Abstract
The aim of our study is to evaluate the results and effectiveness of bilateral decompression via a unilateral approach in the treatment of degenerative lumbar spinal stenosis. We have conducted a prospective study to compare the midterm outcome of unilateral laminotomy with unilateral laminectomy. One hundred patients with 269 levels of lumbar stenosis without instability were randomized to two treatment groups: unilateral laminectomy (Group 1), and laminotomy (Group 2). Clinical outcomes were assessed with the Oswestry Disability Index (ODI) and Short Form–36 Health Survey (SF-36). Spinal canal size was measured pre- and postoperatively. The spinal canal was increased to 4–6.1-fold (mean 5.1 ± SD 0.8-fold) the preoperative size in Group 1, and 3.3–5.9-fold (mean 4.7 ± SD 1.1-fold) the preoperative size in Group 2. The mean follow-up time was 5.4 years (range 4–7 years). The ODI scores decreased significantly in both early and late follow-up evaluations and the SF-36 scores demonstrated significant improvement in late follow-up results in our series. Analysis of clinical outcome showed no statistical differences between two groups. For degenerative lumbar spinal stenosis unilateral approaches allowed sufficient and safe decompression of the neural structures and adequate preservation of vertebral stability, resulted in a highly significant reduction of symptoms and disability, and improved health-related quality of life.
Keywords: Laminectomy, Laminotomy, Lumbar spinal stenosis, Unilateral approach, Vertebral stability
Introduction
Increasing knowledge of pathoanatomy, coupled with the development of magnetic resonance imaging, has allowed a more precise delineation of soft tissue and bony stenosing lesions [48, 53, 57]. Unilateral approach preserves the facet joints and neural arch of the contralateral side, limits postoperative destabilization and protects the nervous structure against posterior scarring [32]. Initially described by Young et al. [57] in 1988 and subsequently modified by McCulloch [34], a microscopic technique characterized by unilateral multifidus retraction, ipsilateral microdecompression, and contralateral microdecompression performed under the midline posterior structures has been used with some modification at the current authors’ center since 1995. The purpose of our prospective study was to compare the safety and the clinical midterm outcomes after unilateral laminectomy and unilateral laminotomy in patients with lumbar spinal stenosis, who were randomized to one of the two treatment groups.
Materials and methods
This prospective observational study was undertaken for analysis of 100 patients with degenerative lumbar spinal stenosis refractory to adequate conservative treatment, who underwent adjacent two or multilevel bilateral decompression via a unilateral approach between January 2000 and January 2002. All patients who had one or more of the following criteria were included in this study: (1) symptoms of neurogenic claudication referable to the lumbar spine (claudicant or radicular symptoms brought on either by walking or by prolonged standing, relieved by sitting or the flexed position, in the absence of vascular or neuropathic pathology), (2) radiological/neuroimaging evidence of degenerative lumbar stenosis (neurologic compression by hypertrophied (infolded) ligamentum flavum, osteophytic facet joints, and annular bulging), (3) failure of conservative measures for a minimum 3 months, (4) the absence of associated pathology such as instability, inflammation or malignancy, and (5) no history of surgery for lumbar stenosis or lumbar fusion. Patients presenting with mild degenerative spondylolisthesis were not excluded. We also did not exclude from outcome analysis 13 patients who required discectomies, which had been identified on preoperative imaging studies.
The assessment of neurologic status of patients was evaluated by physical examination, and preoperative radiological investigations were made with plain roentgenograms, magnetic resonance (MRI) and computed tomographic (CT) images for all patients.
Each patient’s admission number was used to blind the randomization to personal data. If a patient met the inclusion criteria according to the admitting physician and informed consent was obtained, a concealed computer-generated randomization list was used to assign the patient to one of the two treatment groups: unilateral laminectomy (Group 1), unilateral laminotomy (Group 2).
All patients underwent postoperative MR-imaging studies. A single radiologist, blinded to the clinical results of decompression, reviewed all pre- and postoperative studies. For data recording purposes, each vertebral level was divided into four major zones: upper, middle, and lower vertebral bodies, and disk space. Cross-sectional area of each zone of the vertebral level was measured, by using software (General Electric Advantage Windows 4.2). For each zone, measurement was performed thrice. The mean of the three values was calculated, and the mean of the cross-sectional area of four zones was calculated for each spinal segment. Finally, the mean increase in the cross-sectional area of all spinal segments subjected to the surgery was calculated (Fig. 1). All patients were followed-up regularly at intervals of 1, 3, 6, 12 months, and were followed up annually thereafter. Routine radiological investigations including neutral, flexion/extension lateral radiographs, at these time intervals were taken routinely.
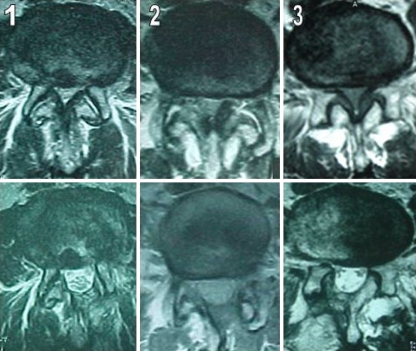
Fig. 1.
Preoperative and postoperative axial MRI of the lumbar spine, demonstrating spinal canal sizes. The postoperative canal size was increased 4.1-fold in 1, 5.2-fold in 2 and, 4.7-fold in 3, compared with the corresponding preoperative canal size
The features studied on these imaging data included: (1) extent of lumbar spinal decompression at each stenotic level, (2) the presence of abnormal motion and/or progression of spondylolisthesis at dynamic roentgenograms (spinal instability was defined as sagittal-plane translation of 5 mm or more documented on flexion–extension radiography [11, 54]) and (3) relationship between the radiological investigations and neurologic status and quality of life of the patients.
The outcomes of surgery in the long-term follow-up were measured for all patients according to the criteria used by the ODI and SF-36. Follow-up data were obtained from the questionnaires forwarded directly to the patients at preoperative term and postoperative third month and 4–7 years. Disability was assessed using the ODI, and physical and mental health status was measured using the SF-36 health survey which has been validated and reported on for Turkish-language speakers [37,55].
Surgical procedures
Unilateral laminectomy for bilateral decompression (Group 1)
The target level is verified by a C-arm scope pre- and peroperatively. The incision is midline and extends over, but is limited to the underlying region of stenosis as documented on magnetic resonance imaging. A 2–6 cm skin incision is made for 2–4 level stenosis. A linear median fascial incision then is made on the patient’s most symptomatic side. The paraspinal muscles are removed from their bony attachments on the spinous process and lamina to expose the bony detail. A modified mini Taylor retractor is then used. A full view of the ipsilateral interlaminar space is now enabled, and the microscope is brought into place. Using Kerrison rongeurs or a high-speed burr, ipsilateral cephalad and then caudal hemilamina is totally resected. The microscope is then angulated into the ipsilateral subarticular zone and, moving cephalad to caudal, soft tissue and bony stenosing pathology is excised using high-speed drill and Kerrison rongeurs. This is done sequentially until the nerve root at the operative level is seen exiting freely into the foramen. Lateral decompression was made via undermining of the hypertrophic facet joint, which was bony stenosing pathology. The medial aspect of the facet joint is resected to decompress the lateral recess. Maximally one third of hypertrophic facet joint was resected. A hemifacetectomy is never performed. Thus, maximal preservation of the pars interarticularis and facet joint were made. If necessary, disk material is removed. Then ipsilateral ligamentum flavum is totally resected. After complete ipsilateral microdecompression, the contralateral side is addressed. The microscope is angulated medially and, the patient tilted contralaterally, to afford visualization across the midline beneath the deepest portion of the interspinous ligament. Resection of portions or all of the interspinous ligaments, and supraspinous ligaments is not performed. Interspinous ligament is retracted medially using with root retractor. A dissector is used to confirm that the anterior surface of the ligamentum flavum is free from adhesion to the dura, and the medial portion of contralateral ligamentum flavum is then resected sequentially from cephalad to caudal with curved curettes and Kerrison rongeurs. The following part of operation cannot be performed without high-speed burr. A vital point in the process, to allow access for contralateral decompression, is the adequate resection of the “wishbone” portion of the cephalad and caudal lamina, i.e., the junction of lamina with the spinous process. Thus, antero-posterior diameter of spinal canal is expanded to afford visualization across the midline beneath the deepest portion of the spinous process. Then, a dissector is used to confirm that the contralateral surface of the dura is free from adhesion to the ligamentum flavum, and the contralateral portion of ligamentum then is resected sequentially from cephalad to caudal with curved curettes and Kerrison rongeurs. The microscope then is angulated into the contralateral subarticular zone and, moving cephalad to caudal, soft tissue and bony stenosing pathology is excised using high-speed drill and Kerrison rongeurs. This is done sequentially until nerve root at the operative level is seen exiting freely into the foramen. If necessary, contralateral disk material can be removed. Both the ipsilateral and contralateral nerve roots are well visualized after the bilateral decompression (Fig. 2). Then same procedure is repeated for each proper level. When decompression is confirmed with direct inspection under surgical microscope, the operation is complete. To reduce postoperative granulation, the decompressed nerve roots are protected with small blocks of fat resected from subfascial tissue. All affected levels can be successfully decompressed through this unilateral approach. Suction drains are not routinely placed.
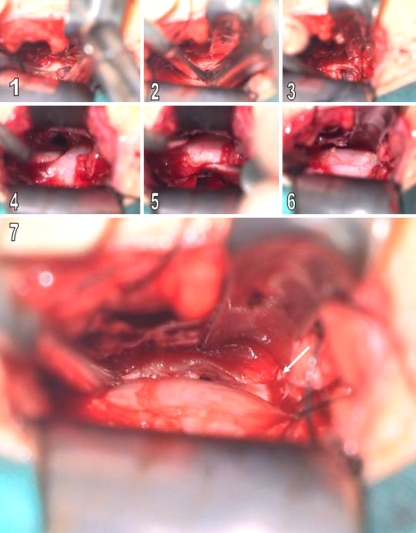
Fig. 2.
Intraoperative views. 1, 2 Removing of the contralateral disc material by using a disc punch. 3 View of after contralateral diskectomy. 4–6 Bilaterally decompressed dural sac. 7 View of contralateral nerve root after the contralateral decompression (white arrow)
Unilateral laminotomy for bilateral decompression (Group 2) [16, 27, 32, 36, 44, 45, 53]
The operation is made in a similar fashion to unilateral hemilaminectomy for bilateral decompression that has been previously described but the approach is made with some modifications. Using Kerrison rongeurs or a highspeed burr, an ipsilateral partial laminectomy of the cephalad hemilamina is performed, angling the microscope upward. A similar but less extensive laminectomy is then performed on the ipsilateral caudal lamina, which allows the removal of intervening ligamentum flavum and affords a midline hemidecompression. Then, the surgical technique is performed as described in surgical procedure of unilateral laminectomy for bilateral decompression.
The patient is allowed out of bed without a lumbosacral corset 4–5 h after surgery and is discharged within 24 h. An exercise program is started after 2 weeks to strengthen the paravertebral muscles and patient advised to return to daily activities.
Statistical analysis
Statistical calculations were performed with GraphPad Prisma V.3 program for Windows. Besides standard descriptive statistical calculations (mean and standard deviation), one way ANOVA was used in the comparison of groups, post Hoc Newman Keuls multiple comparison test was utilized in the comparison of subgroups, unpaired t-test was two treatment values, and Chi square test was performed during the evaluation of qualitative data. Statistical significance level was established at P < 0.05.
Results
Of these 100 patients, 61 were females (61%) and 39 were males (39%) whose mean age was 69.21 ± SD 12.18 (range 55–83 years). Duration of symptoms ranged from 8 to 60 months. Preoperative clinical symptoms and signs were low-back pain (94%), leg pain (88%), neurogenic claudication (99%), sensory change (77%), motor weakness (20%), incontinance (2%). Two levels LSS were present in 52 patients, three levels LSS were present in 27 patients, and four levels LSS were present in 21 patients (in the patients with a second or another stenotic level(s) unrelated to spondylolisthesis). In a total of 269 stenotic levels were decompressed and 13 patients underwent concomitant discectomies at the index level. Seven and six discectomies has been performed in Group 1 and 2 (Table 1).
Table 1.
Clinical and demographic data of patients
| Parameters | Group | |
|---|---|---|
| 1 | 2 | |
| No. Of cases | 50 | 50 |
| Mean age (years) | 69.81 ± 15.15 | 61.84 ± 11.21 |
| Male/female | 21/29 | 18/32 |
| Stenotic level of the lesion | ||
| L1–2 | 12 | 10 |
| L2–3 | 15 | 18 |
| L3–4 | 41 | 39 |
| L4–5 | 53 | 50 |
| L5–S1 | 15 | 16 |
| Number of stenotic levels | ||
| Two | 25 | 27 |
| Three | 14 | 13 |
| Four | 11 | 10 |
| Initial chief complaint | ||
| Leg pain | 45 | 43 |
| Low back pain | 46 | 48 |
| Claudication | 49 | 50 |
| Numbness/tingling | 39 | 38 |
| Weakness | 11 | 9 |
| Incontinence | 2 | 0 |
Follow-up status
The mean follow-up time was 5.4 years (range 4–7 years). The routine radiological investigations at these time intervals were taken and follow-up data were obtained from the ODI, and SF-36 questionnaires in 97 of 100 patients. The remaining two of three patients after 18 months refused to have control radiological investigations, and one of three patient after 20 months died of unrelated cause.
Clinical analysis
There were no perioperative deaths. Of all surgically treated levels accidental duratomy occurred in two patients (4%, Group 1), and three patients (6%, Group 2). All of dural tears occurred on the ipsilateral side, and primary repairing were not performed but covered with fat graft and fibrin glue. These five patients were admitted to the hospital for 48 h of bedrest and duratomies were not noticeably associated with postoperative morbidity, and no subsequent postoperative CSF fistula was observed. Neural injury or any other complication was not observed during operation. No revision surgery was required in early postoperative period. But two wrong levels (instead of stenotic levels) were operated in two patients by mistake so that they underwent reoperation. Only one wound infection was noted in a laminotomy-treated patient requiring antibiotic therapy.
Assessment of outcome
The ODI scores decreased significantly in both early and late follow-up evaluations [Newman–Keuls multiple comparison test, P < 0.0001 (Group 1), P < 0.0001 (Group 2)], from a mean preoperative score of 31.14 ± 9.27, to 14.22 ± 9.88 at third month and 14.02 ± 9.27 at 4–7 years in Group 1, and from a mean preoperative score of 29.62 ± 8.19, to 12.22 ± 6.46 at 3 months and 12.4 ± 6.3 at 4–7 years in Group 2. Most of the changes occurred between preoperative and early follow-up assessments with little changes between early and late follow-up reviews [Newman–Keuls multiple comparison test, P > 0.05 (Group 1), P > 0.05 (Group 2)]. No significant differences in preoperative¹, early postoperative², and late postoperative³ ODI scores were identified between two groups [all according to the unpaired t test, (¹t = 0.87, P = 0.387), (²t = 1.20, P = 0.234), (³t = 1.02, P = 0.309)] (Table 2).
Table 2.
Mean ODI scores preoperatively, at 3 months, and 4–7 years postoperatively
| Group | t | P | ||
|---|---|---|---|---|
| 1 | 2 | |||
| Preop | 31.14 ± 9.27 | 29.62 ± 8.19 | 0.87 | 0.387 |
| Early | 14.22 ± 9.88 | 12.22 ± 6.46 | 1.2 | 0.234 |
| Late | 14.02 ± 9.27a | 12.40 ± 6.30b | 1.02 | 0.309 |
| F | 127 | 136 | ||
| P | 0.0001 | 0.0001 | ||
aP > 0.05 compared with early follow-up in Group 1
bP > 0.05 compared with early follow-up in Group 2
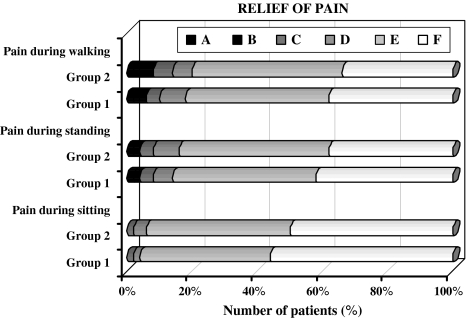
No significant difference in the relief of pain during walking¹, standing² and, sitting³ positions were identified between two groups at 4–7 years postoperatively [all according to the unpaired t test, (¹t = 1.561, P = 0.113), (²t = 1.429, P = 0.128), (³t = 0.556, P = 0.563)], (Fig. 3).
Fig. 3.
Bar graphs showing the relief of pain during walking, standing and, sitting at 4–7 years postoperatively (all according to the subscales of ODI) Walking: A I cannot walk at all without increasing pain. B I cannot walk more than 1/4 mile without increasing pain. C I cannot walk more than 1/2 mile without increasing pain. D I cannot walk more than 1 mile without increasing pain. E I have some pain on walking but it does not increase with distance. F I have no pain on walking. Standing: A I avoid standing because it increases the pain immediately. B I cannot stand for longer than 10 min without increasing pain. C I cannot stand for longer than 0.5 h without increasing pain. D I cannot stand for longer than 1 h without increasing pain. E I have some pain on standing but it does not increase with time. F I can stand as long as I want without pain. Sitting: A I avoid sitting because it increases pain immediately. B Pain prevents me from sitting more than 10 min. C Pain prevents me from sitting more than 0.5 h. D Pain prevents me from sitting more than 1 h. E I can sit only in my favorite chair as long as I like. F I can sit in any chair as long as I like
Comparison of preoperative, early and late postoperative SF-36 scores demonstrated a marked and significant improvement, except in the areas of emotional role. No significant differences in preoperative, early postoperative, and late postoperative scores of emotional role were identified between the two groups [Newman–Keuls multiple comparison test, plate = 0.604 compared with admission (Group 1), Plate = 0.644 compared with admission (Group 2)]. Most of the changes occurred between preoperative and early follow-up assessments with little changes between early and late follow-up reviews, except in the areas of bodily pain. Significant differences were found between early and late follow-up assessments, only in the areas of bodily pain at two groups [Newman–Keuls multiple comparison test, P < 0.05 (Group 1), P < 0.05 (Group 2)]. No significant differences in preoperative, early and late postoperative SF-36 scores were identified between two groups (Table 3).
Table 3.
Mean SF-36 scores preoperatively, at third months, and 4–7 years postoperatively
| Group | P | ||
|---|---|---|---|
| 1 | 2 | ||
| Physical function | |||
| Preop | 55.16 ± 9.03 | 56.12 ± 11.43 | 0.642 |
| Early | 71.80 ± 7.71 | 71.62 ± 8.81 | 0.811 |
| Late | 72.78 ± 10.8 | 70.56 ± 9.90 | 0.776 |
| Physical role | |||
| Preop | 28.50 ± 11.08 | 27.50 ± 11.57 | 0.66 |
| Early | 45.20 ± 10.38 | 44.80 ± 9.57 | 0.841 |
| Late | 46.20 ± 9.70 | 47.62 ± 11.32 | 0.502 |
| Body pain | |||
| Preop | 42.60 ± 10.31 | 43.24 ± 11.77 | 0.773 |
| Early | 62.64 ± 9.52 | 61.78 ± 11.92 | 0.7 |
| Late | 69.64 ± 10.52 | 68.32 ± 9.92 | 0.459 |
| General health | |||
| Preop | 52.66 ± 9.03 | 53.62 ± 10.54 | 0.202 |
| Early | 59.66 ± 10.52 | 60.62 ± 11.28 | 0.202 |
| Late | 60.96 ± 13.98 | 63.12 ± 9.61 | 0.122 |
| Vitality/Energy | |||
| Preop | 42.12 ± 13.90 | 41.84 ± 11.57 | 0.326 |
| Early | 59.38 ± 10.11 | 60.12 ± 10.57 | 0.33 |
| Late | 62.66 ± 11.67 | 61.62 ± 10.65 | 0.202 |
| Social function | |||
| Preop | 42.96 ± 10.16 | 41.88 ± 11.35 | 0.235 |
| Early | 49.67 ± 9.03 | 49.63 ± 10.54 | 0.202 |
| Late | 50.31 ± 11.24 | 50.27 ± 9.65 | 0.202 |
| Emotional role | |||
| Preop | 62.14 ± 11.58 | 61.28 ± 10.23 | 0.459 |
| Early | 63.24 ± 9.85 | 63.54 ± 9.54 | 0.459 |
| Late | 61.95 ± 10.35 | 62.74 ± 12.54 | 0.788 |
| Mental health | |||
| Preop | 61.84 ± 10.35 | 60.98 ± 11.58 | 0.459 |
| Early | 72.24 ± 9.52 | 71.38 ± 12.65 | 0.459 |
| Late | 70.49 ± 12.8 | 71.27 ± 9.68 | 0.776 |
P showing comparison of the mean scores of two groups
Radiographical analysis
Postoperative MR imaging studies demonstrated an increase in lumbar spinal canal size compared with preoperative size. Cross-sectional spinal canal area increased a mean 5.1 ± 0.8-fold (range 4–6.1-fold) in Group 1 and 4.7 ± 1.1-fold (range 3.3–5.9-fold) in Group 2 postoperatively (Figs. 4, 5).
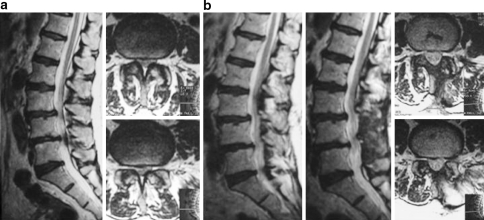
Fig. 4.
Preoperative (a) and postoperative (b) T2-weighted MR images obtained in a patient undergoing two levels decompression
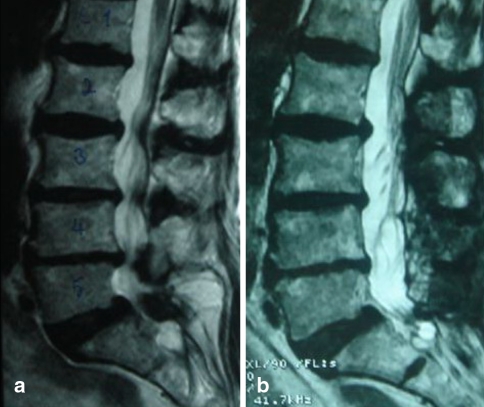
Fig. 5.
Preoperative (a) and postoperative (b) T2-weighted sagittal MR images obtained in a patient undergoing four levels decompression
Reoperation was not required for recurrent spinal stenosis at the same segment(s) within 4–7 years. But two wrong levels (instead of stenotic levels) were operated in the two patients by mistake so that they underwent reoperation. Adjacent level stenosis requiring decompression occurred only in one Group 2 patient.
Asymptomatic degenerative mild spondylolisthesis (Grade I) was observed at 15 different levels in 15 patients (in seven Group 1 patients, in eight Group 2 patients) preoperatively. Abnormal motion in the sagittal plane was not observed on the preoperative X-ray films. No radiograph revealed an increase in the degree of spondylolisthesis in the late postoperative period. No postoperative instability developed requiring instrumentation assisted secondary fusion.
Discussion
Several surgical techniques for lumbar spine decompression have been described over last few decades. The surgical aim of treatment for symptomatic lumbar canal stenosis is relief of symptoms by adequate neural decompression while preserving much of the anatomy and the biomechanical function of the lumbar spine. Traditional treatment of spinal stenosis has involved wide laminectomy and undercutting of the medial facet with foraminotomy. The frequent surgical failures have been attributed to local tissue trauma [4, 53] and to postoperative spinal instability [13, 24, 28, 35, 50, 53], which has led to a dramatic increase in lumbar fusion surgery [9, 30]. Turner’s meta-analysis of 74 published studies of surgery for lumbar spinal stenosis produced good to excellent results ranging from 26 to 100% (mean 64%) [51].
Commonly used techniques of exposure for lumbar decompression that include elevation of the multifidus bilaterally with subsequent wide retraction have potentially serious consequences. Mayer et al. [33] demonstrated a decrease in paraspinal muscle strength with concomitant atrophy on postoperative computed tomography scans. See and Kraft [40] echoed these concerns in their observation of chronic denervation and electromyographic abnormalities of the paraspinal muscles 4 years after open surgery. Sihvonen et al. [42] noted similarly computed tomography and electromyographic abnormalities and correlated these with the postoperative failed back syndrome. Retraction of multifidus beyond the midpoint of the facet joint tethers the medial branch within the mamilloaccessory groove, risking muscular denervation. The described techniques of microdecompression limits ipsilateral retraction to the level of the medial facet border. Contralaterally, no elevation or retraction of the paraspinal musculature is undertaken, thereby minimizing the risk of iatrogenic muscular trauma and thereby proving to be an important tool in decreasing the risk of these undesirable sequelae.
Most surgical approaches to decompression involve excision of the interspinous or supraspinous ligament complexes, altering an already pathologic biomechanical milieu. Loss of the midline supraspinous/interspinous ligament complex can lead to a loss of flexion stability, thereby increasing the risk of delayed spinal instability [48, 49]. Goel et al. [14] found that, under normal conditions, the supraspinous ligament experienced the greatest force when exposed to an external flexion moment across an anatomic segment. Hindle et al. [20] also demonstrated load with flexion in the supra- and interspinous ligaments. Prestar [38] observed similar findings and believed that, in regions lacking this ligamentous support, the paraspinal musculature must come to the aid of stability. The biomechanics of the normal spine have been extensively studied. The supra- and interspinous ligaments resist 19% of flexion forces, with the facet capsular ligaments resisting 39% [2, 3]. Adams and Hutton [3] have also suggested that the muscular attachments to the posterior arch and the insertions of the muscular slips on the facet capsule brace the facets, improving their ability to resist displacement. The muscular and truncal soft-tissue contributions to flexion resistance are critical because the trunk-induced force exerted on the spine in flexion is twofold greater than that required to injure the facet joints [2, 3], which would fail if unaided by other supporting tissues. The supra/intraspinous ligamentous complex has the greatest mechanical advantage because it is farthest from the axis of rotation. It is also the first to fail in flexion [3].
Besides, complete decompression may not be necessary to achieve symptomatic relief as previously suggested by Aryanpur and Ducker [6]. Thomas et al. [46], reported a statistically significant increase in dural sac size after laminotomy or laminectomy but found no statistical relationship between the extent of decompression and clinical outcome. It may only be necessary to bring the patient below a symptomatic threshold. Indeed, in one of the only studies correlating the degree of radiographic with clinical outcome, it was observed that the satisfaction of patients with the results of surgery (e.g., Oswestry score and walking capacity) was more important in surgical outcome than the degree of decompression as seen on a postoperative CT scan [18]. Herno et al. [18] have shown that the clinical results were similar in patients irrespective of whether they had undergone complete decompression of all stenotic levels, complete decompression in one level but no decompression in adjacent stenotic level, or incomplete decompression of all stenotic levels. It seems that the decompression of LSS should be adequate but it does not need to be complete.
Postsurgical dead space has serious potential consequences. Increased volume to be filled results in increased blood loss and provides an ideal bacterial culture medium with potential for increasing the infection rate. The region is inevitably replaced with scar tissue, thereby complicating or necessitating secondary surgical interventions. Resection of portions or all of the spinous processes, interspinous ligaments, and supraspinous ligaments, and iatrogenic damage to the paraspinal musculature results in a large volume of dead space. Dead space and its consequent risks are significantly decreased using the described techniques [53].
Instead of combining fusion with decompression and thus maximizing surgery and associated perioperative risks, other investigators have attempted to decrease the operative failure rate by minimizing the invasiveness of the decompressive procedure. Fenestration with minimal soft tissue dissection and limited bone removal instead of extensive laminectomy to prevent subsequent lumbar instability has become widely accepted for the treatment of spinal stenosis [17, 21, 31, 36, 47, 53]. A unilateral approach for bilateral decompression has been modified and performed successfully by many surgeons [1, 10, 36]. Our experience with bilateral decompression via unilateral laminotomy is that despite surgical decompression resulting in improvement in symptoms, postoperative radiological studies typically show marked multilevel residual stenosis between laminotomies following surgery in these patients. To prevent the residual stenosis, which may cause failure of good surgical results, we began to make hemilaminectomies in very severe cases of stenosis. Hemilaminectomy provides not only good surgical results but also surgical ease and safety. Because when hemilaminectomy is performed at one level, it provides safe anatomical plane that we can follow to the upper and lower levels of stenosis. We therefore undertook a randomized study of these two techniques and in which they were compared with each other.
The main concern of spine surgeons in view of less invasive techniques to decompress lumbar stenosis has been an increased rate of neural injury [12, 39, 41]. Postacchini et al. [39], reported a postoperative increase in radiculopathy in one (1.3%) of 32 patients after laminectomy compared with three (11.5%) of 26 patients after bilateral laminotomy, whereas others have reported this complication in only 1% when using the latter approach [7]. According to our data, actual injury to a nerve root did not occur.
Unintended duratomy is another concern during spinal decompressive procedures, although no association with long-term sequelae has been found [8, 47, 52]. Overall, duratomy rates for laminectomy have been shown to range from 5 to 15% [27, 43, 47, 51, 52]. Bilateral laminotomy is complicated by dural tears in 2–9% [7, 28, 47, 48, 57] and unilateral laminotomy with contralateral decompression in 3.5–12% [10, 36, 45, 47]. The unilateral approach with microscope and tubular retractor system is associated with an incidence of 17.6% (three of 17 patients) [36]. The unilateral microendoscopic approach is associated with an incidence of 16% (four of 25 patients) [27]. The results of the present study are not in accordance with those in the literature. Of all surgically treated levels, accidental duratomy occurred in two patients (4%, Group 1), and three patients (6%, Group 2).
As mentioned previously, total laminectomy is associated with improvement in 64% of patients at 3–6 years after surgery according to a metaanalysis [51]. Postacchini et al. [39], demonstrated good results in 78% (25 of 32 patients) at 4 years. The authors of a study that used standardized patient-derived measures of symptom relief 4 years after decompression reported a success rate of just 57% [26]. In a large retrospective study, Airaksinen et al. [5], found good outcomes after 4 years in 62% of their 438 patients, whereas others have described satisfactory results in approximately 70% [22, 43]. In a literature review Herron and Mangelsdorf [19] reported rates of good outcome ranging from 50 to 86% and stressed that results deteriorated over time. Recently, success rates of 68% (in 27 of 40 patients) [47] have been reported.
Following the description of the bilateral laminotomy technique [29], the authors of clinical case series reported good results in 90% (29 of 32 patients) [6], and 80% patients (in 32 of 40) [47] at 1 year; 87% (13 of 15) [15], 78% (21 of 27) [56], and 68% patients (34 of 50) [48] at 2 years; 85% patients (27 of 32) [57] at 3 years. Nevertheless, Postacchini et al. [39] prospectively and, Thomas et al. [46], and Kalbarczyk et al. [25], retrospectively, compared bilateral laminotomy and laminectomy and found no difference in outcome.
The authors who performed unilateral laminotomy for bilateral decompression, demonstrated good results in 87% patients (26 of 30 patients) [53] at 9 months; in 82% patients (18 of 22) [32] at 1 year; in 88% (22 of 25) [45], 70% patients (in 28 of 40) [47] at 18 months; and in 67.6% patients (in 23 of 34) [23] at 2 years; and 68% patients (in 15 of 22) [32] at 4 years in their studies. In the present study, the ODI scores decreased significantly and, SF-36 scores demonstrated a marked and significant improvement in late follow-up evaluations.
None of our patients showed vertebral hypermobility, or a significant increase in spondylolisthesis after both of two surgical procedures. For degenerative lumbar spinal stenosis with or without asymptomatic mild degenerative spondylolisthesis, unilateral approach usually allows sufficient decompression of the neural structures and adequate preservation of vertebral stability. Long-term follow-up is certainly needed to confirm these results because every decompressive procedure bears the risk of secondary instability, which may require further stabilization.
Conclusion
We think that the goal of the unilateral approach to treat lumbar spinal stenosis is to achieve adequate decompression of the neural elements. An additional benefit of a minimally invasive approach may be the potential to decrease a patient’s postoperative pain and disability as well as to decrease the length of hospital stay and thereby the treatment costs.
Abbreviation
- LSS
Lumbar spinal stenosis
- ODI
Oswestry disability index
- SF-36
36-item short-form health survey
References
- 1.Adachi K, Futami T, Ebihara A, Yamaya T, Kasai N, Nakazawa T, et al (2003) Spinal canal enlargement procedure by restorative laminoplasty for the treatment of lumbar canal stenosis. Spine J 3(6):471–478 [DOI] [PubMed]
- 2.Adams MA, Hutton WC, Stott JR (1980) The resistance to flexion of the lumbar intervertebral joint. Spine 5(3):245–253 [DOI] [PubMed]
- 3.Adams MA, Hutton WC (1983) The mechanical function of the lumbar apophyseal joints. Spine 8(3):327–330 [DOI] [PubMed]
- 4.Airaksinen O, Herno A, Kaukanen E, Saari T, Sihvonen T, Suomalainen O (1996) Density of lumbar muscles 4 years after decompressive spinal surgery. Eur Spine J 5(3):193–197 [DOI] [PubMed]
- 5.Airaksinen O, Herno A, Turunen V, Saari T, Suomlainen O (1997) Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine 22(19):2278–2282 [DOI] [PubMed]
- 6.Aryanpur J, Ducker T (1990) Multilevel lumbar laminotomies: an alternative to laminectomy in the treatment of lumbar stenosis. Neurosurgery 26(3):429–432 discussion 433 [DOI] [PubMed]
- 7.Askar Z, Wardlaw D, Choudhary S, Rege A (2003) A ligamentum flavum-preserving approach to the lumbar spinal canal. Spine 28(19):E385–E390 [DOI] [PubMed]
- 8.Cammisa FP Jr, Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS (2000) Incidental durotomy in spine surgery. Spine 25(20):2663–2667 [DOI] [PubMed]
- 9.Deyo RA, Nachemson A, Mirza SK (2004) Spinal-fusion surgery-the case for restraint. N Engl J Med 350(7):722–726 [DOI] [PubMed]
- 10.diPiero CG, Helm GA, Shaffrey CI, Chadduck JB, Henson SL, Malik JM et al (1996) Treatment of lumbar spinal stenosis by extensive unilateral decompression and contralateral autolougs bone fusion: operative technique and results. J Nuerosurg 84(2):166–173 [DOI] [PubMed]
- 11.Epstein NE (1998) Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord 11(2):116–122 discussion 123 [DOI] [PubMed]
- 12.Epstein NE, Maldonado VC, Cusick JF (1998) Symptomatic lumbar spinal stenosis. Surg Neurol 50(1):3–10 [DOI] [PubMed]
- 13.Fox MW, Onofrio BM, Hanssen AD (1996) Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg 85(5):793–802 [DOI] [PubMed]
- 14.Goel VK, Fromknecht SJ, Nishiyama K, Weinstein J, Liu YK (1985) The role of the lumbar spinal elements in flexion. Spine 10(6):516–523 [DOI] [PubMed]
- 15.Grob D, Humke T, Dvorak J (1995) Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am 77(7):1036–1041 [DOI] [PubMed]
- 16.Guiot BH, Khoo LT, Fessler RG (2002) A minimally invasive technique for decompression of the lumbar spine. Spine 27(4):432–438 [DOI] [PubMed]
- 17.Haba K, Ikeda M, Soma M, Yamashima T (2005) Bilateral decompression of multilevel lumbar spinal stenosis through a unilateral approach. J Clinical Neurosci 12(2):169–171 [DOI] [PubMed]
- 18.Herno A, Saari T, Suomalainen O, Airaksinen O (1999) The degree of decompressive relief and its relation to clinical outcome in patients undergoing surgery for lumbar spinal stenosis. Spine 24(10):1010–1014 [DOI] [PubMed]
- 19.Herron LD, Mangelsdorf C (1991) Lumbar spinal stenosis: results of surgical treatment. J Spinal Disord 4(1):26–33 [PubMed]
- 20.Hindle RJ, Pearcy MJ, Cross A (1990) Mechanical function of the human lumbar interspinous and supraspinous ligaments. J Biomed Eng 12(4):340–344 [DOI] [PubMed]
- 21.Hopp E, Tsou PM (1988) Postdecompression lumbar instability. Clin Orthop Relat Res 227:143–151 [PubMed]
- 22.Javid MJ, Hadar EJ (1998) Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: a prospective study. J Neurosurg 89(1):1–7 [DOI] [PubMed]
- 23.Ji YC, Kim YB, Hwang SN, Park SW, Kwon JT, Min BK (2005) Efficacy of Unilateral Laminectomy for bilateral decompression in elderly lumbar spinal stenosis. J Korean Neurosurg Soc 37:410–415
- 24.Johnsson KE, Willner S, Johnsson K (1986) Postoperative instability after decompression for lumbar spinal stenosis. Spine 11(2):107–110 [DOI] [PubMed]
- 25.Kalbarczyk A, Lukes A, Seiler RW (1998) Surgical treatment of lumbar spinal stenosis in the elderly. Acta Neurochir (Wien) 140(7):637–641 [DOI] [PubMed]
- 26.Katz JN, Lipson SJ, Larson MG, McInnes JM, Fossel AH, Liang MH (1991) The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am 73(6):809–816 [PubMed]
- 27.Khoo LT, Fessler RG (2002) Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery 51(5 Suppl):S146–S154 [PubMed]
- 28.Kleeman TJ, Hiscoe AC, Berg EE (2000) Patient outcomes after minimally destabilizing lumbar stenosis decompression: the “Port-Hole” technique. Spine 25(7):865–870 [DOI] [PubMed]
- 29.Lin PM (1982) Internal decompression for multiple levels of lumbar spinal stenosis: a technical note. Neurosurgery 11(4):546–549 [DOI] [PubMed]
- 30.Lipson SJ (2004) Spinal-fusion surgery-advances and concerns. N Engl J Med 350(7):643–644 [DOI] [PubMed]
- 31.Mackay DC, Wheelwright EF (1998) Unilateral fenestration in the treatment of lumbar spinal stenosis. Br J Neurosurg 12(6):556–558 [DOI] [PubMed]
- 32.Mariconda M, Fava R, Gatto A, Longo C, Milano C (2002) Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: a prospective comparative study with conservatively treated patients. J Spinal Disord Tech 15(1):39–46 [DOI] [PubMed]
- 33.Mayer TG, Vanharanta H, Gatchel RJ, Mooney V, Barnes D, Judge L, et al (1989) Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine 14(1):33–6 [DOI] [PubMed]
- 34.McCulloch JA (1991) Microsurgical spinal laminotomies in the adult spine: principles and practice. J.W. Frymoyer (ed) Raven Press, New York
- 35.Nakai O, Ookawa A, Yamaura I (1991) Long-term roentgenographic and functional changes in patients who were treated with wide fenestration for central lumbar stenosis. J Bone Joint Surg Am 73(8):1184–1191 [PubMed]
- 36.Palmer S, Turner R, Palmer R (2002) Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg 97(2 Suppl):213–217 [DOI] [PubMed]
- 37.Pinar R (2005) Reliability and construct validity of the SF-36 in Turkish cancer patients. Qual Life Res 14(1):259–264 [DOI] [PubMed]
- 38.Prestar FJ (1982) Morphology and function of the interspinal ligaments and the supraspinal ligament of the lumbar portion of the spine. Morphol Med 2:53–58 [PubMed]
- 39.Postacchini F, Cinotti G, Perugia D, Gumina S (1993) The surgical treatment of central lumbar stenosis. Multiple laminotomy compared with total laminectomy. J Bone Joint Surg Br 75(3):386–392 [DOI] [PubMed]
- 40.See DH, Kraft GH (1975) Electromyography in paraspinal muscles following surgery for root compression. Arch Phys Med Rehabil 56(2):80–83 [PubMed]
- 41.Sengupta DK, Herkowitz HN (2003) Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am 34(2):281–295 [DOI] [PubMed]
- 42.Sihvonen T, Herno A, Paljarva L, Airaksinen O, Patanen J, Tapaninaho A (1993) Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine 18(5):575–581 [DOI] [PubMed]
- 43.Silvers HR, Lewis PJ, Asch HL (1993) Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg 78(5):695–701 [DOI] [PubMed]
- 44.Spetzger U, Bertalanffy H, Naujokat C, von Keyserlingk DG, Gilsbach JM (1997) Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part I: Anatomical and surgical considerations. Acta Neurochir 139(5):392–396 [DOI] [PubMed]
- 45.Spetzger U, Bertalanffy H, Reinges MH, Gilsbach JM (1997) Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part II: Clinical experiences. Acta Neurochir 139(5):397–403 [DOI] [PubMed]
- 46.Thomas NW, Rea GL, Pikul BK, Mervis LJ, Irsik R, McGregor JM (1997) Quantitative outcome and radiographic comparisons between laminectomy and laminotomy in the treatment of acquired lumbar stenosis. Neurosurgery 41(3):567–574 discussion 574–75 [DOI] [PubMed]
- 47.Thome C, Zevgaridis D, Leheta O, Bazner H, Pockler-Schoniger C, Wohrle J et al (2005) Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine 3(2):129–141 [DOI] [PubMed]
- 48.Tsai RY, Yang RS, Bray RS Jr (1998) Microscopic laminotomies for degenerative lumbar spinal stenosis. J Spinal Disord 11(5):389–394 [DOI] [PubMed]
- 49.Tuite GF, Stern JD, Doran SE, Papadopoulos SM, McGillicuddy JE, Oyedijo DI et al (1994) Outcome after laminectomy for lumbar spinal stenosis: Part I: Clinical correlations. J Neurosurg 81(5):699–706 [DOI] [PubMed]
- 50.Tuite GF, Doran SE, Stern JD, McGillicuddy JE, Papadopoulos SM, Lundquist CA et al (1994) Outcome after laminectomy for lumbar spinal stenosis. Part II: Radiographic changes and clinical correlations. J Neurosurg 81(5):707–715 [DOI] [PubMed]
- 51.Turner JA, Ersek M, Herron L, Deyo R (1992) Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine 17(1):1–8 [DOI] [PubMed]
- 52.Wang JC, Bohlman HH, Riew KD (1998) Dural tears secondary to operations on the lumbar spine. Management and results after a two-year-minimum follow-up of eighty-eight patients. J Bone Joint Surg Am 80(12):1728–1732 [DOI] [PubMed]
- 53.Weiner BK, Walker M, Brower RS, McCulloch JA (1999) Microdecompression for lumbar spinal canal stenosis. Spine 24(21):2268–2272 [DOI] [PubMed]
- 54.White AA, Panjabi MM (1990) Clinical biomechanics of the spine, 2nd edn. JB Lippincott, Philadelphia
- 55.Yakut E, Duger T, Oksuz C, Yorukan S, Ureten K, Turan D, et al (2004) Validation of the Turkish version of the Oswestry Disability Index for patients with low back pain. Spine 29(5):581–585 discussion 585 [DOI] [PubMed]
- 56.Yone K, Sakou T (1999) Usefulness of Posner’s definition of spinal instability for selection of surgical treatment for lumbar spinal stenosis. J Spinal Disord 12(1):40–44 [DOI] [PubMed]
- 57.Young S, Veerapen R, O’Laoire SA (1988) Relief of lumbar canal stenosis using multilevel subarticular fenestrations as an alternative to wide laminectomy: preliminary report. Neurosurgery 23(5):628–633 [DOI] [PubMed]