Abstract
The aim of this study was to evaluate the efficacy in adult rat completely transected spinal cord of adenovirus vector-mediated brain-derived neurotrophic factor (BDNF) ex vivo gene transfer to bone marrow stromal cells (BMSC). BMSC were infected with adenovirus vectors carrying β-galactosidase (AxCALacZ) or BDNF (AxCABDNF) genes. The T8 segment of spinal cord was removed and replaced by graft containing Matrigel alone (MG group) or Matrigel and BMSC infected by AxCALacZ (BMSC-LacZ group) or AxCABDNF (BMSC-BDNF group). Axons in the graft were evaluated by immunohistochemistry and functional recovery was assessed with BBB locomotor scale. In the BMSC-BDNF group, the number of fibers positive for growth associated protein-43, tyrosine hydroxylase, and calcitonin gene-related peptide was significantly larger than numbers found for the MG and BMSC-LacZ groups. Rats from BMSC-BDNF and BMSC-LacZ groups showed significant recovery of hind limb function compared with MG rats; however, there was no significant difference between groups in degree of functional recovery. These findings demonstrate that adenovirus vector-mediated ex vivo gene transfer of BDNF enhances the capacity of BMSC to promote axonal regeneration in this completely transected spinal cord model; however, BDNF failed to enhance hind limb functional recovery. Further investigation is needed to establish an optimal combination of cell therapy and neurotrophin gene transfer for cases of spinal cord injury.
Keywords: Bone marrow stromal cell, Brain-derived neurotrophic factor, Adenovirus, Spinal cord injury, Gene therapy
Introduction
It has been widely believed that axons in transected adult mammalian spinal cords cannot regenerate. This failure of regeneration is ascribed to the non-permissive environment in the damaged adult mammalian spinal cord, an environment marked by inhibitory molecules in scar tissue and myelin components that block axonal regeneration, a lack of trophic support for axotomized neurons, and post-axotomy intrinsic neuronal changes including cell atrophy and death [1]. Cell transplantation therapy has been attempted to overcome these problems and achieve restoration of damaged spinal cord [2]. Among various types of candidate cells, the bone marrow stromal cell (BMSC) is promising because it has shown potential to repair damaged spinal cord [3–7]. Moreover, BMSC can be easily obtained from adult bone marrow and transplanted autologously, avoiding the immunological and ethical problems that are associated with transplantation of other types of stem cells such as embryonic stem cells [8] or neural stem cells [9].
Neurotrophic factors are keys in modulation of neuronal survival, neurite outgrowth, synaptic plasticity and neurotransmission. Exogenous administration of neurotrophic factors has been proposed as one potential therapeutic treatment for spinal cord injury. Among various neurotrophic factors, we and others have reported the effectiveness of brain-derived neurotrophic factor (BDNF) for spinal cord injury in adult rats. Exogenous administration of BDNF as a protein improves locomotor function after spinal cord injury [10, 11]. We have previously demonstrated that adenovirus vector-mediated in vivo gene transfer of BDNF in completely transected adult rat spinal cord is followed by regeneration of the descending tracts and partial functional recovery [12]. Thus, BDNF may have potential to enhance the regeneration that is promoted by cell transplantation therapy. In fact, retrovirus vector-mediated ex vivo gene transfer of BDNF to fibroblasts [13, 14] or BMSC [15] promotes axonal regeneration and functional recovery, and adenovirus vector-mediated ex vivo BDNF gene transfer enhances the regeneration promoting capacity of olfactory ensheathing glial cells [16].
In the current research, we tested the efficacy of adenovirus-mediated ex vivo BDNF gene therapy to BMSC in completely transected adult rat spinal cord as a candidate for combined therapy involving cell transplantation and gene transfer.
Materials and methods
Primary bone marrow stromal cell (BMSC) cultures
BMSC were collected from femurs of adult male Wistar rats (SLC, Hamamatsu, Japan). Rats were euthanized with pentobarbital overdose, and femurs were removed and placed in cold minimum essential medium with alpha modification (α-MEM, Sigma, St Louis, MO) supplemented with 20% fetal bovine serum (FBS), 2 mM l-glutamine (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 ng/ml amphotericin B (Invitrogen, Carlsbad, CA). After removal of femoral epiphyses, marrow was flushed out with medium using a 25G needle attached to a syringe. Bone marrow cells were cultured with the same medium in a humidified 5% CO2 atmosphere at 37°C under sterile conditions. Twenty-four hours after initial plating, non-adherent cells were removed by replacement of the medium. After the cells had grown to near confluency, they underwent two to five passages by detachment (0.25% trypsin/1 mM EDTA for 5 min), splitting into 1:3–4 dilution, and replating. At the sixth passage, the cells were transferred to Dulbecco’s modified Eagle’s medium (DMEM; Sigma) supplemented with 20% FBS without additional supplementation and were maintained beyond a total of 20 passages [17].
Preparation of the adenovirus vectors
We prepared first-generation, replication-deficient recombinant adenovirus vectors in which the E1 and E3 regions were deleted, encoding either Escherichia coli β-galactosidase (LacZ; AxCALacZ) or mouse BDNF (AxCABDNF), both under the control of CAG [cytomegalovirus IE enhancer, chicken β-actin promoter, and rabbit β-globin poly (A) signal] promoter using the adenovirus expression vector kit (TaKaRa, Tokyo, Japan) as previously described [12]. We obtained recombinant adenoviruses with a titer of 1011 order plaque-forming units (pfu)/ml after concentration and purification.
Transferred gene expression in vitro
To confirm transferred gene expession in vitro, BMSC were infected with adenovirus vectors. The vector dose was multiplicity of infection (MOI) 100 with AxCABDNF (BMSC-BDNF cells) and AxCALacZ (BMSC-LacZ cells). Twenty-four hours after inoculation, the infected BMSC were subjected to X-gal cytochemistry or conditioned medium collection for western blot analysis.
For X-gal cytochemistry, BMSC-LacZ cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min, and incubated with X-gal reagents [1 mg/ml 5-bromo-4-chloro-3-indryl-d-galactoside, 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 2 mM MgCl2] for 3 h at 37°C.
For western blot analysis, BMSC with or without adenovirus infection were washed three times with cold PBS and the medium was changed to DMEM supplemented with 0.05% FBS. The conditioned medium was collected after 48 h of incubation and frozen until use. BDNF contained in the conditioned medium was detected with western blot analysis as described elsewhere [12]. Recombinant human BDNF (kindly provided by Sumitomo Pharmaceuticals Inc., Osaka, Japan) was used as a positive control.
Spinal cord injury and BMSC transplantation
Tissue samples were obtained from 8-week-old male Wistar rats (average weight 200 g; SLC). Animals were anesthetized with inhaled 1–1.2% halothane in 0.5 l/min oxygen. Laminectomy was performed at the T8 level, leaving the dura intact. The T7 and T9 spinous processes were clamped to fix the spine. The spinal cord was completely transected with microsurgical scissors at the T7/8 and T8/9 levels, and the T8 spinal cord segment was removed. For transplantation, a 5-mm-long tube made from ultrafiltration membrane (NMWL: 10,000, Millipore, Bedford, MA) was filled with Matrigel (Becton-Dickinson Biosciences, Bedford, MA; MG rat group) or the mixture of Matrigel and BMSC-LacZ or Matrigel and BMSC-BDNF (BMSC-LacZ rat group and BMSC-BDNF rat group, respectively; density, 1 × 108 cells/ml in both groups). The grafts were transplanted into the gap between the rostral and caudal stumps. After transplantation, the T7 and T9 spinous processes were tightened with 1–0 silk suture to prevent kyphosis and to obtain contact between the graft and spinal cord stumps. Muscle and skin were sutured layer to layer, and the rats were placed in warm cages overnight. Food and water were given ad libitum. Manual bladder expression was performed twice a day until recovery of bladder reflex. The BMSC-transplanted animals showed no apparent abnormal behavior. All experimental procedures were performed in compliance with guidelines established by the Animal Care and Use Committee of Chiba University.
Tissue preparation
For histological evaluation, animals in all groups were perfused transcardially with 4% paraformaldehyde in PBS (pH 7.4) under deep pentobarbital anesthesia 6 weeks after transplantation. Three segments of spinal cords including the graft (T7–9) were removed and post-fixed in the same fixative overnight, stored in 20% sucrose in PBS at 4°C, and embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan). The cryoprotected samples were frozen and kept at −80°C until use. The samples were cut into serial 20-μm sagittal sections with a cryostat and mounted onto poly-l-lysine-coated slides (Matsunami, Tokyo, Japan).
Immunohistochemistry
To evaluate regenerating axons within the graft, we performed immunohistochemistry as previously described [18]. The following primary antibodies were used: anti-growth-associated protein-43 rabbit polyclonal antibody (GAP-43; 1:400; Santa-Cruz) as a marker for regenerating nerve fibers, anti-tyrosine hydroxylase monoclonal antibody (TH; 1:400; Chemicon Int.) as a marker for coeluro-spinal axons, anti-serotonin rabbit polyclonal antibody (5-HT; 1:5,000; Sigma) as a marker for raphe-spinal axons, and anti-calcitonin gene-related peptide rabbit polyclonal antibody (CGRP, 1:1,000; AFFINITI, Exeter, UK) as a marker for sensory afferent axons. Following reaction with primary antibodies, slides were incubated with biotinylated secondary antibodies (HISTOFINE kit, Nichirei, Tokyo, Japan). Then the slides underwent reaction with peroxidase-conjugated streptavidin (Nichirei). The color was developed with diaminobenzidine (DAB) and H2O2.
Every fourth section was reacted with each of the antibodies described above. At least three series were stained with each of the four antibodies, resulting in immunohistochemical examination of a tissue block 240 μm in width, approximately half the width of the graft. For each antibody, immuno-positive fibers that traversed lines perpendicular to the central axis of the graft at the rostral (500 μm caudal to the rostral graft-cord interface), central (center of the graft), and caudal (500 μm rostral to the caudal graft-cord interface) levels within the graft were counted and compared with values for other rats in the MG, BMSC-LacZ, and BMSC-BDNF groups. Statistical analysis between groups was performed with the Mann–Whitney U test.
Next, we performed immunofluorescent double staining to observe the relationship between regenerating fibers and grafted cells in the rats of the BMSC-BDNF group. Anti-fibronectin mouse monoclonal antibody (Chemicon Int.) was used as a marker for transplanted BMSCs and anti-GAP-43 rabbit polyclonal antibody (Santa-Cruz) was used as a marker for regenerating fibers. To detect a positive signal, each slide was incubated with Alexa 488-conjugated anti-mouse IgG and Alexa 594-conjugated anti-rabbit IgG (Molecular Probes, Eugene, OR).
Assessment of locomotor activity
Hind limb function of rats in the MG, BMSC-LacZ, and BMSC-BDNF groups was assessed with the BBB locomotor scale [19] before injury and at 1, 3 days, and 1–6 weeks (once a week) after injury. Statistical analysis of differences between groups was performed with repeated measure analysis of variance (ANOVA). To evaluate whether regenerated nerve fibers contributed to hind limb functional recovery, two rats in the BMSC-BDNF group received re-transection of the graft 6 weeks after transplantation.
Results
Transferred gene expression in vitro
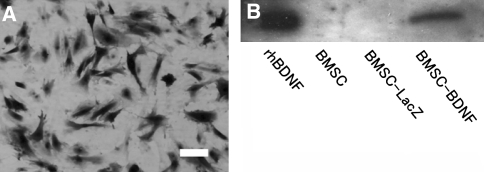
Firstly, we confirmed the infection efficacy of adenovirus vector to BMSC in vitro with X-gal cytochemistry (Fig. 1a). Approximately 80% of the AxCALacZ-infected BMSC were positive for X-gal staining, indicating the high infection efficacy of adenovirus vector to BMSC in this study.
Fig. 1.
X-gal histochemistry of AxCALacZ-infected BMSC. a AxCALacZ was infected to BMSC at multiplicity of infection (MOI) 100. Twenty-four hours after infection, cells were incubated with X-gal reagent at 37°C for 3 h; 80% of AxCALacZ-infected BMSC was positive for X-gal staining, indicating high infection efficacy. b Western blot analysis of BDNF produced by adenovirus AxCABDNF. Bands of BDNF were detected in conditioned medium taken from plates with AxCABDNF-infected BMSC, but not in medium from normal and AxCALacZ-infected BMSC. Recombinant human BDNF (rhBDNF) was used as a positive control. Bar 30 μm
Next, we examined levels of transferred gene expression of BDNF in vitro with western blot analysis (Fig. 1b). The conditioned medium from BMSC-BDNF cells contained a high level of BDNF, whereas there was no detectable level of BDNF in medium from plates with either uninfected BMSC or BMSC-LacZ cells.
Immunohistochemistry
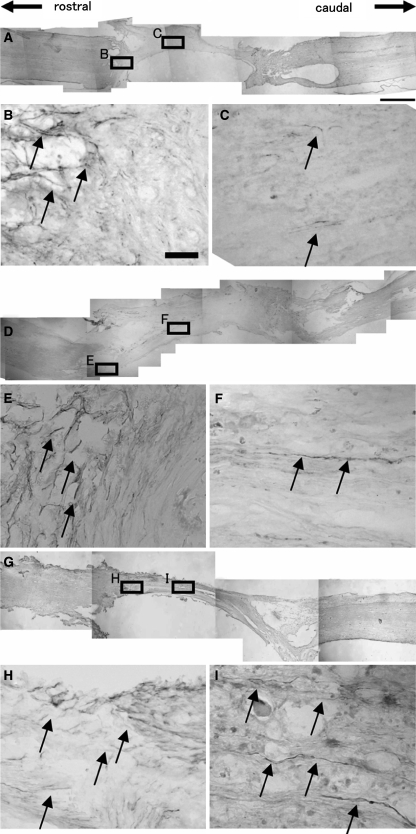
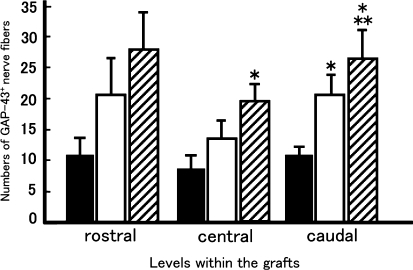
We performed immunohistochemistry for GAP-43 to detect regenerating axons within the graft. Because the T8 spinal cord segment was removed and replaced by the graft, all GAP-43-positive nerve fibers within the graft could be considered regenerated axons. Numerous GAP-43-positive regenerating nerve fibers were detected within the grafts of both the BMSC-LacZ (Fig. 2d–f) and BMSC-BDNF group rats (Fig. 2g–i), whereas few GAP-43-positive fibers were detected within the grafts of the MG group (Fig. 2a–c). A large proportion of these axons were oriented longitudinally within the graft (Fig. 2f, i). The number of GAP-43-positive fibers was significantly larger at the central and caudal levels in rats of the BMSC-transplanted groups (both BMSC-BDNF and BMSC-LacZ groups) than in MG rats (P < 0.05, Fig. 3). Moreover, the number of GAP-43-positive fibers in the BMSC-BDNF group was significantly larger than that in both the BMSC-LacZ and MG groups at the caudal level (P < 0.05, Fig. 3).
Fig. 2.
Immunohistochemistry for GAP-43 in the MG group (a overview; b, c magnified), BMSC-LacZ group (d overview; e, f magnified), and BMSC-BDNF group (g overview; h, i magnified). b In the MG group, numerous GAP-43-positive fibers were detected at the interface between host spinal cord and graft (arrows). c However, few GAP-43-positive regenerating fibers were detected within the graft (arrows). In contrast, a number of GAP-43-positive regenerating fibers were found at the middle of grafts in the f BMSC-LacZ and i BMSC-BDNF groups (arrows) as well as at the interface between host spinal cord and graft (e BMSC-LacZ, h BMSC-BDNF, arrows). Bars 1 mm (a, d, g) and 30 μm (b, c, e, f, h, i)
Fig. 3.
Numbers of GAP-43-positive axons within the grafts. GAP-43-positive fibers that traversed lines perpendicular to the central axis of the grafts at rostral (500 μm caudal to the rostral graft-cord interface), central (center of the graft), and caudal (500 μm rostral to the caudal graft-cord interface) levels within each graft were counted and compared among the MG, BMSC-LacZ, and BMSC-BDNF groups. Statistical analysis of differences between groups was performed with the Mann–Whitney U test. At the central level in the graft, the number of GAP-43-positive axons in the BMSC-BDNF group (hatched column) was significantly larger than that of the MG group (closed column, P < 0.05). At the caudal level in the graft, the number of GAP-43-positive axons in the BMSC-BDNF group was significantly larger than that of the MG and BMSC-LacZ groups (P < 0.05), and the number of GAP-43-positive axons in the BMSC-LacZ group was significantly larger than that in the MG group (P < 0.05). Each value is a mean ± standard error of the mean (SEM). * Significant difference compared with the MG group (P < 0.05), ** significant difference compared with the BMSC-LacZ group (P < 0.05)
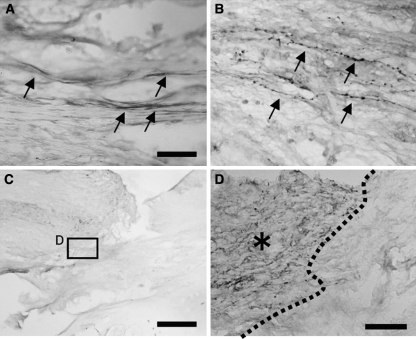
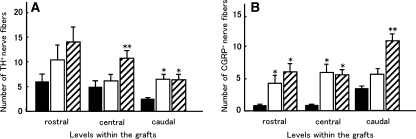
Next we performed immunohistochemistry for nerve fiber markers to elucidate the subpopulation of regenerating axons. TH-positive nerve fibers are considered as coerulo-spinal adrenergic nerve fibers and 5-HT-positive nerve fibers are considered as raphe-spinal serotonergic fibers, both of which contribute to motor function [20]; CGRP is a marker for sensory nerve fibers. Numerous TH-positive nerve fibers were detected within the grafts of BMSC-BDNF rats (Fig. 4a), whereas only a few TH-positive nerve fibers were observed in grafts of MG rats (not shown). The number of TH-positive nerve fibers in the BMSC-BDNF group was significantly larger than numbers in the BMSC-LacZ and MG groups at the central level (P < 0.05, Fig. 5a). The number of TH-positive nerve fibers in both the BMSC-BDNF and BMSC-LacZ groups was significantly larger than that in the MG group at the caudal level (P < 0.05, Fig. 5a). Numerous CGRP-positive fibers were detected in the grafts of BMSC-BDNF and BMSC-LacZ rats (Fig. 4b), whereas few CGRP-positive fibers were observed in grafts of MG rats (not shown). The number of CGRP-positive nerve fibers in the BMSC-BDNF group was significantly larger than that in both the BMSC-LacZ and MG groups at the caudal level (P < 0.05, Fig. 5b); the number of CGRP-positive nerve fibers in the BMSC-BDNF and BMSC-LacZ groups was significantly larger than that in the MG group at the rostral and caudal levels (P < 0.05, Fig. 5b). Only a few 5-HT-positive nerve fibers were observed within the grafts of all three groups, although numerous 5-HT-positive nerve fibers were detected at the rostral stumps of host spinal cords (Fig. 4 c, d).
Fig. 4.
Immunohistochemistry for nerve fiber markers in the grafts. a Numerous TH-positive fibers were detected at the middle of the graft in the BMSC-BDNF group (arrows). b Numerous CGRP-positive fibers were detected at the middle of the graft in the BMSC-BDNF group. c, d Only a few 5-HT-positive fibers extended into the graft in BMSC-BDNF rats, although numerous 5-HT-positive fibers were observed at the rostral stump of the host spinalspinal cord [d; higher magnification of box in c; dotted line indicates interface between host spinal cord (asterisk) and graft]. Bars 30 μm (a, b), 500 μm (c), and 50 μm (d)
Fig. 5.
Numbers of tyrosine hydroxylase-positive and calcitonin gene-related peptide-positive (CGRP-positive) fibers in the grafts. a The number of TH-positive axons in the BMSC-BDNF group (hatched column) was significantly larger than numbers in the MG (closed column) and BMSC-LacZ (open column) groups (P < 0.05) at the central level in the graft. At the caudal level, the numbers of TH-positive axons in the BMSC-BDNF (hatched column) and BMSC-LacZ (open column) groups were significantly larger than that in the MG group (closed column, P < 0.05). b The numbers of CGRP-positive axons in the BMSC-LacZ (open column) and BMSC-BDNF (hatched column) groups were significantly larger than that in the MG group (closed column) at the rostral and central levels (P < 0.05). At the caudal level, the number of CGRP-positive axons in the BMSC-BDNF group (hatched column) was significantly larger than those in the MG (closed column) and BMSC-LacZ (open column) groups (P < 0.05). * Significant difference compared with the MG group (P < 0.05), ** significant difference compared with the BMSC-LacZ group (P < 0.05)
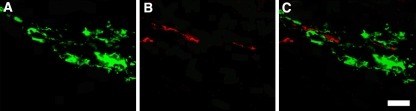
Immunofluorescent double staining for fibronectin (one of markers for BMSC) and GAP-43 (a marker for regenerating or sprouting axons) revealed close contact between GAP-43-positive fibers and grafted cells in the BMSC-BDNF group (Fig. 6a–c). In contrast, there were no fibronectin-positive cells in grafts of MG rats (not shown).
Fig. 6.
Immunofluorescent double staining for fibronectin and GAP-43 in BMSC-BDNF rats. The slides were incubated with anti-fibronectin and anti-GAP-43 antibodies, followed by incubation with Alexa 488-conjugated (fibronectin; green) and Alexa 594-conjugated (GAP-43; red) secondary antibodies. a Fibronectin-positive transplanted BMSCs were longitudinally oriented within the graft. b In addition, some of the GAP-43-positive regenerating fibers were longitudinally oriented within the graft. c Merged view shows the close relationship between transplanted BMSCs and regenerating fibers. Bar 30 μm
Recovery of hind limb function
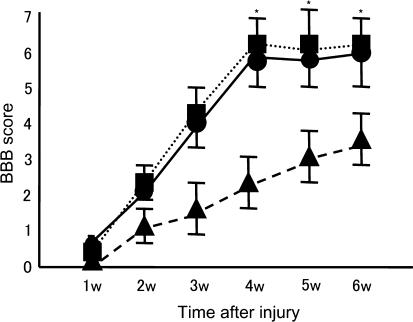
We assessed recovery of hind limb function with the BBB locomotor scale (Fig. 7). Both the BMSC-LacZ and BMSC-BDNF groups showed significant recovery compared with the MG group from 4 weeks after injury onward (Fig. 7, P < 0.03). The average recovery score 6 weeks after transplantation was 6.1 in the BMSC-BDNF group (range 4–8; Fig. 6, circle) and 6.0 in the BMSC-LacZ group (range 4–8; Fig. 7, square), which indicates that two joints of the hind limbs had extensive movement and the third joint had slight movement, whereas the average score in the MG group was 3.6 (range 2–5; Fig. 7, triangle), which indicates that two joints of the hind limbs had extensive movement. There was no significant difference between the BMSC-BDNF and the BMSC-LacZ groups at any time point, indicating that BDNF gene transfer to BMSC had no significant additional effect on the functional recovery promoted by transplantation of BMSC.
Fig. 7.
Hind limb functional assessment with the BBB locomotor scale. Rats from the BMSC-BDNF group (square) and BMSC-LacZ group (circle) showed significant recovery from 4 weeks after injury onward compared with rats from the MG group (triangle). There was no significant difference between the recovery score of the BMSC-BDNF and BMSC-LacZ groups at any time point. Values represent mean ± SEM. * P < 0.03 compared with the MG group. SEM standard error of the mean
Two rats in the BMSC-BDNF group that showed functional recovery received re-transection of the graft 6 weeks after transplantation. Re-transection completely abolished the recovered function, and only a slight functional recovery was observed after re-transection (1–2 points in BBB score).
Discussion
In the present study, we demonstrated that transplantation of BMSC promoted axonal regeneration in completely transected adult rat spinal cord and that ex vivo BDNF gene transfer to BMSC enhanced axonal regeneration or sprouting.
Although the potential of BMSC to trans-differentiate into neural phenotypes is controversial [17, 21], BMSC have been raised as a candidate for cell transplantation therapy with central nervous system diseases because they have a potential to restore damaged neural tissue and they can be transplanted autologously and harvested easily [22]. In fact, some reports indicate the effectiveness of BMSC transplantation for spinal cord injury. Transplantation of BMSC significantly improves hind limb function in the rat spinal cord injury model [3]. BMSC form guiding strands in the injured spinal cord and promote hind limb functional recovery; in addition, transplanted BMSC differentiate toward neural lineage cells in vivo [4]. Finally, BMSC promote regeneration of injured spinal cord [6]. In contrast to previous reports that used contusion- or compression-induced spinal cord injury models, we used a segmental resection and replacement model to make clear the contribution of axonal regeneration to functional recovery. In the present study, the two BMSC-transplanted groups (BMSC-LacZ and BMSC-BDNF) showed significant axonal regeneration and hind limb functional recovery compared with rats in the MG group, a finding that sheds light on the axonal regeneration-inducing properties of BMSC. Immunofluorescent double staining revealed a close relationship between transplanted cells and regenerating axons, suggesting that transplanted BMSC may act as an attractive cue for regenerating axons. Taken together, previous reports and the present results suggest that BMSC have great potential to repair damaged spinal cord.
It is well known that specific neurotrophic factors elicit regrowth of specific axonal populations after spinal cord injury [23]. BDNF promotes regeneration of CGRP-positive sensory fibers, 5-HT-positive serotonergic fibers, TH-positive coerulospinal fibers, as well as reticulospinal and rubrospinal tracts in various experimental paradigms [23]. It has been reported that transplantation of BMSC alone [4] or BMSC engineered to express BDNF by retrovirus vector [15] promote regrowth of 5HT-positive serotonergic axons. In the present study, adenovirus vector-mediated BDNF gene transfer to BMSC enhanced axonal regeneration of TH-positive coerulospinal fibers at the central level and CGRP-positive sensory fibers at the caudal level of grafts; however, BDNF did not promote regeneration of 5-HT-positive raphespinal fibers. The difference in regenerating axonal phenotype between the present results and previously reported ones may be caused by differences in experimental conditions (i.e., injury models, duration and amount of transferred gene expression, number of transplanted cells and scaffold).
In contrast to previous reports using retrovirus vector-mediated ex vivo gene transfer [13–15], we employed adenovirus vector to transfer therapeutic gene ex vivo in the present study, as Ruitenberg has reported [16]. The reasons are as follows. Firstly, adenovirus vector can infect various types of cells with high efficacy. In contrast, retrovirus vector requires selection with antibiotics, a time-consuming procedure, to obtain high transfection efficacy. Because the clinical therapeutic time window for cell therapy in spinal cord injury is limited to a few weeks after initial trauma [9], it is necessary to obtain transfectant within a relatively short time. Secondly, transient transfection of neurotrophic factor with adenovirus vector is sufficient to promote axonal regeneration, something we have previously reported [12]. It is not always necessary to transplant cells that have stably integrated the transferred gene to promote axonal regeneration.
In the present study, ex vivo BDNF gene transfer to BMSC failed to enhance hind limb functional recovery although it did significantly enhance axonal regeneration. Possible explanations for this discrepancy could be as followings; the observation period could be too short to detect significant functional recovery caused by axonal regeneration, greater numbers of regenerating axons are necessary to enhance hind limb functional recovery or regenerating axons cannot grow into the caudal spinal cord. We could not directly prove re-entrance of regenerating axons into the distal cord stump. However, re-transection of the graft in BMSC-BDNF rats completely abolished recovered hind limb function, suggesting that regenerating axons contributed to functional recovery to some extent.
In conclusion, ex vivo BDNF gene therapy to BMSC is a candidate for combined cell-neurotrophic factor therapy, although further investigation is needed to find an optimal condition.
Acknowledgments
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (16390427-00).
References
- 1.Profyris C, Cheema SS, Zang DW, Azari MF, Doyle K, Petratos S (2004) Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15:415–436 [DOI] [PubMed]
- 2.Murray M (2004) Cellular transplantation: steps toward restoration of function in spinal injured animals. Prog Brain Res 143:133–146 [DOI] [PubMed]
- 3.Chopp M, Zhang XH, Li Y et al (2000) Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 11:3001–3005 [DOI] [PubMed]
- 4.Hofstetter CP, Schwarz EJ, Hess D et al (2002) Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 19:2199–2204 [DOI] [PMC free article] [PubMed]
- 5.Lee JB, Kuroda S, Shichinohe H et al (2003) Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology 23:169–180 [DOI] [PubMed]
- 6.Wu S, Suzuki Y, Ejiri Y et al (2003) Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res 72:343–351 [DOI] [PubMed]
- 7.Koda M, Okada S, Nakayama T et al (2005) Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport 16:1763–1767 [DOI] [PubMed]
- 8.McDonald JW, Liu XZ, Qu Y et al (1999) Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5:1410–1412 [DOI] [PubMed]
- 9.Ogawa Y, Sawamoto K, Miyata T et al (2002) Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 69:925–933 [DOI] [PubMed]
- 10.Ikeda O, Murakami M, Ino H et al (2002) Effects of brain-derived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of superoxide dismutase expression and promotes up-regulation of myelin basic protein expression. J Neuropathol Exp Neurol 61:142–153 [DOI] [PubMed]
- 11.Jakeman LB, Wei P, Guan Z, Stokes BT (1998) Brain-derived neurotrophic factor stimulates hind limb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol 154:170–182 [DOI] [PubMed]
- 12.Koda M, Hashimoto M, Murakami M et al (2004) Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J Neurotrauma 21:329–337 [DOI] [PubMed]
- 13.Jin Y, Fischer I, Tessler A, Houle JD (2002) Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol 177:265–275 [DOI] [PubMed]
- 14.Liu Y, Kim D, Himes BT et al (1999) Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci 19:4370–4387 [DOI] [PMC free article] [PubMed]
- 15.Lu P, Jones LL, Tuszynski MH (2005) BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol 191:344–360 [DOI] [PubMed]
- 16.Ruitenberg MJ, Plant GW, Hamers FP et al (2003) Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci 23:7045–7058 [DOI] [PMC free article] [PubMed]
- 17.Woodbury DS, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364–370 [DOI] [PubMed]
- 18.Hashimoto M, Koda M, Ino H et al (2003) Upregulation of osteopontin expression in rat spinal cord microglia after traumatic injury. J Neurotrauma 20:287–296 [DOI] [PubMed]
- 19.Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21 [DOI] [PubMed]
- 20.Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D (1993) Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol 123:3–16 [DOI] [PubMed]
- 21.Neuhuber B, Gallo G, Howard L, Kosture L, Mackay A, Fscher I (2004) Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res 77:192–204 [DOI] [PubMed]
- 22.Chopp M, Li Y (2002) Treatment of neural injury with marrow stromal cells. Lancet Neurol 1:92–100 [DOI] [PubMed]
- 23.Jones LL, Oudega M, Bunge MB, Tuszynski MH (2002) Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J Physiol 533:83–89 [DOI] [PMC free article] [PubMed]