Abstract
The anatomy of the middle layer of lumbar fascia (MLF) is of biomechanical interest and potential clinical relevance, yet it has been inconsistently described. Avulsion fractures of the lumbar transverse processes (LxTP’s) are traditionally attributed to traction from psoas major or quadratus lumborum (QL), rather than transversus abdominis (TrA) acting via the MLF. This attachment is also absent from many biomechanical models of segmental control. The aims of this study were to document: (1) the morphology and attachments of the MLF and (2) the attachments of psoas and QL to the LxTP’s. Eighteen embalmed cadavers were dissected, measuring the thickness, fibre angle and width of the MLF and documenting the attachments of MLF, psoas and QL. The MLF was thicker at the level of the LxTP’s than between them (mean 0.62: 0.40 mm). Psoas attached to the anteromedial surface of each process and QL and TrA to its lateral border; QL at its upper and lower corners and TrA (via the MLF) to its tip. In three cadavers, tension applied to the MLF fractured a transverse process. The MLF has a substantial and thickened attachment to the tips of the LxTP’s which supports the involvement of TrA in lumbar segmental control and/ or avulsion fracture of the LxTP’s.
Keywords: Lumbar fascia, Anatomy, Transverse process, Transversus abdominis, Psoas, Quadratus lumborum
Introduction
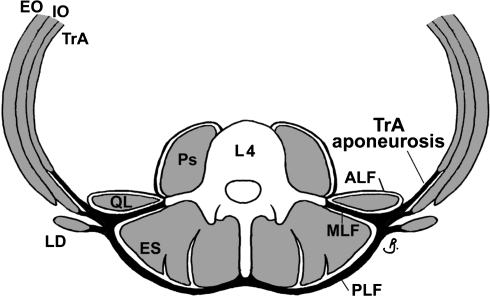
The middle layer of lumbar fascia (MLF) is one of three layers of lumbar fasciae (Fig. 1). Transversus abdominis (TrA), internal oblique (IO) [15, 33] and external oblique (EO) muscles attach to it laterally [3, 32], while medially it attaches to the lumbar transverse processes (LxTP’s) and intertransverse ligaments. Despite its musculoskeletal attachments being of biomechanical interest and potential clinical relevance, several anatomical features of the MLF are unclear.
Fig. 1.
Arrangement of the lumbar fasciae at L4 level. Adapted from Barker et al. [3]. ALF, MLF, PLF anterior, middle and posterior layers of lumbar fascia, Ps psoas, QL quadratus lumborum, TrA transversus abdominis, IO and EO internal and external oblique, ES erector spinae
The vertebral attachments of the MLF are inconsistently described, and quantitative data on the fibre direction and thickness of the fascia are not available. The MLF is reported by some authors to attach to the tips of the lumbar transverse processes [15, 33] and by others to the full length of the LxTP’s [29, 30]. Its fibres are indicated to radiate laterally from the LxTP tips [29, 30], forming ‘arches’ between adjacent processes [10, 31]. Although the MLF is described as being thickest superiorly [26, 31] and thinnest between the LxTP’s [29, 30], no quantitative data confirm this. Similarly, while it is noted as thicker and stronger than the anterior layer of lumbar fascia (ALF) [8, 10, 28, 31], thickness of the ALF has not been reported.
Different methods of measuring the width of the MLF may have resulted in conflicting findings. Measured posteriorly, from its transverse process attachments to where it meets the PLF at the lateral raphe (Fig. 1), the MLF is reported to be 2.6 cm [3]. In contrast, anterior measurements from the transverse process attachments to the musculotendinous junction of TrA [31] technically also measure the width of the TrA aponeurosis (incorporating, proximally, the width of the MLF). These are variably reported to range between 7.1 cm [3] and 10–11 cm [31].
Both MLF and PLF can transmit tension from the lateral abdominal muscles to the lumbar spine [3] and thereby influence segmental motion of the lumbar vertebrae. Simulated tension on the TrA aponeurosis was recently shown to primarily act via the MLF to influence movement within the ‘neutral zone’ [4], a region of particular importance in biomechanical models of segmental control [23, 24]. However, TrA and the lumbar fasciae are frequently excluded from such models. Morphologic data on the structure (and tensile capacity) of the MLF may contribute to more accurate spinal modelling.
TrA’s vertebral attachments may also be of interest in fractures of the LxTP’s, a relatively common orthopaedic injury. Avulsion fractures are a subgroup of LxTP fractures that tend to occur at the L2–L4 process tips [7, 22]. They are generally attributed to contraction of psoas (major) or quadratus lumborum (QL) [1, 9, 16, 17, 19, 20, 22, 25], however involvement of TrA and the abdominal obliques (EO, IO) might also contribute to this injury [18]. Documentation and comparison of the vertebral attachments of these muscles may help clarify their role in avulsion fractures of the LxTP’s.
The aims of this study were to document and quantify the:
Morphologic features of the MLF; including its fibre angles, thickness and width of the TrA aponeurosis.
Attachments of the MLF, psoas and QL to the LxTP’s.
Materials and methods
The lateral and posterior abdominal walls of eighteen cadavers (embalmed in 4% formaldehyde; 10M, 8F; mean age 83, range 60–96 years) were dissected bilaterally. Psoas and QL were removed, preserving their attachments to the transverse processes. All thickness measures were taken bilaterally using a manual micrometer (Mitutoyo, Japan; accuracy ± 0.005 mm). Thickness of the ALF was measured at L3 before its removal. Thickness of the MLF was measured adjacent to as well as between the L2–L4 transverse processes, by piercing the intertransverse ligaments and removing underlying fatty tissue.
Psoas, QL and MLF attachments to the L2–L4 transverse processes were measured on all specimens. The dimensions and location of each attachment were documented using a dial caliper (Mitutoyo, Japan; accuracy ± 0.5 mm) and micrometer. Fascicle angles were also measured. In many cases QL had two anterior tendinous attachments to each process (to the superolateral and inferolateral corners), so the total cross-sectional area (CSA = width × thickness) per transverse process was calculated as the sum of these. The CSA of each MLF attachment was similarly determined using its attachment length and thickness measures.
Fascicle directions of psoas and QL and fibre orientation of the MLF were measured bilaterally using a goniometer (L.S Starrett, USA, accuracy ± 0.5°), which was aligned along a reference ‘horizontal’ axis between the tips of left and right transverse processes. The width of the TrA aponeurosis was measured from each (L2–L4) transverse process using a tape measure (±1 mm).
Paired t tests were used to identify differences in MLF thickness values between sides and for cadavers of different sex and age. In the absence of differences between sides, left and right values were pooled and paired t tests (df = 17, P = 0.05) were used to determine thickness differences at the level of the LxTP’s compared with those between the transverse processes.
Results
Lumbar fascia morphology
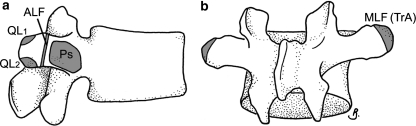
The ALF was thin and membranous, attaching towards the lateral end of each transverse process, between the attachments of psoas and QL (Fig. 2a). The mean thickness of the ALF at L3 was 0.10 (range 0.06–0.14) mm. In all cadavers the MLF attached to the entire lateral edge of each of the (L2–L4) transverse processes (on their posterior aspect; Fig. 2b). Laterally, fibres of the MLF were continuous with the aponeurosis and fascicles of TrA. EO and IO originated from muscular attachments behind the upper and lower parts of the TrA aponeurosis (above and below L3, respectively). IO’s attachment was via short fascicles, oriented perpendicular to the MLF fibres.
Fig. 2.
Attachments to transverse processes at L3. a The attachment of the ALF divides the attachments psoas (medially) and QL (laterally). QL has tendinous attachments to each corner (QL1 and QL2). b The MLF attaches to the lateral edge and tip of the transverse process and is more extensive posteriorly. Legends as for Fig. 1
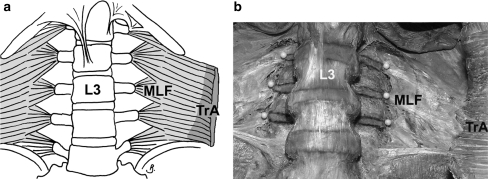
The majority of MLF fibres radiating from each transverse process were inferolateral, although these became more horizontal towards the iliac crest (Fig. 3a, b). The mean (range) fibre angle below reference ‘horizontal’ was 24 degrees (10–50°) at L2, 19 (5–33°) degrees at L3, and 8 (−15 to 26°) degrees at L4. Superolateral fibres were oriented up to 30° above the transverse process before joining inferolateral fibres from the transverse process above. The average width of the TrA aponeurosis at the L2, L3 and L4 transverse processes was 9.5 (5.0–11.3) cm, 9.3 (5.7–11.7) cm and 8.3 (5.0–11.4) cm, respectively (Fig. 3a).
Fig. 3.
Middle layer of lumbar fascia. Note the MLF is continuous with TrA and the borders and tips of the transverse processes have been marked and pinned (respectively). Legends as for Fig. 1
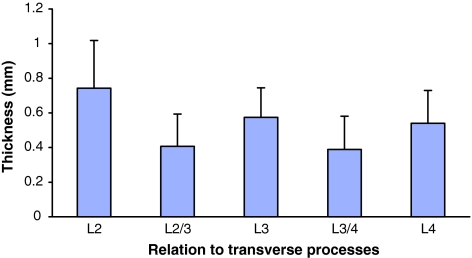
The MLF ranged in thickness from 0.11 to 1.34 mm. It was thicker at the level of the transverse process (mean 0.62 mm) than between (mean 0.40 mm) the transverse processes (P = 0.01, Fig. 4). The mean thickness of the MLF was also greatest superiorly and on the right side (mean difference between sides at L3 was 0.10 mm, P = 0.04). There were no significant differences in MLF thickness between sexes, nor any correlation between thickness and age.
Fig. 4.
Mean MLF thickness at and between transverse processes. Note greater mean thickness of MLF at the level of the transverse processes compared to between the processes. Error bars on the histogram indicate standard deviations
The mean CSA of MLF attachment to each transverse process was 7.1 mm2 at L2, 5.3 mm2 at L3, and 4.5 mm2 at L4 (Table 1). In three female cadavers, transverse processes were accidentally fractured while measuring the thickness of the adjacent MLF, when the end of the micrometer was ‘hooked’ behind the MLF, shearing this attachment anteroposteriorly. Each fracture occurred vertically across the shaft of the transverse process, 5–10 mm from the tip.
Table 1.
Comparison of cross-sectional areas (CSA) of quadratus lumborum (QL) and the middle layer or lumbar fascia (MLF) at their attachments to lumbar transverse processes
| Tendinous attachment | Location on process | Mean (range) total cross-sectional area (mm2) | ||
|---|---|---|---|---|
| L2 | L3 | L4 | ||
| QL | At corners | 2.0 (0.8–4.2) | 8.2 (1.6–9.0) | 8.1 (1.0–16) |
| MLF (TrA) | Along lateral edge | 7.1(2.8–17.2) | 5.3 (3.3–8.4) | 4.5 (1.9–8.2) |
| Collective QL + MLF | Corners + lateral edge | 9.1 | 13.4 | 12.6 |
Muscle attachments
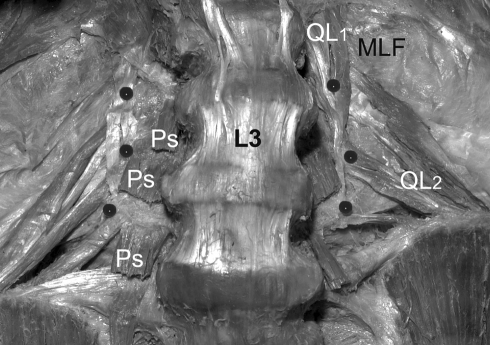
Psoas originated from the vertebral bodies and medial three-quarters of the transverse processes (L2–L4, anteriorly) in all specimens, but did not attach to the transverse process tips (Figs. 2a, 5). QL’s main transverse process attachments were tendinous and arranged in two layers. The first layer descended almost vertically from fascicles arising from the twelfth rib, with tendons attaching to the superolateral corner of each transverse process. The second (deeper) layer was oblique, fascicles originating from the iliac crest and ascending superomedially to insert to the inferolateral corner of each transverse process (Fig. 5). These oblique attachments were most consistent at L3 and L4 transverse processes and ranged in CSA from 2 to 6 mm2, with the inferior attachments generally being greater in size (Fig. 2a).
Fig. 5.
Dissection of transverse process attachments. Note the relation of psoas (Ps) and quadratus lumborum (QL) attachments to the L2–L4 transverse processes (pinned at their tips). QL’s attachments are in two layers (QL1 vertical and QL2 oblique) to the corners of the TP’s, while psoas’ attachments lie medial to the tips
Discussion
The results of this study indicated that the MLF provides a substantial attachment between TrA and the commonly avulsed (L2–L4) transverse process tips. It was considerably thicker at the transverse processes and was capable of transmitting tension resulting in fracture in these specimens. QL provided a more variable attachment to the process tips and psoas only attached medial to them. The findings have implications for understanding the role of TrA and the MLF in mechanisms of both avulsion fracture of the LxTP’s and lumbar segmental control.
Morphologic features of the ALF and MLF
The thickness of the ALF was approximately one-fifth that of the MLF. Its membranous structure suggests this layer has minimal capacity for tensile transmission. Findings regarding attachments of the MLF concurred with descriptions that it is attached to the lateral border [10, 28], rather than length [29, 30] of each transverse process (Fig. 2b).
Continuity of the MLF with the attachments of the LxTP’s medially and with TrA fascicles laterally support its primary role as an aponeurosis of TrA [5, 10, 15, 18, 26, 30, 33]. The attachments of EO and IO to the MLF (above and below L3, respectively) are consistent with existing findings [3]. IO’s attachment (via short fascicles, oriented perpendicular to the MLF fibres) indicates a reduced capacity for tensile transmission between IO and the MLF. Fibre directions noted for the MLF were closer to the reported fascicle orientations of TrA than to those of the obliques [32].
The mean width of the TrA aponeurosis (∼9 cm) was similar to that reported from CT scans of young adult males [29]. Previous reports indicated the aponeurosis to be slightly wider (10–11 cm; [31]) and narrower (7.1 cm at L3 [3]) than in our results. While the latter report was also from an elderly sample, its measures were taken from two-dimensional photographs, rather than using a flexible tape measure (as in our study), which accounts for the plane of fascial curvature.
Thickness of the MLF at its transverse process attachments was both statistically greater as well as capable of (accidental) fracture of the process tip. While these elderly cadavers were likely to have increased bone fragility, one might also expect the fascia to tear rather than fracture the process if it were weaker. Capacity for tensile transmission between these attachments and the transverse processes requires formal testing using physiologic forces. This is the subject of further investigation.
Consistent with early descriptions that the MLF is the ‘strongest’ [8, 28] layer of lumbar fascia, the average thickness of the MLF (0.55 mm) was slightly greater than that reported for the PLF (0.52 mm) in a previous study on a similar sample [2]. The mean increase in thickness of the MLF at transverse processes was far greater than the increased thickness of PLF reported at the spinous processes (55 cf. 6%). The finding of greater MLF thickness on the right was unexpected, yet on reviewing the specimens, the right fascia appeared in many cases visibly more opaque and fibrous than the left. The difference could possibly be associated with hand dominance but this requires further investigation.
It is unlikely that the fascial thickness measures from this study accurately represent those in the general population, due to attrition of the attached muscles associated with age and disuse as well as potential shrinkage of tissue associated with fixation [27]. Thickness of TrA in elderly, embalmed specimens [32] is less than one-quarter of that in-vivo (using ultrasound) on young, healthy subjects [6]. Nonetheless, the findings are useful to provide relative differences in thickness between regions of the MLF and between the three layers of fascia.
Muscle attachments: implications for transverse process fracture and stability
The attachments of psoas reported in this study are not consistent with current reports that psoas avulses the lumbar transverse processes [1, 9, 17, 22]. However, both TrA (via the MLF) and QL demonstrated consistent attachments to the tips of L2–L4 (typically avulsed) transverse processes and had some fascicles oriented inferolaterally. Of interest, the cumulative attachments of TrA and QL appear most consistent with the incidence of avulsion fracture noted clinically [20, 22, 25], being greatest at L3 (Table 1).
Of TrA and QL, TrA had more consistent attachments to the three most commonly avulsed (L2–L4) transverse processes (QL’s attachments being greatly reduced at L2; Table 2) and a more horizontal fascicle orientation (Table 2). TrA also has an uninterrupted attachment to the lateral border of the process that was observed to fracture the process tips. The fascicle orientation of TrA might also contribute to lateral displacement of fragments following lumbar transverse process fracture, an observation previously attributed only to QL [11]. These findings indicate that future descriptions of avulsion fracture of the LxTP’s should consider involvement of TrA.
Table 2.
Fibre angle of quadratus lumborum at its transverse process attachments (at lateral border)
| Attachment to corners of transverse processes | Mean angle (degrees) |
|---|---|
| L2 superolateral | +87 |
| L2 inferolateral | −49 |
| L3 superolateral | +87 |
| L3 inferolateral | −45 |
| L4 superolateral | +88 |
| L4 inferolateral | −28 |
Angle measured in degrees above/below ‘horizontal’ (between left and right transverse process tips)
Applied tension to TrA is transmitted to vertebrae mainly via the MLF [3, 4] and reported to limit both gross movement (in the coronal plane [29, 30]) and segmental motion (in the sagittal plane, particularly affecting the neutral zone [4]). The current study documents the MLF as having suitable morphology to transmit tension between TrA and the lumbar vertebrae and potentially contribute to segmental control. Altered timing of recruitment of TrA in people with low back pain [13, 14] is associated with recurrent low back injury [12, 21]. Reduced effectiveness of the musculofascial support mechanism may help to explain this.
Conclusions
The MLF forms a strong aponeurotic attachment between TrA and the lumbar vertebrae and provides a suitable mechanism for force transmission to them. These forces may contribute to lumbar segmental stability and may also play a role in avulsion fractures of the LxTP’s.
Acknowledgments
The authors sincerely thank Dr Colin Anderson (Department of Anatomy and Cell Biology, The University of Melbourne), Mr. Robert Marshall (Surgeon’s Rooms: 24 Collins Street, Melbourne) and Dr. Alex Pitman (Diagnostic Imaging, Peter MacCallum Cancer Institute) for valued advice. Thanks also to Stuart Thyer for photography, Mathew Jackson and Diana Keshtiar (Department of Anatomy and Cell Biology, The University of Melbourne) for technical assistance.
References
- 1.Armstrong JR (1965) Lumbar disc lesions; pathogenesis and treatment of low back pain and sciatica, 3rd edn. E. & S. Livingstone, Edinburgh
- 2.Barker PJ, Briggs CA (1999) Attachments of the posterior layer of lumbar fascia. Spine 24(17):1757–1764 [DOI] [PubMed]
- 3.Barker PJ, Briggs CA, Bogeski G (2004) Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine 29(2):129–138 [DOI] [PubMed]
- 4.Barker PJ, Guggenheimer KT, Grkovic I, Briggs CA, Jones DC, Thomas CD et al (2006) Effects of tensioning the lumbar fasciae on segmental stiffness during flexion and extension: young investigator award winner. Spine 31(4):397–405 [DOI] [PubMed]
- 5.Basmajian JV, Slonecker CE (1989) Grant’s method of anatomy: a clinical problem-solving approach, 11th edn. Williams & Wilkins, Baltimore, p 37
- 6.Cresswell AG, Grundstrom H, Thorstensson A (1992) Observations on intra-abdominal pressure and patterns of abdominal intra-muscular activity in man. Acta Physiol Scand 144(4):409–418 [DOI] [PubMed]
- 7.Dandy DJ, Edwards DJ (1998) Essentials of orthopaedics and trauma, 3rd edn. Churchill Livingstone, New York, p 157
- 8.Davies-Colley JNC (1894) The muscles. J & A Churchill, New York, pp 433–434
- 9.Debrunner HU (1971) Biomechanics of the spine. Z Unfallmed Berufskr 64(4):245–254 [PubMed]
- 10.Frazer JE (1933) The anatomy of the human skeleton, 3rd edn. J. & A. Churchill, London, pp 31–43
- 11.Gilsanz V, Miranda J, Cleveland R, Willi U (1980) Scoliosis secondary to fractures of the transverse processes of lumbar vertebrae. Radiology 134(3):627–629 [DOI] [PubMed]
- 12.Hides JA, Jull GA, Richardson CA (2001) Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine 26(11):E243–E248 [DOI] [PubMed]
- 13.Hodges PW, Richardson CA (1996) Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine 21(22):2640–2650 [DOI] [PubMed]
- 14.Hodges PW, Richardson CA (1998) Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J Spinal Disord 11(1):46–56 [DOI] [PubMed]
- 15.Hollinshead WH (1962) Textbook of anatomy. Harper & Row Publishers, New York, pp 348–431
- 16.Howarth MB, Petrie JG (1964) Injuries of the spine. Williams & Wilkins, Baltimore, p 265
- 17.Krueger MA, Green DA, Hoyt D, Garfin SR (1996) Overlooked spine injuries associated with lumbar transverse process fractures. Clin Orthop 327:191–195 [DOI] [PubMed]
- 18.Marshall R (2001) Living anatomy: structure as the mirror of function. Melbourne University Press, Melbourne, pp 280–283
- 19.McRae R (1998) Practical fracture treatment, 3rd edn. Churchill Livingstone, Edinburgh, p 230
- 20.Miller CD, Blyth P, Civil ID (2000) Lumbar transverse process fractures- a sentinel marker of abdominal organ injuries. Injury 31(10):773–776 [DOI] [PubMed]
- 21.O’Sullivan PB, Twomey LT, Allison GT (1997) Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine 22(24):2959–2967 [DOI] [PubMed]
- 22.Otte D, Sandor L, Zwipp H (1990) Significance and mechanism of thoracic and lumbar spine injuries in traffic accidents. Unfallchirurg 93(9):418–425 [PubMed]
- 23.Panjabi M, Abumi K, Duranceau J, Oxland T (1989) Spinal stability and intersegmental muscle forces. A biomechanical model. Spine 14(2):194–200 [DOI] [PubMed]
- 24.Panjabi MM (1992) The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord 5(4):390–396 [DOI] [PubMed]
- 25.Patten RM, Gunberg SR, Brandenburger DK (2000) Frequency and importance of transverse process fractures in the lumbar vertebrae at helical abdominal CT in patients with trauma. Radiology 215(3):831–834 [DOI] [PubMed]
- 26.Poirier P (1901) Myologie. In: Poirer P, Charpy A (eds) Traite d’Anatomie Humaine. Masson et Compagnie, Paris, pp189–190, 497
- 27.Salmhofer W, Rieger E, Soyer HP, Smolle J, Kerl H (1996) Influence of skin tension and formalin fixation on sonographic measurement of tumor thickness. J Am Acad Dermatol 34(1):34–39 [DOI] [PubMed]
- 28.Sharpey W, Thomson A, Cleland J (eds) (1867) Quain’s elements of anatomy, 7th edn. James Walton Publishing, London, pp 248–253, 266
- 29.Tesh KM (1986) The abdominal muscles and vertebral stability. PhD Thesis, University of Strathclyde, Glasgow, pp 166–349
- 30.Tesh KM, Dunn JS, Evans JH (1987) The abdominal muscles and vertebral stability. Spine 12(5):501–508 [DOI] [PubMed]
- 31.Testut L, Latarjet A (1948) Traite d’anatomie humaine, 9th edn. G. Doin & Compagnie, Paris, pp 944–952, 1107–1109
- 32.Urquhart DM, Barker PJ, Hodges PW, Story IH, Briggs CA (2005) Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech (Bristol, Avon) 20(3):233–241 [DOI] [PubMed]
- 33.Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE et al (eds) (1995) Gray’s anatomy, 38th edn. Churchill Livingstone, New York, pp 809–829