Abstract
PMMA is the most common bone substitute used for vertebroplasty. An increased fracture rate of the adjacent vertebrae has been observed after vertebroplasty. Decreased failure strength has been noted in a laboratory study of augmented functional spine units (FSUs), where the adjacent, non-augmented vertebral body always failed. This may provide evidence that rigid cement augmentation may facilitate the subsequent collapse of the adjacent vertebrae. The purpose of this study was to evaluate whether the decrease in failure strength of augmented FSUs can be avoided using low-modulus PMMA bone cement. In cadaveric FSUs, overall stiffness, failure strength and stiffness of the two vertebral bodies were determined under compression for both the treated and untreated specimens. Augmentation was performed on the caudal vertebrae with either regular or low-modulus PMMA. Endplate and wedge-shaped fractures occurred in the cranial and caudal vertebrae in the ratios endplate:wedge (cranial:caudal): 3:8 (5:6), 4:7 (7:4) and 10:1 (10:1) for control, low-modulus and regular cement group, respectively. The mean failure strength was 3.3 ± 1 MPa with low-modulus cement, 2.9 ± 1.2 MPa with regular cement and 3.6 ± 1.3 MPa for the control group. Differences between the groups were not significant (p = 0.754 and p = 0.375, respectively, for low-modulus cement vs. control and regular cement vs. control). Overall FSU stiffness was not significantly affected by augmentation. Significant differences were observed for the stiffness differences of the cranial to the caudal vertebral body for the regular PMMA group to the other groups (p < 0.003). The individual vertebral stiffness values clearly showed the stiffening effect of the regular cement and the lesser alteration of the stiffness of the augmented vertebrae using the low-modulus PMMA compared to the control group (p = 0.999). In vitro biomechanical study and biomechanical evaluation of the hypothesis state that the failure strength of augmented functional spine units could be better preserved using low-modulus PMMA in comparison to regular PMMA cement.
Keywords: Vertebroplasty, Adjacent fractures, PMMA, Low-modulus cement, Biomechanics
Introduction
Percutaneous vertebroplasty, cement augmentation of vertebral bodies, is an effective treatment option for painful vertebral fractures in the presence of osteoporosis. Vertebral body fractures are among the most common fractures associated with osteoporosis [10]. Compression fractures of the osteoporotic spine represent an important indication for vertebroplasty. The most widely used bone substitute material for vertebroplasty is polymethylmethacrylate (PMMA), which has stiffness seven to ten times higher than osteoporotic vertebral cancellous bone. While vertebroplasty greatly increases the failure strength of augmented vertebrae [6, 18], a significantly increased risk of adjacent vertebral body fractures was found in both clinical [12, 16, 19] and experimental studies [7]. Uppin et al. [19] reviewed 177 patients treated with vertebroplasty. They found that 22 patients (12.4%) developed 36 new vertebral body fractures, 24 of them (67%) involved vertebrae adjacent to a treated vertebral body, and 20 fractures occurred within 31 days after treatment. Pérez-Higueras et al. [16] observed 13 patients after vertebroplasty: three patients (23%) developed four new vertebral fractures in which two (50%) were adjacent vertebrae. This may be the consequence of the natural course of osteoporosis since existing fractures are strong, independent predictors of the risk of future vertebral fracture [1, 20]. But these fractures may also have been provoked by the rigid reinforcement of the adjacent vertebral body with PMMA cement. It was shown [7] that the ultimate failure load of a functional spine unit (FSU, two adjacent vertebral bodies and the disc between them) augmented with PMMA amounted to 3.7 ± 1.3 kN, which was 19% lower than that of the non-augmented FSU (4.4 ± 1.4 kN). The increased rigidity of the augmented vertebral body has been postulated as a possible reason for the reduction in failure load. It is not known whether the fracture risk of the adjacent body can be better reduced by the degree of filling or by the choice of augmentation material. It has been suggested that merely restoring the initial strength of osteoporotic vertebrae after fracture is not sufficient as a treatment goal in vertebroplasty, but rather attempts should be made to restore the strength of the vertebral body to healthy, normal values [5]. Finite element studies showed that the compressive stiffness of the entire spinal unit increased by approx. 12% and the hydrostatic pressure within the nucleus by approx. 15% after simulated vertebroplasty with regular PMMA [3, 4, 17]. The increased nucleus pressure can be explained by the decrease in deformability of the endplate next to the cement in comparison to the endplate of a non-augmented vertebral body. To preserve this deformability, the stiffness of the augmented vertebral body should be reduced. This might be achieved by means of cement with stiffness values comparable to normal cancellous bone. The optimal stiffness of a bone substitute material for vertebroplasty in osteoporosis is unknown. In a recent study [8], low-modulus bone cement based on porous PMMA was reported. The stiffness of the cement could be modified between 50 and 930 MPa and the failure strength between 1 and 40 MPa, which is in the range of the stiffness (100–600 MPa) and failure strength (0.5–4 MPa) reported for osteoporotic vertebral cancellous bone [2, 11]. This development enables an evaluation whether augmentation material with properties comparable to normal cancellous bone reduces the fracture risk of adjacent vertebral bodies. The purpose of this study therefore was to assess the failure of adjacent vertebral bodies in FSUs with or without augmentation by means of low-modulus PMMA.
Materials and methods
The study comprised 12 osteoporotic human thoracic–lumbar spines (age 79 ± 11.2 years) from five females and seven males. They were stored frozen at −20°C and screened radiologically to ensure that there were no previous fractures or abnormalities. Thirty-six FSUs (T9–L4) were prepared from the specimens and all soft tissues dissected with the exception of the intervertebral ligaments and facet joint capsules. To minimize the effects of variability between the FSUs, assignment to three groups was based on an equal distribution of bone mineral density (BMD), spine level, FSU height, FSU cross-section and disc height.
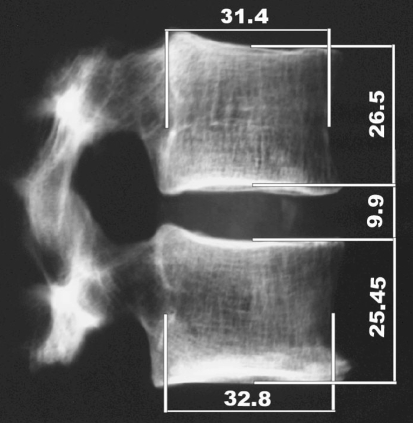
The BMD of an FSU was defined as the average of the BMD values from each of the two vertebral bodies. The BMD of a vertebral body was measured by means of an XtremeCT scanner (Scanco Medical AG, Bassserdorf, Switzerland) using a voxel size of 123 μm3 (average value measured within a circular region in the center of all transverse scans). All slices between the endplates were used for the BMD measurements, which comprised around 85% of the whole vertebral cancellous bone volume. BMD was measured as hydroxyapatite density (dHA) in mg/cm3, because the machine was calibrated using a standard hydroxyapatite phantom. According to our in house reference database, a spine specimen (age > 70 years) is considered osteoporotic if dHA is <97.1 mg/cm3. The spine level of the FSUs ranged from (T9/T10) to (L3/L4). Antero-posterior and lateral radiographs were taken to check specimens regarding bone abnormalities and to determine the widths and heights of the vertebral bodies and the disc (Fig. 1). Radiographs were made using a Faxitron X-ray system (Model 43855A, Faxitron X-Ray Corp., Buffalo Grove, IL, USA) using 60 kV (lateral view), 65 kV (antero-posterior view) and 450 mAs, 5 min exposure time on films (Agfa, Structurix D4DW, Fisch AG, Dübendorf, Switzerland). FSU height was calculated as the average of the four mid-line vertebrae heights measured on the lateral and a-p projections. The cross-sectional area of the vertebral bodies was estimated using the lateral and a-p widths of the vertebral bodies as major and minor diameters of an ellipse. The average of these two areas defined the FSU cross-section. Disc height was calculated as the average of the lateral and a-p disc height (Fig. 1).
Fig. 1.
Lateral radiograph of an FSU showing the widths and heights of the vertebral bodies and the disc measured in the horizontal and vertical mid-line of the projection
The first group of specimens comprised the untreated control group. The second group was assigned to augmentation with low-modulus cement (group C_35) and the third to regular cement (group C_0). Because the incidence of adjacent fracture is the same for the upper vertebral body as for the lower one [19], we decided to always assign the caudal vertebral body of the FSUs to augmentation. Subsequent to preparation, the FSUs were wrapped in saline-soaked bandages, protected from dehydration by means of double plastic bags and frozen at −20°C until the time of augmentation and testing.
From an initial 12 specimens per group, a total of 11 remained for testing. One FSU in the control group reached the chosen cut off setting of the testing device (load limit = 10 kN) before failure. In each of the two augmentation groups (C_35/C_0) one FSU was excluded because initial damage did not occur in the vertebral body, but in the neural arcs, during compression. A group size of eight FSUs for each group was calculated to be sufficient to reach a statistical power of 0.8 for an effect size of 1.66 to be detected in an ANOVA of three levels.
Cement preparation
The materials used for augmentation were a commercial polymethylmethacrylate (Vertecem, Synthes Inc., Oberdorf, Switzerland), which is certified for medical applications, and a porous, low-modulus cement [8]. The regular cement was prepared according to the manufacturer’s instructions. The low-modulus cement was prepared with Vertecem by addition of an aqueous fraction of 35% sodium hyaluronate (1.5%, Batch number 2415A, Hyaltech Ltd., Edinburgh, UK) as described in a recent study [8]. The stiffness and yield strengths of the resulting cements were 1,840 ± 30 and 91 ± 2 MPa for the regular and 470 ± 30 and 11.5 ± 2 MPa for the porous cement [8] according to ISO 5833.
Augmentation technique
For the bipedicular augmentation, two 3.2 mm holes were drilled transpedicularly into the centre of the caudal vertebral body of the FSU using a trephine. Drilling direction was defined by anatomical landmarks and controlled by a-p and lateral fluoroscopy. Traplok™ bone marrow biopsy needles (8 gauge; Medical Device Technologies Inc. Gainesville, FL, USA) were guided through the holes. The final position was documented using fluoroscopy. Four minutes after mixing the cement, the vertebral body was injected under fluoroscopic control. Cement filling from the upper to the lower endplate was obtained. Cement volume injected was determined using the scale of the syringes. Cement filling in percentage of the idealized vertebral body volume (elliptical cross-section multiplied by vertebral body height) was correlated to BMD. The idealized vertebral body volume was used for calculations, because it showed overestimation of around 8% (n = 3) compared to the vertebral cancellous bone region (received from 3d-CT reconstructions). Therefore the time saving method was chosen.
Mechanical testing
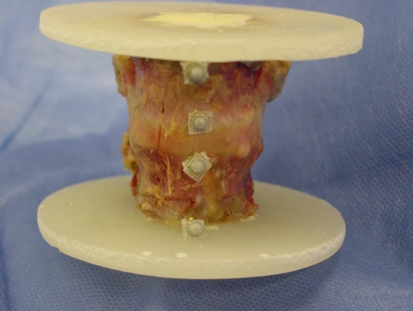
For mechanical testing, molds of the cranial and caudal ends of each FSU (Fig. 2) were made using PMMA cement (Beracryl, Troller AG, Fuhlenbach, Switzerland), ensuring parallel orientation of the outer surfaces of each mold as well as a perpendicular orientation of the FSU with respect to the loading axis. During axial testing, rotation was blocked but horizontal motion remained unconstrained. The tests were carried out on a servohydraulic testing machine (Bionix; MTS Systems Cooperation, Eden Prairie, MN, USA) using a 25 kN load cell with an accuracy of 0.1%. The testing protocol was the same as described by Berlemann et al. [7]. The specimens were subjected to sinusoidal dynamic compression (amplitude 50–450 N, frequency 1 Hz, total of 600 cycles) before and after treatment by vertebroplasty. Immediately after the second dynamic load series, the FSU’s were compressed at a rate of 0.5 mm/s until failure (maximum 10 mm). The load and cross-head displacement data were recorded at a frequency of 100 Hz. The FSU stiffness and failure load were determined from the force-displacement curves. Stress and strain values were calculated using the initial height of the FSU and the elliptical cross-section of the fractured vertebral body. The stiffness was determined as the slope of the linear region of the curves. Failure of the FSU was defined as the first peak load followed by an increase in displacement. Additionally, during the compression test to failure, a video was taken using a C-arm image intensifier (ARCOSi 100, Schweizer, Zürich, Switzerland; 50 keV, 0.5 mA), to determine which vertebral body failed first and to identify the failure mechanism and resulting fracture pattern.
Fig. 2.
Photograph of the optical reflection markers mounted on small screws inserted anteriorly and identifying the top and bottom of each vertebral body
In order to determine the individual stiffness values of the caudal (stiffness VBcaudal) and cranial vertebral bodies (stiffness VBcranial), their displacements were recorded using an optical motion tracking system (Qualisys motion capture systems, ProReflex MCU 120, Gothenburg, Sweden). Retroreflective markers were attached to small screws at the antero-cranial and antero-caudal aspect of each vertebral body (Fig. 2). Translations of the markers in the direction of the machines crosshead axis were used for calculations. The noise level of the motion tracking data was 10 μm per coordinate direction (average of all markers and measurements).
Individual stiffness was determined as the slope of the linear region of the stress–strain curve for the corresponding vertebral body. Stress was calculated as the ratio of the loads measured and the cross-section of the corresponding vertebral body. Strain was derived from the individual displacement divided by the vertebral body height. Difference between the caudal and cranial stiffness was determined as an additional parameter (stiffnessdifference). Statistical analysis was carried out using ANOVA for the parameters FSU stiffness, failure strength, stiffness VBcaudal, stiffness VBcaudal, and stiffnessdifference after ensuring normal distribution and variance homogeneity. A Tukey HSD (honestly significant difference) was used for post hoc testing. All statistical tests were performed at a probability level of 95% (α = 0.05). The software package SPSS version 13 was used for analysis.
Results
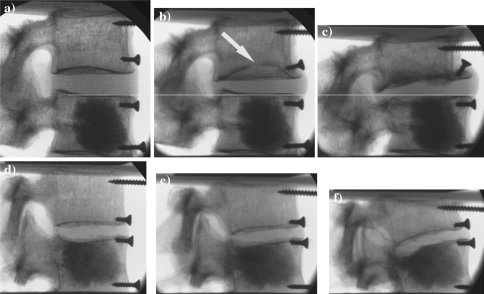
BMD of the FSUs ranged from 27.3 to 105.5 mg/cm3. One FSU of each group was higher than the osteoporosis limit of 97.1 mg/cm3. Therefore, 92% of the specimens used for study were osteoporotic. The properties of the specimen groups and the cement volumes injected are given in Table 1. The cement volume injected was approximately 38 ± 12 and 35 ± 23% of the total volume of the vertebral body for the C_35 and the C_0 groups, respectively. There was a negative linear correlation between degree of filling and the BMD, i.e. a lower degree of filling in the vertebrae with denser bone in both groups (C_35: R2 = 0.67, C_0: R2 = 0.578). The pattern of filling was spherical and similar for all vertebrae (Fig. 3a, d). A-p and lateral radiographs showed an even distribution on both sides of the vertebral body. No extrusion of cement from the vertebral bodies was observed. No deformations were apparent in the regions augmented with regular cement (Fig. 3a–c). Segments augmented with low-modulus cement showed large deformations in the cranial vertebral body and smaller deformations in the caudal cancellous bone region where the low-modulus cement had been injected (Fig. 3d–f). In the untreated control FSUs, failure occurred in both cranial (5) and caudal (6) vertebrae. In the C_35 group failure occurred at the cranial (7) and at the caudal (4) vertebrae. For the C_0 group, ten failures occurred in the cranial vertebrae, and one in the caudal vertebrae. The FSUs showed endplate and wedge-shaped fractures as seen clinically. The distribution of endplate and wedge-shaped fractures was different for the three groups. The ratio of endplate to wedge-shaped fracture was 3:8; 4:7 and 10:1 for the control, C_35 and C_0 groups, respectively (Fig. 3b, c, e, f).
Table 1.
Dimensions and cement volumes injected (mean ± SD) for the three specimen groups
| Control | C_35 | C_0 | |
|---|---|---|---|
| BMD (mg/cm3) | 68.7 ± 25.5 | 68.5 ± 23.1 | 69.2 ± 22.6 |
| Height (mm) | 25.7 ± 2.9 | 23.4 ± 2.9 | 25.0 ± 2.8 |
| Cross-section (mm2) | 1310 ± 257 | 1153 ± 180 | 1217 ± 365 |
| Spine level | 4.3 ± 1.8 | 4.0 ± 2.3 | 4.0 ± 1.9 |
| Disc height (mm) | 7.0 ± 2.1 | 7.1 ± 2.2 | 8.2 ± 1.6 |
| Cement volume injected (ml) | – | 10.4 ± 1.4 | 10.8 ± 1.3 |
Fig. 3.
Fluoroscopic images of FSUs augmented with regular cement (above) and low-modulus cement (below) during mechanical testing. a Before testing, b taken subsequent to endplate fracture (arrow) and c after testing. The white line shows that there is less deformation in the augmented vertebral body. d Before testing, e at the time a wedge-shaped fracture developed in the cranial vertebral body and f after testing
The values for FSU stiffness and failure load, as well as stiffness of the cranial and caudal vertebral bodies are listed in Table 2.
Table 2.
Mechanical parameters of entire FSU and individual vertebral bodies (mean ± SD)
| Control | C_35 | C_0 | |
|---|---|---|---|
| Stiffness FSU (N/mm) | 2,920 ± 948 | 2,531 ± 1087 | 2,007 ± 618 |
| Stiffness FSU (Mpa) | 132 ± 51 | 111 ± 35 | 98 ± 34 |
| Failure load FSU (N) | 4,435 ± 139 | 3,719 ± 1395 | 3,463 ± 1281 |
| Stiffness VBcranial (MPa) | 321 ± 150 | 177 ± 71 | 264 ± 220 |
| Stiffness VBcaudal (MPa) | 337 ± 146 | 191 ± 95 | 477 ± 273 |
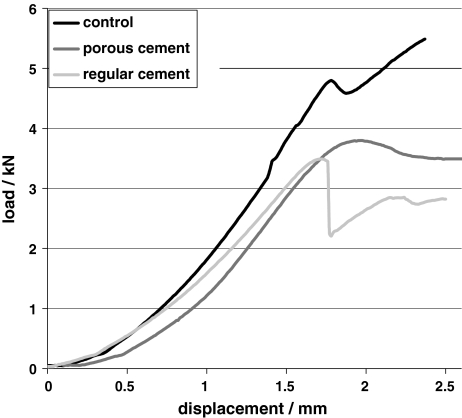
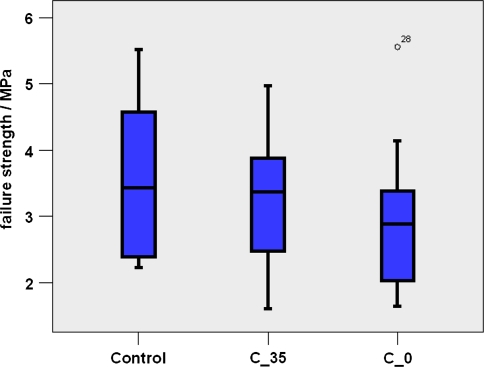
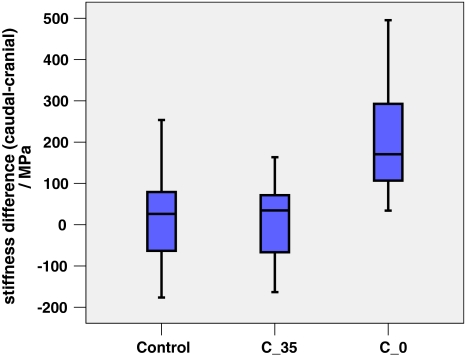
No significant difference in FSU stiffness was found. Figure 4 shows representative load–displacement curves for each group. Mean values of FSU stiffness, which includes the one from the disc, were around 20–55% of the stiffness of the individual vertebral bodies. The mean failure strength was 3.3 ± 1 MPa with low-modulus cement, 2.9 ± 1.2 MPa with regular cement and 3.6 ± 1.3 MPa for the control group. Difference between the groups was not significant (p = 0.754 and p = 0.375, respectively, for low-modulus cement vs. control and regular cement vs. control) (Figs. 4, 5). Significant differences were observed for the parameters stiffness VBcranial, stiffness VBcaudal and stiffnessdifference between the three groups (Table 2, Fig. 6). The parameter stiffnessdifference was significantly higher for the C_0 group compared to the other groups (control to C_0, p = 0.003; C_35–C_0, p = 0.002). Stiffnessdifference was similar for the control and the FSU group treated with the low-modulus cement (p = 0.999).
Fig. 4.
Load–displacement curves of representative FSUs from each group
Fig. 5.
Failure strengths (box-plots) of the FSU groups: control, treated with low-modulus cement (C_35), and treated with regular cement (C_0)
Fig. 6.
Difference of the cranial and caudal vertebral stiffness (box-plots) of the FSU groups, control, treated with low-modulus cement (C_35), and treated with regular cement (C_0)
Discussion
Vertebroplasty using PMMA offers a successful treatment for osteoporotic vertebral body fractures. A laboratory study [7] clearly showed the reduction in failure load of functional spine units after vertebroplasty using regular PMMA cement. Because the failure always occurred in the adjacent non-augmented vertebral body, the reduction in failure load may be useful to quantify the fracture risk of the adjacent vertebral body. In a previous study [8], we characterized a low-modulus PMMA cement, which showed similar mechanical properties to cancellous bone in compression. Using this low-modulus cement instead of the regular PMMA cement we hypothesized that the reduction of failure strength could be minimized or reduced due to augmentation. Entire FSUs augmented with low-modulus and regular PMMA cement were investigated and compared to an untreated control group. In this study a non-fractured model was used as a first approach for several reasons. A similar protocol was used by Berlemann et al. [7], enabling comparison of results. Also, the amount of sample variation was reduced if compared to a pre-fractured model, allowing us to better express the differences between filling materials. A definition and creation of a fracture pattern would contribute to clinical relevance of the study, but would also lead to more variance in results. So far, no accepted fracture-model exists for osteoporotic vertebral body fractures. Furthermore, prophylactic reinforcement of adjacent, non-fractured vertebrae is increasing in popularity [13]. For this application, a less stiff material could provide a better transition in terms of load-transfer to the untreated vertebrae. Due to the used model of non-fractured FSUs, the clinical relevance of this study is restricted to prophylactic vertebroplasty. In order to maximize the effect of material properties, the injected volume (approx. 10 ml) was higher than clinically recommended (4–8 ml) [14], but still comparable to prophylactic vertebroplasty in the lumbar region. A critical element of this study was the use of a fixed plate at the cranial aspect of the FSU during testing. The model would be more realistic if rotational motion of the specimen was also possible during axial compression. However, due to morphological variation and the existing small to medium-sized osteophytes, it was difficult to locate the centre of rotation at which axial compression would have to be applied in order to avoid any non-reproducible moments acting on the vertebrae.
The displacement measurements using optical markers are restricted to selected locations on the surface of the vertrebral body. With constrained endplates, a homogeneous deformation along the axis of the vertebra in the elastic region could be assumed. Therefore, optical motion tracking was exclusively used for stiffness evaluation during elastic deformation. Fractures occurring consequently after elastic deformation, normally in the inner part of the vertebral body, could not be assessed. Fracture type is therefore excluded to be influential on the stiffness evaluation. Accuracy of optical motion tracking systems is hard to judge [15]. The measured noise-level represents a hypothetical maximum of the system-accuracy. However, the effective accuracy is assumed a magnitude lower. However, the local accuracy converges to the noise level with decreasing volume of interest (maximum range of actual marker motion, here approx. 1 mm3) even though the total observation volume is greater (depending on camera distance and focal length, here approx. 60 × 60 × 60 mm) [21]. The accuracy remains to be quantified and can only be estimated as barely but sufficient with regard to the law of error propagation. Therefore the outcome of optical measurements has to be considered critical. For further investigations, high-resolution X-ray data acquisition might provide an opportunity to improve the quantification of vertebral body stiffness and fracture behavior.
Our findings in terms of FSU stiffness and failure load of both the controls and the FSUs augmented with regular cement agreed with the results of Berlemann et al. [7]. We obtained the same difference between the groups, but with higher data scatter and therefore lower significance levels presumably due to greater heterogeneity of the specimens. Even though the filling volume was maximized, we could not show significance in the reduction of failure strength using the low-modulus cement in comparison with the regular cement.
The specimens showed high variances regarding the degeneration state of the disc, which could be considered as the most influencing parameter for FSU stiffness evaluation. In order to exclude this disturbing factor, the individual vertebral body stiffness was considered. Unfortunately the stiffness values of the cranial, and non-augmented vertebrae differed between all the groups (Table 2) and might be confusing. Therefore, the difference of caudal and cranial vertebral body stiffness was the only useable parameter for comparing material effects. Although the caudal (augmented) vertebral body stiffness of the low-modulus cement group was found to be lower compared to the regular cement group, the failure strength was even higher (not significant). Augmentation effect of that low-modulus cement showing a slight increase in stiffness and a significant increase in failure strength compared to non-augmented bone was demonstrated using osteoporotic vertebral body cancellous bone biopsies [9].
The low stiffness difference in the low-modulus group demonstrates a reduced mechanical alteration to the FSUs. Because the stiffness of both the vertebral bodies is similar, the deformation under load will also be similar. Therefore, as demonstrated by the results, a vertebral body augmented with low-modulus cement is more likely to be re-fractured than a vertebral body with regular PMMA. Although not statistically significant, this re-fracture would occur at an ultimate higher load, and therefore reduce the overall fracture risk. An influence of the degeneration state of the vertebral disc on the failure strength cannot be excluded.
A difference in fracture patterns, i.e. a higher incidence of endplate fractures occurring at lower loads in the C_0 group and a greater similarity between the fracture patterns/failure loads in the control and the C_35 groups, demonstrates the change in load transfer along the spinal column due to regular cement and the lesser alteration of the mechanical system after augmentation with low-modulus cement. Individual stiffness values and local deformations showed the stiffening effect of the regular cement in the caudal vertebral body in contrast to the low-modulus group. The increased vertebral body stiffness due to regular cement augmentation prevents the disc from extending in the direction of the augmented vertebral body, which results in asymmetrical endplate deflection under loading. This leads to a higher extension of the adjacent endplate of the non-augmented vertebral body under loading. Higher local strains in the adjacent non-augmented vertebral bodies after vertebroplasty may lead to a higher incidence of endplate fractures with lower failure strength than cancellous bone (wedge-shaped fracture) measured at the smallest cross-section of the construct at uniform loading as obtained for the control group. Utilization of low-modulus cement might help to overcome this problem.
Conclusion
The objective of the study, to demonstrate that cement with mechanical properties similar to those of cancellous bone may be beneficial in terms of reducing the fracture risk of adjacent vertebrae after vertebroplasty could not be reached. Due to the study model on non-fractured FSUs, the clinical relevance is restricted to prophylactic vertebroplasty. Results showed that an outcome discussion based on clinically relevant fracture patterns seems to be more important than determining mechanical parameters like failure strength to obtain a better understanding of how to achieve improvement in the human application. The success of such a low-modulus cement in stabilization of the fractures remains to be demonstrated.
Acknowledgments
The authors thank Beat Schenk and Kurtis Wheeler from Synthes Biomaterials in Oberdorf, Switzerland, for providing the material components and proof reading.
References
- 1.Ananthakrishnan D, Berven S, Deviren V, Cheng K, Lotz JC, Xu Z, Puttlitz CM (2005) The effect on anterior column loading due to different vertebral augmentation techniques. Clin Biomech 20:25–31 [DOI] [PubMed]
- 2.Banse X, Sims TJ, Bailey AJ (2002) Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res 17(9):1621–1628 [DOI] [PubMed]
- 3.Baroud G, Nemes J, Heini P, Steffen T (2003) Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J 12:421–426 [DOI] [PMC free article] [PubMed]
- 4.Baroud G, Nemes J, Ferguson SJ, Steffen T (2003) Material changes in osteoporotic human cancellous bone following infiltration with acrylic bone cement for a vertebral cement augmentation. Comput Methods Biomech Biomed Eng 6(2):133–139 [DOI] [PubMed]
- 5.Belkoff SM, Mathis JM, Erbe EM, Fenton DC (2000) Biomechanical evaluation of a new bone cement for use in vertebroplasty. Spine 25:1061–1064 [DOI] [PubMed]
- 6.Belkoff SM, Maroney M, Fenton DC, Mathis JM (1999) An in vitro biomechanical evaluation of bone cements used in percutaneous vertebroplasty. Bone 25(2):23S–26S [DOI] [PubMed]
- 7.Berlemann U, Ferguson SJ, Nolte LP, Heini PF (2002) Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br 84:748–752 [DOI] [PubMed]
- 8.Boger A, Bohner M, Heini P, Verrier S, Schneider E (2005) Properties of a low-modulus PMMA bone cement for osteoporotic bone. Eur Cell Mater 10(1):17 [DOI] [PubMed]
- 9.Boger A, Heini P, Bohner M, Schneider E (2006) Vertebral cancellous bone augmented with stiffness-adapted pmma cement does not show acute failure under dynamic loading. Eur Cell Mater 11(1):29
- 10.Cooper C, Melton LJ 3rd (1992) Vertebral fractures. BMJ 304(6842):1634–1635 [DOI] [PMC free article] [PubMed]
- 11.Fyhrie DP, Vashishth D (2000) Bone stiffness predicts strength similarly for human vertebral cancellous bone in compression and for cortical bone in tension. Bone 26(2):169–173 [DOI] [PubMed]
- 12.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P (2000) Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 39:1410–1414 [DOI] [PubMed]
- 13.Heini PF, Orler R (2004) Vertebroplasty in severe osteoporosis. Technique and experience with multi-segment injection. Orthopäde 33(1):22–30 [DOI] [PubMed]
- 14.Heini PF, Wälchli B, Berlemann U (2000) Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results; A prospective study for the treatment of osteoporotic compressionfractures. Eur Spine J 9:445–450 [DOI] [PMC free article] [PubMed]
- 15.Jobbágy Á, Furnée EH, Romhányi B, Gyöngy L, Soós G (1998) Resolution and accuracy of passive marker-based motion analysis. In: Proceedings of the 8th international IMEKO TC-13 conference on measurement in clinical medicine, Dubrovnik, Croatia, 16–19 September 1998, pp 2.3–2.6
- 16.Pérez-Higueras A, Alvarez L, Rossi RE, Quinones D, Al-Assir I (2002) Percutaneous vertebroplasty : long-term clinical and radiological outcome. Neuroradiology 44:950–954 [DOI] [PubMed]
- 17.Polikeit A, Nolte LP, Ferguson SJ (2003) The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit. Spine 28(10):991–996 [DOI] [PubMed]
- 18.Schildhauer TA, Bennett AP, Wright TM, Lane JM, O’Leary PF (1999) Intravertebral body reconstruction with an injectable in situ-setting carbonated apatite: biomechanical evaluation of a minimally invasive technique. J Orthop Res 17(1):67–72 [DOI] [PubMed]
- 19.Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS (2003) Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology 226(1):119–124 [DOI] [PubMed]
- 20.Wasnich RD (1996) Vertebral fracture epidemiology. Bone 18:179–183 [DOI] [PubMed]
- 21.Windolf M,Götzen N, Morlock MM (2005) Genauigkeitsanalyse des Vicon Motion-Analysis-Systems—Experimentelle Analyse unter Einsatz eines Kalibrier- und Messroboters. Biomechanika, Hamburg