Abstract
During the process of lymphocyte recirculation, lymphocytes bind via L-selectin to sulfated sialyl-Lewisx (sLex)–containing carbohydrate ligands expressed on the surface of high endothelial venules (HEV). We have examined the expression of sLex on HEV using a panel of mAbs specific for sLex and sLex-related structures, and have examined the function of different sLex-bearing structures using an in vitro assay of lymphocyte rolling on HEV. We report that three sLex mAbs, 2F3, 2H5, and CSLEX-1, previously noted to bind with high affinity to glycolipid-linked sLex, vary in their ability to stain HEV in different lymphoid tissues and bind differentially to O-linked versus N-linked sLex on glycoproteins. Treatment of tissue sections with neuraminidase abolished staining with all three mAbs but slightly increased staining with MECA-79, a mAb to a sulfation-dependent HEV-associated carbohydrate determinant. Treatment of tissue sections with O-sialoglycoprotease under conditions that removed the vast majority of MECA-79 staining, only partially reduced staining with the 2F3 and 2H5 mAbs. Using a novel rolling assay in which cells bind under flow to HEV of frozen tissue sections, we demonstrate that a pool of O-sialoglycoprotease–resistant molecules is present on HEV that is sufficient for attachment and rolling of lymphocytes via L-selectin. This interaction is not inhibited by the mAb MECA-79. Furthermore, MECA-79 mAb blocks binding to untreated sections by only 30%, whereas the sLex mAb 2H5 blocks binding by ∼60% and a combination of MECA-79 and 2H5 mAb blocks binding by 75%. We conclude that a pool of O-glycoprotease-resistant sLex-like L-selectin ligands exist on human HEV that is distinct from the mucin-associated moieties recognized by MECA-79 mAb. We postulate that these ligands may participate in lymphocyte binding to HEV.
Through L-selectin, recirculating lymphocytes bind to and form rolling adhesions on high endothelial venules (HEV)1 in secondary lymphoid organs. This brings lymphocytes into intimate contact with the vessel wall and enables signaling and adhesive interactions through other molecules responsible for development of firm adhesion and extravasation through the vessel wall (5, 16, 60). L-selectin–deficient mice have decreased numbers of lymphocytes localized to peripheral lymph nodes and are deficient in antigen surveillance in peripheral sites (3, 18, 67).
The L-selectin ligands identified in HEV thus far are mucin-like molecules bearing fucosylated, sialylated, and sulfated carbohydrates (6, 7, 13, 36, 52, 53). These include MAdCAM-1 and CD34. The rat mAb MECA-79 recognizes an antigenic determinant that is closely associated with the L-selectin ligand; this mAb binds to HEV, inhibits lymphocyte binding to HEV, and immunoprecipitates a group of proteins termed peripheral node addressin (PNAd) that bind to L-selectin (9, 32, 34, 62). Sialylation of carbohydrate ligands is required for L-selectin binding but not for MECA-79 mAb recognition, whereas binding of both L-selectin chimera and MECA-79 requires sulfation (9, 32, 33, 53). Although the location and number of sulfates required for L-selectin binding is still unknown, sLex sulfated on the 6 position of galactose, i.e., Siaα2→ 3(SO4-6)Galβ1→ 4(Fucα1→ 3)GlcNAc, was found to be a major capping group of the O-linked chains of the L-selectin ligand GlyCAM-1 and may serve as an important L-selectin recognition structure (31). MECA-79 has been reported to block lymphocyte binding to murine peripheral lymph node HEV by 95% (62), and it inhibits homing into lymph nodes in vivo by ∼80% (62, 63); however, subsequent studies have demonstrated incomplete inhibition of lymphocyte binding to human PNAd (38, 52). Moreover, MECA-79 mAb inhibited binding of human peripheral blood mononuclear cells (PBMC) to human peripheral lymph node HEV by only 47% (44). PNAd has been defined as a vascular addressin involved in directing lymphocyte homing to peripheral lymph nodes (62). At the same time, the MECA-79 mAb has been stated to define this vascular addressin in the mouse (62) and human (9) and PNAd has been defined as “the isolated complex of MECA-79–reactive proteins” (32).
Synthetic sLex on glycolipid and protein carriers can serve as an in vitro ligand for L-selectin (2, 54). Immunohistochemical studies with mAb to sLex or related structures have demonstrated the presence of sLex-like moieties on HEV of human tissues (47, 50, 57), but the relationship of these moieties to L-selectin ligands has been unclear. These mAb include: HECA-452, raised against stromal components of human lymph nodes and that recognizes an epitope common to both sLex and sLea (10, 22); CSLEX-1, raised against the cell surface proteins of adenocarcinoma tissue; 2F3, raised against a synthetic sLex glycolipid, and 2H5, raised against complex sLex-containing glycolipids isolated from colon carcinoma tissue (26, 48, 57). Each of these mAbs to sLex-like structures has been shown to block some aspect of selectin-mediated adhesion. E-selectin, expressed on cutaneous vessels in inflamed skin, mediates binding of CLA+ T lymphocytes and this binding is blocked by the HECA-452 mAb (11, 51). The 2H5 mAb inhibits L-selectin–dependent adhesion of lymphocytes to human lymph node HEV, and 2F3 mAb blocks binding of sLex-expressing T cell leukemia cells to endothelial E-selectin (48, 57). More recently, both 2F3 and 2H5 have been reported to inhibit binding of L-selectin–transfected cells to human lymph node HEV in Stamper-Woodruff assays (45). Lastly, depletion of CSLEX-1+ T cells was shown to remove the population capable of binding to E-selectin (21).
In this study we examine the expression of sLex-like molecules on HEV of human tissues, show differences in the types of protein backbones that present different sLex-related structures, and demonstrate the presence of sLex-like molecules distinct from PNAd that can mediate L-selectin binding. Expression of sLex by HEV is examined by immunohistochemical staining of human tissues with a panel of sLex-specific mAbs. We report that three sLex mAbs, 2F3, 2H5, and CSLEX-1, vary in their ability to stain HEV in various tissues and to recognize sLex presented on different O- and N-linked carbohydrates, although all three mAbs bind with high affinity to sLex presented on glycolipids (26, 48, 57). Furthermore, HEV staining with the mAbs 2F3 and 2H5 persisted after the majority of HEV staining with MECA-79 was removed with O-sialoglycoprotease treatment, suggesting that additional, nonmucin structures may present sLex on HEV. Using a rolling assay in which cells attach to HEV of frozen tissue sections under flow conditions, we show that O-sialoglycoprotease–resistant L-selectin ligands exist on HEV that can mediate attachment and rolling of lymphocytes and that this interaction is not inhibited by the mAb MECA-79. Lastly, we demonstrate that the sLex mAb 2H5 inhibits lymphocyte binding to untreated HEV by ∼60%, whereas MECA-79 inhibits binding by only 30%.
Materials and Methods
Antibodies
2F3 (48) and 2H5 (57) mAbs were kindly provided by R. Kannagi (Aichi Cancer Center, Nagoya, Japan). CSLEX-1 (26) and CSLEA-1 mAbs were generous gifts of P. Terasaki (University of California Los Angeles, Los Angeles, CA). FH6 mAb (25) was provided by S. Hakomori (University of Washington, Seattle, WA). HECA-452 (22) and MECA-79 (62) were generous gifts of E. Butcher (Stanford University, Stanford, CA). DREG-56 (35) was a kind gift of T.K. Kishimoto (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT). P-selectin mAb SZ-51, E-selectin mAb HAE-1a, and PECAM-1 mAb SG134 were obtained from the Fifth International Workshop on Human White Cell Differentiation Antigens (Kobe, Japan).
sLex-Bearing Glycoproteins
The β-glycyl amine of sLex conjugated to BSA was purchased from Oxford Glycosystems (Rosedale, NY). F2b and glycoprotein respiratory mucin (41) from cystic fibrosis (CF) patients were provided to our laboratory by G. Lamblin (Unité INSERM, Lille, France). L-selectin/human IgG1 chimera (7) was a gift of L. Lasky and S. Watson (both from Genentech Inc., South San Francisco, CA). sLex-Decorated L-selectin chimera was produced by transient co-transfection of COS cells with L-selectin chimera and fucosyl transferase III expression plasmids (24). Culture supernatants from these cells were collected and L-selectin chimera was purified using protein A–Sepharose (Sigma Chemical Co., St. Louis, MO). sLex-Decorated L-selectin chimera produced in this way bound CSLEX-1 mAb and supported the tethering and rolling of E-selectin–transfected CHO cells via E-selectin when immobilized on plastic (24). E-selectin–transfected CHO cells were a generous gift of R. Lobb (Biogen Inc., Cambridge, MA) (17).
Immunohistochemistry
Human tissues from surgical procedures were obtained from Brigham and Women's Hospital (Boston, MA) and snap frozen in liquid nitrogen. Thymus was obtained from pediatric patients undergoing cardiac surgery. Inflamed appendix was obtained from patients with appendicitis and normal appendix was obtained from a patient with ulcerative colitis undergoing colectomy; the appendix from this patient was not histologically inflamed. Mesenteric lymph nodes were also obtained from this patient and were taken from areas of colon that were not histologically inflamed. “Less reactive” versus “Highly reactive” tonsil was a qualitative distinction based on the observation that highly reactive tonsils contained greater numbers of HEV and had HEV that expressed high levels of P-selectin by immunofluorescence; Less reactive tonsils contained sparse numbers of HEV that expressed low to undetectable levels of P-selectin. Cryostat sections (5 μm) were cut and fixed by one of three methods: room temperature acetone for 5 min, 1:1 mixture of acetone and methanol for 2 min at room temperature, or 2% paraformaldehyde for 10 min at room temperature. Sections were fixed with acetone unless otherwise noted. Sections were incubated for 45 min with a 40 μg/ml concentration of primary mAb in PBS, rinsed three times for 5 min each time in PBS, 1% BSA (GIBCO BRL, Gaithersburg, MD), and incubated for 30 min with a 1:40 dilution of FITC-conjugated goat anti–rat Ig (Tago Inc., Burlingame, CA) for rat primary mAbs (HECA-452 and MECA-79) or FITC-conjugated goat anti– mouse IgG+A+M (Zymed Labs Inc., South San Francisco, CA) for mouse primary mAbs. Sections were subsequently rinsed three times for 5 min each time in PBS, 1% BSA, and mounted under glass coverslips (Fisher Scientific Co., Pittsburgh, PA) using mounting media (Fluoromount-G; Southern Biotechnology Associates, Birmingham, AL). Sections were observed by fluorescence microscopy and photographed immediately after staining.
Immunoblot Analysis of sLex Antibodies
sLex-Conjugated proteins were spotted onto nitrocellulose and allowed to dry overnight at room temperature. Blots were blocked for 1 h in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20 (TBST) with 1% BSA, and incubated with 20 μg/ml solution of primary antibodies for 1 h at room temperature. Blots were then rinsed three times for 7 min each in TBST and incubated for 30 min in a 1:1,000 dilution of alkaline phosphatase-conjugated goat anti–mouse IgG+A+M (Zymed Labs Inc.) in TBST for mouse primary mAbs, or alkaline phosphatase-conjugated rabbit anti–rat IgM (Zymed Labs Inc.) in TBST for rat primary mAbs. Blots were rinsed three times for 7 min each in TBST and developed with Western blue stabilized alkaline phosphatase substrate (Promega Corp., Madison, WI). Reaction was stopped by washing with distilled water.
Enzyme Treatment of Tissue Sections
Sets of two serial sections of mesenteric lymph node or tonsil (5 μm thickness for immunofluorescence experiments, 9 μm thickness for flow chamber experiments) were prepared and used immediately without fixation. One of each set of two sections was treated with 100 μl control medium consisting of HBSS with 1 mM Ca2+ with 1 U/ml DNaseI (from bovine pancreas; Boehringer Mannheim, Mannheim, Germany) for either 1 h (neuraminidase control) or 2 h (O-sialoglycoprotease control) at 37°C in a humidified chamber. The second serial section was treated with 100 μl of either 0.1 U/ml of neuraminidase (from Vibrio cholerae; Sigma Chemical Co.) for 1 h, or 60 μg/ml (0.3 U/ml) O-sialoglycoprotease (Accurate Chemical and Scientific Corporation, Westbury, NY) for 2 h at 37°C in a humidified chamber. Heparitinase I (from Flavobacterium heparinum; Sigma Chemical Co.; 600 mU/ml) was added to the solution containing O-sialoglycoprotease and incubated with sections for 2 h at 37°C. Enzymes were diluted into HBSS, 1 mM Ca2+, containing 1 U/ml DNase. Sections to be stained with all sLex mAbs except CSLEX-1 were rinsed briefly in PBS, fixed in 2% paraformaldehyde for 10 min at room temperature, and stained as described above. Sections to be stained with CSLEX-1 were rinsed briefly in PBS and fixed in a 1:1 mixture of methanol and acetone for 2 min at room temperature because fixation in paraformaldehyde eliminated HEV staining with this mAb. The same HEV were identified in both serial sections so that the reactivity of these HEV with sLex mAbs with and without enzyme treatment could be directly compared. Photographs of each set of serial HEV were taken using the same exposure time. Sections to be used in flow experiments were not fixed; sections were rinsed briefly in PBS and stored at 4°C in PBS for up to 1 h before use.
Flow HEV Binding Assay
PBMC were isolated as previously described (56). For experiments using desialylated cells, cells (7.5 × 106 in 150 μl of HBSS with 2 mM Ca2+, 0.1% BSA) were treated with 0.1 U/ml of neuraminidase for 40 min at 37°C. For antibody blocking experiments using DREG-56 mAb, cells (7.5 × 106 cells in 150 μl of HBSS with 2 mM Ca2+) were pre-incubated for 40 min on ice with a final concentration of 50 μg/ml blocking mAb. Freshly cut 9 μm sections of human tonsil identified as “highly reactive” (see above) were placed on 70 × 50 mm glass slides (Corning Glass Works, Corning, NY) pre-coated with 0.1 mg/ml poly-L-lysine (Sigma Chemical Co.), allowed to dry 1–3 h at room temperature, and then stored at 4°C for up to 2 h until use. A parallel plate flow chamber was attached directly to the glass slide such that the tissue section made up the floor of the chamber (19, 38). PBMC were introduced into the chamber under flow, and the tethering of cells to HEV at 25°C was observed and recorded using video microscopy. Flow experiments used the following shear stresses: cells were accumulated at 0.75 dyn/cm2 for 80 s, followed by rinsing with cell-free medium at 1.2, 1.7, 2.1, 3.0, 3.9, 4.8, 6.3, 7.8, 9.3, 10.8, and 12.3 dyn/cm2 for 10 s each. Binding was evaluated at the end of the 1.7 dyn/cm2 step and the higher shears were used to remove all adherent lymphocytes from the sections. In general, an individual section could be used for up to four consecutive evaluations of cell binding without diminution of lymphocyte attachment to HEV; after the fourth run, a progressive decrease in lymphocyte binding was usually observed. Individual sections were therefore used for only three to four runs. For Figs. 4–6, video images were captured using NIH Image software version 1.59 and unbound cells were darkened by comparing two consecutive frames and using the minimum value for each pixel. For antibody-blocking experiments using mAbs MECA-79, 2H5, and 2F3, cells were first bound to the HEV in the absence of mAb. The mAb (50 μg/ml of MECA-79 or culture supernatants of 2H5 or 2F3) was then infused into the flow chamber for 15 min at a rate of 0.02 ml/min. Binding of cells to the same HEV was then re-evaluated at the end of mAb infusion. For MECA-79 experiments, mAb at a final concentration of 50 μg/ml was added to the cell suspension before infusion into the flow chamber and wash medium was also supplemented with 50 μg/ml MECA-79.
Figure 4.
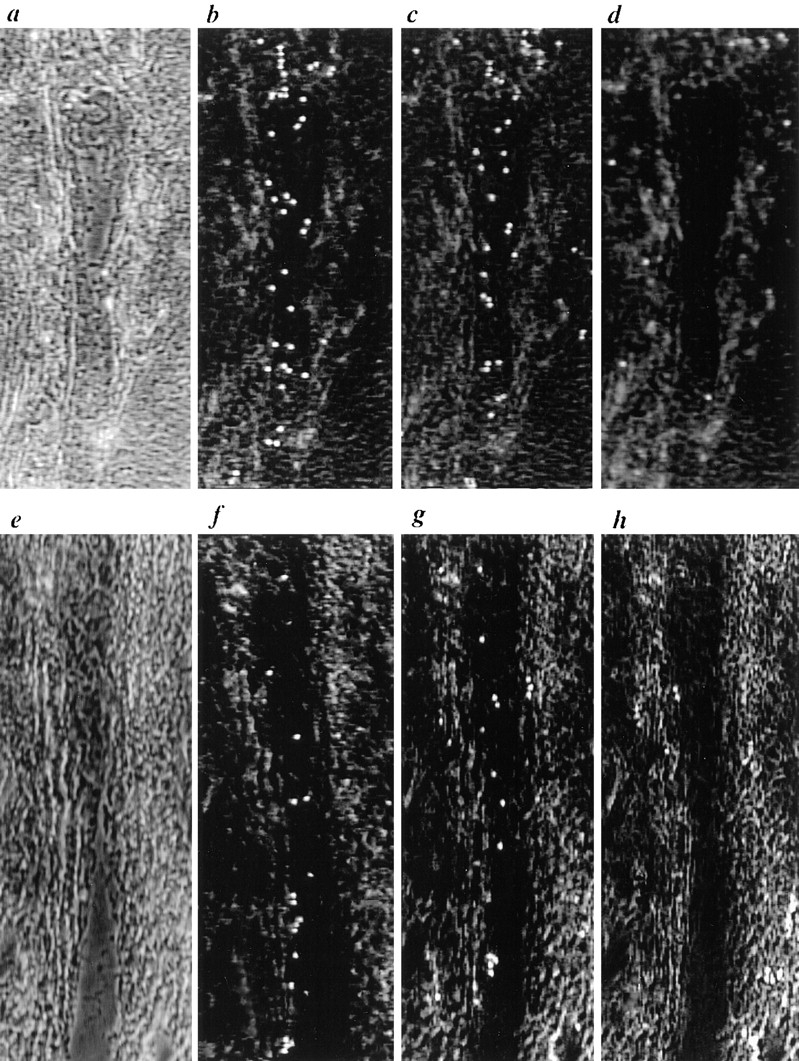
Binding of PBMC under flow conditions to HEV of frozen sections of human tonsil. To clearly delineate HEV boundaries, phase contrast images taken before binding of lymphocytes and focused in the plane of the section are shown in (a and e). Other panels used a focal plane in the plane of the binding lymphocytes and the venule structure is less clearly seen. Binding of (b) untreated PBMC, (c) DREG-56–treated PBMC, and (d) PBMC in the presence of 5 mM EDTA to an HEV. Binding to (e), a second HEV, was evaluated using (f) untreated PBMC, (g) neuraminidase-treated PBMC, and (h) neuraminidase-treated PBMC pre-incubated with DREG-56. Cells were accumulated at 0.75 dyn/cm2 for 80 s, followed by rinsing with cell-free medium at 1.2, 1.7, and 2.1 dyn/cm2 for 10 s each. Under increasing shear, cells were observed to roll along the walls of HEV oriented in the direction of flow. Video images were captured at the end of the 1.7 dyn/cm2 step using NIH Image software version 1.59; unbound cells were darkened by comparing two consecutive frames and using the minimum value for each pixel. The direction of flow was from the bottom of the figure to the top.
Figure 6.
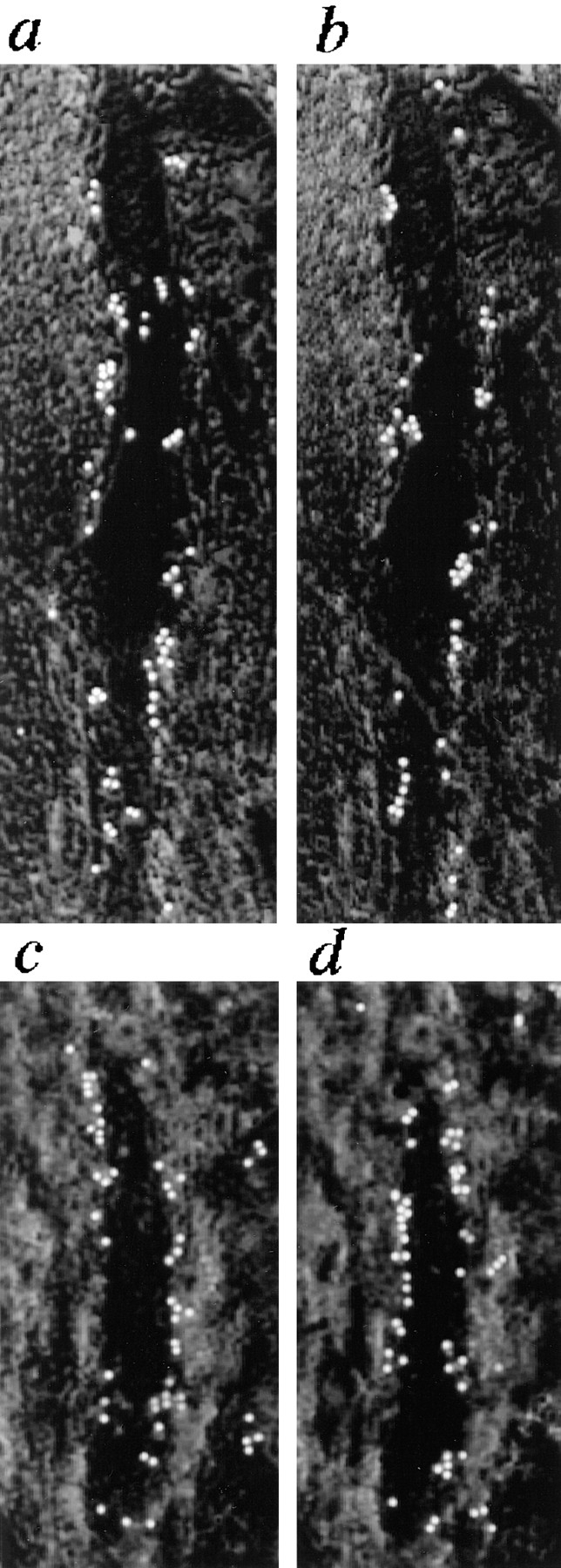
Effect of MECA-79 mAb on binding of neuraminidase-treated PBMC under flow conditions to HEV of (a and b) untreated and (c and d) O-sialoglycoprotease– treated tonsil sections. Binding to an individual HEV was assessed before (a and c) and (b and d) after incubation of sections with MECA-79 mAb. Flow parameters were identical to those used in Fig. 4. The direction of flow was from the bottom of the figure to the top.
Results
Staining Patterns of mAbs Recognizing sLex and Related Stuctures
The 2F3, 2H5, and CSLEX-1 sLex mAbs stained HEV in two distinct patterns (Table I). 2F3 and 2H5 mAb brightly stained the HEV of all lymphoid tissues tested; postcapillary venules in the thymus were not stained. Staining with these antibodies was intense both in inflamed and in noninflamed lymphoid tissues. HEV-staining using CSLEX-1 was much more limited; staining was absent in tonsil, moderate in mesenteric lymph nodes (10–60% of the HEV were positive), and present at low levels in mucosal lymphoid tissue. Staining of appendix HEV with CSLEX-1 mAb correlated with the degree of inflammation as indicated by both patient history and expression by HEV of P-selectin and E-selectin (Tables I and II). Unlike the other two mAbs, staining of HEV with CSLEX-1 depended on the method of fixation. Sections fixed in acetone had the brightest staining of HEV, sections fixed in methanol and acetone had less bright staining, and sections fixed in paraformaldehyde showed no staining of HEV with CSLEX-1.
Table I.
High Endothelial Venule (HEV) Staining* in Human Tissues
| sLex | di-sLex | sLea | HECA-452 | MECA-79 | P-selectin | PECAM-1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2H5 | 2F3 | CSLEX-1 | FH6 | CSLEA-1 | ||||||||||||||
| Tonsil: less reactive | +++ | +++ | − | − | − | ++ | ++ | + | +++ | |||||||||
| Tonsil: highly reactive | +++ | +++ | − | − | − | +++ | +++ | +++ | +++ | |||||||||
| Mesenteric lymph node | +++ | +++ | ++ | − | − | +++ | +++ | ++ | +++ | |||||||||
| Appendix: normal | +++ | +++ | − | − | − | ++ | + | + | +++ | |||||||||
| Appendix: inflamed | +++ | +++ | ++ | − | − | +++ | ++ | +++ | +++ | |||||||||
| Colon lymphoid aggregates | +++ | +++ | + | − | − | ++ | ++ | + | +++ | |||||||||
| Thymus | − | − | − | − | − | − | − | + | +++ | |||||||||
+++ Intense staining, ++ moderate staining, + low staining, − no staining.
Table II.
Staining of Appendix HEV with CSLEX-1 Is Increased at Sites of Inflammation*
| Specimen number | 1‡ | 2‡ | 3§ | 4§ | 5§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSLEX-1 | + | ++ | + | − | − | |||||
| E-selectin | − | ++ | − | − | − | |||||
| P-selectin | +++ | +++ | ++ | ++ | ++ | |||||
| PECAM-1 | +++ | +++ | +++ | +++ | +++ |
+++ Intense staining, ++ moderate staining, + low staining, − no staining.
Histologically inflamed specimen from patient with acute appendicitis.
Histologically normal specimen from patient with ulcerative colitis.
Both the sLea mAb CSLEA-1 and the di-fucosyl sLex mAb FH6 showed no staining of HEV in any tissue tested. However, CSLEA-1 did brightly stain the basillar cells of the tonsillar epithelium and FH6 stained dendritic-like cells in tonsillar T cell areas, confirming that these mAbs were active. In agreement with earlier findings that HECA-452 recognizes an epitope common to both sLex and sLea (10), the staining pattern of this mAb was found to be a superposition of that seen with the sLea mAb CSLEA-1 and the sLex mAb 2H5 in tonsil, mesenteric lymph node, thymus, skin, colon, appendix, and breast, with the exception that skin-associated lymphocytes were also stained by HECA-452 but not by the other mAbs. MECA-79 staining of HEV was brightest on peripheral HEV, i.e., tonsil, but present on mucosal HEV, i.e., appendix as well, as has been previously described (62). Mesenteric lymph nodes were mosaic for expression of MECA-79 Ag, and contained local clusters of HEV that stained either brightly or weakly with MECA-79 mAb. P-selectin expression on HEV correlated with the degree of inflammation as determined by inflammatory infiltrate and patient history for appendix, and as determined by lymphoid hyperplasia for tonsil. PECAM-1 (CD31) staining was universally high on HEV of all tissues and was used as a positive control.
Immunoblot Analysis of Ligand Specificity of sLex mAbs
All three of the mAbs, 2F3, 2H5, and CSLEX-1 react strongly with sLex presented by glycolipids (26, 48, 57), yet CSLEX-1 did not stain HEV at the high levels observed for the other two mAbs. Using immunoblotting of nitrocellulose-immobilized proteins, we examined whether this differential reactivity with HEV might result from differences in the structure or presentation of sLex-bearing glycans on glycoproteins bearing O- or N-linked carbohydrates.
Fractions of purified mucins from CF patients were used as a source of sLex bound to mucins. The F2b mucin fraction has been chemically characterized, and contains, among other compounds, sLex conjugated to mucins via a type II core structure (41) and the same structure sulfated on the 6 position of GlcNAc, i.e., Siaα2→ 3Galβ1→ 4(Fucα1→ 3) GlcNAc-Core type 2 and Siaα2→ 3Galβ1→ 4(Fucα1→ 3), (SO4-6)GlcNAc-Core type 2. The GP mucin was prepared by ion-exchange chromatography of the F2b fraction and contains the less acidic, less sulfated portion of the eluate. When immobilized on plastic, both mucin fractions supported rolling of E-selectin–transfected CHO cells and this rolling was abrogated by treatment of the mucins with neuraminidase (Ronen Alon, personal communication). MECA-79 does not bind to the sLex bearing mucins in these preparations (Fig. 1). sLex sulfated on the 6 position of galactose, i.e., Siaα2→ 3(SO4-6)Galβ1→ 4(Fucα1→ 3) GlcNAc, was found to be a major capping group of the O-linked chains of GlyCAM-1, an L-selectin ligand that is recognized by MECA-79 (32, 34, 36). Undersulfated GlyCAM-1 and other L-selectin ligands were not recognized by MECA-79, suggesting that binding of this mAb to sLex requires sulfation (32). The absence of sulfation of the sLex galactose residue in the CF mucins used in this study may explain their lack of reactivity with MECA-79.
Figure 1.
Binding of sLex antibodies to sLex-containing glycoproteins examined by immunoblotting. The indicated amounts of (a) chemically conjugated BSA-sLex and sLex-presenting respiratory mucins from CF patients and (b) BSA-sLex and L-selectin chimera decorated with N-linked sLex were blotted on nitrocellulose and incubated with 20 μg/ml of indicated antibodies.
L-selectin chimera decorated with N-linked sLex was purified from Jurkat cells co-transfected with the L-selectin chimera and fucosyl-transferase 3. This chimera supported rolling of E-selectin–transfected CHO cells (24). L-selectin and the Fc portion of IgG1 contain only N-linked glycosylation sites (8, 15, 58).
Immunoblotting revealed that at identical antibody concentrations, mAb CSLEX-1 preferentially bound to L-selectin sLex containing N-linked sLex and BSA-conjugated sLex but bound weakly to sLex on less acidic mucins (GP) and did not bind to sLex on the acidic, more highly sulfated mucins (F2B) (Fig. 1 a and b). The 2H5 mAb was found to recognize sLex on less acidic but not on more acidic mucins or N-linked structures; reactivity with BSA-sLex was weak but present (Fig. 1 a). In contrast, mAb 2F3 reacted strongly with sLex on all substrates tested (Fig. 1 a and b), suggesting that it can recognize sLex on glycolipids, both 6-sulfated and unsulfated sLex-bearing mucins, and glycoproteins containing N-linked sLex (48). The ability of this mAb to recognize sLex in a broad variety of contexts makes it the practical choice for studies that examine tissues or cells for sLex expression.
Treatment of Tissue Sections with Neuraminidase and O-sialoglycoprotease
To additionally characterize staining by these mAbs, freshly cut frozen sections of human mesenteric lymph node were treated with neuraminidase or O-sialoglycoprotease and then stained with one of the three sLex mAbs. One of the two serial sections of lymph node was treated with control medium and the other was treated with enzyme. Particular HEV were then identified in both sections and their staining with sLex mAbs was compared in the presence and absence of enzyme treatment.
Neuraminidase treatment of sections abolished staining of the three sLex mAbs, 2H5, 2F3, and CSLEX-1, but slightly increased HEV staining with MECA-79 (Fig. 2). O-sialoglycoprotease is an enzyme isolated from the bacterium Pasteurella hemolytica that selectively degrades O-sialomucins (1, 64) and reduces binding of lymphocytes to immobilized MECA-79 Ag by >90% (52). In keeping with the ability of this enzyme to cleave PNAd, O-sialoglycoprotease treatment of tissue sections greatly reduced the staining of HEV with MECA-79 (Fig. 3). Staining by mAb directed to the mucin-like region of CD34 (29) was reduced by O-sialoglycoprotease to the same extent as for mAb MECA-79 (data not shown). In contrast, O-sialoglycoprotease treatment of sections only partially removed HEV reactivity with 2F3 and 2H5 and left CSLEX-1 reactivity unchanged (Fig. 3). Results are illustrated for mesenteric lymph node to include CSLEX-1 mAb, which is negative on tonsil as described above; however, mAb staining and sensitivity to enzyme treatment were otherwise identical on tonsil (data not shown). Staining of HEV in O-sialoglycoprotease-treated sections with 2F3 and 2H5 was always of greater intensity than with MECA-79. These findings suggest that 2F3 and 2H5 recognize both a species of sLex associated with O-sialoglycoprotease–sensitive mucin-like glycoproteins and a species of sLex presented by O-sialoglycoprotease–resistant glycoproteins or glycolipids. As a control, neuraminidase and O-sialoglycoprotease treatment of sections had no effect on staining of a non-sLex control mAb recognizing PECAM-1 (not shown).
Figure 2.
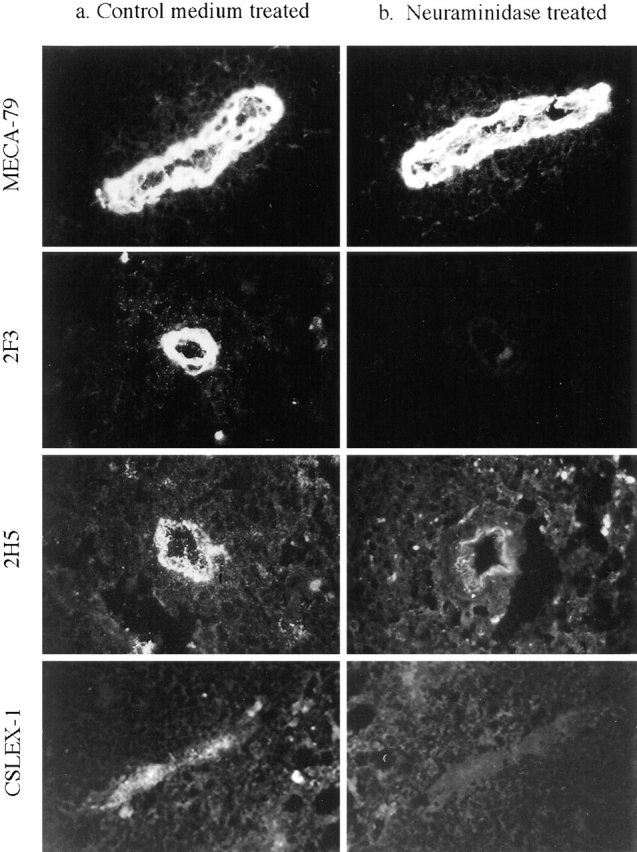
Binding of antibodies to HEV of neuraminidase-treated sections of human mesenteric lymph node. Freshly cut serial sections were treated for 1 h with (a) control medium or (b) neuraminidase, fixed, and stained with 40 μg/ml of indicated mAbs. Identical HEV were observed in each serial section to directly compare the effects of enzyme treatment. Photographs of each pair of serial sections were taken and printed using the same exposure times. Results were identical using tonsil sections except that CSLEX-1 did not stain tonsillar HEV.
Figure 3.
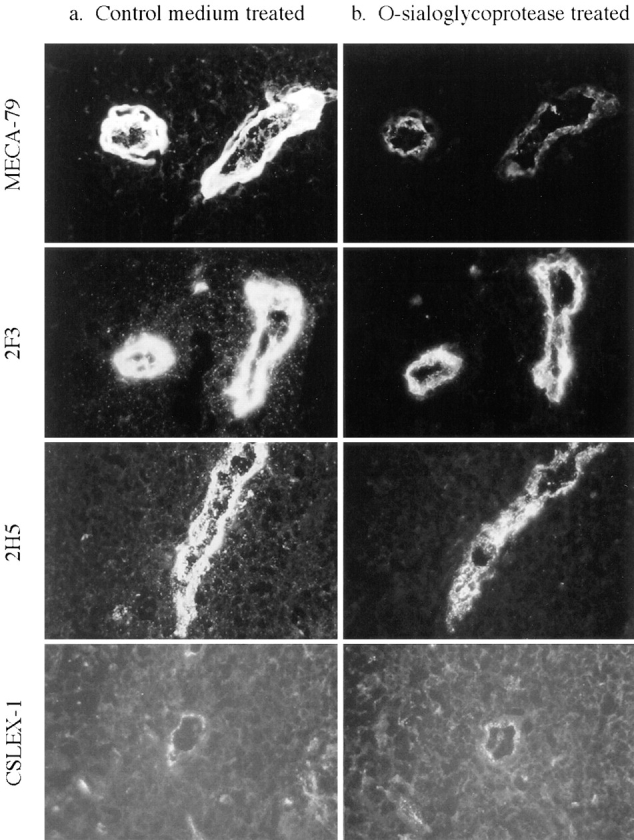
Binding of sLex antibodies to HEV of O-sialoglycoprotease–treated sections of human mesenteric lymph node. Freshly cut serial sections were treated for 2 h with (a) control medium or (b) O-sialoglycoprotease, fixed, and stained with 40 mg/ml of indicated sLex mAbs. Identical HEV were compared in each serial section and photographs of each set of serial sections were taken using identical exposure times. Results using tonsil sections were identical except that CSLEX-1 did not stain tonsillar HEV.
Non-PNAd Structures Resistant to O-sialoglycoprotease Support Lymphocyte Rolling
The results presented above suggested that a significant amount of sLex is present on HEV that is not recognized by MECA-79, but is detected using mAbs, 2F3 and 2H5. Whether this non-PNAd sLex can support lymphocyte rolling via L-selectin on HEV is not known. To investigate this question, we used a novel flow assay to study lymphocyte binding to the HEV of human frozen tonsil sections. Although sections of mesenteric lymph nodes also worked well in these assays, tonsil was chosen as the preferred rolling substrate because of the presence of large, distinctive HEV that could be easily identified in serial sections. Freshly cut sections of human tonsil were attached to glass slides and placed into a parallel plate flow chamber such that the tissue section comprised the floor of the chamber. HEV profiles in the sections could easily be identified by phase contrast microscopy. Human PBMC were introduced into the flow chamber at a shear stress of 0.75 dyn/cm2; this shear stress is permissive for efficient L-selectin tethering (23). Cells tethered to HEV under flow, and either became firmly adherent to the HEV or rolled along the wall of the HEV in the direction of flow. Once rolling cells reached the end of an HEV profile, they detached from the section and occasionally re-attached to an HEV further downstream. Most striking was the ability of cells to actually roll down the length of the HEV endothelial wall; this was particularly apparent for longitudinally cut HEV that were oriented in the direction of flow, as shown in Figs. 4 and 5. A shear strength of 0.75 dyn/cm2 was routinely used for cell attachment; at this shear strength, virtually all of the cell attachment to the section was to HEV profiles. At lower shear strengths in the range of 0.2–0.5 dyn/cm2, some tethering of lymphocytes was also seen to extracellular matrix associated with reticular fibers. Prior studies have shown that this binding likely represents interaction of lymphocytes with hyaluronan or tenascin (19, 20).
Figure 5.
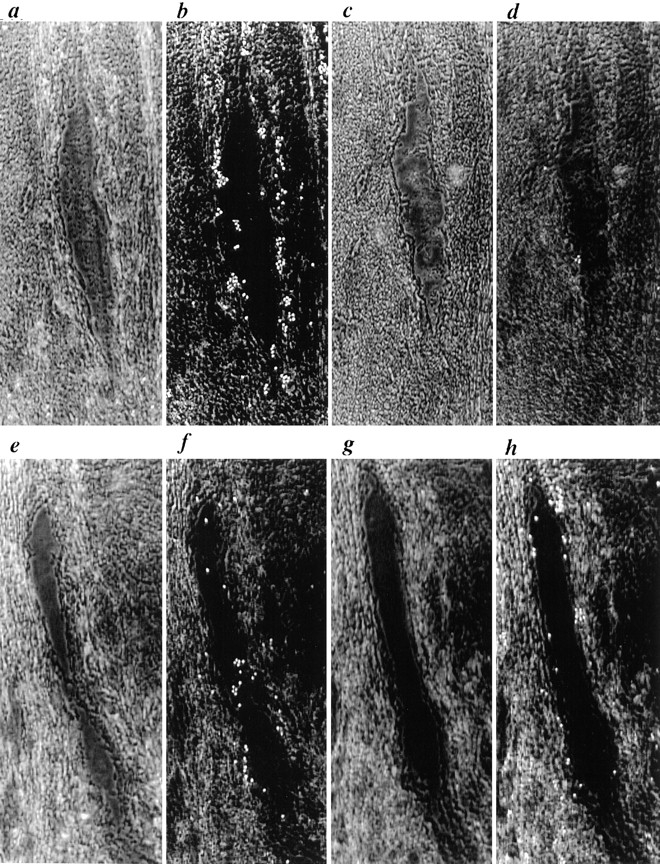
Binding of neuraminidase-treated PBMC under flow conditions to HEV of human tonsil sections treated with neuraminidase or O-sialoglycoprotease. To clearly delineate HEV boundaries, phase contrast images focused in the plane of the section taken before lymphocyte binding are shown in (a, c, e, and g). Binding of cells to HEV of serial sections treated for 1 h with (a and b) control medium or (c and d) neuraminidase or for 2 h with (e and f) control medium or (g and h) O-sialoglycoprotease. Binding to identical HEV in the control and enzyme-treated serial sections were examined in order to directly compare the effects of enzyme treatment. Flow parameters were identical to those used in Fig. 4. The direction of flow was from the bottom of the figure to the top.
Binding of PBMC to HEV under flow (Fig. 4) was only partially blocked by L-selectin mAb DREG-56 (Fig. 4 c), but completely blocked by EDTA (Fig. 4 d). A subset of lymphocytes express ligands for P-selectin and E-selectin (54, 60); staining of tonsil sections with mAbs revealed that most HEV expressed P-selectin (Table I). All selectin ligands require sialylation for activity (54). We, therefore, treated mononuclear cells with neuraminidase to eliminate any binding contribution by P-selectin or E-selectin (Fig. 4 g) and found that binding was then almost completely blocked by L-selectin mAb DREG-56 (Fig. 4 h). To examine the effects of neuraminidase and O-sialoglycoprotease on cell binding, one of two serial sections of human tonsil were treated with control medium and the other was treated with enzyme under the same conditions as used in Figs. 2 and 3. Binding of cells to profiles of the same HEV was then compared in the enzyme-treated and control-treated serial sections (Fig. 5). Binding of neuraminidase-treated cells (Fig. 5 b) was virtually eliminated by neuraminidase treatment of the section (Fig. 5 d). However, under conditions that almost completely removed MECA-79 reactivity (Fig. 3), treatment of sections with O-sialoglycoprotease only partially inhibited the binding of neuraminidase-treated cells to HEV (compare Fig. 5, f and h). In the experiment shown, 33 cells bound to the HEV treated with control medium and 21 cells bound to the HEV treated with O-sialoglycoprotease. In experiments with 10 different pairs of serial sections, binding to O-sialoglycoprotease–treated sections was 71 ± 11% of binding to control sections (Table III). Inclusion of DREG-56 mAb blocked lymphocyte binding to HEV after O-sialoglycoprotease treatment of sections, confirming that this binding remained L-selectin dependent (data not shown). Prior studies have demonstrated the presence of glycoprotein L-selectin ligands on arterial endothelium that are partially destroyed by heparin lyases (28); we, therefore, incubated sections with a combination of heparitinase I and O-sialoglycoprotease, but found no additional inhibition of binding from heparitinase treatment (see Fig. 7).
Table III.
Effect of O-sialoglycoprotease Treatment on Lymphocyte Binding to HEV*
| Section | Treatment | Number of lymphocytes bound to HEV | Percent of control binding , ± SD | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | Exp. 7 | Exp. 8 | Exp. 9 | Exp. 10 | |||||||||||||||
| Serial section 1 | Control medium | 33 | 40 | 23 | 45 | 47 | 36 | 37 | 28 | 33 | 31 | 100 | ||||||||||||
| Serial section 2 | O-sialo- | 21 | 33 | 17 | 26 | 37 | 29 | 24 | 19 | 28 | 17 | 71 ± 11 | ||||||||||||
| glycoprotease | ||||||||||||||||||||||||
The same HEV in serial sections was identified for comparison of cell binding.
Figure 7.
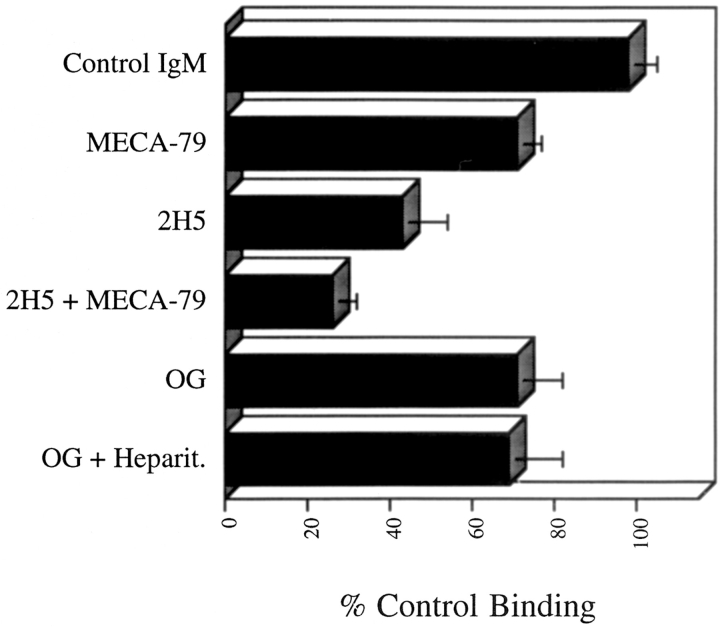
Effect of treatment of tonsil sections with sLex mAbs, O-sialoglycoprotease (OG), and heparitinase (Heparit.) on binding of neuraminidase-treated PBMC. Flow parameters for all experiments were identical to those used in Fig. 4. Binding to an individual HEV was assessed before and after incubation of sections with sLex mAb. Enzyme experiments were conducted as described in Fig. 5. Mean values of at least six experiments are shown with the standard deviation indicated by bars.
Because treatment of sections with O-sialoglycoprotease did not completely remove HEV staining with the MECA-79 mAb, we could not rule out the possibility that the remaining PNAd was contributing to lymphocyte binding. MECA-79 mAb was therefore used to block binding of neuraminidase-treated lymphocytes to both untreated and O-sialoglycoprotease–treated sections. When HEV were tested for lymphocyte binding both before and after infusion of blocking mAb, MECA-79 mAb reduced lymphocyte binding to HEV of untreated sections to 60–80% of control levels (Fig. 6 b). In the experiment shown, 62 cells bound to HEV before MECA-79 treatment (Fig. 6 a) and 44 cells bound after mAb treatment (Fig. 6 b). Infusion of a control CD31 IgM mAb in duplicate experiments had no effect on lymphocyte binding to HEV (data not shown). In sections treated with O-sialoglycoprotease, MECA-79 mAb did not affect binding of lymphocytes to HEV (Fig. 6 c and d). In the experiment shown, 44 cells bound to the HEV before mAb treatment (Fig. 6 c) and 46 cells bound after treatment (Fig. 6 d). Six experiments confirmed that although MECA-79 mAb did decrease binding of cells to untreated HEV, the mAb had no effect on lymphocyte binding to HEV treated with O-sialoglycoprotease (Table IV). Similar results were obtained when cells were accumulated at a lower shear stress of 0.38 dyn/cm2 (data not shown). With respect to the concentration of MECA-79 used for blocking, these experiments used MECA-79 at 50 μg/ml; additional experiments using MECA-79 mAb at a concentration of 100 μg/ml produced similar results (data not shown). In vitro flow studies have demonstrated >85% inhibition of lymphocyte binding to purified human PNAd using MECA-79 mAb at 20 μg/ml (52). The results of these experiments suggest that PNAd mediates only part of lymphocyte binding to untreated HEV and does not contribute to lymphocyte binding to HEV in O-sialoglycoprotease–treated sections.
Table IV.
Effect of MECA-79 mAb on Lymphocyte Binding to HEV*
| Section treatment | Ab treatment | Lymphocyte binding to HEV | Percent of binding ± SD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | |||||||||||
| Control medium | −MECA-79 | 62 | 56 | 40 | 47 | 60 | 48 | 100 | ||||||||
| Control medium | +MECA-79 | 44 | 34 | 31 | 34 | 45 | 32 | 71 ± 6.1 | ||||||||
| O-sialoglycoprotease | −MECA-79 | 44 | 47 | 38 | 66 | 29 | 41 | 100 | ||||||||
| O-sialoglycoprotease | +MECA-79 | 46 | 45 | 39 | 63 | 30 | 43 | 101 ± 4.3 | ||||||||
Binding to a particular HEV was counted before and after antibody addition.
sLex mAb 2H5 Inhibits Binding to Human HEV under Shear Flow
To determine if MECA-79-resistant lymphocyte binding in the flow assay to HEV was indeed mediated by sLex-like molecules, tonsil sections were treated with the sLex mAb 2H5. Whereas MECA-79 mAb reduced lymphocyte binding to HEV by 29 ± 6%, mAb 2H5 reduced binding by 57 ± 11%. Furthermore, a combination of the two mAbs reduced binding by 74 ± 6%. These results demonstrate that sLex-like molecules, distinct from PNAd, support L-selectin–mediated attachment of lymphocytes to human HEV.
Discussion
The adhesion of lymphocytes to the surface of HEV via L-selectin is a crucial step in lymphocyte recirculation. Although the only known ligands of L-selectin on HEV are mucin-like molecules, sLex-containing glycolipids and proteins have been shown to bind L-selectin in vitro (2, 12, 54). Previous immunohistochemical studies of the expression of sLex by HEV have produced conflicting reports, only some of which can be explained on the basis that different sLex mAbs were used for staining. For example, in studies using the mAb CSLEX-1, different groups found expression of sLex by both peripheral lymph nodes and mucosal lymphoid tissue (47), by peripheral lymph nodes but not mucosal lymphoid tissue (50), and by neither peripheral lymph nodes nor mucosal lymphoid tissue, except for a faint staining of peripheral lymph nodes at high antibody concentrations (57). Similar disagreement exists about the expression of the structurally related selectin ligand sLea (12), which has been reported to be expressed by HEV (30, 50) and not expressed by HEV (57).
In this study, we stained a common set of tissues with a panel of mAbs against sLex and related structures. We found that three sLex mAbs, CSLEX-1, 2F3, and 2H5, stained the HEV of lymphoid organs in two distinct patterns. 2F3 and 2H5 stained the HEV of all lymphoid tissues tested at a constitutively high level that was not increased by the presence of inflammation. In contrast, CSLEX-1 weakly stained the HEV of mucosal lymphoid tissues and a subset of HEV in mesenteric lymph nodes, and this staining was enhanced when these organs were inflamed. Staining of HEV with mAb 2H5 has been previously reported (57). Our finding that 2F3 mAb stains peripheral lymph node HEV is in agreement with a recent report (45). However, this study failed to detect CSLEX-1 staining of HEV, most likely because tissue sections were fixed with paraformaldehyde before staining. Indeed, the staining pattern we observed with CSLEX-1 mAb was found to depend upon the method of tissue fixation. HEV staining with CSLEX-1 has also been shown to depend on the concentration of mAb used in staining; in one study, 1 μg/ml mAb produced no staining of HEV in the lymph node but 10 μg/ml mAb did produce positive lymph node staining (57). Variation among reports (47, 50, 57) on abilities of CSLEX-1 mAb to stain HEV are most likely related to both the concentration of mAb and the different methods of fixation used in these studies. mAbs recognizing sLea (CSLEA-1) and di-fucosyl sLex (FH6) did not stain the HEV of any tissue tested, in agreement with studies by Sawada et al. (57). The mAb HECA-452 stained HEV and non-HEV structures in a pattern that was the sum of the sLex staining pattern with 2H5 and the sLea staining pattern with CSLEA-1, with the exception that lymphocytes in the skin were also positive (51). MECA-79 mAb stained the HEV of both peripheral and mucosal lymphoid tissues, although staining of peripheral lymphoid HEV was brighter, in agreement with earlier reports (42, 63).
Immunoblotting of immobilized mucins bearing sLex, glycoprotein bearing N-linked sLex, and BSA chemically conjugated to sLex showed that mAb CSLEX-1 preferentially bound N-linked and BSA-sLex, reacted weakly with a population of sLex-bearing mucins, and did not react with acidic mucin fractions known to contain sLex sulfated on the 6 position of GlcNAc. These findings are consistent with recent studies showing that CSLEX-1 recognizes sLex but not 6-sulfated sLex (45). In contrast, 2H5 mAb was found to recognize sLex on less acidic mucins but not on more acidic mucins or on N-linked structures, and mAb 2F3 recognized sLex on all substrates tested. The mAb 2F3, therefore, has the broadest sLex specificity, recognizing sLex when conjugated to glycolipids (57), mucins containing sLex and 6-sulfo-sLex, and sLex N-linked to glycoproteins. In support of our findings that the 2F3 mAb binds to forms of sLex not recognized by CSLEX-1, skin-infiltrating adult T cell leukemia cells have been shown to express high levels of sLex as measured by reactivity with the 2F3 mAb, but these cells exhibited no reactivity with the CSLEX-1 mAb (27).
The 2H5 and CSLEX-1 mAbs are known to require sialic acid for binding (26, 57). In agreement with these reports, treatment of tissue sections with neuraminidase completely eliminated binding of the 2F3, 2H5, and CSLEX-1 mAbs to HEV. Binding of MECA-79 to HEV was slightly enhanced by neuraminidase treatment, consistent with in vitro studies showing that binding of MECA-79 to its epitope is increased by neuraminidase treatment, although desialylation destroys L-selectin ligand activity (9, 32, 55).
O-sialoglycoprotease recognizes O-linked carbohydrate groups and cleaves the polypeptide chain nearby (43). It cleaves mucin-like surface molecules such as CD43 and glycophorin A, and selectively cleaves mucin-like regions of CD34, CD44, and CD45. Afterwards, the membrane-proximal nonmucin–like regions of CD34 and CD45 remain on the cell surface, and the membrane-distal nonmucin–like region of CD44 is released (1, 29, 64). Nonmucin proteins that are O-glycosylated, such as fetuin, are not cleaved (43). The NH2-terminal but not COOH-terminal threonine and proline-rich repeats of the P-selectin glycoprotein ligand are cleaved by O-sialoglycoprotease (40, 46). It appears safe to conclude that all glycoproteins cleaved by O-sialoglycoprotease contain mucin-like regions; however, by no means will all proteins containing O-linked carbohydrate be cleaved. O-sialoglycoprotease treatment of immobilized PNAd that was purified with MECA-79 mAb from tonsil reduces its ability to support L-selectin–dependent lymphocyte binding by >90% (52). Here, treatment of sections with O-sialoglycoprotease almost completely eliminated staining with MECA-79 mAb, and completely eliminated the MECA-79 mAb-inhibitable component of lymphocyte binding. Thus, almost all MECA-79 antigen is associated with mucin-like proteins. By contrast, treatment of sections with O-sialoglycoprotease had no effect on CSLEX-1 staining, and only partially decreased staining with 2H5 and 2F3 mAb. The results with CSLEX-1 are in agreement with immunoblotting results that show that this mAb recognizes sLex-bearing mucins very poorly, and preferentially binds to sLex presented on different structures such as N-linked glycoproteins. The partial decrease in HEV staining with the 2F3 and 2H5 mAbs with O-sialoglycoprotease treatment indicated that the high levels of HEV staining we observed with these mAbs represent two pools of sLex, one associated with mucin-like glycoproteins, and another associated with glycolipids or O-sialoglycoprotease–resistant glycoproteins. The recognition of sLex associated with mucin-like glycoproteins by 2F3 and 2H5 is consistent with our immunoblotting results that show recognition of sLex-bearing CF mucins by these two mAbs. Earlier studies have shown that the 2F3 and 2H5 mAbs recognize glycoprotein-linked sLex, although not necessarily presented by mucins. The 2F3 mAb recognized both sLex presented on glycolipids and a distinct set of O-linked oligosaccharides from glycoproteins derived from human cancer tissues, and 2H5 immunoprecipitated a group of glycoproteins from preparations of human lymph node in addition to reaction with sLex on glycolipids (48, 57).
The finding that 2F3 and 2H5 mAbs recognize a pool of HEV sLex that is resistant to O-sialoglycoprotease, and thus distinct from the mucin-associated carbohydrate structures recognized by MECA-79 mAb, raised the possibility that other molecules bearing sLex on HEV may act as L-selectin ligands. The 2H5 mAb recognizes a functional L-selectin ligand on HEV as shown by its ability to partially block the adhesion of lymphocytes to sections of human lymph node (57). This functional effect could result either from blocking by 2H5 of the same mucin-sLex sites as MECA-79, or from blocking of additional L-selectin ligand sites recognized by 2H5, but not by MECA-79. The latter possibility is supported by the finding that 2H5 immunoprecipitates a set of glycoproteins bearing O-linked sLex from lymph nodes that is distinct from the set of proteins immunoprecipitated from the same tissue using MECA-79 (9, 57). 2F3 has also recently been reported to block adhesion of L-selectin–transfected cells to human HEV, although the dependence of this effect on L-selectin was not clearly established (45).
To examine the possibility that 2F3 and 2H5 staining of HEV may reflect the presence of ligands for L-selectin that are related to sLex but distinct from PNAd, we used a novel rolling assay in which peripheral blood lymphocytes attached under flow to the HEV of sections prepared from frozen human tonsil. In the Stamper-Woodruff assay (61), lymphocytes are overlaid onto frozen sections of human lymph nodes, and incubated with gentle agitation. Lymphocyte binding to morphologically distinct HEV is then examined by light microscopy. The flow assay used in this study resembles the Stamper-Woodruff assay in that it uses frozen sections, but has the additional advantages of selectively visualizing interactions that are capable of mediating tethering and rolling under flow, and allowing use of defined shear stresses. The initial tethering and rolling steps of lymphocyte adhesion to HEV can be directly observed, and are therefore separable from steps involving firm attachment. Furthermore, our assay is suitable for use on human specimens and allows in vitro manipulations. Intravital microscopy offers many advantages for studies of lymphocyte homing (4, 39, 65), but is limited to animal models and to the degree of experimental manipulation. In contrast to intravital microscopy, in both the standard and flow version of the Stamper-Woodruff assay, it is possible that the cells attaching to HEV are binding to ligands on the cell surface of the HEV or to molecules in intracellular compartments. P-selectin, usually confined to storage vesicles in unstimulated endothelium, may mediate some binding of lymphocytes in this assay; E-selectin, often present on the surface of stimulated HEV, could also contribute to binding. However, binding to both P- and E-selectin was eliminated in our studies by pretreatment of the lymphocytes with sialidase. Furthermore, the 2F3 and 2H5 mAbs to sLex clearly stained the surface of HEV, with the greatest concentration of staining present on the lumenal surface of the HEV. Despite possible limitations, the Stamper-Woodruff assay has been remarkably robust, and has enabled identification of all known receptors and ligands involved in homing to date, including L-selectin, PNAd, the integrin α4β7, and MAdCAM-1 (16, 60).
Using the flow assay described here, we found that binding of neuraminidase-treated PBMC to HEV was completely blocked by EDTA and by the L-selectin mAb DREG-56, confirming that L-selectin alone contributed to binding under these conditions. Neuraminidase treatment of sections completely blocked lymphocyte binding to HEV, in agreement with studies done using Stamper-Woodruff assays in the mouse (55). In contrast, treatment of sections with O-sialoglycoprotease under conditions that virtually eliminated MECA-79 staining, only partially inhibited cell attachment to HEV. Cell binding to O-sialoglycoprotease–treated sections averaged 71% of binding to sections treated with control medium. Incubation of O-sialoglycoprotease–treated sections with MECA-79 mAb did not further diminish lymphocyte binding to HEV, suggesting that the residual MECA-79 antigen present after enzyme treatment did not significantly contribute to lymphocyte binding. This functional pool of O-sialoglycoprotease–resistant L-selectin ligands may include the sLex detected by mAb 2F3 and 2H5 on O-sialoglycoprotease– treated sections.
Studies on untreated HEV sections also supported a distinction between the L-selectin ligands associated with 2H5 epitopes and MECA-79 epitopes, and showed that the former are more functionally important. Approximately one-half of lymphocyte binding through L-selectin was blocked by 2H5 mAb, one-quarter was blocked by MECA-79 mAb, and blocking by these mAbs was additive. These results clearly demonstrate the presence of sLex-like L-selectin ligands on HEV that are capable of binding lymphocytes and that are distinct from the moieties recognized by MECA-79. In agreement with our hypothesis that 2H5-reactive material can support lymphocyte binding, this mAb has recently been shown to block binding of L-selectin–transfected cells to HEV of human lymph node sections in classical Stamper-Woodruff assays (45).
We conclude from these studies that L-selectin ligands distinct from those recognized by MECA-79 exist on human HEV, and that these ligands are capable of supporting rolling lymphocyte adhesion in flow. Furthermore, structural moieties that are O-glycoprotease resistant, and that bear sLex and L-selectin ligand activity but lack MECA-79 antigen, are present on HEV. Our studies dissociate for the first time on HEV ligands for L-selectin that are O-glycoprotease resistant from ligands for L-selectin that are associated with mucin-like counter receptors and MECA-79 antigen. Our studies have been confined to human tissue. Inhibition by MECA-79 mAb of the Woodruff-Stamper assay in the mouse is greater than in the human yet still incomplete, as is inhibition of homing to peripheral lymph nodes in the mouse (14, 38, 44, 62, 63). Therefore, two classes of L-selectin ligands may also be present in mouse, although that associated with MECA-79 antigen appears to be more predominant.
Historically, two independent approaches have been used to identify ligands important in homing on peripheral lymph node HEV. The first used MECA-79 mAb (62), and the second used L-selectin itself as a probe for ligand activity (66). The mAb MECA-79 has been an extremely useful tool in characterizing this ligand both in the mouse and human. In the course of work with MECA-79 mAb, it was described as “an anti-peripheral lymph node addressin mAb” and peripheral node addressin was defined as “the isolated complex of MECA-79-reactive proteins,” even in studies that directly compared materials affinity isolated with MECA-79 mAb and L-selectin (32). The current study demonstrates that MECA-79 antigen and material that functions in L-selectin-dependent rolling on HEV sections are overlapping but distinct moieties. This complexity in L-selectin ligands is in agreement with the complexity of O- and N-glycans in general, and with the presence of an L-selectin ligand on leukocytes, despite absence of reactivity of MECA-79 mAb with leukocytes (5, 24, 49, 59). Our work suggests that it would be best to return to the original, functional definition of addressins (63), and to associate the term peripheral node addressin with L-selectin ligand activity rather than MECA-79 antigen.
Acknowledgments
We would like to thank R. Kannagi, P. Terasaki, S. Hakamori, E. Butcher, M. Hemler, and T. K. Kishimoto for contributing antibodies. We also thank G. Lamblin for providing sLex-bearing CF mucins, L. Lasky and S. Watson for supplying L-selectin/human IgG1 chimera, R. Lobb for contributing E-selectin–transfected CHO cells, and R. Alon for advice and assistance.
This work was supported by National Institutes of Health grant CA31798.
Abbreviations used in this paper
- CF
cystic fibrosis
- HEV
high endothelial venules
- PBMC
peripheral blood mononuclear cells
- PNAd
peripheral node addressin
- TBST
Tween-20
Footnotes
Address all correspondence to Timothy A. Springer, The Center for Blood Research and Harvard Medical School, Department of Pathology, 200 Longwood Avenue, Boston, MA 02115. Tel.: (617) 278-3200. Fax: (617) 278-3232.
References
- 1.Abdullah KM, Udoh EA, Shewen PE, Mellors A. A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect Immun. 1993;60:56–62. doi: 10.1128/iai.60.1.56-62.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alon R, Feizi T, Yuen C-T, Fuhlbrigge RC, Springer TA. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J Immunol. 1995;154:5356–5366. [PubMed] [Google Scholar]
- 3.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 4.Bargatze RF, Butcher EC. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 7.Baumhueter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin, CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- 8.Beale D, Feinstein A. Structure and function of the constant regions of immunoglobulins. Q Rev Biophys. 1976;9:135–180. doi: 10.1017/s0033583500002390. [DOI] [PubMed] [Google Scholar]
- 9.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Lea and sialyl Lexis recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 11.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg EL, Magnani J, Warnock RA, Robinson MK, Butcher EC. Comparison of L-selectin and E-selectin ligand specificities: the L-selectin can bind the E-selectin ligands sialyl Lex and sialyl Lea . Biochem Biophys Res Commun. 1992;184:1048–1055. doi: 10.1016/0006-291x(92)90697-j. [DOI] [PubMed] [Google Scholar]
- 13.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 14.Bergelson JM, St. John NF, Kawaguchi S, Pasqualini R, Berdichevsky F, Hemler ME, Finberg RW. The I domain is essential for echovirus 1 interaction with VLA-2. Cell Adhes Commun. 1994;2:455–464. doi: 10.3109/15419069409004455. [DOI] [PubMed] [Google Scholar]
- 15.Bowen BR, Nguyen T, Lasky LA. Characterization of a human homologue of the murine peripheral lymph node homing receptor. J Cell Biol. 1989;109:421–427. doi: 10.1083/jcb.109.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 17.Carlos T, Kovach N, Schwartz B, Rosa M, Newman B, Wayner E, Benjamin C, Osborn L, Lobb R, Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991;77:2266–2271. [PubMed] [Google Scholar]
- 18.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark RA, Alon R, Springer TA. CD44 and hyaluronan-dependent rolling interactions of lymphocytes on tonsillar stroma. J Cell Biol. 1996;134:1075–1087. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA, Erickson HP, Springer TA. Tenascin supports lymphocyte rolling. J Cell Biol. 1997;137:755–765. doi: 10.1083/jcb.137.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diacovo T, Roth SJ, Morita CT, Rosat J-P, Brenner MB, Springer TA. Interactions of human αβ and γδ T lymphocyte subsets in shear flow with E-selectin and P-selectin. J Exp Med. 1996;183:1193–1203. doi: 10.1084/jem.183.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duijvestijn AM, Horst E, Pals ST, Rouse BN, Steere AC, Picker LJ, Meijer CLM, Butcher EC. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–155. [PMC free article] [PubMed] [Google Scholar]
- 23.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 24.Fuhlbrigge RC, Alon R, Puri KD, Lowe JB, Springer TA. Sialylated, fucosylated ligands for L-selectin expressed on leukocytes mediate tethering and rolling adhesions in physiologic flow conditions. J Cell Biol. 1996;135:837–848. doi: 10.1083/jcb.135.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukushi Y, Nudelman E, Levery SB, Hakomori S-I. Novel fucolipids accumulating in human adenocarcinoma. J Biol Chem. 1984;259:10511–10517. [PubMed] [Google Scholar]
- 26.Fukushima K, Hiroto M, Terasaki PI, Wakisaka A. Characterization of sialosylated Lewis X as a new tumor-associated antigen. Cancer Res. 1984;44:5279–5285. [PubMed] [Google Scholar]
- 27.Furukawa Y, Tara M, Ohmori K, Kannagi R. Variant type of sialyl Lewis X antigen expressed on adult T cell leukemia cells is associated with skin involvement. Cancer Res. 1994;54:6533–6538. [PubMed] [Google Scholar]
- 28.Giuffre L, Cordey AS, Monai N, Tardy Y, Schapira M, Spertini O. Monocyte adhesion to activated aortic endothelium: role of L-selectin and heparan sulfate proteoglycans. J Cell Biol. 1997;136:945–956. doi: 10.1083/jcb.136.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greaves, M.F., I. Titley, S.M. Colman, H.-J. Buhring, L. Campos, G.L. Castoldi, F. Garrido, G. Gaudernack, J.-P. Girard, J. Ingles-Esteve et al. 1995. Report on the CD34 cluster workshop. In Leucocyte Typing V: White Cell Differentiation Antigens. S. Schlossman, L. Boumsell, W. Gilks, J. Harlan, T. Kishimoto, C. Morimoto, J. Ritz, S. Shaw, R. Silverstein, T. Springer, T. Tedder, and R. Todd, editors. Oxford University Press, New York. 840–846.
- 30.Green PJ, Tamatani T, Watanabe T, Miyasaka M, Hasegawa A, Kiso M, Yuen C-T, Stoll MS, Feizi T. High affinity binding of the leucocyte adhesion molecule L-selectin to 3′-sulphated-Lea and -Lexoligosaccharides and the predominance of sulphate in this interaction demonstrated by binding studies with a series of lipid-linked oligosaccharides. Biochem Biophys Res Commun. 1992;188:244–251. doi: 10.1016/0006-291x(92)92376-9. [DOI] [PubMed] [Google Scholar]
- 31.Hemmerich S, Rosen SD. 6′-Sulfated sialyl Lewis x is a major capping group of GlyCAM-1. Biochemistry. 1994;33:4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- 32.Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai Y, Lasky LA, Rosen SD. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature. 1993;361:555–557. doi: 10.1038/361555a0. [DOI] [PubMed] [Google Scholar]
- 34.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasky LA, Singer MS, Dowbenko D, Imai Y, Henzel WJ, Grimley C, Fennie C, Gillett N, Watson SR, Rosen SD. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992;69:927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 39.Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- 40.Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 41.Lo-Guidice J-M, Wieruszeski J-M, Lemoine J, Verbert A, Roussel P, Lamblin G. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J Biol Chem. 1994;269:18794–18813. [PubMed] [Google Scholar]
- 42.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 43.Mellors A, Lo R. O-sialoglycoprotease from pasteurella haemolytica. Methods Enzymol. 1995;248:728–740. doi: 10.1016/0076-6879(95)48049-8. [DOI] [PubMed] [Google Scholar]
- 44.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin: an inducible endothelial antigen involved in lymphocyte homing. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsuoka C, Kawakami-Kimura N, Kasugai-Sawada M, Hiraiwa N, Toda K, Ishida H, Kiso M, Hasegawa A, Kannagi R. Sulfated sialyl Lewis X, the putative L-selectin ligand, detected on endothelial cells of high endothelial venules by a distinct set of anti-sialyl Lewis X antibodies. Biochem Biophys Res Commun. 1997;230:546–551. doi: 10.1006/bbrc.1996.6012. [DOI] [PubMed] [Google Scholar]
- 46.Moore KL, Patel KD, Bruehl RE, Fugang L, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munro JM, Lo SK, Corless C, Robertson MJ, Lee NC, Barnhill RL, Weinberg DS, Bevilacqua MP. Expression of sialyl-Lewis X, an E-selectin ligand, in inflammation, immune processes, and lymphoid tissues. Am J Pathol. 1992;141:1397–1408. [PMC free article] [PubMed] [Google Scholar]
- 48.Ohmori K, Takada A, Ohwaki I, Takahashi N, Furukawa Y, Maeda M, Kiso M, Hasegawa A, Kannagi M, Kannagi R. A distinct type of sialyl Lewis X antigen defined by a novel monoclonal antibody is selectively expressed on helper memory T cells. Blood. 1993;82:2797–2805. [PubMed] [Google Scholar]
- 49.Oxley SM, Sackstein R. Detection of an L-selectin ligand on a hematopoietic progenitor cell line. Blood. 1994;84:3299–3306. [PubMed] [Google Scholar]
- 50.Paavonen T, Renkonen R. Selective expression of sialyl-Lewis X and Lewis A epitopes, putative ligands for L-selectin, on peripheral lymph-node high endothelial venules. Am J Pathol. 1992;141:1259–1264. [PMC free article] [PubMed] [Google Scholar]
- 51.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin–homing T cells. Nature. 1991;349:796–798. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 52.Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen SD. Cell surface lectins in the immune system. Semin Immunol. 1993;5:237–247. doi: 10.1006/smim.1993.1028. [DOI] [PubMed] [Google Scholar]
- 54.Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 55.Rosen SD, Singer MS, Yednock TA, Stoolman L M. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science. 1985;228:1005–1007. doi: 10.1126/science.4001928. [DOI] [PubMed] [Google Scholar]
- 56.Roth SJ, Carr MW, Rose SS, Springer TA. Characterization of transendothelial chemotaxis of T lymphocytes. J Immunol Methods. 1995;100:97–116. doi: 10.1016/0022-1759(95)00208-1. [DOI] [PubMed] [Google Scholar]
- 57.Sawada M, Takada A, Ohwaki I, Takahashi N, Tateno H, Sakamoto J, Kannagi R. Specific expression of a complex sialyl Lewis X antigen on high endothelial venules of human lymph nodes: possible candidate for L-selectin ligand. Biochem Biophys Res Commun. 1993;193:337–347. doi: 10.1006/bbrc.1993.1629. [DOI] [PubMed] [Google Scholar]
- 58.Siegelman MH, Weissman IL. Human homologue of mouse lymph node homing receptor: evolutionary conservation at tandem cell interaction domains. Proc Natl Acad Sci USA. 1989;86:5562–5566. doi: 10.1073/pnas.86.14.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon SI, Rochon YP, Lynam EB, Smith CW, Anderson DC, Sklar LA. β2-integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood. 1993;82:1097–1106. [PubMed] [Google Scholar]
- 60.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 61.Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Streeter PR, Lakey-Berg E, Rouse BTN, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 64.Sutherland DR, Abdullah KM, Cyopick P, Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. . J Immunol. 1992;148:1458–1464. [PubMed] [Google Scholar]
- 65.von Andrian UH, Hansell P, Chambers JD, Berger EM, Filho IT, Butcher EC, Arfors KE. L-selectin function is required for β2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol. 1992;263:H1034–H1044. doi: 10.1152/ajpheart.1992.263.4.H1034. [DOI] [PubMed] [Google Scholar]
- 66.Watson S, Imai Y, Fennie C, Geoffroy JS, Rosen SD, Lasky LA. A homing receptor-IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990;110:2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183(2):589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]