5 | 1 | |
| Urinary catheter usage | 2(12) | 0(12) | |
*Statistical significance
Man-Whitney U-test except **Pearson chi-square test
Relationship between length of time to surgery and measured outcomes
There was no correlation (Spearman Ro) between the number of hours from the onset of symptoms to surgery and all outcome measures at both early and late follow up. There was no statistically significant difference (P ≤ 0.05) in all observed outcome measures between the three groups of patients with respect to length of time to surgery (Man-Whitney U-test).
Age
The only statistically significant correlation for age was with the VAS score for back pain at early follow up (P = 0.02, Spearman Rho). This may reflect increasing incidence with age.
Discussion
CES is an uncommon entity, accounting for 2–6% of all lumbar disc herniations [2, 10, 19]. The term describes a diverse spectrum of symptoms and signs caused by compression of nerve roots in the lumbar spinal canal. Despite surgical intervention, varied outcomes have been reported. Consequently, there has been a significant impetus to identify the pre-operative variables associated with improved post-operative outcomes. We have prospectively evaluated outcomes at defined time points using validated patient based outcome measures of spine and urological function [3, 13] as like others [6], we believe patient orientated goals represent the ultimate evaluation of treatment.
Determining when exactly CES is deemed to be present is difficult as the evolving analogue symptoms and signs cannot easily be placed in to categorical boxes. As in previous studies [14, 20], we have shown delays in diagnosis still exist and this probably reflects the diagnostic uncertainty as to what constitutes CES. Depending on the nerve roots involved the patient may exhibit lower back pain, unilateral or bilateral sciatica, saddle anaesthesia, sensory and/or motor deficit in the lower limbs and evidence of impaired pelvic visceral dysfunction manifesting as urinary and/or fecal incontinence. In the present study the most consistent findings in CES were urological dysfunction (91%), which encompassed loss of sensation during micturition, painful retention and incontinence; back pain with sciatica (85%) and perineal sensory disturbance (82%). Of greater note, all 33 patients had either urological dysfunction and/or a subjective or objectively identifiable perineal sensory deficit. We believe these two features represent the most consistent identifying features of CES. The presence of either of these in clinical context necessitates urgent assessment and imaging.
Patient based outcome measures, such as VAS for pain and the ODI, are principally used to measure response to a treatment between two set time points usually pre and post-intervention [3]. The acute presentation of CES precluded pre intervention outcome measures in our multicentre study. However, we have measured these outcomes at two timepoints post-operatively and there was no statistically significant difference between early and late follow up for each of the measured outcomes. Our results, like others, show persisting back pain, sciatica and disability to be an ongoing problem post-decompression [20].
The most distressing sequelae of CES relates to loss of sphincter control which can be socially disruptive and emotionally distressing. There are mixed reports of the recovery of bladder function in CES with some studies reporting universally poor outcomes [7, 21]. Urodynamic studies suggest recovery of complete bladder paralysis does not occur [18]. Interestingly, in the absence of detrusor contractions on such studies, patients may deny all symptoms as they may be unaware that they are able to void through straining and prevent stress incontinence by regularly passing urine [15, 18]. Consequently, some studies suggest that the consequences of persistent paralysis may be much less severe than earlier authors have stated [16, 18]. Our findings demonstrate that urological impairment does persist as despite only three patients reporting the need for catheterization, 48% of patients were experiencing urinary incontinence at early follow up. Of greater note, a significant number of patients were at the very least unhappy with their urinary pattern (11 of 23) at early follow up with no statistically significant change in all urological outcomes between successive follow up.
Controversy exists throughout the literature regarding the question of timing of surgical decompression in CES and its influence on outcome. In his retrospective review of 44 patients with CES [20], Shapiro noted the delayed surgery group (>48 h) demonstrated a significantly greater chance of permanent motor weakness, urological dysfunction, chronic severe pain and sexual dysfunction. Ahn’s meta-analysis of 322 cases of CES [1] has similarly shown a significant difference in outcome in those cases decompressed in under 48 h and those decompressed after 48 h. This study has been critiqued for its inappropriate methodology and flawed statistical analysis. A repeat analysis of the data by Kohles et al. [9] still demonstrated a significant improvement in outcome with earlier decompression. Although several authors support the critical importance of timing of surgery, these studies have been retrospective in nature with limitations of subjectively ascertaining outcomes with variable length of follow up and often incomplete data gathering. Previously held beliefs regarding the importance of timeliness of surgical decompression and the consensus opinion that delays in decompression negatively affect outcome should be questioned.
We have shown no statistically significant difference in any of the measured outcomes comparing those who underwent decompression within 24, 24–48 and after 48 h of developing symptoms.
Certain studies, although supporting emergency decompression in CES, have failed to demonstrate any correlation between the timing of surgery and clinical outcome [10–16]. Gleave and MacFarlane [4, 5] argue that this may be explained by recovery being more dependent on the nature of the prolapse rather than the speed at which the nerve roots are compressed. A spectrum of impairment exists with respect to urological dysfunction caused by cauda equina compression [8]. Initial irritative symptoms give way to loss of bladder and urethral sensation and poor stream with progressive compression eventually culminating in painless retention with overflow incontinence. This endpoint of an insensate incontinent bladder may constitute a complete cauda equina lesion which is unlikely to improve regardless of the time to decompression [4, 5]. In contrast, the prognosis may be more favourable when the syndrome remains incomplete at the time of decompression. Pre-operative urodynamic studies represent the most satisfactory method of distinguishing these two groups through the detection of detrusor activity [15, 17]. We have compared the outcomes of those patients who were incontinent of urine at the time of presentation with those who were continent. The latter group had a statistically significant likelihood of less leg and back pain and a better urological outcome at late follow up. This supports the view that the major determinant of outcome may not be timing but the severity or density of deficit [4, 5, 21].
The question arises as to whether timing has a role to play in management of CES. Prompt decompression is still advisable to prevent an incomplete lesion progressing to complete sphincter paralysis [4, 5]. However, any potential benefit in this should be balanced against the morbidity of the procedure. Surgical decompression in CES is technically more challenging than elective lumbar disc surgery and our data suggests a higher co-morbidity and complication rate. The surgery should be undertaken by experienced surgeons to avoid a theoretical increase in the risk of post-operative complications [4]. Urgent decompression should be performed at the earliest opportunity but probably not in the middle of the night when circumstances may not be optimal. The length of acceptable delay is not known for an evolving surgical emergency. The evidence from this study suggests that timing in terms of number of hours from symptom onset to surgical decompression may not be the most important determinant of outcome. Despite this controversy will remain regarding the medico-legal implications of perceived delays in diagnosis or treatment [11, 12].
Although we have not shown a relationship between the timing of surgery and outcome in CES, this current study has limitations. The number of cases is small which reflects the difficulties in evaluating a relatively infrequent emergency presentation. Although our small cohort is comparable to other studies in the literature, this factor in association with the usage of appropriate, but less sensitive, non-parametric statistical tests may have a type 1 error. Lack of proof of benefit does not equate to proof of lack of benefit [9]. Further study is required specifically to assess the impact of varying grades of urological deficit in CES, determined by urodynamic studies, on post-operative outcome. Despite the failings this study does contribute to the knowledge base on cauda equina syndrome due to prolapsed intervertebral disc. The influence of delays in treatment may have historically been overestimated. Our data suggests that the timing of surgical decompression does not adversely affect the outcome especially when surgery occurs within 48 h and under optimal conditions. Our findings although imperfect are the result of prospectively gathered data at specific sequential time points and represent the highest level of evidence available to date on this subject matter.
Acknowledgements
We would like to acknowledge the contribution by the following surgeons and the British Association of Spinal Surgeons. Mr. Allerton, Mr. Craig, Mr. Cross, Mr. Davies, Mr. Douglas, Mr. Floyd, Mr. Green, Mr. Grevitt, Mr. Kumar, Mr. Marshall, Mr. Mehdian, Mr. Newey, Mr. Pennie, Mr. Trivedi, Mr. Schranz, Mr. Scofield and Mr. Wilson-Macdonald.
References
- 1.Ahn UM, Ahn NU, Buchowski MS, Garrett ES, Sieber AN, Kostuik JP (2000) Cauda equina syndrome secondary to lumbar disc herniation. A meta-analysis of surgical outcomes. Spine 25:1515–1522 [DOI] [PubMed]
- 2.Dining TAR, Schaeffer HR (1993) Discogenic compression of the cauda equina: a surgical emergency. Aust NZ J Surg 63:927–934 [DOI] [PubMed]
- 3.Fairbank JCT, Pynsent PB (2000) The oswestry disability index. Spine 25(22):2940–2953 [DOI] [PubMed]
- 4.Gleave JRW, Macfarlane R (1990) Prognosis of recovery of bladder function following lumbar central disc prolapse. Br J Neurosurg 4:205–210 [DOI] [PubMed]
- 5.Gleave JRW, Macfarlane R (2002) Cauda equina syndrome: what is the relationship between timing of surgery and outcome? Br J Neurosurg 16(4):325–328 [DOI] [PubMed]
- 6.Hussain SA, Gullan RW, Chitnavas BP (2003) Cauda equina syndrome: outcome and implications for management. Br J Neurosurg 17(2):164–167 [DOI] [PubMed]
- 7.Jennett WB (1956) A study of 25 cases of compression of the cauda equina by prolapsed intervertebral discs. J Neurol Neurosurg Psychiatry 19:106–116 [DOI] [PMC free article] [PubMed]
- 8.Jones DL, Moore T (1973) The types of neuropathic bladder dysfunction associated with prolapsed lumbar intervertebral discs. Br J Urol 45:39–43 [PubMed]
- 9.Kohles SS, Kohles DA, Karp AP, Erlich VM, Polissar NL (2004) Time dependent surgical outcomes following cauda equina syndrome diagnosis: comments on a meta-analysis. Spine 29(11):1281–1287 [DOI] [PubMed]
- 10.Kostuik JB, Harrington I, Alexander D, Rand W, Evans D (1986) Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg 68A:386–391 [PubMed]
- 11.Kostuik JP (2004) Medicolegal consequences of cauda equina syndrome: an overview. Neurosurg Focus 16(6):39–41 [DOI] [PubMed]
- 12.Markham DE (2004) Cauda equina syndrome: diagnosis, delay and litigation risk. J Orthop Med 26(3):103–105
- 13.McGrother CW, Donaldson MMK, Shaw C, Matthews RJ, Hayward TA, Dallosso HM, Jagger C, Clarke M, Castleden CM, the MRC Incontinence Study Team (2004) Storage symptoms of the bladder: prevalence, incidence and need for services in the UK. BJU Int 93:763–769 [DOI] [PubMed]
- 14.Ng LCL, Tafazal S, Longworth S, Sell P (2004) Cauda equina syndrome: an audit. Can we do better? J Orthop Med 26(2):98–101
- 15.Nielsen B, de Nully M, Schmidt K, Hansen RI (1980) A urodynamic study of cauda equina syndrome due to lumbar disc herniation. Urol Int 35:167–170 [DOI] [PubMed]
- 16.O’Laoire SA, Crockard HA, Thomas DG (1981) Prognosis for sphincter recovery after operation for operation for cauda equina compression owing to lumbar disc prolapse. Br Med J 282:1852–1854 [DOI] [PMC free article] [PubMed]
- 17.Rosomoff HL, Johnston JD, Gallo AE, Ludmer M, Givens FT, Carney FT, Kuehn CA (1963) Cystometry in the evaluation of nerve root compression in the lumbar spine. Surg Gynecol Obstet 117:263–270 [PubMed]
- 18.Scott PJ (1965) Bladder paralysis in cauda equina lesions from disc prolapse. J Bone Joint Surg 47B(2):224–235 [PubMed]
- 19.Shapiro S (1993) Cauda equina syndrome secondary to lumbar disc herniation. Neurosurgery 32:743–747 [DOI] [PubMed]
- 20.Shapiro S (2000) Medical realities of cauda equina syndrome secondary to lumbar disc herniation. Spine 25:348–352 [DOI] [PubMed]
- 21.Shephard RH (1959) Diagnosis and prognosis of cauda equina syndrome produced by protrusion of lumbar disc. Br Med J 2:1434–1439 [DOI] [PMC free article] [PubMed]
- 22.Todd NV (2005) Cauda equina syndrome: the timing of surgery probably does influence outcome. Br J Neurosurg 19(4):301–306 [DOI] [PubMed]