Abstract
Rationale behind motion preservation devices is to eliminate the accelerated adjacent-level effects (ALE) associated with spinal fusion. We evaluated multidirectional flexibilities and ALEs of StabilimaxNZ® and simulated fusion applied to a decompressed spine. StabilimaxNZ® was applied at L4–L5 after creating a decompression (laminectomy of L4 plus bilateral medial facetectomy at L4–L5). Multidirectional Flexibility and Hybrid tests were performed on six fresh cadaveric human specimens (T12–S1). Decompression increased average flexion–extension rotation to 124.0% of the intact. StabilimaxNZ® and simulated fusion decreased the motion to 62.4 and 23.8% of intact, respectively. In lateral bending, corresponding increase was 121.6% and decreases were 57.5 and 11.9%. In torsion, corresponding increase was 132.7%, and decreases were 36.3% for fusion, and none for StabilimaxNZ® ALE was defined as percentage increase over the intact. The ALE at L3–4 was 15.3% for StabilimaxNZ® versus 33.4% for fusion, while at L5–S1 the ALE were 5.0% vs. 11.3%, respectively. In lateral bending, the corresponding ALE values were 3.0% vs. 19.1%, and 11.3% vs. 35.8%, respectively. In torsion, the corresponding values were 3.7% vs. 20.6%, and 4.0% vs. 33.5%, respectively. In conclusion, this in vitro study using Flexibility and Hybrid test methods showed that StabilimaxNZ® stabilized the decompressed spinal level effectively in sagittal and frontal planes, while allowing a good portion of the normal rotation, and concurrently it did not produce significant ALEs as compared to the fusion. However, it did not stabilize the decompressed specimen in torsion.
Introduction
Low back pain is one of the most common painful conditions in the industrialized countries. About 70–85% of all people have back pain sometime in life [3]. It has been estimated that the cost of low back pain in the USA alone was about 90.7 billion dollars in 1998 [11]. An important pathology of low back pain is the degenerative disc disease, which is associated with decreased disc height, facet joint arthropathy, spondylolysthesis, and spinal stenosis with narrowing of the neural foramen. The spinal fusion, sometimes in association with neural decompression, has been the gold standard for the treatment of low back pain, once the conservative treatment has failed. However, the spinal fusion is associated with several morbidities; the most important being the accelerated adjacent-level degeneration [9, 17], also called adjacent-level effects (ALE). Non-fusion or motion preservation devices, such as artificial discs, nucleus and facet-joint replacements, extension stops, and flexible stabilizers, are the latest treatment options for the low back problem.
An important advantage claimed for the non-fusion devices is that they will eliminate or decrease the ALE. However, it will take many years of use in a patient before it can be shown if the non-fusion device succeeded or failed in this important function. An in vitro method, called Hybrid test method, has been especially developed to help estimate the ALE of any fusion or non-fusion device in a reliable and repeatable manner [14]. The purpose of this study was to evaluate the multidirectional flexibilities and ALEs due to the application of StabilimaxNZ®, a new flexible stabilizer, and to contrast the results with those due to simulated solid fusion, using the Flexibility and Hybrid test methods.
Methods
Nomenclature: a certain nomenclature is necessary for clear presentation of the experimental design and procedures.
Operated-level: spinal level where a surgical operation is performed.
Non-operated levels: all spinal levels other than the operated-level.
Construct: spine specimen with the operated device.
ROM: rotation recorded at the maximum moment on the third load cycle from the neutral position in the same direction as the applied moment.
iROM: intervertebral ROM at a spinal level.
tROM: total ROM of the specimen, i.e., T12–S1 for the lumbar spine.
Adjacent-level effects (ALE): change in iROM at any non-operated level due to a change at the operated-level. The ALE at a level was quantified by the formula:
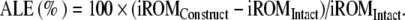 |
The specimens and their preparation
Six fresh cadaveric whole lumbar spine specimens (T12–S1, three males and three females with average age of 67.5 years, range 55–80 years) were used. The specimens showed normal degenerative changes consistent with this age group as seen on lateral and frontal radiographs. Specific grading of specimens was not performed. Each specimen was oriented with L3 vertebral body horizontal, and provided with horizontal mounts at its ends. Motion measuring flags containing three light emitting diodes each were rigidly attached to all vertebrae. Through the pedicles, vertebral bodies were injected with quick-setting epoxy to provide solid anchoring for the pedicle screws and the screws for the 360° simulated fusion.
Experimental design
The experimental design consisted of the following sequential steps.
Intact specimen is tested with Flexibility test method.
Decompression specimen is tested with Flexibility test method.
StabilimaxNZ® construct is tested with Flexibility and Hybrid test methods.
Simulated fusion construct is tested with Flexibility and Hybrid test methods.
Flexibility test method
The Flexibility test method has been described [13], and used in many studies of fusion devices [2, 4, 7, 12], and non-fusion devices [5, 10]. In the present study, the input was unconstrained pure moment applied in flexion/extension: maximum of 8 Nm in the presence of 400 N follower load [16]. Bilateral lateral bending and axial torsion were produced with a maximum moment of 6 Nm, without the follower. (For the choice of these loads, please see Discussion.) Two preconditioning cycles were used, and the motion measurements were taken on the third load cycle. Three-dimensional intervertebral motions were measured by an optoelectronic system (Optotrak 3020, Northern Digital Inc., Waterloo, ON, Canada).
Hybrid test method
The Hybrid test method has been described and used elsewhere [14, 15]. Briefly, the rationale behind the Hybrid test method is that after the alteration at the operated-level, e.g., spinal fusion surgery, the spinal column of the patient is subjected to additional stresses due to the activities of daily living, which overtime result in motion re-distribution within the spine. Further, it is assumed that the motion re-distribution is such that the total spinal motion (tROM rotation of T12–S1) is conserved. Therefore, the input in the Hybrid test method is pure rotation. In the present study, the rotation-input was generated by unconstrained pure moment applied to the spinal construct, using the Flexibility test method briefly described above. The moment was increased continuously until the T12–S1 rotation of the construct (tROMConstruct) equaled the total rotation of the intact specimen (tROMIntact).
Decompression
The decompression consisted of bilateral transactions of the L4 lamina, superaspinous and interspinous ligaments and ligamentum flavum, and ∼50% medial facetectomy at 4-L5 [1]. This was consistent with the clinical indications for the use of the StabilimaxNZ® as defined by the manufacturer.
The StabilimaxNZ®
This flexible stabilizer, developed by Applied Spine Technology, New Haven, CT, USA, consists of two concentric springs designed and arranged specifically to provide well-defined non-linear load-displacement curve. The spring assembly on each end is attached to the pedicle screws via ball and socket joints. A pair of StabilimaxNZ® with 6.5 mm screws was applied to each decompressed specimen at L4–L5 after the specimen was placed in neutral posture as defined by the intact state of the specimen (Fig. 1a). This particular device is designed to simultaneously stabilize the spine and provide controlled motions.
Fig. 1.
Photographs. a Bilateral application of the StabilimaxNZ® and b the multidirectional spine tester
Simulate fusion
To simulate a solid spinal fusion, posteriorly a pedicle screw fusion device with 6 mm screws was applied bilaterally, while anteriorly a 4 mm thick custom-made plate with 4 mm screws immobilized the L4–L5 vertebrae via screws anchored into the L4 and L5 vertebral bodies.
Multidirectional spine tester
The spine tester, with the capability to continuously apply unconstrained pure moment and simultaneously monitor the total specimen rotation tROM, has been described [15], and is shown in Fig. 1b. The iROM at all levels were measured by the optoelectronic motion measurement system.
Data analysis and statistics
Repeated measures analysis of variance (ANOVA) and Bonferroni post hoc analysis were performed at each spinal level to assess statistically significant differences in the iROM of each spinal construct (StabilimaxNZ® and simulated fusion) with respect to the intact spine. For the Flexibility data, the analysis was performed for the iROM at L4–L5. The significance was set at P < 0.05.
Results
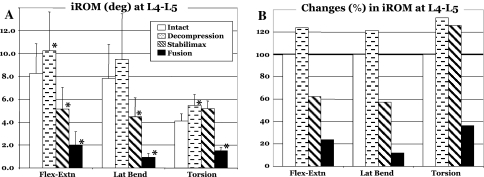
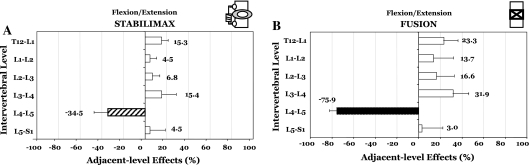
The Flexibility testing showed that at L4–L5, the decompression increased the average rotation by 2.0° (to 124.0% of the intact), while the application of the StabilimaxNZ® and simulated fusion to the decompressed spine decreased the rotation to 62.4 and 23.8% of the intact, respectively (Fig. 2a, b). Using the Hybrid testing, the average results with standard deviation for flexion–extension are presented in Table 1A, together with indicators for statistically significant differences (P < 0.05) between the iROM of the two constructs versus intact. The average ALE for the StabilimaxNZ® at the proximal adjacent-level L3–L4 was 15.4%, while for the simulated fusion it was 31.9% (Fig. 3a, b). At the distal adjacent-level L5–S1, the average ALE values were 4.5% for the StabilimaxNZ® and 3.0% for the fusion.
Fig. 2.
Results of flexibility testing in three planes. a Intervertebral ranges of motion (iROM) in degrees at L4–L5 for the Intact, Decompression, StabilimaxNZ® and simulated fusion. Statistical significance with respect to the Intact are indicated by asterisks (*). b The iROM at L4–L5 in percentage of the Intact
Table 1.
Hybrid testing results
| Ranges of motion (degrees) | T12–L1 | L1–L2 | L2–L3 | L3–L4 | L4–L5 | L5–S1 |
|---|---|---|---|---|---|---|
| A. Flexion/extension | ||||||
| Intact | 5.82 ± 1.86 | 6.78 ± 2.02 | 5.90 ± 1.43 | 7.34 ± 1.41 | 8.26 ± 2.57 | 4.73 ± 3.40 |
| StabilimaxNZ® | 6.65 ± 1.88* | 7.05 ± 2.04 | 6.34 ± 1.83 | 8.49 ± 1.81 | 5.44 ± 1.95* | 4.97 ± 3.62 |
| Simulated fusion | 7.13 ± 2.11 | 7.53 ± 1.76 | 6.91 ± 2.04 | 9.79 ± 2.77 | 2.04 ± 1.20* | 5.27 ± 4.88 |
| B. Bilateral lateral bending | ||||||
| Intact | 6.58 ± 2.22 | 7.21 ± 2.25 | 9.25 ± 1.61 | 8.32 ± 2.84 | 7.81 ± 2.99 | 5.31 ± 3.44 |
| StabilimaxNZ® | 7.04 ± 2.25 | 8.22 ± 3.09 | 10.00 ± 1.81 | 8.57 ± 2.68 | 4.85 ± 1.65* | 5.91 ± 3.75 |
| Simulated fusion | 7.39 ± 2.36* | 9.00 ± 3.59* | 10.95 ± 1.98* | 9.91 ± 3.11* | 0.89 ± 0.34* | 7.21 ± 4.93* |
| C. Bilateral torsion | ||||||
| Intact | 3.11 ± 2.30 | 3.56 ± 2.48 | 4.02 ± 1.95 | 5.19 ± 2.31 | 4.12 ± 1.26 | 2.85 ± 1.52 |
| StabilimaxNZ® | 3.01 ± 2.28 | 3.7 ± 2.66 | 3.93 ± 1.84 | 5.38 ± 2.36 | 4.86 ± 1.46 | 2.73 ± 1.29 |
| Simulated fusion | 3.63 ± 2.61* | 4.32 ± 2.89* | 4.70 ± 2.36* | 6.26 ± 2.58* | 1.49 ± 1.09* | 3.80 ± 1.71* |
Average ± 1 SD of intervertebral ranges of motion (iROM) in degrees for the Intact, StabilimaxNZ®, and simulated fusion
*Significant (P < 0.05) difference with respect to the corresponding Intact values
Fig. 3.
Results of Hybrid testing in flexion–extension. Adjacent-level effects (ALE) due to a StabilimaxNZ® and b simulated fusion. For the definition of ALE, please see text
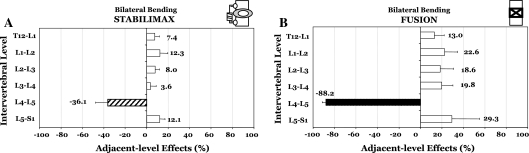
In bilateral lateral bending, the Flexibility testing showed that the decompression at L4–L5 produced an average increase in motion of 1.7° or to 121.6% of the intact rotation (Fig. 2a, b). Both StabilimaxNZ® and simulated fusion decreased the decompressed spine rotation, respectively, to 57.5 and 11.9% of the intact motion. Results for Hybrid testing, averages with standard deviations, are presented in Table 1B, together with indicators for statistically significant differences (P < 0.05) between the iROM of the two constructs versus intact. The Hybrid testing found the average ALE at L3–L4 to be 3.6 and 19.8%, respectively, for the StabilimaxNZ® and simulated fusion (Fig. 4a, b). At the L5–S1, the average ALE values were 12.1 and 29.3%, respectively, for the StabilimaxNZ® and simulated fusion.
Fig. 4.
Results of Hybrid testing in bilateral lateral bending. Adjacent-level effects (ALE) due to a StabilimaxNZ® and b simulated fusion. For the definition of ALE, please see text
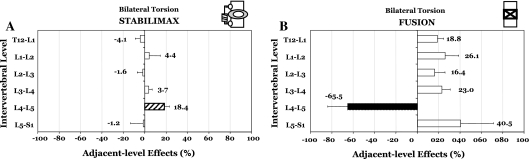
Finally, in bilateral torsion, the Flexibility testing showed the decompression at L4–L5 to increase the average rotation by 1.35° (to 132.7% of the intact), Fig. 2a, b. The StabilimaxNZ® showed an increase of 1.07°, while the simulated fusion decreased the rotation to 36.3% of the intact. For Hybrid testing, the average results with standard deviation are presented in Table 1c, together with indicators for statistically significant differences (P < 0.05) between the iROM of the two constructs versus intact. The ALE at L3–L4 was 3.7% for StabilimaxNZ® and 23.0% for the simulated fusion, while at L5–S1, the respective values were −1.2 and 40.5% (Fig. 5a, b).
Fig. 5.
Results of Hybrid testing in bilateral torsion. Adjacent-level effects (ALE) due to a StabilimaxNZ® and b simulated fusion. For the definition of ALE, please see text
Discussion
Non-fusion motion preservation devices are being introduced at ever increasing rate. An important rationale for the development of these devices is to avoid the clinically relevant long-term adverse effects of spinal fusion, namely the accelerated adjacent-level degeneration. StabilimaxNZ® is a new flexible stabilizer that is designed to simultaneously stabilize the unstable spine (decompressed/degenerated), and allow sufficient spinal motion to avoid the accelerated adjacent-level effects or ALE. Using a recently introduced in vitro Hybrid test method [14], especially designed to evaluate the ALE, and the well-established Flexibility test method results for the StabilimaxNZ® and simulated fusion were compared. We found that the StabilimaxNZ® stabilized the clinically relevant posterior decompression at L4–L5 such that, in flexion–extension, it retained 62.4% of the intact motion, and at the same time produced, on average, only about one-half of the ALE at the clinically relevant proximal adjacent level (L3–L4), as compared to the simulated solid fusion. The corresponding result for lateralbending was that the StabilimaxNZ® retained 57.5% of the intact motion, while it produced only about one-sixth of the ALE due to the simulated fusion at L3–L4. In torsion, the device was not effective in stabilizing the spine. However, its ALE compared to the fusion simulation, was still one-sixth at L3–L4.
A limitation of this in vitro study is that of the Hybrid test method itself. This method, being an in vitro method, does not, and cannot, simulate in a laboratory the actual effects of adaptation that occur over many years after the implantation of the device in a patient. Presently, we lack such detailed knowledge. As mentioned earlier, the Hybrid test method is based upon the assumption that the post-surgery adaptation in a patient overtime, in response to altered stresses at the non-operated spinal levels, results in motion re-distribution such that the overall motion, i.e., T12–S1 in the present study, is conserved. There is some evidence to support this assumption. In a recent clinical study, patients with upper cervical instability were fused at C1–C2 in 17° of lodosis [18]. At 6.7-year follow-up, compensatory kyphosis was observed in sub-axial region (C2–C7), so that the overall neck (C0–C7) lordosis was conserved to the pre-fusion level. Presently there is no equivalent long-term study of the changes in the intervertebral and overall ranges of motion after the fusion. Another limitation is that we did not use the follower load in lateral bending and torsion. Several preliminary studies performed in our laboratory convinced us that the resulting spinal motions, for these two loading directions in the presence of the follower load, were un-physiological. We used the follower load during testing to enhance the biofidelity of our experiment as done by others [16]. The follower-load cables traveled over miniature ball-bearings placed on rods passing laterally through the vertebral bodies, so that cable centerlines passed through each intervertebral center of rotation of the spine specimen. During lateral bending and torsion, the follower load cable on the contra lateral side resisted the applied moment, which violates the in vivo observations of relaxing of the contra-lateral muscles. For this reason we limited the follower load to flexion–extension. In some cases, we observed significant ALE at the most proximal level T12–L1, i.e., StabilimaxNZ® in flexion–extension, and fusion in lateral bending and torsion. However, the rotations in these cases were quite small (average 0.7°), close to the overall accuracy of the test machine and the motion measurement system. Finally, the number of specimens was limited to six due to shortages of good human cadaveric spine specimens. This is most likely due to the increased number of motion preservation devices being developed. Each of these devices needs to be tested prior to the FDA approval for the start of the clinical trials, requiring human cadaveric specimens. This is a good thing that the new devices are tested before they are implanted in a patient. However, it does limit the availability of the spine specimens.
The methodology used was state-of-the-art. The newly designed multidirectional spine tester incorporated six of freedom arrangement, where any one of the three rotation degrees of freedom could be locked to apply the unconstrained pure moment to the specimen [15]. The machine also included appropriate balancing weights so that the generation of un-wanted moments during testing was eliminated. Further, it provided continuous real-time measure of the total rotation (T12–S1) of the specimen, which is needed to carry out the Hybrid test method. Finally, low-friction linear and rotary bearings were used together with a vibration generating motor to further reduce the friction and the unwanted loads. The magnitude of the moment applied to the spine specimen is important. Its upper limit was defined so that it did not cause injury to the specimen and that the specimen could be used for sub-sequent tests. The lower limit was defined by the spinal motions produced, which were close to the physiological motions as reported in the literature. The moments chosen in the present study did just that. In our preliminary studies, we found that 8 Nm in the presence of a 400 N follower load was sufficient for flexion/extension, while 6 Nm in the absence of the follower load was sufficient for the other two directions.
Although there are some biomechanical studies of ALE of artificial discs [6, 8, 15], to our knowledge, there are no ALE studies of any flexible stabilizer.
Conclusions
We studied multidirectional ALEs of StabilimaxNZ®, a flexible stabilizer, and compared the results to those due to simulated fusion, using multidirectional Flexibility and Hybrid test methods. The StabilimaxNZ® applied at L4–L5, stabilized the L4–L5 decompressed spine adequately in flexion–extension and lateral bending but not in torsion. At the same time, it allowed sufficient motion so that the ALEs produced were small compared to those due to the solid fusion simulation.
Acknowledgments
We gratefully acknowledge a gift from Applied Spine Technology, New Haven, CT, USA which made this research possible.
References
- 1.Abumi K, Panjabi MM, Kramer KM, et al (1990) Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine 15:1142–1147 [DOI] [PubMed]
- 2.Ames CP, Acosta FL Jr, Chamberlain RH, Larios AE, Crawford NR (2005) Biomechanical analysis of a newly designed bioabsorbable anterior cervical plate. Invited submission from the joint section meeting on disorders of the spine and peripheral nerves, March 2005. J Neurosurg Spine 3:465–470 [DOI] [PubMed]
- 3.Andersson GB (1999) Epidemiological features of chronic low-back pain. Lancet 354:581–585 [DOI] [PubMed]
- 4.Cunningham BW, Lewis SJ, Long J, et al (2002) Biomechanical evaluation of lumbosacral reconstruction techniques for spondylolisthesis: an in vitro porcine model. Spine 27:2321–2327 [DOI] [PubMed]
- 5.Cunningham BW, Lowery GL, Serhan HA, et al (2002) Total disc replacement arthroplasty using the AcroFlex lumbar disc: a non-human primate model. Eur Spine J 11(Suppl 2):S115–S123 [DOI] [PMC free article] [PubMed]
- 6.Dmitriev AE, Cunningham BW, Hu N, et al (2005) Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine 30:1165–1172 [DOI] [PubMed]
- 7.Erulkar JS, Grauer JN, Patel TC, Panjabi MM (2001) Flexibility analysis of posterolateral fusions in a New Zealand white rabbit model. Spine 26:1125–1130 [DOI] [PubMed]
- 8.Goel V, Grauer J, Patel T, et al (2005) Effects of charite artificial disc on the implanted and adjacent spinal segment mechanics using a hybrid testing protocol. Spine 30:2755–2764 [DOI] [PubMed]
- 9.Hilibrand AS, Robbins M (2004) Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 4:190S–194S [DOI] [PubMed]
- 10.Kotani Y, Cunningham BW, Abumi K, et al (2005) Multidirectional flexibility analysis of cervical artificial disc reconstruction: in vitro human cadaveric spine model. J Neurosurg Spine 2:188–194 [DOI] [PubMed]
- 11.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L (2004) Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 29:79–86 [DOI] [PubMed]
- 12.Nibu K, Panjabi MM, Oxland T, Cholewicki J (1997) Multidirectional stabilizing potential of BAK interbody spinal fusion system for anterior surgery. J Spinal Disord 10:357–362 [DOI] [PubMed]
- 13.Panjabi MM (1988) Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine 13:1129–1134 [DOI] [PubMed]
- 14.Panjabi MM (2007) Hybrid multidirectional test method to evaluate spinal adjacent-level effects. Clin Biomech 22(3):257–265 [DOI] [PubMed]
- 15.Panjabi MM, Malcolmson G, Teng E, Tominaga Y, Henderson G (2006) Hybrid adjacent-level testing of lumbar Charite discs versus fusions. Spine 32:959–966 [DOI] [PubMed]
- 16.Patwardhan AG, Havey RM, Carandang G, et al (2003) Effect of compressive follower preload on the flexion-extension response of the human lumbar spine. J Orthop Res 21:540–546 [DOI] [PubMed]
- 17.Schlegel JD, Smith JA, Schleusener RL (1996) Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine 21:970–981 [DOI] [PubMed]
- 18.Yoshimoto H, Ito M, Abumi K, et al (2004) A retrospective radiographic analysis of subaxial sagittal alignment after posterior C1-C2 fusion. Spine 29:175–181 [DOI] [PubMed]