Abstract
Endocytosis and intracellular transport of ricin were studied in stable transfected HeLa cells where overexpression of wild-type (WT) or mutant dynamin is regulated by tetracycline. Overexpression of the temperature-sensitive mutant dynG273D at the nonpermissive temperature or the dynK44A mutant inhibits clathrin-dependent endocytosis (Damke, H., T. Baba, A.M. van der Blieck, and S.L. Schmid. 1995. J. Cell Biol. 131: 69–80; Damke, H., T. Baba, D.E. Warnock, and S.L. Schmid. 1994. J. Cell Biol. 127:915–934). Under these conditions, ricin was endocytosed at a normal level. Surprisingly, overexpression of both mutants made the cells less sensitive to ricin. Butyric acid and trichostatin A treatment enhanced dynamin overexpression and increased the difference in toxin sensitivity between cells with normal and mutant dynamin. Intoxication with ricin seems to require toxin transport to the Golgi apparatus (Sandirg, K., and B. van Deurs. 1996. Physiol. Rev. 76:949–966), and this process was monitored by measuring the incorporation of radioactive sulfate into a modified ricin molecule containing a tyrosine sulfation site. The sulfation of ricin was much greater in cells expressing dynWT than in cells expressing dynK44A. Ultrastructural analysis using a ricin-HRP conjugate confirmed that transport to the Golgi apparatus was severely inhibited in cells expressing dynK44A. In contrast, ricin transport to lysosomes as measured by degradation of 125I-ricin was essentially unchanged in cells expressing dynK44A. These data demonstrate that although ricin is internalized by clathrin-independent endocytosis in cells expressing mutant dynamin, there is a strong and apparently selective inhibition of ricin transport to the Golgi apparatus. Also, in cells with mutant dynamin, there is a redistribution of the mannose-6-phosphate receptor.
Clathrin-dependent endocytosis has been characterized in detail during recent years, and a number of molecules required for this process has been identified (for reviews see references 39, 49, 61, 64). Recently, the 100-kD GTPase dynamin was shown to play an important role in clathrin-mediated endocytosis (for reviews see references 9, 13, 68, 77). Three mammalian dynamin isoforms, dynI (neuron-specific) (62), dynII (ubiquitously expressed) (7, 65), and dynIII (found in testis, brain and lung) (6, 43) have been found so far. Transiently and stable transfected cell lines overexpressing GTPase-defective mutants of dynI have recently been used to study its function in vivo (10, 26, 69). These studies revealed that the GTPase activity of dynamin is necessary for clathrin-dependent endocytosis, and a model for the function of dynamin in endocytosis has been proposed. The model suggests that after recruitment of GDP-bound dynamin to clathrin-coated pits, a GDP–GTP exchange induces the distribution of dynamin in rings surrounding and constricting the neck of the pit. Finally, a second conformational change after GTP hydrolysis could be involved in the formation of the coated vesicle (9, 77). In addition to uptake by clathrin-dependent endocytosis, cells can internalize membrane, ligands, and fluid also by clathrin-independent mechanisms (for reviews see references 33, 54, 72). Several treatments, such as K+ -depletion or cytosol acidification, have been used to interfere selectively with clathrin- dependent endocytosis, and the cells thus treated can still endocytose bulk membrane markers (41, 56). Furthermore, clathrin-independent endocytosis has been demonstrated without such perturbations in HEp-2 cells (23). In these cells, clathrin-dependent and -independent endocytic pathways seem to merge at the endosomal level (24).
An alternative approach to study clathrin-independent endocytosis is to use stable cell lines where the overexpression of either wild-type dynamin or mutant dynamin can be regulated by tetracycline. The substitution of lysine 44 (located in the nucleotide-binding motif) by an alanine generates a dynamin molecule defective in GTP binding and hydrolysis that selectively blocks clathrin-mediated endocytosis (10). Furthermore, substitution of glycine 273 with aspartic acid leads to a temperature-sensitive mutant of dynamin that when overexpressed inhibits clathrin- dependent endocytosis at the nonpermissive temperature (11). Although clathrin-dependent uptake is strongly inhibited in the cells expressing dynamin mutant, bulk membrane markers are still endocytosed (10, 11, 74). Thus, these cells can be used to study the endocytic pathway used by different molecules, the role of clathrin-dependent endocytosis, and the importance of dynamin for intracellular trafficking.
Ricin is a plant toxin that consists of two polypeptide chains linked by a disulfide bond. The B-chain is responsible for the binding to glycolipids and glycoproteins with terminal galactose (44), and the A-chain inhibits protein synthesis enzymatically (14, 15). Intoxication with ricin requires its endocytosis (53), its transport to the Golgi apparatus (70, 71), and its subsequent retrograde transport to the endoplasmic reticulum from where translocation to the cytosol may occur (75, 76). In this study we have used ricin as a bulk membrane marker for endocytosis, and modified ricins carrying a sulfation site with or without N-glycosylation sites as markers to monitor intracellular transport to the Golgi. Furthermore, we have used ricin toxicity as an indicator of toxin translocation to the cytosol. When clathrin-dependent endocytosis is inhibited by several methods, ricin endocytosis continues, although to a lower extent (56). In agreement with this we found that when clathrin-dependent endocytosis was blocked by overexpression of mutant dynamin (dynK44A or dynG273D), ricin was still endocytosed. Internalized ricin can be recycled to the cell surface, degraded in the lysosomes, or transported to the trans-Golgi apparatus (for review see reference 55). We show here that there was no significant reduction of ricin degradation when mutant dynamin was overexpressed. However, we found that transport of endocytosed ricin to the Golgi apparatus as well as intoxication with ricin were strongly reduced. The inhibition of transport from endosomes to the Golgi apparatus was not restricted to ricin. Thus, immunofluorescence studies revealed that at steady state the distribution of the mannose-6-phosphate receptor (M6PR)1 after expression of mutant dynamin was also changed. Taken together, these results suggest that overexpression of dominant-negative mutants of dynI directly or indirectly affect endosome to Golgi transport.
Materials and Methods
Reagents
Tetracycline, puromycin, geneticin, pronase, type VIA HRP, butyric acid, nocodazole, ricin, transferrin, cycloheximide, bafilomycin A1, Hepes, and lactose were obtained from Sigma Chemical Co. (St. Louis, MO). Trichostatin A (TSA) was from Wako Chemicals GmbH (Neuss, Germany). [3H]Leucine, Na2 35SO4, and Na125I were obtained from the Radiochemical Centre (Amersham International, Buckinghamshire, UK). [35S]Methionine was from NEN Research Products (Wilmington, DE). Brefeldin A was obtained from Epicentre Technologies (Madison, WI). Ricin and transferrin were labeled by the iodogen method (16) to a specific activity of 30,000–40,000 and 20,000–30,000 cpm/ng respectively. Ricin-HRP conjugates were prepared by the SPDP method as previously described (70).
Cells
The HeLa cell line (tTA-HeLa [19]) stably transformed with the cDNAs for dynWT, dynK44A, and dynG273D were cultured as previously described (10–12). Briefly, the cells were grown in Falcon (Franklin Lakes, NJ) or Nunc (Naperville, IL) flasks and maintained in DME (Flow Laboratories, Irvine, Scotland) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 400 μg/ml geneticin, 200 ng/ml puromycin, and 1 μg/ml tetracycline. For experiments, dynWT and dynK44A cells (4–6 × 104 cells/ml) were seeded with and without tetracycline and used 48 h later. In some experiments, butyric acid (2 mM) or TSA (0.1 or 1 μM) were added to the growth medium 24 h before the start of the experiment. For experiments, dynG273D cells were grown with and without tetracycline for 3 d at 30°C.
Measurements of Endocytosis and Degradation
Endocytosis of transferrin was measured after a 5-min incubation with 125I-transferrin (∼100 ng/ml) in Hepes medium essentially as described by Ciechanover et al. (5). After incubation with transferrin, the cells were washed three times with ice-cold Hepes medium and treated with pronase (2 mg/ml) in Hepes medium for 1 h at 0°C. The mixture was transferred to Eppendorf tubes and centrifuged for 2 min. Pellet and supernatant were separated and the radioactivity was measured. Endocytosed ricin was measured as the amount of 125I-labeled toxin that could not be removed by incubating the cells with a 0.1 M lactose solution for 1 h at 4°C (52). Degradation of ricin was measured as the amount of radioactivity that could not be precipitated by trichloroacetic acid after 2 h (52).
Measurements of Protein Synthesis
Protein synthesis was measured by incubating the cells in a Hepes medium (no unlabeled leucine) with 1–2 μCi/ml [3H]leucine for 30 min at 37°C. The medium was then removed and 5% (wt/vol) trichloroacetic acid was added. 10 min later, the cells were washed once with the same solution. Finally, the cells were solubilized in KOH (0.1 M) and the acid-precipitable radioactivity was measured. The results are expressed in percent of [3H]leucine incorporated in cells incubated without toxin. Deviations between duplicates did not vary by more than 10%.
Measurements of Protein Secretion
HeLa cells were washed in DME without methionine (Flow Laboratories) and then incubated with [35S]methionine (∼40 μCi/ml) in the same medium. After 15 min, the cells were washed and further incubated with Hepes medium containing FCS (5%) and methionine (4 mM) for 1 h. Trichloroacetic acid was then added to the cells and the medium, the precipitates were dissolved in KOH (0.1 M), and the radioactivity was measured by liquid scintillation.
Sulfation of Ricin A-sulf-1 and Ricin A-sulf-2
Ricin A-sulf-1 and ricin A-sulf-2 reconstituted with ricin B (ricin sulf-1 and ricin sulf-2, respectively) were produced, purified, and reconstituted as recently described (48). Ricin A-sulf-1 contains a sulfation site, whereas ricin A-sulf-2 contains both a sulfation site and glycosylation sites. The cells were washed in DME without sulfate and incubated with 100 μCi/ml Na35SO4 in the same medium. After 4 h, ricin sulf-1 or ricin sulf-2 (∼200 ng/ml) were added, and the incubation was continued for 3–4 h. The medium was then removed and in some experiments ricin was immunoprecipitated as described below to investigate whether sulfated ricin had been secreted. The cells were washed twice (5 min) with a 0.1 M lactose solution in PBS at 37°C and then with cold PBS, lysed (lysis buffer: 0.1 M NaCl, 10 mM Na2HPO4, 1 mM EDTA, 1% Triton X-100, and 1 mM PMSF, pH 7.4), and centrifuged to remove the nuclei for 10 min at 5,000 rpm in a centrifuge (model 5415; Eppendorf Scientific, Inc., Madison, WI). The supernatant was immunoprecipitated with rabbit anti–ricin antibodies immobilized on CNBr–Sepharose 4B for 3 h at 4°C. Finally, the beads were washed twice with cold PBS containing 0.35% Triton X-100, and the adsorbed material was analyzed by SDS-PAGE (12%) under reducing conditions.
Electron Microscopy
Cells growing in 25-cm2 flasks were washed with Hepes medium and incubated with a ricin-HRP conjugate for 1 h at 37°C in the same medium. The cells were then washed twice with PBS and fixed with 2% glutaraldehyde in 0.1 M Na-cacodylate buffer, pH 7.2, for 30 min at room temperature. After careful washing with PBS (five times), the cells were incubated in PBS containing 0.5 mg/ml diaminobenzidine and 0.5 μl 30% H2O2/ml at room temperature. The cells were then scraped off the flasks and pelleted. The pellets were postfixed with OsO4, treated with 1% uranyl acetate in distilled water, embedded in Epon, cut, and examined in an electron microscope (model 100; Philips Electron Optics, Mahwah, NJ).
Western Blot Analysis
Cells were washed with PBS and lysed in sample buffer with mercaptoethanol. SDS-PAGE (7.5%) of the lysate was performed, and the proteins in the gel were transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The membrane was blocked with 2% nonfat dry milk powder in PBS containing 0.1% Tween 20 and incubated with antidynamin antibody (0.08 μg/ml) (Upstate Biotechnology Inc., Lake Placid, NY) in a solution containing 1% nonfat dry milk powder in PBS with 0.1% Tween 20 for 1 h at room temperature. The membrane was then washed three times for 10 min with 0.1% Tween 20 and finally incubated with goat anti–mouse IgG antibody conjugated to horseradish peroxidase for 1 h at room temperature. The result was developed with a chemiluminescent detection kit (SuperSignalTM CL-HRP; Pierce, Rockford, IL).
Immunofluorescence
DynK44A cells were grown for 48 h in the presence or absence of tetracycline. Subconfluent cultures were then fixed with 2% formaldehyde, permeabilized with 0.2% saponin in 5% goat serum, and incubated with a polyclonal anti-M6PR antibody (kindly provided by Bernard Hoflack, EMBL, Heidelberg, Germany) followed by a Texas red–conjugated goat anti–rabbit IgG (Southern Biotechnology Associates, Inc., Birmingham, AL).
SDS-PAGE
SDS-PAGE was done as described by Laemmli (32). The gels were fixed in 4% acetic acid and 27% methanol for 30 min. In addition, when 35S- labeled proteins were analyzed, the gels were treated with 1 M Na-salicylate, pH 5.8, in 2% glycerol for 15 min. Dried gels were exposed for fluorography to Kodak XAR-5 films (Rochester, NY) at −80°C.
Results
Ricin Endocytosis and Degradation in Cells Overexpressing DynK44A and DynG273D
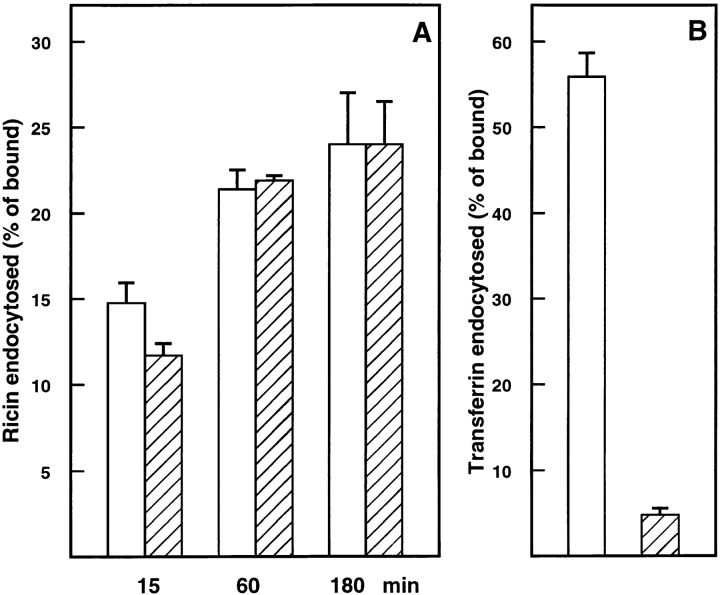
It has been shown earlier that clathrin-mediated endocytosis of both transferrin and EGF was severely inhibited by dynK44A expression, whereas fluid phase endocytosis, measured by HRP uptake, was not affected (10). Similarly, when clathrin-dependent endocytosis was blocked in HeLa dynK44A cells by mutant overexpression, ricin endocytosis continued (Fig. 1 A). 125I-ricin internalization was slightly inhibited after 15 min. However, ricin accumulation at late time points was unaffected by overexpression of mutant dynamin. Furthermore, ricin endocytosis was unchanged by overexpression of wild-type dynamin (data not shown). 125I-transferrin endocytosis was measured to ensure that clathrin-dependent endocytosis was in fact >90% inhibited by overexpression of mutant dynamin in dynK44A cells (Fig. 1 B). Similarly, in cells expressing the temperature-sensitive mutant of dynamin, dynG273D, ricin endocytosis was unaffected when clathrin-dependent endocytosis was blocked by incubation at the nonpermissive temperature (not shown).
Figure 1.
Ricin and transferrin endocytosis in dynK44A cells. DynK44A cells were grown with (open bars) and without (hatched bars) tetracycline for 2 d and transferred to Hepes medium for the experiment. Then 125I-ricin (A) or 125I-transferrin (B) were added to the cells, and endocytosis was measured after the indicated times (A) or after 5 min (B) as described in Materials and Methods. Bars represent the mean +SD (A, n = 4; B, n = 11).
To investigate whether the transport to endosomes and further on to lysosomes was affected by mutant dynamin, dynK44A cells cultured with (uninduced) and without (induced) tetracycline were incubated for 1 h with ricin-HRP (Figs. 2 and 3, respectively). There were not apparent differences in the number, the morphology, or the extent of labeling of endosomes and lysosomes in induced and uninduced cells. Furthermore, 125I-ricin degradation was measured and, as shown in Fig. 4, overexpression of dynK44A did not change toxin degradation to any significant extent. Bafilomycin, an inhibitor of the vacuolar H+-ATPase (4, 79), inhibited ricin degradation, indicating that the process was taking place in a low-pH compartment. Similar results were obtained at the nonpermissive temperature in cells overexpressing the temperature-sensitive mutant of dynamin (data not shown).
Figure 2.
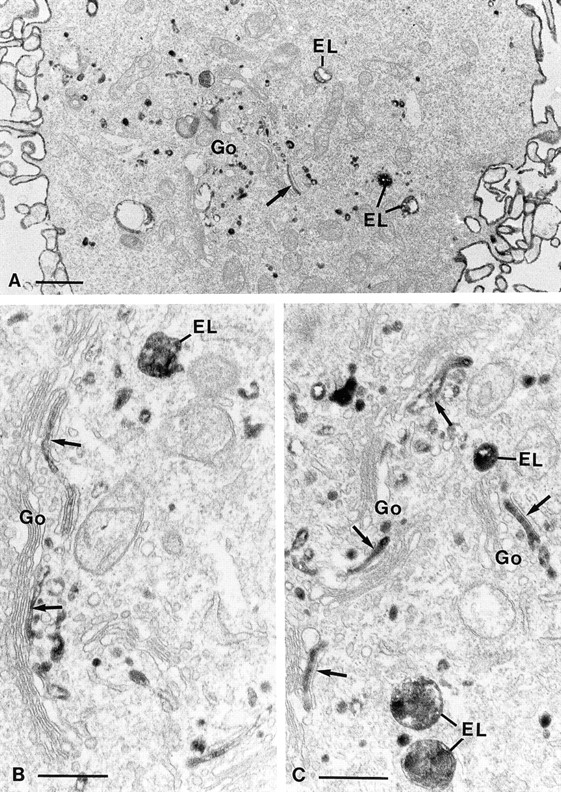
Electron microscopical pictures of dynK44A cells with endogenous dynamin. DynK44A cells grown for 2 d in the presence of tetracycline were incubated for 1 h in a Hepes-containing medium with a ricin-HRP conjugate before fixation. Processing for electron microscopy was done as described in Materials and Methods. Ricin-HRP binds to the cell surface and is seen intracellularly in endosomes–lysosomes (EL) as well as in association with the Golgi stacks (Go) in presumptive TGN elements (arrows). Bars: (A) 1 μm; (B and C) 0.5 μm.
Figure 3.
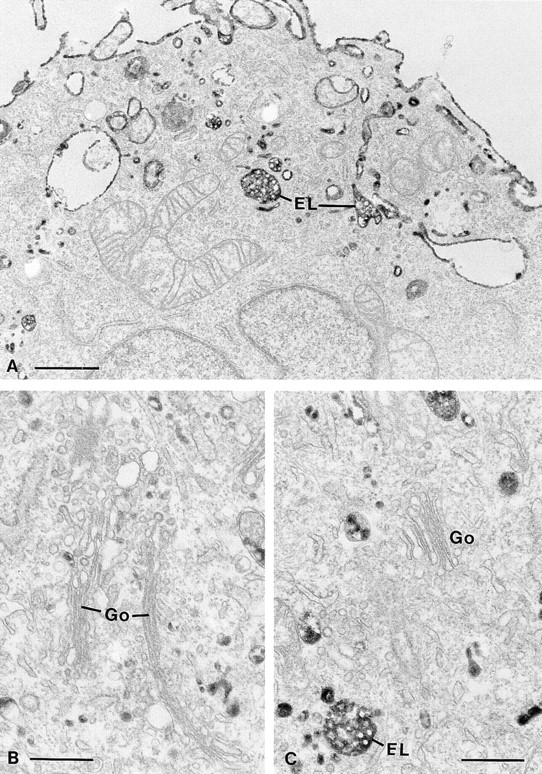
Electron microscopical pictures of dynK44A cells with mutant dynamin. DynK44A cells grown for 2 d in the absence of tetracycline were incubated for 1 h in a Hepes-containing medium with a ricin-HRP conjugate before fixation. Processing for electron microscopy was done as described in Materials and Methods. Ricin-HRP on the cell surface as well as in endosomes– lysosomes (EL) is readily observed. However, no labeling was seen in association with the Golgi complexes (Go). Bars: (A) 1 μm; (B and C) 0.5 μm.
Figure 4.
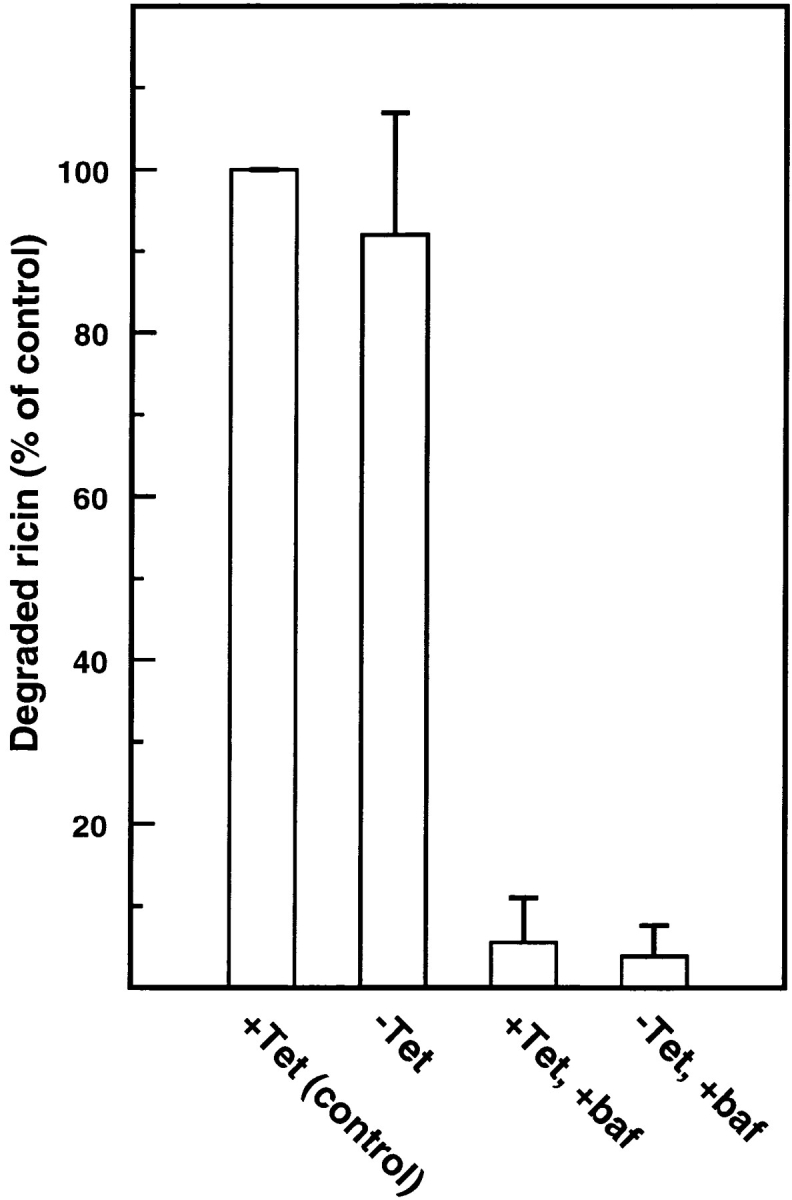
Degradation of ricin in HeLa dynK44A cells. HeLa dynK44A cells were grown with (+Tet) and without (−Tet) tetracycline for 2 d. The cells were then transferred to a Hepes-containing medium without serum and preincubated without or with (+baf) bafilomycin A1 (1 μM) for 30 min at 37°C. 125I-ricin was then added, and 15 min later surface-bound toxin was removed with a 0.1 M lactose solution at 37°C. The incubation was continued in the presence or in the absence of bafilomycin A1, and after 2 h further incubation ricin degradation was measured as described in Materials and Methods. Bars represent the mean +SD (n = 2–6).
Effect of DynK44A and DynG273D Overexpression on Ricin Cytotoxicity
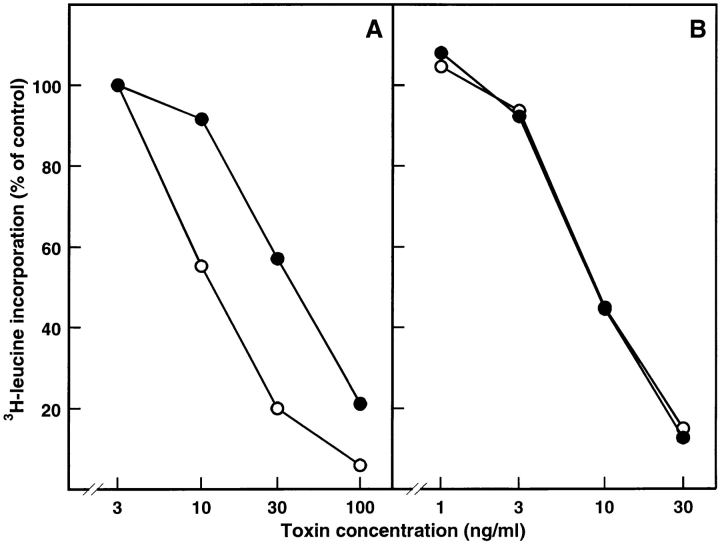
Ricin binds to glycoproteins and glycolipids containing terminal galactose. After endocytosis and presumably after transport to the Golgi apparatus and the endoplasmic reticulum (75, 76), a small number of ricin molecules reach the cytosol and are sufficient to inhibit protein synthesis by removing an adenine from the 28S RNA of the 60S ribosomal subunit (14, 15). To investigate whether overexpression of dynK44A changes the ability of ricin to intoxicate the cells, we measured protein synthesis 3 h after addition of increasing concentrations of ricin to dynK44A (Fig. 5 A) and dynWT (Fig. 5 B) cells grown with tetracycline (only endogenous dynamin is expressed) or without tetracycline (overexpression of dynK44A or dynWT, respectively). Surprisingly, cells overexpressing dynK44A were more resistant to ricin than either dynWT or dynK44A cells expressing endogenous dynamin. The difference in toxicity was the same whether serum (3%) was present or not. As shown in Fig. 5 B, overexpression of dynWT had no effect on the sensitivity of the cells to ricin.
Figure 5.
Ability of ricin to inhibit protein synthesis in HeLa dynK44A and dynWT cells grown with and without tetracycline. DynK44A (A) and dynWT (B) cells were grown with (open circles) and without (filled circles) tetracycline for 2 d. Then the cells were transferred to Hepes medium (no unlabeled leucine) without serum and increasing concentrations of toxin were added. 3 h later, protein synthesis was measured as described in Materials and Methods. The figure shows the average between duplicates of a representative experiment.
In some experiments, HeLa cells with a temperature-sensitive defect in clathrin-dependent endocytosis were used. In these cells, the expression of a temperature-sensitive mutant of dynamin, dynG273D, homologous to the Drosophila shibirets1 allele (21), is also regulated by tetracycline. To induce mutant expression, the cells were incubated for 3 d at 30°C (permissive temperature) without tetracycline. It has previously been shown that 30 min after transfer of the cells to 38°C (nonpermissive temperature), transferrin endocytosis was inhibited, whereas fluid phase endocytosis of HRP had recovered to control levels (11). In our experiments, we incubated the dynG273D cells for 1 h at 38°C before addition of ricin to allow ricin endocytosis to recover. After incubation with the toxin for 3 h, protein synthesis was measured. Also, cells expressing the temperature-sensitive dynamin mutant dynG273D were more resistant to ricin than cells with endogenous dynamin (not shown).
Transport of ricin from the plasma membrane to the Golgi apparatus is important for intoxication since disruption of the Golgi apparatus by brefeldin A leads to complete protection against ricin in a number of cell lines (58). Therefore, we wondered whether brefeldin A could also protect HeLa cells expressing dynK44A against intoxication. For this experiment, ricin was added to brefeldin A–pretreated (2 μg/ml) HeLa cells with and without mutant dynamin, and protein synthesis was measured 3 h later. In both cases, the cells were completely protected against ricin by brefeldin A (data not shown), indicating that ricin internalized by the clathrin-independent pathway in these cells has to be transported through the Golgi apparatus to intoxicate the cells.
Butyric Acid and TSA Enhance the Effect of DynK44A on Ricin Cytotoxicity
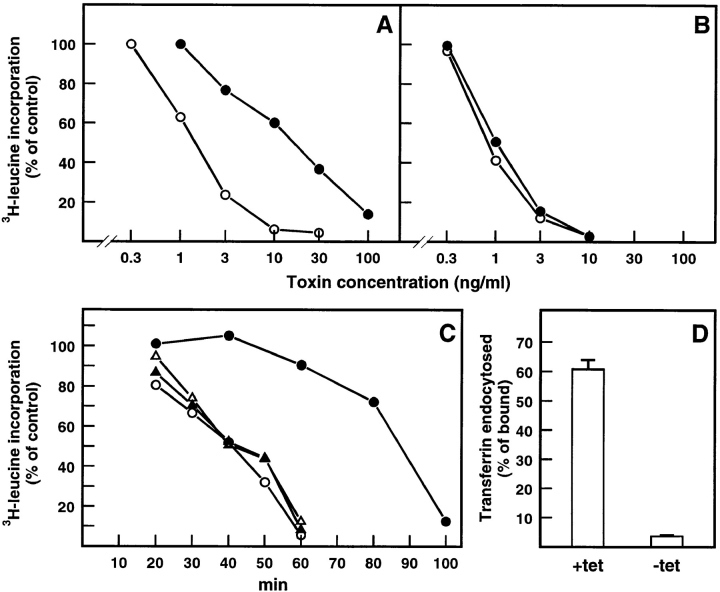
We have shown that butyric acid increases the sensitivity of A431 cells to ricin without affecting binding and endocytosis (59). We therefore decided to investigate whether butyric acid can also sensitize HeLa cells and what effect this might have on the ricin resistance exhibited by dynK44A cells. To first examine the effect of butyric acid in uninduced cells, dynK44A cells were grown with tetracycline in the presence of increasing concentrations of butyric acid (0.5–2 mM) for 24 and 48 h. Increasing concentrations of ricin were then added and the effect on protein synthesis was determined. It was shown that pretreatment with 2 mM butyric acid for 24 h sensitized HeLa cells to ricin without significantly affecting protein synthesis or cell growth (data not shown). Uninduced dynK44A and dynWT cells treated under these conditions were about five times more sensitive to ricin than untreated cells (compare Fig. 6, A and B, with Fig. 5, A and B, open circles). Cells overexpressing dynK44A were again more resistant to ricin than cells expressing endogenous dynamin (uninduced dynK44A and dynWT cells) or cells overexpressing wild-type dynamin (induced dynWT cells) (Fig. 6, A and B). Pretreatment with butyric acid also increased the difference in ricin sensitivity between induced and uninduced dynK44A cells (Fig. 6 A). Expression of dynK44A in the absence of butyric acid results in an approximately threefold increase in resistance to ricin (Fig. 5 A), whereas dynK44A expression in the presence of butyric acid resulted in an ∼10-fold increase in resistance (Fig. 6 A). Again, no differences were observed with dynWT cells grown with or without tetracycline (Fig. 6 B). Also, as shown in Fig. 6 C, ricin internalized in uninduced dynK44A and in induced and uninduced dynWT cells pretreated with butyric acid decreased protein synthesis after a shorter time than ricin internalized in induced dynK44A cells. Finally, control experiments showed that the presence of butyric acid did not affect the extent of inhibition of clathrin-dependent endocytosis of transferrin by overexpression of dynK44A (Fig. 6 D).
Figure 6.
Butyric acid enhances the difference in ricin toxicity between cells with endogenous dynamin and mutant dynamin without affecting the inhibition of clathrin-dependent endocytosis caused by dynK44A overexpression. DynK44A (A) and dynWT (B) cells grown with (open circles) and without (filled circles) tetracycline for 2 d and with butyric acid (2 mM) for 1 d were incubated with increasing concentrations of ricin in Hepes medium (no unlabeled leucine) without serum. Protein synthesis was measured 3 h later as described in Materials and Methods. The figure shows a representative experiment. In C, dynK44A (open and filled circles) and dynWT (open and filled triangles) cells grown with (open symbols) and without (closed symbols) tetracycline and with butyric acid (2 mM) for 1 d were incubated with ricin (2 μg/ml) in Hepes medium (no unlabeled leucine) for the indicated periods of time. Protein synthesis was measured then as indicated in Materials and Methods. The figure shows the average between duplicates of a representative experiment. Finally, in D, dynK44A cells grown with or without tetracycline and with butyric acid as previously indicated were incubated with 125I-transferrin in Hepes medium for 5 min. Then endocytosed transferrin was measured as described in Materials and Methods. Bars represent the mean +SD (n = 3).
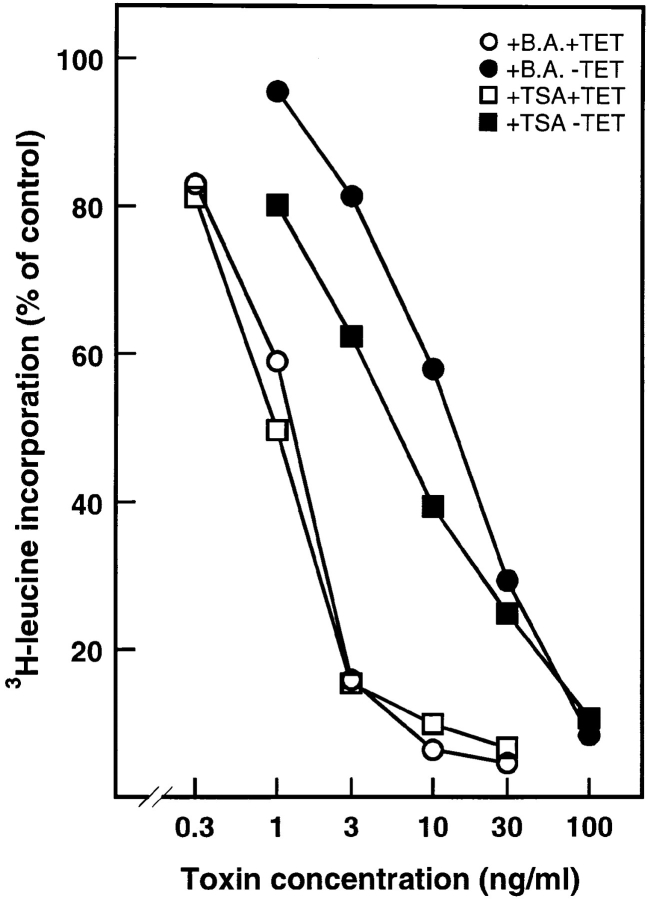
Glycolipid synthesis seems to be important for butyric acid–induced sensitization of A431 cells to Shiga toxin since several inhibitors of glycosphingolipid synthesis, such as threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) (1 μM), an inhibitor of glucosylceramide synthetase, prevent sensitization of A431 cells to Shiga toxin (60). Similarly, PDMP prevented butyric acid–induced sensitization to Shiga toxin in HeLa cells as well (data not shown). However, PDMP did not counteract the ability of butyric acid to sensitize cells to ricin, indicating that formation of new glycolipids is not required. Interestingly, TSA—a compound that like butyric acid, albeit in a more specific manner—inhibits the enzyme histone deacetylase (78), also increases the sensitivity of HeLa cells to ricin and enhances the difference in ricin toxicity between cells with endogenous dynamin and mutant dynamin (Fig. 7). The effect of TSA (0.1 μM) on ricin sensitization was almost identical to the one seen with butyric acid. Thus, butyric acid most likely exerts its sensitization effect by inhibiting the enzyme histone deacetylase.
Figure 7.
Effects of TSA on ricin toxicity in cells with endogenous dynamin and mutant dynamin. DynK44A cells grown with (open symbols) and without (closed symbols) tetracycline (TET) for 2 d and either with butyric acid (B.A.) (2 mM) or TSA (0.1 μM) for 1 d as indicated were incubated with increasing concentrations of ricin in Hepes medium (no unlabeled leucine) without serum. Protein synthesis was measured 3 h later as described in Materials and Methods. The figure shows the average between duplicates of a representative experiment.
It is known that microtubules bind and stimulate the GTPase activity of dynamin in vitro (62, 63). Although the significance of this interaction in vivo is not clear, the possibility existed that dynK44A had an effect on an interaction of dynamin with microtubules. However, we found that pretreatment of butyric acid–treated cells with nocodazole (33 μM), a microtubule disrupter (51), did not change the differences in ricin sensitivity seen in dynK44A cells (data not shown). Thus, it is unlikely that a disturbance in dynamin–microtubule interactions is responsible for the differences in toxicity.
Effect of Butyric Acid and TSA on the Overexpression of DynWT and DynK44A
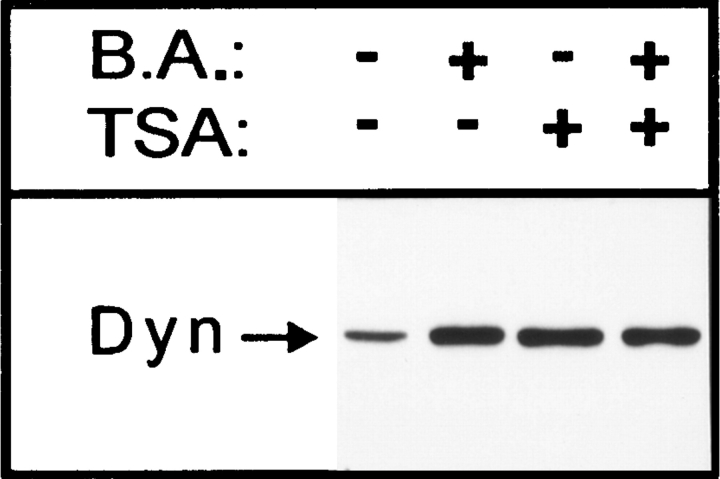
It is known that butyric acid increases histone acetylation by inhibiting the enzyme histone deacetylase (8) and that one of the proposed roles for histone acetylation is to diminish histone/DNA interactions, thereby increasing accessibility of transcriptional factors to DNA (34). Butyric acid has often been used to increase expression from cytomegalovirus (CMV) promoter–driven vectors transfected into cells (45, 46, 73). Therefore, we decided to investigate by Western blot analysis whether butyric acid treatment increased the expression of dynamin that is under control of a minimal CMV promoter in the pUHD10-3 plasmid. Mutant dynamin is clearly overexpressed relative to endogenous dynamin after removal of tetracycline from the growth medium of dynK44A cells (10 and our unpublished results). Furthermore, butyric acid increased the already high expression of both mutant (Fig. 8) and wild-type dynamin (data not shown). To investigate the possibility that histone acetylation is involved in the butyric acid–induced overexpression of dynamin, we studied the effect of a more specific histone deacetylase inhibitor, TSA (78). As shown in Fig. 8, TSA also stimulates overexpression of mutant dynamin. Quantification of Western blots revealed a three- to fourfold increase in the level of expression after treatment either with butyric acid or TSA. Furthermore, when both compounds were added together, the expression was not enhanced more than when the compounds were added separately (Fig. 8), suggesting that both TSA and butyric acid regulate a common mechanism. The increased expression of mutant dynamin may explain the enhanced effects of dynK44A on ricin intoxication observed both with butyric acid and with TSA.
Figure 8.
Effect of butyric acid and TSA on mutant dynamin overexpression. DynK44A cells grown without tetracycline for 2 d and with and without butyric acid (B.A.) (2 mM), TSA (1 μM), or both for 1 d were washed with PBS and lysed in sample buffer with mercaptoethanol. The lysate was run on a 7.5% gel, a Western blot was prepared, and the data were analyzed as described in Materials and Methods.
Sulfation of a Modified Ricin Is Strongly Inhibited by DynK44A Overexpression
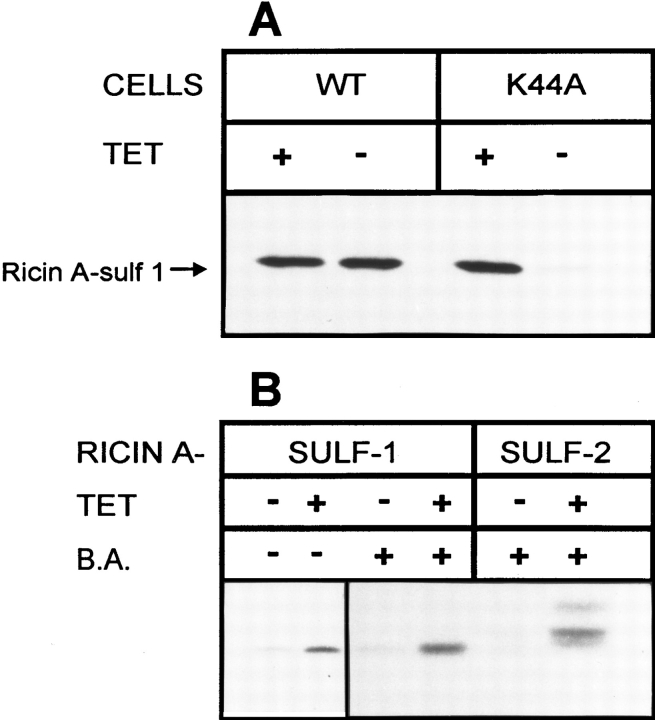
Ultrastructural analysis of dynK44A cells grown in the presence of tetracycline and incubated with ricin-HRP for 1 h revealed that labeling of the Golgi apparatus (the presumptive TGN) was frequently seen in all cell profiles (Fig. 2). However, after removal of tetracycline, no such Golgi labeling could be detected (Fig. 3), suggesting that overexpression of the dynamin mutant inhibited transport of ricin to the Golgi apparatus. To further analyze this notion, we used a modified ricin that can be sulfated as a probe for transport to the TGN. Protein sulfation is a trans-Golgi modification found mainly in secretory proteins (3, 27–30). Considering that [35S]sulfate labeling of proteins has proven useful to study secretion of sulfated molecules, Leitinger et al. (35) decided to use this method to study the secretion of nonnaturally sulfated proteins by adding to these proteins the COOH-terminal nonapeptide of rat cholecystokinin precursor, a tyrosine containing peptide that can be sulfated. This approach has been used by Rapak et al. (48) to generate ricin A-sulf-1, a ricin molecule that can be sulfated. In this case, the tyrosine sulfation signal was added to the COOH-terminal end of ricin A-chain. In addition, a second modified ricin, ricin A-sulf-2, was made by adding a nonapeptide containing three partially overlapping N-glycosylation sites to the COOH terminus of ricin A-sulf-1. Both constructs have been used to study retrograde transport of ricin to the TGN (the sulfation site) and to the endoplasmic reticulum (the glycosylation site) (48).
To investigate changes in ricin trafficking due to overexpression of dynK44A, sulfation of ricin A-sulf-1 and sulfation and glycosylation of ricin A-sulf-2 were studied. When dynK44A cells grown with and without tetracycline were incubated with Na2 35SO4 and reconstituted ricin A-sulf-1, the sulfation of the ricin A-chain was strongly inhibited in cells overexpressing the dynK44A mutant (Fig. 9 B). Similar results were obtained when the cells had been treated with butyric acid (Fig. 9 B). In that case, the labeling of ricin in the presence of tetracycline was somewhat stronger, in agreement with our previous finding that butyric acid treatment increases the efficiency of ricin intoxication (59). Furthermore, the differences in the extent of sulfation between induced and uninduced cells were larger than in non-butyric acid–treated cells. Quantification of sulfated ricin in autoradiograms by densitometry revealed that the difference in sulfation corresponded to the difference in toxicity. No inhibition of the extent of sulfation of endogenous proteins was observed in cells expressing dynK44A. This was investigated by SDS-PAGE of TCA-precipitated proteins from the cell lysates where ricin (and sulfated ricin) had been removed by immunoprecipitation. Thus, the inhibition of ricin sulfation in cells overexpressing mutant dynamin was not due to a general defect in sulfation. Since ricin in itself inhibits protein synthesis, the sulfation experiment was also done in the presence of a protein synthesis inhibitor, cycloheximide (10 μg/ml), to investigate whether the difference in sulfation was dependent on protein synthesis. However, the presence of cycloheximide did not alter the results. When dynWT cells were used, no difference in ricin sulfation between cells grown with and without tetracycline was observed (Fig. 9 A). The lower amount of sulfated ricin found in cells overexpressing dynK44A was not due to an increased secretion to the medium of sulfated ricin. This was investigated by immunoprecipitation of ricin from the medium. Similarly, secretion of [35S]methionine-labeled proteins was not affected by dynamin mutant overexpression (data not shown).
Figure 9.
Sulfation of ricin A-sulf-1 and ricin A-sulf-2. DynWT (A) and dynK44A (A and B) cells were grown with and without tetracycline (TET) for 2 d and with (A and B) and without (B) butyric acid (B.A.) (2 mM) as indicated for 1 d. The cells were then washed with DME without sulfate and incubated with Na35SO4 (100 μCi/ml) for 4 h. Then, reconstituted ricin sulf-1 (A and B) or ricin sulf-2 (B) was added, and the incubation was continued for 3–4 h. Butyric acid and tetracycline were present during the incubation. The cells were washed with a 0.1 M lactose solution in PBS at 37°C and with cold PBS and then lysed. The nuclei were removed by centrifugation and the supernatant was immunoprecipitated by incubating with rabbit anti–ricin antibodies immobilized on CNBr–Sepharose 4B for 3 h at 4°C. Finally, the adsorbed material was analyzed by SDS-PAGE (12%) under reducing conditions and the gel was subjected to autoradiography.
To study trafficking of ricin not only to the Golgi apparatus but also to the endoplasmic reticulum, we investigated the modification of ricin sulf-2 in cells with and without overexpression of mutant dynamin. When ricin sulf-2 was added to the cells, three bands were visible after immunoprecipitation of ricin and SDS-PAGE (Fig. 9 B). The band with the highest molecular weight represented both sulfated and glycosylated ricin since it has been shown earlier that after pretreatment of several cell lines with the glycosylation inhibitor tunicamycin, this band disappears (48). A band representing sulfated ricin could also be observed (middle band). The band with the lowest molecular weight has not been observed in other cell lines (48) and is probably a degradation product. As expected, the bands were very weak in cells expressing dynK44A.
M6PR Redistributes in Cells Overexpressing DynK44A
To investigate whether the dynK44A mutant also influences transport to the Golgi apparatus of an endogenous protein, we decided to study the steady-state distribution of a molecule that under normal conditions constitutively shuttles between the TGN and late endosomes (18, 20). The M6PR is concentrated in the TGN and serves to deliver several lysosomal enzymes from this compartment to late endosomes. In the acidic environment of late endosomes, lysosomal enzymes are released from the M6PR, and the receptor is recycled back to the TGN for other rounds. If mutant dynamin inhibits the transport from endosomes to the Golgi apparatus, it should be possible to observe a change in the intracellular distribution of the M6PR after expression of this protein. As shown in Fig. 10, this was indeed the case. Thus, expression of mutant dynamin caused a much more coarse-dotted (“late endosome/lysosome-like”) M6PR fluorescence (Fig. 10, B–D) than observed in controls (Fig. 10 A).
Figure 10.
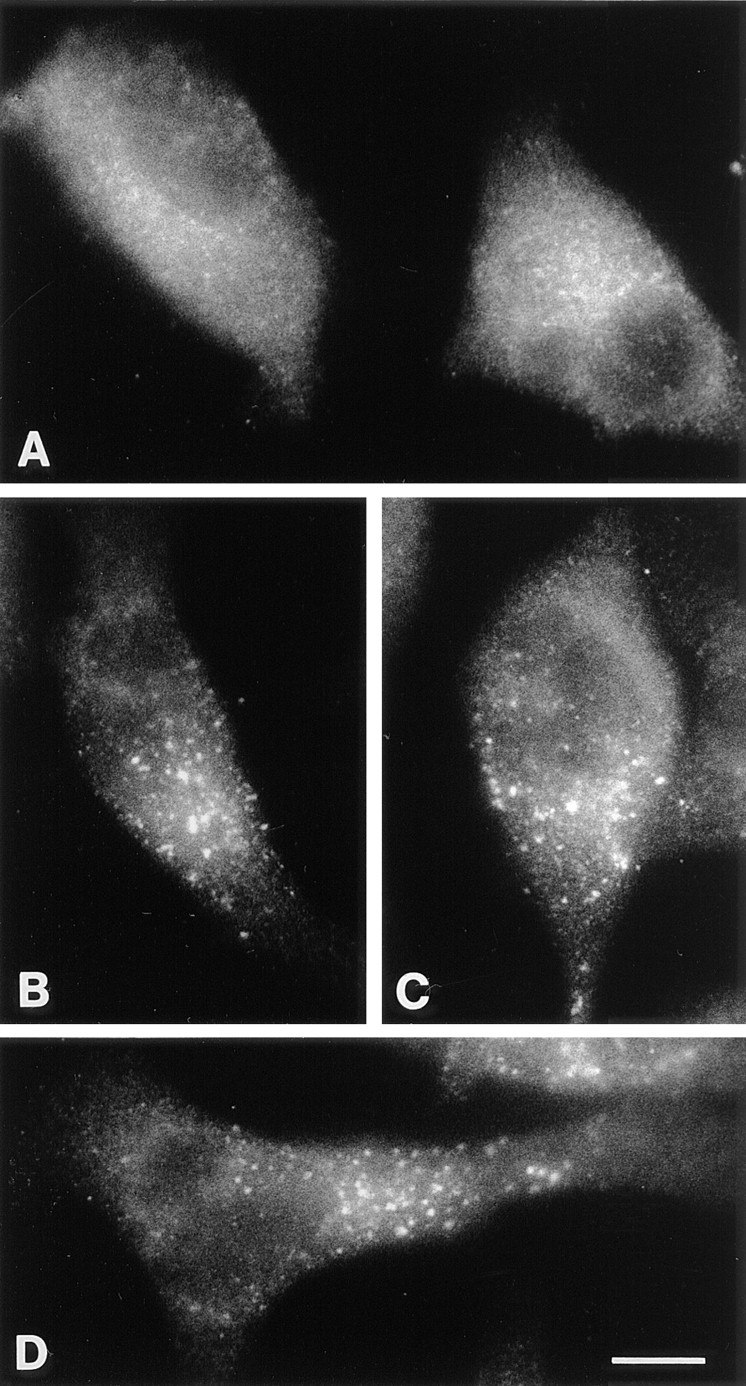
Localization of the M6PR in dynK44A cells by immunofluorescence. (A) Cells from a subconfluent culture grown in the presence of tetracycline. (B–D) Cells from a subconfluent culture grown for 48 h in the absence of tetracycline before fixation. Bar, 10 μm.
Discussion
We here demonstrate that transport of internalized ricin to the Golgi apparatus is strongly inhibited in HeLa cells overexpressing dominant-negative mutants of dynamin. This inhibition is not due to a defect in endocytosis, as ricin internalization is unaffected. Clathrin-dependent endocytosis can be inhibited both by overexpression of a GTPase mutant of dynamin (dynK44A) (10) or by expression of a temperature-sensitive mutant (dynG273D) and subsequent incubation of the cells at the nonpermissive temperature (11). In both cases, receptor-mediated endocytosis of transferrin is potently inhibited, whereas bulk-phase endocytosis of fluid and, as shown here, ricin continues. We had previously shown that selective inhibition of clathrin- mediated endocytosis by cytosol acidification or K+-depletion resulted in only a small decrease in uptake of ricin and that ricin endocytosis continued via clathrin-independent mechanisms (41, 56, 57). Our findings here that ricin endocytosis occurs at normal levels in cells expressing dynK44A support the conclusion that mutant dynamin is a selective and potent inhibitor of clathrin-mediated endocytosis.
In HEp-2 cells, we showed that clathrin-dependent and clathrin-independent endocytic pathways merge at the level of endosomes containing transferrin receptors (24). We have also reported that inhibition of clathrin-mediated endocytosis by cytosolic acidification did not significantly affect the ability of endocytosed ricin to intoxicate Vero cells (56). Thus, one might expect that if mutant dynamin selectively affected only clathrin-dependent endocytosis, then ricin taken up through the clathrin-independent pathway would be equally toxic to cells. However, this was clearly not the case. Strikingly, we found that the toxicity of ricin is reduced in cells overexpressing mutant dynamin. Intoxication by endocytosed ricin seems to require its transport to the Golgi apparatus and retrograde transport to the ER before translocation of the toxic A-chain to the cytosol (75, 76), and ricin intoxication in dynK44A cells still requires retrograde transport through the Golgi, as it remains completely sensitive to brefeldin A treatment. We also directly monitored transport of ricin to the Golgi apparatus using both a ricin-HRP conjugate for electron microscopic studies and a ricin molecule modified with a sulfation site and/or glycosylation sites (48) for biochemical analysis of retrograde transport to the TGN and/or endoplasmic reticulum. Whereas in control cells expressing endogenous dynamin ricin-HRP could easily be seen in the Golgi apparatus, ricin-HRP could only be observed in endosomes and lysosomes in cells overexpressing mutant dynamin. Furthermore, both sulfation, which occurs in the TGN, and N-linked glycosylation, which occurs in the ER of the modified ricin molecules, were severely reduced in cells overexpressing mutant dynamin. Thus, by several criteria, we have demonstrated that overexpression of mutant dynamin inhibits transport of endocytosed ricin to the Golgi apparatus. Since mutant dynamin inhibits intracellular transport of ricin, one could expect to see inhibition of the endosome to Golgi transport of some endogenous molecules as well. That is indeed what we saw when we looked at the intracellular distribution of the M6PR in the presence of mutant dynamin. There was a clear change in the steady-state distribution of this receptor.
The block in transport of endocytosed ricin to the Golgi apparatus was not due to general effects on membrane transport along either the endocytic or exocytic pathways. We found, for example, that ricin degradation was not inhibited, suggesting that dynamin is not required for transport to lysosomes. This is in agreement with the finding that transport of cathepsin D to lysosomes was normal in the same cells (10). We also found that there were no significant changes in transport of sulfated ricin from the Golgi apparatus to the medium. These results are in agreement with earlier data obtained from cells isolated from shits flies (cells with a temperature-sensitive dynamin mutant) and from cells overexpressing GTPase-deficient dynamin mutants. In these cells, there is normal transport out of the Golgi apparatus of the VSV-G protein, fluorescent Golgi lipids, cathepsin D, and the transferrin receptor (10) (Radhakrisna, H., R.E. Pagano, C.E. Machamer, and T.F. Roth. 1993. J. Cell Biol. 4:211a).
It has recently been shown that EGF-induced signaling was altered in cells overexpressing mutant dynamin (74), and one might therefore wonder whether the decreased transport of ricin to the Golgi apparatus and the change in ricin toxicity could be due to a defect in dynamin-dependent signaling of growth factors or hormones. It is clear from previous studies that transport of ricin from endosomes to the Golgi apparatus is a regulated process (36, 40, 47). However, we consider this unlikely because the differences in ricin toxicity were the same in the absence and presence of serum, and the transport to the Golgi apparatus was studied in the absence of serum. Taken together, our results raise the question of whether overexpression of mutant dynamin specifically affects an additional transport step from endosomes to the Golgi apparatus.
Three mammalian dynamin isoforms have been identified, although there is only one isoform expressed in Drosophila. HeLa cells express the ubiquitous dynII isoform and in these and previous studies (10, 11) are transfected with WT and mutant dynI, the neuronal isoform. Electron microscopic immunolocalization of both ubiquitously expressed dynII and overexpressed dynI has established that both proteins are localized to clathrin-coated pits on the plasma membrane (2, 10). Thus, it has been proposed that both dynI and dynII are required for endocytic coated vesicle formation but that dynI exhibits functional properties specific for rapid endocytosis at the synapse. Recent support for this hypothesis derives from the effects of microinjection of dynII-specific antibodies (Henley, J.R., E.W. Krueger, and M.A. McNiven. 1996. Mol. Biol. Cell. 83a). It is possible that the effects of overexpression of a dominant-negative mutant of dynamin, although specific, might be indirect, and that neither dynI nor dynII per se are required for transport between the endosomal compartment and the Golgi apparatus (see below).
Dynamin is a member of a functionally diverse family of GTPases that share a high degree of homology, principally restricted to their NH2-terminal GTPase domains (for reviews see references 68, 77). These include the interferon-inducible mx proteins that confer viral resistance (66), Mgm1p involved in maintenance of the mitochondrial genome (31), and phragmoplastin involved in cell plate formation in plants (22). Two dynamin-related proteins in yeast, Vsp1p and Dnm1p, have been implicated in trafficking to lysosomes along the exocytic and endocytic pathways, respectively (17, 50). A dynamin-related molecule has also been detected in the Golgi apparatus of fibroblasts and melanocytes (25), and in HepG2 cells (38) using antibodies directed against conserved regions in the GTPase domain. The identity and function of this GTPase is not known. Both dynamin and the distantly related mx proteins exist as homooligomers. Assembly of these homooligomers is dependent both on conserved sequences within the GTPase domain and more divergent sequences in the COOH-terminal region (42). Thus, it is possible that at high levels of overexpression, dynI might coassemble and interfere with the function of an as yet unidentified dynamin-related molecule required for trafficking of endocytosed ricin to the Golgi.
Support for this hypothesis derives from the observed differences in the dose responses seen for inhibition of endocytosis and ricin intoxication by mutant dynamin. While overexpression of dynK44A resulted in an ∼10-fold reduction in receptor-mediated endocytosis, both the extent and rate of sensitivity to ricin intoxication were reduced by only two- to threefold. Increasing overexpression levels of mutant dynamin by butyric acid treatment led, however, to an even greater decrease in sensitivity of these cells to ricin. These results establish that inhibition of ricin toxicity and transport to the Golgi apparatus requires significantly higher levels of overexpression of mutant dynamin than inhibition of receptor-mediated endocytosis. The finding that a potent histone deacetylation inhibitor, TSA, has similar effects to butyric acid concerning ricin sensitization and dynamin expression, and also increases the difference in toxicity between cells with endogenous and mutant dynamin to the same extent, suggests that butyric acid and TSA exert their effects on these parameters by inhibiting the enzyme histone deacetylase.
The results presented here further demonstrate that care must be taken in interpreting a functional change in response to a ligand in cells overexpressing mutant dynamin, as this change may not necessarily be due to inhibition of endocytosis of that ligand.
So far, little is known about transport of ricin from endosomes to the Golgi apparatus. Is ricin transported to late endosomes from where it enters the Golgi apparatus by a rab9-dependent transport step (37), or can it also be transported to the Golgi apparatus from an earlier compartment? Recently both COP-coated and clathrin-coated structures have been identified on endosomes (1, 67), but their complete composition, the molecular mechanisms for vesicle formation and their destination are still not known in detail. It is possible that one of these structures could be involved in transport to the Golgi apparatus and that this transport step might require an as yet unidentified dynamin-like GTPase.
Acknowledgments
We would like to thank Tove Lie Berle (The Norwegian Radium Hospital, Norway), Kirsten Pedersen (The Panum Institute, Denmark) and Mette Ohlsen (The Panum Institute) for expert technical assistance and Frederik Vilhardt for helping us with the immunofluorescence work.
This work was supported by the Norwegian Research Council for Science and the Humanities (NAVF), The Norwegian Cancer Society, The Danish Cancer Society, The Danish Medical Research Council, the Novo-Nordisk Foundation, Blix legacy, Torsteds legacy, Odd Fellow, the Jahre foundation, the Nordic Cancer Union, a NATO Collaborative Research Grant (CRG 900517) and a Human Frontier Science Program grant (RG404/96M).
Abbreviations used in this paper
- CMV
cytomegalovirus
- M6PR
mannose-6-phosphate receptor
- TSA
trichostatin A
Footnotes
Address all correspondence to Kirsten Sandvig, Institute for Cancer Research, The Norwegian Radium Hospital, Montebello, 0310 Oslo, Norway. Tel.: 47 22934294. Fax: 47 22508692. E-mail: ksandvig@radium.uio.no
References
- 1.Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba T, Damke H, Hinshaw JE, Ikeda K, Schmid SL, Warnock DE. Role of dynamin in clathrin-coated vesicle formation. Cold Spring Harbor Symp Quant Biol. 1995;60:235–242. doi: 10.1101/sqb.1995.060.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle PA, Huttner WB. Tyrosine sulfation is a trans-Golgi–specific protein modification. J Cell Biol. 1987;105:2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciechanover A, Schwartz AL, Dautry-Varsat A, Lodish HF. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 6.Cook T, Mesa K, Urrutia R. Three dynamin-encoding genes are differentially expressed in developing rat brain. J Neurochem. 1996;67:927–931. doi: 10.1046/j.1471-4159.1996.67030927.x. [DOI] [PubMed] [Google Scholar]
- 7.Cook TA, Urrutia R, McNiven MA. Identification of dynamin 2, an isoform ubiquitously expressed in rat tissues. Proc Natl Acad Sci USA. 1994;91:644–648. doi: 10.1073/pnas.91.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousens LS, Gallwitz D, Alberts BM. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979;254:1716–1723. [PubMed] [Google Scholar]
- 9.Damke H. Dynamin and receptor-mediated endocytosis. FEBS Lett. 1996;389:48–51. doi: 10.1016/0014-5793(96)00517-0. [DOI] [PubMed] [Google Scholar]
- 10.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damke H, Gossen M, Freundlieb S, Bujard H, Schmid SL. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995;257:209–220. doi: 10.1016/s0076-6879(95)57026-8. [DOI] [PubMed] [Google Scholar]
- 13.De Camilli P, Takei K, McPherson PS. The function of dynamin in endocytosis. Curr Opin Neurobiol. 1995;5:559–565. doi: 10.1016/0959-4388(95)80059-x. [DOI] [PubMed] [Google Scholar]
- 14.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 15.Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- 16.Fraker PJ, Speck JC., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 17.Gammie AE, Kurihara LJ, Vallee RB, Rose MD. DNM1, a dynamin-related gene, participates in endosomal trafficking in yeast. J Cell Biol. 1995;130:553–566. doi: 10.1083/jcb.130.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goda Y, Pfeffer SR. Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans Golgi network in vitro. Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- 19.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 21.Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 22.Gu X, Verma DP. Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. EMBO (Eur Mol Biol Organ) J. 1996;15:695–704. [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen SH, Sandvig K, van Deurs B. The preendosomal compartment comprises distinct coated and noncoated endocytic vesicle populations. J Cell Biol. 1991;113:731–741. doi: 10.1083/jcb.113.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen SH, Sandvig K, van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol. 1993;123:89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henley JR, McNiven MA. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herskovits JS, Burgess CC, Obar RA, Vallee RB. Effects of mutant rat dynamin on endocytosis. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hille A, Rosa P, Huttner WB. Tyrosine sulfation: a post-translational modification of proteins destined for secretion? . FEBS Lett. 1984;177:129–134. doi: 10.1016/0014-5793(84)80996-5. [DOI] [PubMed] [Google Scholar]
- 28.Hille A, Braulke T, von Figura K, Huttner WB. Occurrence of tyrosine sulfate in proteins—a balance sheet. 1. Secretory and lysosomal proteins. Eur J Biochem. 1990;188:577–586. doi: 10.1111/j.1432-1033.1990.tb15438.x. [DOI] [PubMed] [Google Scholar]
- 29.Huttner WB. Sulphation of tyrosine residues—a widespread modification of proteins. Nature. 1982;299:273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- 30.Huttner WB. Tyrosine sulfation and the secretory pathway. Annu Rev Physiol. 1988;50:363–376. doi: 10.1146/annurev.ph.50.030188.002051. [DOI] [PubMed] [Google Scholar]
- 31.Jones BA, Fangman WL. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 1992;6:380–389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Lamaze C, Schmid SL. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 35.Leitinger B, Brown JL, Spiess M. Tagging secretory and membrane proteins with a tyrosine sulfation site. Tyrosine sulfation precedes galactosylation and sialylation in COS-7 cells. J Biol Chem. 1994;269:8115–8121. [PubMed] [Google Scholar]
- 36.Llorente A, Garred Ø, Holm PK, Eker P, Jacobsen J, van Deurs B, Sandvig K. Effect of calmodulin antagonists on endocytosis and intracellular transport of ricin in polarized MDCK cells. Exp Cell Res. 1996;227:298–308. doi: 10.1006/excr.1996.0279. [DOI] [PubMed] [Google Scholar]
- 37.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO (Eur Mol Biol Organ) J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier O, Knoblich M, Westermann P. Dynamin II binds to the trans-Golgi network. Biochem Biophys Res Commun. 1996;223:229–233. doi: 10.1006/bbrc.1996.0876. [DOI] [PubMed] [Google Scholar]
- 39.McClure SJ, Robinson PJ. Dynamin, endocytosis and intracellular signaling. Mol Membr Biol. 1996;13:189–215. doi: 10.3109/09687689609160598. [DOI] [PubMed] [Google Scholar]
- 40.Melby EL, Prydz K, Olsnes S, Sandvig K. Effect of monensin on ricin and fluid phase transport in polarized MDCK cells. J Cell Biochem. 1991;47:251–260. doi: 10.1002/jcb.240470311. [DOI] [PubMed] [Google Scholar]
- 41.Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhlberg AB, Warnock DE, Schmid SL. Domain structure and intramolecular regulation of dynamin GTPase. EMBO (Eur Mol Biol Organ) J. 1997;16:6676–6683. doi: 10.1093/emboj/16.22.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakata T, Takemura R, Hirokawa N. A novel member of the dynamin family of GTP-binding proteins is expressed specifically in the testis. J Cell Sci. 1993;105:1–5. doi: 10.1242/jcs.105.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Olsnes S, Refsnes K, Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974;249:627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- 45.Oster T, Thioudellet C, Chevalot I, Masson C, Wellman M, Marc A, Siest G. Induction of recombinant human gamma-glutamyl transferase by sodium butyrate in transfected V79 and CHO Chinese hamster cells. Biochem Biophys Res Commun. 1993;193:406–412. doi: 10.1006/bbrc.1993.1638. [DOI] [PubMed] [Google Scholar]
- 46.Palermo DP, DeGraaf ME, Marotti KR, Rehberg E, Post LE. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J Biotechnol. 1991;19:35–47. doi: 10.1016/0168-1656(91)90073-5. [DOI] [PubMed] [Google Scholar]
- 47.Prydz K, Hansen SH, Sandvig K, van Deurs B. Effects of brefeldin A on endocytosis, transcytosis and transport to the Golgi complex in polarized MDCK cells. J Cell Biol. 1992;119:259–272. doi: 10.1083/jcb.119.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rapak A, Falnes PØ, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to the cytosol. Proc Natl Acad Sci USA. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 50.Rothman JH, Raymond CK, Gilbert T, O'Hara PJ, Stevens TH. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990;61:1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- 51.Samson F, Donoso JA, Heller-Bettinger I, Watson D, Himes RH. Nocodazole action on tubulin assembly, axonal ultrastructure and fast axoplasmic transport. J Pharmacol Exp Ther. 1979;208:411–417. [PubMed] [Google Scholar]
- 52.Sandvig K, Olsnes S. Effect of temperature on the uptake, excretion and degradation of abrin and ricin by HeLa cells. Exp Cell Res. 1979;121:15–25. doi: 10.1016/0014-4827(79)90439-7. [DOI] [PubMed] [Google Scholar]
- 53.Sandvig K, Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982;257:7504–7513. [PubMed] [Google Scholar]
- 54.Sandvig K, van Deurs B. Endocytosis without clathrin. Trends Cell Biol. 1994;4:275–277. doi: 10.1016/0962-8924(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 55.Sandvig K, van Deurs B. Endocytosis, intracellular transport, and cytotoxic action of shiga toxin and ricin. Physiol Rev. 1996;76:949–966. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- 56.Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandvig K, Olsnes S, Petersen OW, van Deurs B. Inhibition of endocytosis from coated pits by acidification of the cytosol. J Cell Biochem. 1988;36:73–81. doi: 10.1002/jcb.240360108. [DOI] [PubMed] [Google Scholar]
- 58.Sandvig K, Prydz K, Hansen SH, van Deurs B. Ricin transport in brefeldin A–treated cells: correlation between Golgi structure and toxic effect. J Cell Biol. 1991;115:971–981. doi: 10.1083/jcb.115.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandvig K, Ryd M, Garred Ø, Schweda E, Holm PK, van Deurs B. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J Cell Biol. 1994;126:53–64. doi: 10.1083/jcb.126.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandvig K, Garred Ø, van Helvoort A, van Meer G, van Deurs B. Importance of glycolipid synthesis for butyric acid-induced sensitization to shiga toxin and intracellular sorting of toxin in A431 cells. Mol Biol Cell. 1996;7:1391–1404. doi: 10.1091/mbc.7.9.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmid SL. The mechanism of receptor-mediated endocytosis: more questions than answers. Bioessays. 1992;14:589–596. doi: 10.1002/bies.950140903. [DOI] [PubMed] [Google Scholar]
- 62.Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 63.Shpetner HS, Vallee RB. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature. 1992;355:733–735. doi: 10.1038/355733a0. [DOI] [PubMed] [Google Scholar]
- 64.Smythe E, Warren G. The mechanism of receptor-mediated endocytosis. Eur J Biochem. 1991;202:689–699. doi: 10.1111/j.1432-1033.1991.tb16424.x. [DOI] [PubMed] [Google Scholar]
- 65.Sontag JM, Fykse EM, Ushkaryov Y, Liu JP, Robinson PJ, Sudhof TC. Differential expression and regulation of multiple dynamins. J Biol Chem. 1994;269:4547–4554. [PubMed] [Google Scholar]
- 66.Staeheli P, Haller O, Boll W, Lindenmann J, Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986;44:147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- 67.Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? . Proc Natl Acad Sci USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Deurs B, Tonnessen TI, Petersen OW, Sandvig K, Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986;102:37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Deurs B, Petersen OW, Olsnes S, Sandvig K. Delivery of internalized ricin from endosomes to cisternal Golgi elements is a discontinuous, temperature–sensitive process. Exp Cell Res. 1987;171:137–152. doi: 10.1016/0014-4827(87)90257-6. [DOI] [PubMed] [Google Scholar]
- 72.van Deurs B, Petersen OW, Olsnes S, Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]
- 73.Van Hove JL, Yang HW, Wu JY, Brady RO, Chen YT. High-level production of recombinant human lysosomal acid α-glucosidase in Chinese hamster ovary cells which targets to heart muscle and corrects glycogen accumulation in fibroblasts from patients with Pompe disease. Proc Natl Acad Sci USA. 1996;93:65–70. doi: 10.1073/pnas.93.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 75.Wales R, Chaddock JA, Roberts LM, Lord JM. Addition of an ER retention signal to the ricin A chain increases the cytotoxicity of the holotoxin. Exp Cell Res. 1992;203:1–4. doi: 10.1016/0014-4827(92)90032-4. [DOI] [PubMed] [Google Scholar]
- 76.Wales R, Roberts LM, Lord JM. Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J Biol Chem. 1993;268:23986–23990. [PubMed] [Google Scholar]
- 77.Warnock DE, Schmid SL. Dynamin GTPase, a force-generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 79.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H (+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]