Abstract
This study evaluated spatial and temporal extracellular matrix changes, induced by controlled surgical defects in the outer third of the annulus fibrosus (AF) of ovine intervertebral discs (IVDs). Thirty-two 4 year old sheep received a 4 mm deep × 10 mm wide standard annular surgical incision in the L1L2 and L3L4 IVDs (lesion group), 32 sheep were also subjected to the same surgical approach but the AF was not incised (sham-operated controls). Remodeling of the IVD matrix in the lesion and sham discs was assessed histochemically at 3, 6,12 and 26 month post operation (PO). Discs were also dissected into annular lesion site and contra-lateral AF and NP and equivalent zones in the sham sheep group, extracted with GuHCl, dialysed, freeze dried, digested with chondroitinase ABC/keratanase-I and aliquots examined for small leucine repeat proteoglycan (SLRP) core protein species by Western blotting using C-terminal antibodies to decorin, biglycan, lumican and fibromodulin and monoclonal antibody (Mab) 2B6 to unsaturated stub epitopes on chondroitin-4-sulphate generated by chondroitinase ABC. Masson Trichrome and Picrosirius red staining demonstrated re-organisation of the outermost collagenous lamellae in the incised discs 3–6 month PO. Toluidine blue staining also demonstrated a focal loss of anionic proteoglycan (PG) from the annular lesion 3–6 month PO with partial recovery of PG levels by 26 month. Specific fragments of biglycan and fibromodulin were associated with remodeling of the AF 12–26 month PO in the lesion IVDs but were absent from the NP of the lesion discs or all tissue zones in the sham animal group. Fragments of decorin were also observed in lesion zone extracts from 3 to 6 months but diminished after this. Isolation and characterization of the biglycan/fibromodulin fragments may identify them as prospective biomarkers of annular remodeling and characterization of the enzyme systems responsible for their generation may identify therapeutic target molecules.
Keywords: IVD, Annular remodelling, Experimental disc degeneration, SLRP fragmentation
Introduction
The small leucine rich repeat family of proteoglycans (SLRPs), decorin, biglycan, fibromodulin and lumican are all intervertebral disc (IVD) components [9, 23, 58]. The core proteins of the SLRPs are characterised by a series of central leucine-rich repeat domains and C-terminal disulphide-bonded domains. The glycosaminoglycan (GAG) side chains on the SLRPs are composed of dermatan sulphate (DS) or chondroitin sulphate (CS) in the case of decorin and biglycan and keratan sulphate (KS) in fibromodulin and lumican. Both the GAG and core protein of the SLRPs are interactive with extracellular matrix (ECM) components [47, 48]. The SLRPs have diverse functions in musculoskeletal tissues as modulators of tissue organisation, cellular proliferation and matrix adhesion and influence cellular responses to growth factors and cytokines [10]. Direct evidence for the importance of the SLRPs in musculoskeletal tissues has been demonstrated using single and double knockout mice models [1, 2, 7]. Non-glycanated forms of decorin and biglycan have also been identified in IVD [23].
The IVD undergoes profound cellular and matrix changes with ageing, and degenerates earlier than other weight-bearing cartilaginous tissues [5]. Systematic cadaveric studies have shown that discs of older spines exhibit a range of pathologies including injuries to the AF. These annular defects are in addition to the vertebral “rim lesion” described by Hilton and Ball [21] which arise from discontinuities in the vertebral bony attachment to the AF. These annular lesions are invariably associated with degenerative changes in the NP and it has been questioned whether AF lesions lead to NP degeneration or vice-versa [33–35, 43, 60]. In contrast, the concentric (circumferential) tear is seen as a separation of the annular lamellae resulting from the propagation of clefts initiating from within the NP and is considered to be an age-related degenerative phenomenon [4, 15, 42]. Despite vascular in-growth around annular tears [36], evidence from human post-mortem studies indicate that these show a very limited ability to undergo spontaneous repair.
Artificially created controlled annular defects such as those described in the present study have provided some important insights into the temporal extracellular matrix changes which occur following annular injury. Compositional changes previously noted in the ovine and porcine annular lesion models include an alteration in the amount of, and in the types of collagens synthesised by cells of the lesion site [26], loss of large high-buoyant density aggrecan type proteoglycans (PGs) and an elevation in levels of the small leucine repeat proteoglycans (SLRPs) decorin and biglycan in the injured disc [25, 34, 35]. The SLRPs play critical roles in controlling collagen fibrillogenesis and cross-linking and may protect collagen fibres from enzymatic attack. Altered proteolysis and turnover of the SLRPs may therefore have important consequences for the integrity of fibrous connective tissues such as the AF. In the present investigation we have used histological and Western blotting methods to identify temporal matrix changes and SLRP degradation in the disc degeneration induced by controlled surgical defects of the outer AF. These studies extend our previous biochemical investigations on matrix changes following disc injury using the ovine model and implicate specific SLRP proteolytic events in the degenerative process [33–35].
Materials and methods
Materials
C-terminal affinity purified rabbit polyclonal antibodies to decorin (PR-84), biglycan (PR-85) lumican (PR-353) and fibromodulin (PR-184) [38, 65] and a commercial monoclonal antibody (Mab 2-B-6, Seikegaku Corporation, Japan) to the unsaturated stub epitopes generated by the action of chondroitinase ABC on chondroitin-4-sulphate side chains were used in this study [6, 8]. Chondroitinase ABC (Proteus vulgaris) and keratanase-I (Pseudomonas sp) were Sigma-Aldrich products, Castle Hill, NSW, Australia. Alkaline phosphatase conjugated goat anti-rabbit or mouse IgG and NBT/BCIP blotting development kits were purchased from Biorad, Regents Park, NSW, Australia. All electrophoresis and Western blotting consumables were purchased from Invitrogen, Mount Waverley, VIC, Australia.
The ovine annular lesion model of experimental disc degeneration
Pure-bred merino wethers aged 4 years at the inception of the study as assessed by their dentition were used. All experimental, pain relief, analgesia, antibiotic prophylaxis protocols and animal maintenance procedures were approved by the Animal Ethics Research Committee of The University of Sydney. The animals (n = 64) were randomly divided into two groups. The lesion group of animals received a controlled 4 mm deep × 10 mm wide surgical lesion in the left antero-lateral annulus of the L1L2 and L3L4 IVDs using a modified customised scalpel with a cuff which permitted a maximum blade penetration depth into the AF of 4 mm (Fig. 1). The lesion was parallel, and adjacent to, the inferior CEP of the superior vertebral body located close to the vertebral body rim. The sham operated control sheep were subjected to the same retro-peritoneal surgical approach but the AF was not incised. Animals were sacrificed at 3, 6, 12 and 26 month. PO by intravenous overdose of pentobarbitone (Euthal, Delta Veterinary laboratories, NSW, Australia). Lumbar (T14L1 to L4L5) spinal segments were removed within 30 min of death and individual IVDs isolated by a medial cut through adjacent superior and inferior vertebral bodies using a bone saw.
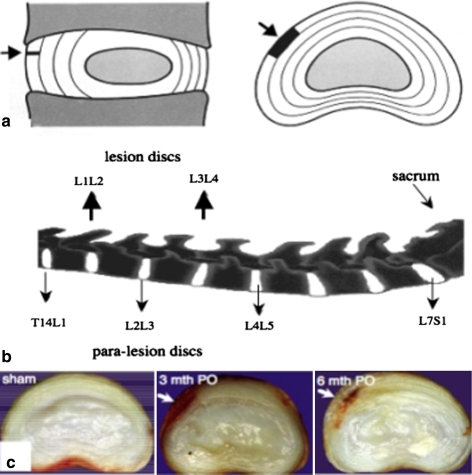
Fig. 1.
a Diagrammatic representation in sagittal and transverse horizontal section of the anatomical site and extent of the anterolateral annular lesion (arrow, dark area). b The L1L2 and L3L4 discs received the lesion depicted in segment a. Para-lesion discs (T14L1, L2L3 and L4L5) were also processed for histology. c Transverse horizontal sections of sham control and lesion discs 3 and 6 month PO depicting the extent of the well defined experimental anterolateral lesion (arrow)
Histology
Intact IVDs were fixed en-bloc in 10% neutral buffered formalin for 1 week then decalcified in several changes of 10% formic acid in 5% neutral buffered formalin for 2 weeks with constant agitation. Vertical sagittal slabs (3–4 mm) of the vertebral body-IVD specimens were dehydrated in graded alcohols and double embedded in paraffin-celloidin, 5 μm tissue sections were cut using a rotary microtome, and mounted on star frost Plus glass slides. These were dried at 75°C for 10 min followed by 55°C in an oven overnight. Following de-paraffinisation in xylene (4 changes × 2 min), and re-hydration through graded ethanol washes (100–70% v/v) to water, the tissue sections were stained with Harris’ haematoxylin and eosin, Masson’s-Trichrome [30], toluidine blue/fast green [12] or picro-sirius red [56], the latter stain was examined by polarised light microscopy, the others by bright field microscopy. The Masson-Trichrome method used [30] was a combination of the method of Masson [30] as modified by Bancroft and Cook [3]. Tissue sections were initially incubated in Bouins’ mordant/fixative for 1 h at 60°C, rinsed in running tap water for 10 min, and stained with Weigerts Iron-haematoxylin reagent for 30 min. The slides were then rinsed, differentiated in 1% HCl in 70% ethyl alcohol, and stained in Ponceau-de-xylidene: acid-fuchsin then differentiated in 5% w/v phosphotungstic acid solution until the collagen was decolourised (∼5 min). The sections were counter-stained with 2% w/v Fast Green FCF in 1% Acetic acid (2 min), dehydrated in absolute ethanol, cleared in xylene and mounted in Eukitt mounting medium. The picrosirius red staining method of Sweat et al. [56] was used after initial removal of tissue proteoglycans as advocated by Junquiera et al. [24] by pre-digestion with bovine testicular hyaluronidase (1,000 U/ml) in 0.1 M phosphate buffer pH 5.0 for 2 h at 37°C. The slides were initially stained in Wiegert’s Iron haematoxylin for 30 min, and stained in 0.1% Sirius red F3BA in saturated aqueous picric acid for 2 h at room temp. The sections were not rinsed in water since sirius red is soluble in water but were washed rapidly in several changes of absolute ethanol, cleared in xylene and mounted in Eukitt. Tissue proteoglycans were stained in 0.04% w/v toluidine blue O in 0.1 M sodium acetate buffer pH 4.0 for 10 min and the sections counterstained with 0.1% w/v aqueous fast green FCF for 2 min. The tissue sections were examined independently by three of the chief investigators (JM, CBL, MS), two of them blinded (JM, CBL) and representative sections selected for photographic data presentation.
Extraction of disc tissues
A total of 32 IVDs from the lesion and sham operated sheep IVDs were zonally dissected and the tissues extracted. Tissues were pooled from eight of the IVDs at each time point. The IVDs from the lesion affected and sham operated sheep groups were initially bisected by a mid horizontal incision and the AF dissected into left and right halves (AF zones 1 and 2) and the NP (Fig. 4). The tissues were finely diced and weighed then extracted with 10 vol 4 M GuHCl 100 mM acetate buffer pH 5.8 containing a cocktail of proteinase inhibitors (20 mM EDTA, 100 mM 6-amino hexanoic acid, 25 mM benzamidine) for 48 h at 4°C with constant end-over end stirring. The tissue extracts were recovered by centrifugation, the tissue residues washed and the washings combined with the tissue extracts, these were dialysed against distilled water then freeze dried.
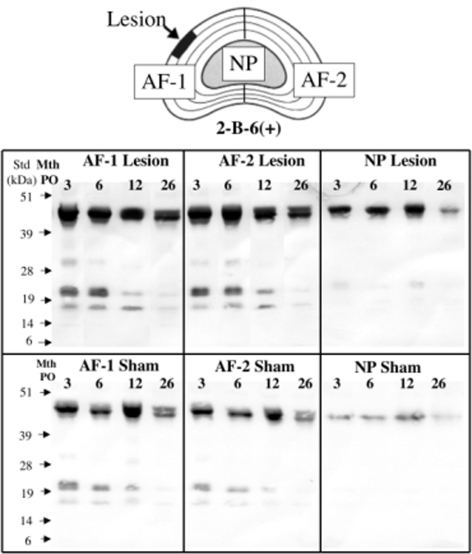
Fig. 4.
Diagrammatic representation of the annular lesion (AF zone 1) and contralateral AF zone (AF zone 2) and NP of a lesion affected disc which were the tissue zones extracted and examined for proteoglycan species. Immunoblot of proteoglycan species containing the unsaturated chondroitinase-4-sulphate stub epitope generated by chondroitinase ABC digestion and detected using Mab 2-B-6 and present in the respective disc tissue extracts. The 45–48 kDa free core proteins of decorin/biglycan are the most prominent bands at the top of each blot, however their levels are significantly lower in the NP extracts compared to AF and also relatively lower in the sham animal group compared to the lesion group
Pre-digestion of tissue extracts with chondroitinase ABC and keratanase-I
The freeze dried, dialysed tissue extracts (from 5 mg wt weight of tissue) were re-dissolved in 0.1 M Tris–acetate buffer pH 6.5 (2 ml), chondroitinase ABC (500 mU/250 μl) and keratanase-1 (50 mU/50 μl) were added and the samples were digested overnight at 37°C then the samples were dialysed and freeze dried.
Electrophoresis and electroblotting
Chondroitinase ABC/keratanase-I digested samples (1 mg) were re-dissolved in 4× LDS PAGE application buffer (50 μl), 10× NuPAGE reducing agent (20 μl) and deionised water (0.13 ml) and heated at 70°C for 10 min, and 10 μl aliquots electropheresed/well in 26 well Xcell4 Midi 4–12% polyacrylamide Bis-Tris slab gels (Novex) in NuPAGE MOPS SDS running buffer containing NuPAGE antioxidant at 200 V constant voltage for 55 min. Selected gels were stained directly with colloidal Coomassie stain, other gels were electroblotted to nitrocellulose membranes using a a Biorad Transblot SD semi-dry transfer cell at 20 V constant voltage for 1 h in NuPAGE transfer buffer containing 10% methanol.
Immunoblotting
The nitrocellulose membranes were blocked overnight in 5% BSA in 50 mM Tris–HCl pH 7.2 containing 150 mM NaCl (TBS). Primary antibodies (1/1,000 dilution) (see Table 1 for details) were diluted in TBS containing 2%BSA and allowed to bind for 1 h. The blots were then briefly rinsed (3 × 1 min) in TBS and alkaline phosphatase conjugated goat anti-rabbit (or anti mouse IgG) 1/5,000 dilution in TBS containing 2% BSA was added. After a further hour the blots were washed with TBS (4 × 10 min). Immune complexes were visualized using an NBT/BCIP visualization kit (Bio-rad). After 20 min colour development the membranes were transferred into distilled water to stop the reaction. Blots were also developed using secondary antibody only to confirm an absence of cross-reactive IgG species which can accumulate in severely degenerate IVD tissues depleted of their space filling aggrecan molecules in which blood vessels can penetrate [36] and serum components gain access [13, 14, 44]. The blots were scanned using a flat bed-scanner at 1,000 dpi and saved as high-resolution jpeg files using Adobe Photoshop software then imported into a Powerpoint worksheet for assembly of the composite blotting figures. SeeBlue-2 pre-stained protein standards were used for molecular weight calibration and as a visual measure of the transfer efficiency of the electroblotting.
Table 1.
Antibodies used for Western blotting to identify SLRP core protein species
| Antibody (clone) | Epitope recognized | References |
|---|---|---|
| C-4-S Δ stub (2-B-6) | Chondroitin-4 sulphate unsaturated stub epitopes generated by chondroitinase ABC | [8] |
| Decorin (PR-84) | -YVRSAIQLGNYK C-terminus of decorin | [38, 65] |
| Biglycan (PR-85) | -TDRLAIQFGNYKK C-terminus of biglycan | [38, 65] |
| Fibromodulin (PR-184) | -LRLASLIEI C-terminus of fibromodulin | [38, 65] |
| Lumican (PR-353) | -LRVANEVTLN C-terminus of lumican | [38, 65] |
Results
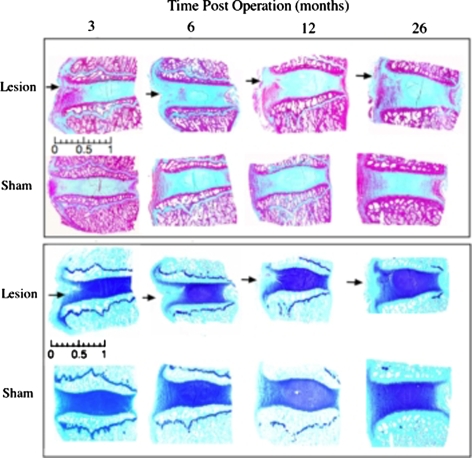
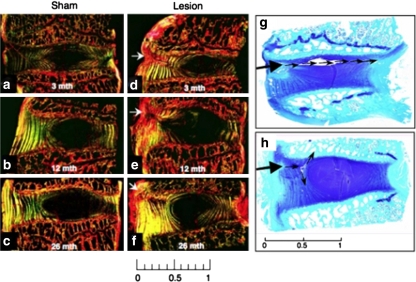
Histology results obtained for the sham operated control discs were similar to those obtained for the para-lesion discs (Fig. 1) of the lesion animal group with no overt degenerative pathology evident in any of the discs examined. Masson-trichrome stained tissue sections for the lesion group discs demonstrated a focal re-organisation of collagenous structure along the plane of the lesion (Fig. 2). At 3 month PO this response was moderate but a progressive loss in collagenous structure was evident up to 12 month PO. Toluidine blue/fast green staining demonstrated a focal loss of PGs from the outer AF in the vicinity of the lesion 3 to 12 month PO with a partial recovery in PG levels by 26 month PO (Fig. 2). Polarised light microscopic examination of picrosirius red stained intervertebral disc tissue sections is a sensitive method for the evaluation of collagen tertiary organisation. Highly ordered native collagen fibres have strong refractile properties in this technique and appear as bright regions in the tissue sections, whereas areas devoid of this type of collagenous organization are non-refractile and appear as dark or black areas. Crimp patterns in collagen fibres are also well delineated using this technique. Picro-sirius red stained tissue sections evaluated by polarised light microscopy confirmed the changes in the collagenous structure suggested by Masson-trichrome staining (Fig. 3a–f). Early re-organisation of the outer AF lamellae 3 month PO was clearly seen and this progressed 6–26 month PO (Fig. 3d–f). This disturbed collagen birefringence in the annular lamellae of the lesion affected discs contrasted with the well organised bright/dark appearance of adjacent annular lamellae observed in the sham operated disc samples. These results were consistent with alternate stressed and relaxed layers of annular lamellae in the control samples and with a disruption in lamellar structure in the lesion samples. The NP in contrast appeared as a dark non-refractile central region indicative of an absence of collagen fibril organisation in this region appropriate for the provision of polarised light refractive properties. In some cases (3/16) the controlled experimental outer annular defects which initially only affected the outer third of the AF developed into radiating tears which extended through the whole depth of the AF to the NP and sometimes even extended past this to the contralateral AF (Fig. 3g). Circumferential tears involving the separation of adjacent annular lamellae (de-lamellation) were also observed in some cases (Fig. 3h). Two of the 16 disc specimens examined at 12 month PO displayed circumferential tears whereas four cases were evident 26 month PO.
Fig. 2.
Sagittal sections of intervertebral discs and adjacent vertebral body segments. Masson Trichrome staining (upper panel) depicts focal changes in the collagenous organisation of the outer AF in lesion affected discs (black arrows). Toluidine blue/fast green staining (lower panel) depicts early focal depletion 3–6 month PO of anionic proteoglycan associated with lesion development and some recovery of AF proteoglycan levels by 26 month PO. The lesion site is depicted by an arrow at the left hand side of each disc section. Scale bar 1 cm
Fig. 3.
Sagittal sections of IVD and adjacent vertebral body segments stained with picro-sirius red and examined under polarised light to visualise changes in collagenous organization (a–f) and with toluidine blue-fast green to depict anionic proteoglycan (g, h). Thickening of the anterior and posterior longitudinal ligaments is evident 26 month PO in a lesion affected disc (f). Arrows indicate the site of the initial annular lesion. In g and h controlled outer annular defects, initially involving only the outer third of the AF (large arrow), have developed into a radiating tear (g) which has propagated through the inner AF and NP to the contralateral AF 12 month PO (small arrows), and a circumferential tear involving separation of adjacent annular lamellae 26 month PO (h). Scale bars 1 cm
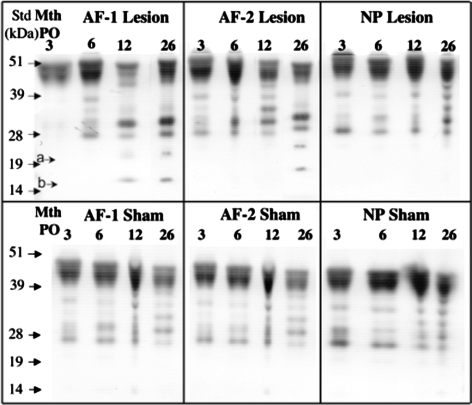
Immunoblotting of extracts of ovine disc tissues (AF zone 1, 2 and NP) was initially conducted using an antibody (Mab 2-B-6) which detects the unsaturated stub epitopes of the chondroitin-4-sulphate side chains which are left on the proteoglycan core proteins after digestion with chondroitinase ABC (Fig. 4). Decorin and biglycan have free core protein sizes of 45–47 kDa thus the number of smaller GAG-peptide fragments (20–25 kDa) as well as 45–48 kDa species detected in the present study likely represent the free core proteins of decorin and biglycan and fragments of these core proteins but they could also have possibly arisen from fragmentation of the aggrecan core protein. While the staining of all 2-B-6 positive bands was more intense in all regions in lesion versus sham discs, there was no change in the overall banding pattern between operated groups. In both sham and lesion discs there were more intense 2-B-6 positive bands in the AF compared with the NP, and more evidence of fragments in the AF. In both lesion and sham discs, the intensity of all 2-B-6 positive bands decreased from 3 to 26 months post operatively. Immunoblotting using a C-terminal decorin Ab identified the intact core protein (∼45 kDa) in both sham and lesion discs with more intense staining in the AF compared with the NP (Fig. 5). The staining intensity of the intact decorin core protein generally increased with age in both the lesion and sham discs. In both lesion and sham discs a second band just below the intact core protein (Fig. 5, band a, 42 kDa) was evident in both AF and NP and increased with age. In lesion but not sham discs, four other faintly stained decorin core-protein species ranging in size from 14 to 35 kDa were identified in the AF but not NP at 3 and 6 months (Fig. 5). There was little difference in abundance of these decorin fragments in the two AF zones of lesion discs, and in both regions the staining intensity diminished with time post surgery. Biglycan core protein (45–48 kDa) was also evident in discs with similar distribution in AF and NP, in lesion and sham discs and with time post operatively. A number of biglycan core protein fragments were present in most of the lesion and sham IVD AF and NP extracts at all time points (Fig. 6). In contrast with decorin however, these fragments increased with time after surgery in the lesion but not sham discs. Two biglycan fragments of 17 and 25 kDa were specifically associated only with the lesion zone annular extracts (AF zone 1 and 2), were not present in the NP samples and increased with time post surgery.
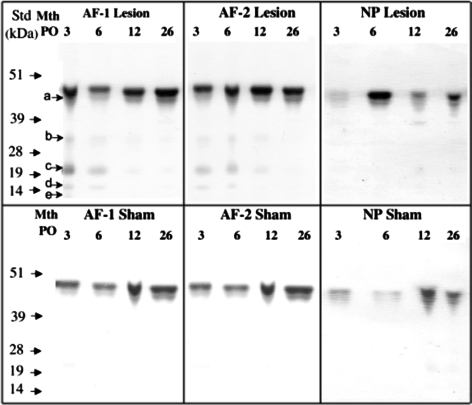
Fig. 5.
Identification of decorin core protein species by Western blotting using the C-terminal anti-decorin Ab PR-84. Tissue extracts were pre-digested with chondroitinase ABC/keratanase-I and electrophoresed at equivalent tissue weights per lane. For an explanation of the tissue zones see the legend to Fig. 4. Intact core protein of 48 kDa and smaller (20–45 kDa) core protein species (a–e) were observed particularly in the lesion affected tissue zones
Fig. 6.
Detection of biglycan core protein species by Western blotting using the C-terminal biglycan antibody PR-85. The intact biglycan core protein of 48–51 kDa was prominently detected, however five to seven smaller core protein species were also detected. Many of these were found in both the sham and lesion affected samples groups. Two species of ∼17 and 25 kDa were observed localized specifically to the lesion affected AF zones only (arrowsa, b)
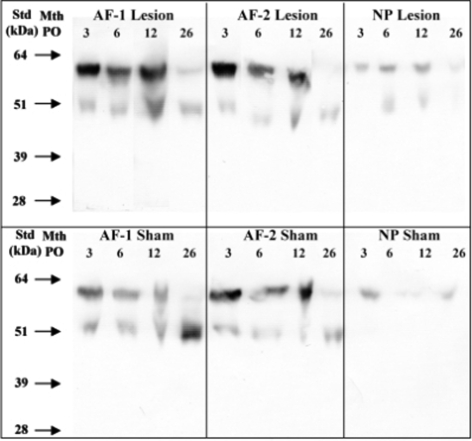
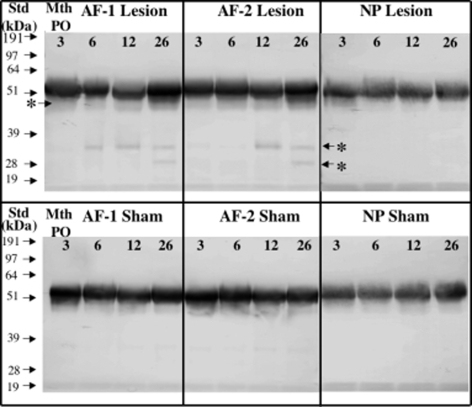
Lumican core protein was detected in both lesion and sham discs, particularly in the AF (Fig. 7). Two predominant bands of 60 and 52 kDa were detected in all zones representing glycosylated and non-glycosylated species respectively [65] with no smaller core protein fragments observed. There was an increase in the proportion of the non-glycosylated lumican species in the AF of both sham and lesion discs with ageing. Fibromodulin was detected in all regions of both sham and lesion discs with somewhat more intense staining in the AF compared with NP (∼54 kDa, Fig. 8). There was little change in intact fibromodulin core protein with ageing in either AF or NP in sham or lesion discs. A 48–50 kDa fibromodulin band was observed in both AF zones of lesion discs and increased in intensity from 6 to 26 months (Fig. 8, asterisk). Furthermore, two smaller fibromodulin fragments of approximately 28 and 35 kDa were specifically associated with annular lesion extract samples 6–26 month PO and their relative abundance increased over this time period (Fig. 8).
Fig. 7.
Detection of lumican core protein species by Western blotting using an anti C-terminal lumican antibody (PR-353). The samples were pre-digested with chondroitinase ABC and keratanase-I, dialysed and freeze dried prior to electrophoresis Samples were loaded at equivalent tissue wet weights per lane
Fig. 8.
Detection of fibromodulin core protein species by Western blotting using an anti C-terminal fibromodulin antibody (PR-184). The samples were pre-digested with chondroitinase ABC and keratanase-I, dialysed and freeze dried prior to electrophoresis Samples were loaded at equivalent tissue wet weights per lane. The fibromodulin free core protein ∼53 kDa was observed in each case however two additional core protein species, (labeled with an asterisk and arrows) were observed in the lesion affected discs (AF zones 1 and 2)
Discussion
The present investigation has confirmed and extended earlier studies with the ovine annular lesion model (Fig. 1–3) and found that in response to a controlled peripheral annular injury, (i) the outer regions of lesions underwent structural re-organisation consistent with an active repair mechanism, (ii) the inner margin of the annular lesions did not undergo repair but were capable of propagating radially and circumferentially to also involve the NP [43], (iii) fragmentation of decorin, biglycan and fibromodulin was also associated with the lesion site. Extracellular matrix remodelling consistent with an attempted repair response was universally seen in all IVDs examined histologically in this study however this response was confined to the outer AF only thus it was an incomplete repair response. Repair did not extend into the inner two-thirds of the AF and the outer annular lesions were observed to propagate along the track of the original defect site as far as the contralateral AF in three out of sixteen animals. In some cases (6/16) the original annular defect which was initially confined to the outer one-third of the AF was also observed to propagate between adjacent annular lamellae leading to delammellation of these structures and these were presumably forerunners of the circumferential defects which have been described by other research groups. In most cases the annular defect propagated into the NP as a radiating defect (7/16 cases). Therefore, while there was a universal attempted repair response in the outermost AF this response was incomplete in the inner AF/NP and the types of defects which propagated from the initial controlled standard outer 4 × 10 mm lesion affecting the outer third of the AF was variable. Presumably this reflects the biological variability inherent in our system but is nevertheless consistent with the types of annular defects which have been described in man.
Picro-sirius red staining of disc/vertebral body sections confirmed that remodeling of vertebral bone and changes in endplate vascularity adjacent to outer annular defects were a feature of this model as noted earlier (Fig. 3d–f) [40]. Localised hyperplasia of the anterior and posterior longitudinal ligaments in response to the introduction of the annular lesion was also observed (Fig. 3e, f) a finding which may relate to the active repair process localised in the outer AF. The lamellae of the outermost AF were also significantly thicker in lesion-affected discs at 26 month PO compared to age-matched sham operated control samples (Fig. 3e, f). These structural changes were visualised particularly well by picro-sirius red staining, examined by polarised light microscopy. This indicated that the collagen fibres in the AF had a high degree of structural organisation and were highly refractile when stained with Picrosirius red and viewed under polarized light. This is presumably related to an active repair response localised to the outer regions of the AF in this experimental model.
Earlier studies from our laboratory using the ovine annular lesion model immunolocalised transforming growth factor-beta (TGF-β) and basic fibroblast growth factor (bFGF) with blood vessel and nerve in-growth around experimental annular lesions [36, 37]. At 3 month PO penetration of granulation tissue and blood vessels was evident along the plane of the original surgical lesion and a large influx of fibroblastic cells was observed along its margins. Cells of a more rounded chondrocytic morphology and a few larger tissue macrophages were also present adjacent to the blood vessels. A large proportion of the cells in the vicinity of the annular lesions undergoing matrix remodelling expressed α-smooth muscle cell actin indicative of a myofibroblastic phenotype [37]. By 6 month PO penetration of blood vessels and the influx of cells into the inner AF was more advanced however by 12 month PO the blood vessels had commenced resorption and the number of associated cells had decreased [36, 37]. This increased cellularity in the mid and inner annular lesion may have resulted from increased proliferation of endogenous disc cell populations in response to TGF-β, and FGF-2 which stimulate cellular proliferation and matrix synthesis [59].
We have previously shown increased synthesis of decorin and biglycan by AF cells in lesion discs [35]. The present study has for the first time identified fragments of decorin, biglycan and fibromodulin associated with the ovine annular lesion site. Initial analysis with Mab 2-B-6 identified intact core proteins and N-terminal fragments with associated chondroitin/dermatan sulphate attachment. These non-specific blots showed age related changes but little difference between lesion and sham discs. In contrast, using SLRP-specific antibodies to the core protein C-termini, we identified novel fragmentation of some but not all proteoglycans in the AF of the lesion discs. Decorin fragment levels were highest 3 month PO and then decreased suggesting that this may be associated with early post-operative remodeling and repair. In contrast, biglycan and fibromodulin fragments increased with lesion development 12–26 month PO and may therefore be associated with progressive degeneration of the AF. Some changes in SLRP core proteins and fragments identified in this study, such as the progressive increase in intact decorin core protein, most biglycan fragments and non-glycosylated lumican appeared to be associated with ageing as there was no difference between sham and lesion discs. However, specific biglycan and fibromodulin fragments were only found in the lesion discs suggesting that novel proteolytic pathways may be activated with progressive degeneration in these discs. Given the diverse functions of the SLRPs, their catabolism could result in altered tissue responses to growth factors and cytokines, abnormal collagen fibril formation, and modulation of cell adhesion and growth [7, 17, 19, 20, 22, 34, 54, 55, 61, 62], events which are important not only in tissue remodeling but also in repair responses which might relate to the incomplete repair potential of the controlled outer annular defects used in the ovine model in the present study. Fragments of biglycan and fibromodulin were detected in the contralateral AF of the lesion affected IVD levels in this study, the relative abundances of these SLRP fragments (and their temporal appearance) were slightly lower (and later) than in the AF 1 zone but nevertheless mirrored the AF1 lesion zone extracts. This presumably is indicative of a disruption in the functional properties of the intact AF by the introduction of an anterolateral defect. The propogation of outer annular defects (development of transverse and circumferential tears) to previously unaffected regions of the IVD is also further evidence of the resolution of biomechanical forces in this system.
Cleavage of SLRPs may precede destruction of the collagen networks in tissues in disease and thus may represent an early marker of this destructive process. Decorin, fibromodulin and lumican all have specific binding sites with the surfaces of type I and II collagen fibrils and through such interactions they provide a protective coating which prevents degradation of type I and II collagen by MMP-1 and 13 in vitro [11]. Decorin, biglycan and fibromodulin can all be degraded by MMP-13 in vitro however fragmentation of biglycan and fibromodulin is more extensive [38]. MMP-13 has also been shown to degrade fibromodulin attached to collagen in vitro with the generation of a 37–39 kDa C-terminal fragment but fibromodulin in free solution was not degraded [18]. This cleavage product was shown to be identical to a fibromodulin fragment produced in interleukin-1 (IL-1) stimulated cartilage explant cultures or cartilage explants digested with MMP-13 [18]. In our study however, we observed elevated levels of 48–50, 35 and 28 kDa fibromodulin bands 6–26 month PO in the AF of lesion discs. An earlier study from this laboratory also detected a similar 28 kDa fibromodulin fragment associated with cartilage degeneration induced by the altered biomechanics and joint destabilization induced by meniscal removal in an ovine model of OA [65]. While the enzyme responsible for generation of these novel fibromodulin fragments have not been identified, we have previously shown that IL-1 induces MMP-2 and MMP-3 production by ovine NP cells in vitro and this represents a potential pathway of IVD degeneration [49]. In addition to the 28 kDa biglycan catabolite generated by MMP-13 cleavage [38], aggrecanases-1, 2 have been shown to degrade biglycan in vitro at the start of it’s fifth leucine rich domain between an asparagine and cysteine residue [32] generating a 27 kDa C-terminal core protein fragment of similar size to a biglycan fragment identified in the present study. The amino acid sequence in this region of the biglycan core protein is highly conserved in mouse, rat, human, bovine, dog, sheep and horse [32], suggesting that aggrecanases could be implicated in the biglycan breakdown seen in our sheep AF. Previous reports however have indicated that the aggrecanases are less active in IVD than cartilage [45, 57] and may be associated with infiltrating macrophages in herniated disc tissues [16]. Neither aggrecanase or MMP-13 digestion of biglycan [32, 38], generated any smaller biglycan fragments such as we detected in the lesion AF at 12–26 months (Table 1).
There are no publications that have examined SLRP fragmentation in pathology of human IVD, however in a study of age-related changes, little fragmentation of fibromodulin was seen with a 50 kDa fragment demonstrated in the AF of a single 61 year old individual and no catabolites evident in the NP [58]. Similarly we did not observe any small fibromodulin fragments with the onset of age in our sham-operated group. Fibromodulin and lumican do undergo a transformation from a proteoglycan form in the neonate to a glycoprotein form in the adult [58]. In our study we also observed proteoglycan and glycoprotein forms of lumican in the sham and lesion group samples, both forms were evident in the 4 year old sheep samples at the inception of the study but by 6.5 year of age the glycoprotein form of lumican only was detectable in the AF and both forms in the NP, but in lower abundance. We have previously observed similar proteoglycan and glycoprotein forms of lumican in an ovine model of OA in association with cartilage degeneration rather than ageing [65]. In contrast to aged human discs where two lumican fragments (38 and 32 kDa) were seen in the AF, no catabolites of the lumican core protein was detected in the present study.
Disc degeneration is of significant socioeconomic impact and difficult to treat clinically [27]. Until relatively recently research into this important clinical problem was hampered by a lack of an appropriate animal model. While none of the many animal models of IVD degeneration so far developed are ideal [25, 28, 29, 31, 33, 43, 46, 51–53] the ovine annular lesion model reproduces many of the pathological features evident in degenerate human IVDs [33–37, 39–41, 43] and is a particularly useful large animal model which has provided some important insights into ECM reorganization processes associated with IVD degeneration. Changes which have been observed in lesion affected IVDs such as the controlled well defined outer annular defects used in the present study therefore mimic many of the matrix changes observed in human IVD degeneration. In the ovine annular lesion model (i) aggrecan is catabolised and decorin and biglycan synthesis are both focally elevated in the vicinity of the lesion site leading to a loss in disc height and in it’s functional properties which equip it with the ability to act as a weight bearing cushion. (ii) Blood vessel and nerve in-growth into lesion affected IVDs depleted of their space-filling aggrecan molecules is elevated in lesion affected IVDs. (iii) An alteration in the types of and amounts of collagens I, II, III, IV, VI has also been observed in lesion affected ovine and porcine IVDs. (iv) Changes in the end-plate vascularity underlying outer annular rim lesions and focal remodeling of subchondral bone adjacent to the lesion site has also been noted. Critical evaluation of the anatomy and biomechanical properties of ovine and human spines has shown many similarities [63, 64] and the use of quadrupeds for spinal studies has also been validated [50]. Experimental induction of controlled outer AF defects in sheep discs reproduces a sequence of events in the AF which closely mimics pathologically and biochemically, many of the characteristics of disc degeneration in man [33–37, 39–41, 43]. The present study has used this model to identify specific degradation of SLRPs which occurred during the healing response of the AF to peripheral injury and then at later times with ongoing IVD degeneration. Identification of the enzyme systems responsible for SLRP proteolysis may identify potential therapeutic target molecules, provide a better understanding of annular remodeling events at the molecular level, which if translated into a clinical application might lead to better treatment for IVD degeneration.
Acknowledgments
Funding for this project was provided by the National Health and Medical Research Council of Australia (Project grants 211266 and 352562) whose support is gratefully acknowledged.
References
- 1.Ameye L, Young MF (2002) Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 12(9):107R–116R [DOI] [PubMed]
- 2.Ameye LG, Young MF (2006) Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol 18(5):537–547 [DOI] [PubMed]
- 3.Bancroft JD, Cook HC (eds) (1994) Manual of histological techniques and their diagnostic application, Chapt. 3. Churchill-Livingstone, London, p 42
- 4.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG (2002) Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo award in basic science. Spine 27(23):2631–2644 [DOI] [PubMed]
- 5.Buckwalter JA (1995) Aging and degeneration of the human intervertebral disc. Spine 20(11):1307–1314 [DOI] [PubMed]
- 6.Caterson B, Christner JE, Baker JR, Couchman JR (1985) Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc 44(2):386–393 [PubMed]
- 7.Chakravarti S (2002) Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J 19(4–5):287–293 [DOI] [PubMed]
- 8.Christner JE, Caterson B, Baker JR (1980) Immunological determinants of proteoglycans. Antibodies against the unsaturated oligosaccharide products of chondroitinase ABC-digested cartilage proteoglycans. J Biol Chem 255(15):7102–7105 [PubMed]
- 9.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS (2002) Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine 27(20):2212–2219 [DOI] [PubMed]
- 10.Flannery CR (2006) Usurped SLRPs: novel arthritis biomarkers exposed by catabolism of small leucine-rich proteoglycans? Arthritis Res Ther 8(2):106 [DOI] [PMC free article] [PubMed]
- 11.Geng Y, McQuillan D, Roughley PJ (2006) SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol 25(8):484–491 [DOI] [PubMed]
- 12.Getzy LL, Malemud CJ, Goldberg VM, Moskowitz RW (1982) Factors influencing metachromatic staining in paraffin embedded sections of rabbit and human articular cartilage: a comparison of the Safranin O and toluidine blue techniques. J Histotechnol 5:111–116
- 13.Guner A, Oktay G, Kerman M, Guner G (1995) Immunoglobulins and alpha-1-proteinase inhibitor in human intervertebral disc material. Biochem Soc Trans 23(2):212S [DOI] [PubMed]
- 14.Habtemariam A, Gronblad M, Virri J, Seitsalo S, Ruuskanen M, Karaharju E (1996) Immunocytochemical localization of immunoglobulins in disc herniations. Spine 21(16):1864–1869 [DOI] [PubMed]
- 15.Haefeli M, Kalberer F, Saegesser D, Nerlich AG, Boos N, Paesold G (2006) The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine 31(14):1522–1531 [DOI] [PubMed]
- 16.Hatano E, Fujita T, Ueda Y, Okuda T, Katsuda S, Okada Y, Matsumoto T (2006) Expression of ADAMTS-4 (aggrecanase-1) and possible involvement in regression of lumbar disc herniation. Spine 31(13):1426–1432 [DOI] [PubMed]
- 17.Hausser H, Groning A, Hasilik A, Schonherr E, Kresse H (1994) Selective inactivity of TGF-beta/decorin complexes. FEBS Lett 353(3):243–245 [DOI] [PubMed]
- 18.Heathfield TF, Onnerfjord P, Dahlberg L, Heinegard D (2004) Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem 279(8):6286–6295 [DOI] [PubMed]
- 19.Hedbom E, Heinegard D (1993) Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem 268(36):27307–27312 [PubMed]
- 20.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302(Pt 2):527–534 [DOI] [PMC free article] [PubMed]
- 21.Hilton RC, Ball J (1984) Vertebral rim lesions in the dorsolumbar spine. Ann Rheum Dis 43(2):302–307 [DOI] [PMC free article] [PubMed]
- 22.Iozzo RV (1999) The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 274(27):18843–18846 [DOI] [PubMed]
- 23.Johnstone B, Markopoulos M, Neame P, Caterson B (1993) Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem J 292(Pt 3):661–666 [DOI] [PMC free article] [PubMed]
- 24.Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11(4):447–455 [DOI] [PubMed]
- 25.Kaapa E, Holm S, Inkinen R, Lammi MJ, Tammi M, Vanharanta H (1994) Proteoglycan chemistry in experimentally injured porcine intervertebral disk. J Spinal Disord 7(4):296–306 [PubMed]
- 26.Kaapa E, Han X, Holm S, Peltonen J, Takala T, Vanharanta H (1995) Collagen synthesis and types I, III, IV, and VI collagens in an animal model of disc degeneration. Spine 20(1):59–66; discussion 66–67 [DOI] [PubMed]
- 27.Katz JN (2006) Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am 88(Suppl 2):21–24 [DOI] [PubMed]
- 28.Lipson SJ, Muir H (1981) 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine 6(3):194–210 [DOI] [PubMed]
- 29.Lotz JC (2004) Animal models of intervertebral disc degeneration: lessons learned. Spine 29(23):2742–2750 [DOI] [PubMed]
- 30.Masson P (1929) Some histological methods. Trichrome stainings and their preliminary technique. Bulletin of The International Association of Medicine. J Tech Methods 12:75
- 31.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS (2005) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30(1):5–14 [DOI] [PubMed]
- 32.Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ (2006) The cleavage of biglycan by aggrecanases. Osteoarthr Cartil 14(11):1147–1154 [DOI] [PubMed]
- 33.Melrose J, Ghosh P, Taylor TK, Hall A, Osti OL, Vernon-Roberts B, Fraser RD (1992) A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res 10(5):665–676 [DOI] [PubMed]
- 34.Melrose J, Ghosh P, Taylor TK, Latham J, Moore R (1997) Topographical variation in the catabolism of aggrecan in an ovine annular lesion model of experimental disc degeneration. J Spinal Disord 10(1):55–67 [DOI] [PubMed]
- 35.Melrose J, Ghosh P, Taylor TK, Vernon-Roberts B, Latham J, Moore R (1997) Elevated synthesis of biglycan and decorin in an ovine annular lesion model of experimental disc degeneration. Eur Spine J 6(6):376–384 [DOI] [PMC free article] [PubMed]
- 36.Melrose J, Roberts S, Smith S, Menage J, Ghosh P (2002) Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine 27(12):1278–1285 [DOI] [PubMed]
- 37.Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P (2002) Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine 27(16):1756–1764 [DOI] [PubMed]
- 38.Monfort J, Tardif G, Reboul P, Mineau F, Roughley P, Pelletier JP, Martel-Pelletier J (2006) Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther 8(1):R26 [DOI] [PMC free article] [PubMed]
- 39.Moore RJ, Osti OL, Vernon-Roberts B, Fraser RD (1992) Changes in endplate vascularity after an outer anulus tear in the sheep. Spine 17(8):874–878 [DOI] [PubMed]
- 40.Moore RJ, Vernon-Roberts B, Osti OL, Fraser RD (1996) Remodeling of vertebral bone after outer anular injury in sheep. Spine 21(8):936–940 [DOI] [PubMed]
- 41.Moore RJ, Crotti TN, Osti OL, Fraser RD, Vernon-Roberts B (1999) Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine 24(6):519–525 [DOI] [PubMed]
- 42.Nerlich AG, Schleicher ED, Boos N (1997) 1997 Volvo award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine 22(24):2781–2795 [DOI] [PubMed]
- 43.Osti OL, Vernon-Roberts B, Fraser RD (1990) 1990 Volvo award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine 15(8):762–767 [DOI] [PubMed]
- 44.Pennington JB, McCarron RF, Laros GS (1988) Identification of IgG in the canine intervertebral disc. Spine 13(8):909–912 [DOI] [PubMed]
- 45.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM (2000) Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine 25(23):3005–3013 [DOI] [PubMed]
- 46.Rousseau MA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC (2007) Stab incision for inducing intervertebral disc degeneration in the rat. Spine 32(1):17–24 [DOI] [PubMed]
- 47.Scott JE (2003) Elasticity in extracellular matrix ‘shape modules’ of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol 553(Pt 2):335–343 [DOI] [PMC free article] [PubMed]
- 48.Scott JE, Stockwell RA (2006) Cartilage elasticity resides in shape module decoran and aggrecan sumps of damping fluid: implications in osteoarthrosis. J Physiol 574(Pt 3):643–650 [DOI] [PMC free article] [PubMed]
- 49.Shen B, Melrose J, Ghosh P, Taylor F (2003) Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J 12(1):66–75 [DOI] [PubMed]
- 50.Smit TH (2002) The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J 11(2):137–144 [DOI] [PMC free article] [PubMed]
- 51.Smith JW, Walmsley R (1951) Experimental incision of the intervertebral disc. J Bone Joint Surg Br 33-B(4):612–625 [DOI] [PubMed]
- 52.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG (2005) A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine 30(1):15–24 [DOI] [PubMed]
- 53.Alini M, Eisenstein S, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ (2007) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J, 14 Jul 2007; [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 54.Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A (1999) Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem 274(14):9636–9647 [DOI] [PubMed]
- 55.Svensson L, Narlid I, Oldberg A (2000) Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett 470(2):178–182 [DOI] [PubMed]
- 56.Sweat F, Puchtler H, Rosenthal SI (1964) Sirius Red F3ba as a stain for connective tissue. Arch Pathol 78:69–72 [PubMed]
- 57.Sztrolovics R, Alini M, Roughley PJ, Mort JS (1997) Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J 326(Pt 1):235–241 [DOI] [PMC free article] [PubMed]
- 58.Sztrolovics R, Alini M, Mort JS, Roughley PJ (1999) Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine 24(17):1765–1771 [DOI] [PubMed]
- 59.Thompson JP, Oegema TR Jr, Bradford DS (1991) Stimulation of mature canine intervertebral disc by growth factors. Spine 16(3):253–260 [DOI] [PubMed]
- 60.Vernon-Roberts B (1988) Pathology of the intervertebral disc. In: Ghosh P (ed) Biology of the intervertebral disc, Vol II. CRC, Boca Raton, pp 73–120
- 61.Vogel KG, Paulsson M, Heinegard D (1984) Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223(3):587–597 [DOI] [PMC free article] [PubMed]
- 62.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M (2003) Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem 278(39):37698–37704 [DOI] [PubMed]
- 63.Wilke HJ, Kettler A, Claes LE (1997) Are sheep spines a valid biomechanical model for human spines? Spine 22(20):2365–2374 [DOI] [PubMed]
- 64.Wilke HJ, Kettler A, Wenger KH, Claes LE (1997) Anatomy of the sheep spine and its comparison to the human spine. Anat Rec 247(4):542–555 [DOI] [PubMed]
- 65.Young AA, Smith MM, Smith SM, Cake MA, Ghosh P, Read RA, Melrose J, Sonnabend DH, Roughley PJ, Little CB (2005) Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res Ther 7(4):R852–R861 [DOI] [PMC free article] [PubMed]