Abstract
The small GTPase ADP-ribosylation factor (ARF) is absolutely required for coatomer vesicle formation on Golgi membranes but not for anterograde transport to the medial-Golgi in a mammalian in vitro transport system. This might indicate that the in vivo mechanism of intra-Golgi transport is not faithfully reproduced in vitro, or that intra-Golgi transport occurs by a nonvesicular mechanism. As one approach to distinguishing between these possibilities, we have characterized two additional cell-free systems that reconstitute transport to the trans-Golgi (trans assay) and trans-Golgi network (TGN assay). Like in vitro transport to the medial-Golgi (medial assay), transport to the trans-Golgi and TGN requires cytosol, ATP, and N-ethylmaleimide–sensitive fusion protein (NSF). However, each assay has its own distinct characteristics of transport. The kinetics of transport to late compartments are slower, and less cytosol is needed for guanosine-5′-O-(3-thiotriphosphate) (GTPγS) to inhibit transport, suggesting that each assay reconstitutes a distinct transport event. Depletion of ARF from cytosol abolishes vesicle formation and inhibition by GTPγS, but transport in all assays is otherwise unaffected. Purified recombinant myristoylated ARF1 restores inhibition by GTPγS, indicating that the GTP-sensitive component in all assays is ARF. We also show that asymmetry in donor and acceptor membrane properties in the medial assay is a unique feature of this assay that is unrelated to the production of vesicles. These findings demonstrate that characteristics specific to transport between different Golgi compartments are reconstituted in the cell-free system and that vesicle formation is not required for in vitro transport at any level of the stack.
Much of what is known about transport within the Golgi complex has come from the analysis of a cell-free system that reconstitutes a single step in the intra-Golgi transport of vesicular stomatitis viral glycoprotein (VSV-G protein)1 (2). This system is believed to measure transport between the cis and medial compartments of two distinct populations of Golgi-enriched membranes. The “donor” Golgi population is isolated from cells that are deficient in N-acetylglucosaminyl (GlcNAc) transferase I activity and that have been infected with vesicular stomatitis virus. The “acceptor” Golgi population is prepared from uninfected wild-type cells containing the functional enzyme. When donor and acceptor membranes are incubated at physiological temperature with cytosol, ATP, and UDP-GlcNAc, VSV-G protein from donor membranes is mobilized and subsequently glycosylated by acceptor-derived GlcNAc transferase I. This system has been extensively used to analyze the mechanism of intra-Golgi transport and to identify numerous factors required for transport (for review see reference 49). From these studies, a model has been proposed in which coated vesicles transport anterograde-directed protein cargo between the individual cisternae of the Golgi complex (for review see reference 50).
The small GTPase ADP-ribosylation factor (ARF) plays a central role in the coated vesicle transport hypothesis. ARF is required for coated vesicle formation on Golgi cisternae (40, 56). In addition, constitutive activation of ARF by the slowly hydrolyzable analogue of GTP, guanosine-5′-O-(3-thiotriphosphate) (GTPγS), results in the accumulation of nonfunctional Golgi vesicles (35, 58) and inhibits in vitro Golgi transport (35, 55). From these and other findings, a model for ARF as the initiator of vesicle formation has been proposed (for review see reference 6). Initiation requires the interaction of soluble ARF-GDP with a brefeldin A–sensitive exchange factor, exchange of GDP for GTP, and association of ARF with the Golgi membrane (17, 20). ARF-GTP then recruits a cytosolic coat protein complex, coatomer (16, 43) or the adaptor AP-1 (52, 57), to the membrane, possibly through activation of a Golgi-associated phospholipase D (32). Assembly of the coat proteins causes deformation of the membrane to form a coated bud, which pinches off to form a coated vesicle containing protein to be transported. Hydrolysis of ARF-bound GTP is required for subsequent disassembly of the coat before vesicle fusion with the target membrane (54). GTPγS prevents nucleotide hydrolysis by ARF, thereby blocking vesicle uncoating and leading to excessive accumulation of coated vesicles (35, 58).
Although this model for ARF function in vesicle formation is compelling, the relationship between ARF-induced vesicle formation and intra-Golgi transport is less certain. A reexamination of ARF-GTPγS–induced (35) and ARF NH2-terminal peptide–induced (30) inhibition of in vitro Golgi transport has provided evidence for an inhibitory mechanism involving induction of generalized Golgi membrane disfunction, rather than specific inhibition of transport per se (58, 60). Moreover, the vesicular transport hypothesis is seemingly in contradiction with the observation that ARF is not required for cell-free Golgi transport (18, 56). Only one distinction has been discovered between in vitro transport in the presence or absence of ARF. When ARF is present and vesicles are formed, glycosylation-incompetent Golgi compete with wild-type acceptor Golgi for transported VSV-G protein, as expected for vesicular transport (18). In contrast, no competition is observed in the absence of ARF and vesicles, suggesting that cisternae fuse and their enzyme contents mix. This finding led to the proposal that transport is vesicular when vesicles are formed and then switches to direct fusion of cisternae in the absence of vesicles (18).
There are at least two alternative hypotheses that are also consistent with this and all other in vitro transport data. One is that this particular in vitro transport assay may actually measure fusion between the cis-Golgi and transport intermediates derived from endoplasmic reticulum vesicles (ERGIC) that have matured beyond the stage that requires ARF and coat proteins (56). Removal of ARF might cause Golgi cisternae to fuse with one another, abolishing the competition for fusion with ERGIC donor membranes. However, the mechanism of transport from ERGIC to Golgi would be otherwise unaffected. An alternative hypothesis is that transport may occur via transient tubular connections between cisternae (59). For example, tubules extending from one cisterna could fuse with another cisterna. Scission of a tubule close to the originating cisterna would result in transfer of the tubule and its contents to the second cisterna and would also prevent mixing of Golgi resident enzymes. Although ARF may be required to initiate tubule formation in vivo (59), transport could be ARF independent in vitro if preexisting tubules on the isolated Golgi cisternae can participate in transport. On the other hand, if ARF is also required for tubule scission, removal of ARF would allow mixing of cisternal enzymes during the in vitro reaction.
As one approach to distinguish between these various hypotheses, we have characterized two additional intra-Golgi transport assays that measure transport to the trans-Golgi and the TGN (48). If the original assay is in fact measuring fusion of ERGIC with the cis-Golgi, then transport to later Golgi compartments might require vesicles and exhibit distinct characteristics. On the other hand, if the mechanism of transport at all levels of the stack is the same, then the characteristics of transport to all Golgi compartments should be similar. Here we show that the characteristics of in vitro transport to late Golgi compartments are distinct from those of transport to early Golgi compartments. Despite these distinctions, ARF is not required for in vitro transport to any compartment within the Golgi, and no evidence was found for a switch in the mechanism of transport upon removal of ARF.
Materials and Methods
Reagents
UMP-kinase, N-ethylmaleimide (NEM), and 2-mercaptoethanesulfonic acid (MESNA) were purchased from Sigma Chemical Co. (St. Louis, MO). GTPγS and ATP-regeneration system components were purchased from Boehringer Mannheim Corp. (Indianapolis, IN). Tritiated nucleotide sugars were purchased either from Amersham Life Science (Cleveland, OH), New England Nuclear (Boston, MA), or American Radiolabeled Chemicals (St. Louis, MO). Protein concentrations were determined by BCA protein assay using BSA standards (Pierce Chemical Co., Rockford, IL). Anti-ARF antibody and myristoylated recombinant ARF1 were kind gifts of R. Kahn (Department of Biochemistry, Emory University, Atlanta, GA). Nonmyristolyated recombinant ARF1 was a kind gift of Scott Berger and Paul Melançon (Department of Cell Biology and Anatomy, University of Alberta, Canada).
Cell Culture and Biological Materials
CHO wild-type (Pro−5) and mutant cell lines Lec 1 (defective in GlcNAc transferase I; reference 53), Lec 8 (defective in UDP-galactose translocase; references 14, 53), and Lec 2 (defective in CMP-sialic acid translocase; references 15, 53) were purchased from American Type Culture Collection (Rockville, MD) and cultured in suspension with α-MEM, 7% Fetalclone II serum (Hyclone Laboratories, Logan, UT), and penicillin/streptomycin (GIBCO BRL, Gaithersburg, MD). Donor membranes were prepared from each mutant cell line after infection with VSV, as previously described (10). Acceptor membranes were prepared from uninfected wild-type cells. Golgi-enriched membranes were prepared from the cell homogenates by sucrose density flotation (2). CHO cytosol was prepared from homogenates of wild-type or Lec 1 cells as described (5) without the NEM-sensitive fusion protein (NSF) inactivation step. Bovine brain cytosol was prepared according the method of Clary and Rothman (9). CHO and bovine brain cytosols were essentially interchangeable in all assays. Cytosol buffer consists of 10 mM Tris, pH 7.4, 50 mM KCl, and 1 mM DTT.
Intra-Golgi Transport Assays
The basic components of each 25-μl transport reaction were 25 mM Hepes, pH 7.4, 2.5 mM Mg acetate, 20 mM KCl, an ATP-regeneration system (50 μM ATP, 250 μM UTP, 5 mM creatine phosphate, 8 IU/ml creatine phosphokinase), 10 μM palmitoyl-CoA, 2.5 μl acceptor membranes (∼60 μg/ml final), and 2.5–10 μl cytosol (CHO = 0.5–2 mg/ml final, bovine brain = 1.2–4.8 mg/ml final). In addition to the basic components, the medial assay also contained 2.5–5 μCi/ml UDP-[3H]GlcNAc and 2.5 μl Lec 1 donor membranes (∼60 μg/ml) and was incubated at 37°C for 60–80 min.
In addition to the basic components, the trans assay also contained 20 μCi/ml UDP-[3H]galactose, 2.5 μl Lec 8 donor membranes (∼60 μg/ml), 1 mg/ml soybean trypsin inhibitor (STI), and 10 μM MnCl2 and was incubated at 37°C for 90 min. Galactosyl transferase has an absolute requirement for Mn2+ (21), and inclusion of Mn2+ in this assay increased the incorporation of [3H]Gal into VSV-G protein by three- to fourfold. Although 30 mM Mn2+ can induce cytosol and ATP-independent fusion of Golgi membranes (42), transport in this assay was absolutely cytosol- and ATP-dependent at 10 μM Mn2+.
In addition to the basic components, the TGN assay also contained 6 μCi/ml CMP-[3H]sialic acid, 2.5 μl Lec 2 donor membranes (∼60 μg/ml), and 1 mg/ml STI and was incubated at 37°C for 2.5 h. It has previously been reported that optimal in vitro transport to the TGN requires higher concentrations of Mg2+ and DTT than present in the medial assay (48). In our hands, no stimulatory effect of Mg2+ was observed in the TGN assay, and DTT was found to inhibit incorporation of sialic acid into VSV-G protein. Addition of STI to the trans and TGN assays appeared to stabilize the membranes during the longer incubations, resulting in a 1.2–1.4 fold increase in the incorporation of labeled sugar. At the end of a transport incubation, VSV-G protein was immunoprecipitated as previously described (2). Immune complexes were collected by filtration, and incorporation of tritiated label was quantitated by scintillation counting.
Two-Stage Kinetic Assays
The two-stage kinetic assay was a modification of the method of Hiebsch and Wattenberg (28), where transport is allowed to occur in the absence of nucleotide sugar in the first stage, and then the transported protein is glycosylated in a second stage incubation. In the trans and TGN assays, we found it necessary to allow initiation of glycosylation during the first stage of the assay to obtain complete glycosylation of transported protein in the second stage. In the modified assay, the first stage consisted of a large transport reaction mixture containing the appropriate nucleotide-[3H]sugar, ATP mix, cytosol, and membranes that was incubated at 37°C to permit transport. At various times, transport was stopped by removing 25 μl of reaction mixture, diluting it 1:4 with 25 mM Hepes, pH 7.4, and 0.3 M sucrose at room temperature, and collecting the membranes by centrifugation at 16K rpm in a microfuge at room temperature for 2.5 min. The supernatant was withdrawn, and the membrane pellet was covered with glycosylation buffer (25 mM Hepes, pH 7.4, 20 mM KCl, 2.5 mM Mg (OAc)2, ATP-regenerating system, 0.2 mg/ml UMP-kinase, 0.3 M sucrose, and appropriate nucleotide-[3H-]sugar). The incubation was then continued at 37°C for a total of 60, 90, or 150 min for the medial, trans, and TGN assays, respectively, to allow complete glycosylation of the VSV-G protein transported in the first stage of the reaction. In the absence of a first stage incubation or when cytosol was omitted from the first incubation, no incorporation of [3H]sugar was obtained in the second stage. The maximum incorporation of label in the modified two-stage assay was identical to that obtained in a normal one-stage transport incubation.
Preparation of ARF-depleted Cytosol
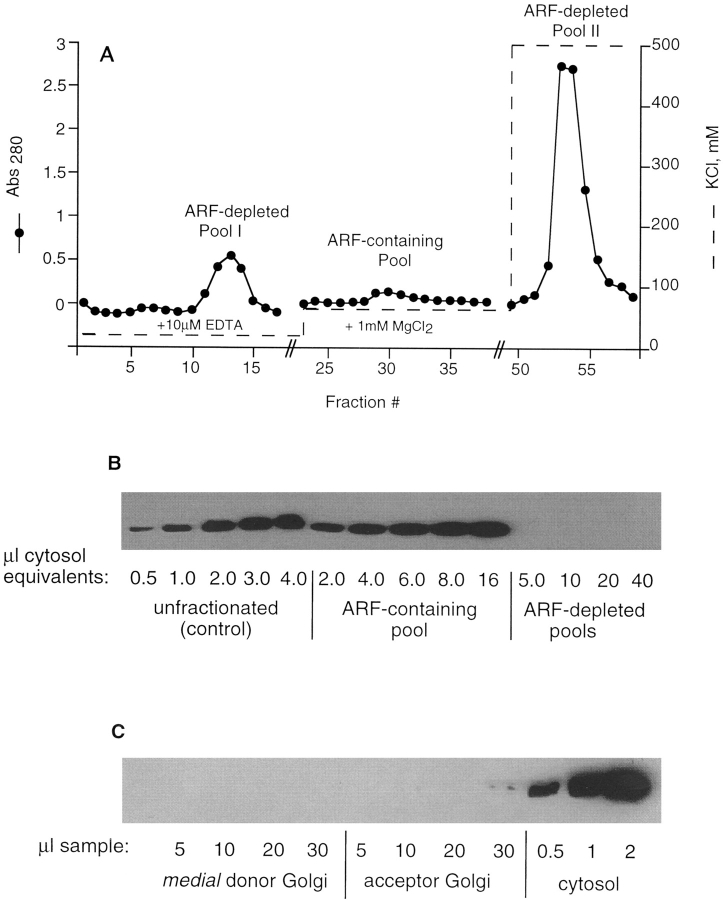
ARF was removed from CHO or bovine brain cytosol by anion exchange chromatography. Desalted cytosol (at 4–6 mg/ml for CHO or 10–12 mg/ml for bovine brain, in 10 mM Tris, pH 7.4, at room temperature, 50 mM KCl, and 1 mM DTT) was diluted in TD buffer (10 mM Tris, pH 7.4, 1 mM DTT) containing 25 μM EDTA to give 10 μM EDTA and 20 mM KCl after dilution. This was loaded in the cold onto a Fast-Flow Q anion exchange matrix (Pharmacia Biotech, Piscataway, NJ), such that the protein/matrix ratio was ∼0.9 mg/ml. The column was washed with TD buffer containing 20 mM KCl and 10 μM EDTA. The unbound protein constituted ARF-depleted pool I. ARF was eluted from the column with TD buffer containing 65 mM KCl and 1 mM MgCl2. The remaining protein was eluted with 500 mM KCl in TD buffer. This constituted ARF-depleted pool II. ARF-depleted pools I and II were combined, concentrated to approximately half the original volume of starting cytosol in either an Amicon pressure cell (Danvers, MA) with a YM10 membrane or a centrifugal ultrafilter (model Centricel 20, 10,000 MW cutoff; Polysciences, Warrington, PA), and desalted on disposable desalting columns (Bio-Rad Laboratories, Hercules, CA). The ARF-containing pool was similarly concentrated to half the original volume of starting cytosol. Therefore, 1 μl starting cytosol was equivalent to 0.5 μl ARF-depleted cytosol plus 0.5 μl ARF-containing pool. The amount of ARF in each pool was determined after SDS-PAGE (15% acrylamide) by Western blotting with 1D9 mouse monoclonal antibody directed against all human isoforms of ARF (8), using rabbit anti– mouse IgG peroxidase and chemiluminescent reagent from Pierce Chemcial Co. for detection.
NEM Sensitivity Assays
The sensitivity of membranes to NEM was measured by preincubating either donor or acceptor membranes alone in a 25-μl standard transport reaction mixture containing NEM at concentrations ranging from 0 to 1 or 2 mM. Reactions were preincubated for 15 min at room temperature, and then unreacted NEM was quenched with 2 or 4 mM MESNA. The complementary untreated membrane, in a 25-μl standard reaction mixture, was added to provide NSF, other NEM-sensitive cytosolic components, and a functional membrane partner for transport. The incubation was continued as described for a normal transport reaction.
NSF-dependent Transport to the medial-Golgi with CHO Lec 8 and Lec 2 Donors
Lec 8 and Lec 2 donor membranes contain functional GlcNAc transferase I and, therefore, are capable of incorporating GlcNAc into VSV-G protein that already resides in the medial compartment. This can give rise to transport-independent incorporation of GlcNAc into VSV-G protein already residing in the medial compartment (56). To specifically measure transport to the medial-Golgi by incorporation of 3H-GlcNAc, we took advantage of the NSF dependence of transport. The rate, NEM sensitivity, and GTPγS sensitivity of transport to the medial-Golgi were measured in transport reactions containing UDP-[3H]GlcNAc and either CHO Lec 8 or Lec 2 donor membranes in the presence or absence of NSF. NSF was selectively inactivated by NEM pretreatment of both donor and acceptor membranes on ice, as previously described (5). The amount of VSV-G protein transported was determined from the total [3H]GlcNAc incorporated into VSV-G protein with NSF, less the amount incorporated in the absence of NSF. Similar experiments were performed using CHO Lec 2 donor membranes (containing active galactosyl translocase) to measure NSF-dependent transport to the trans-Golgi by incorporation of [3H]galactose.
Electron Microscopy
Transmission electron microscopy of replicas from quick-frozen, vacuum-dried Golgi membranes was carried out as previously described (56, 58, 60). Briefly, freshly isolated Golgi membranes were attached to polylysine- and glutaraldehyde-treated coverslips and subjected to in vitro transport incubations for 15 min at 37°C with various preparations of bovine cytosol and purified recombinant ARF1. Carbon and platinum replicas of the membranes were prepared after fixation, quick freezing, and vacuum drying. Replicas were viewed in a transmission electron microscope, and 20–40 micrographs at 20,000–33,000 magnification were taken of each sample. The micrographs were numbered and randomized for measurement of the area encompassed by the Golgi membranes and the numbers of buds and vesicles with or without a punctate coating (coated or uncoated), as previously described (56, 58). The micrographs were also scored for the number of cisternae per micrograph, the number and estimated average size of fenestrae in each cisternae, and the presence of long (>100 nm) blunt-ended tubules extending from the periphery of cisternae. The range of fenestrae sizes were determined from measurements taken from five representative micrographs for each size class. The randomized data were entered into a computer plotting program (Deltagraph 4.0; Deltapoint, Monterey, CA), sorted, and the various parameters computed for each sample.
Results
Cell-free Transport to Three Distinct Golgi Compartments Displays Similar Biochemical Requirements
We have characterized two cell-free transport assays that measure transport to either the trans-Golgi or the TGN. These assays are based on the original in vitro Golgi transport assay that is believed to reconstitute transport to the medial compartment (the medial assay; reference 2). In this assay, transport between GlcNAc transferase–deficient VSV-infected donor membranes and uninfected wild-type acceptor membranes is measured by the incorporation of labeled GlcNAc into the carbohydrate chains of VSV-G protein. To measure transport from the medial-Golgi to the trans-Golgi (the trans assay), VSV-infected donor membranes were prepared from CHO Lec 8 cells, which lack UDP-galactose translocase activity (14). In the absence of this activity, UDP-galactose cannot be transported into the Golgi lumen, where it normally serves as substrate for the predominantly trans-Golgi enzyme, galactose transferase (14, 46, 47). As a result, Lec 8 donor Golgi are unable to add galactose to the carbohydrate chains on VSV-G protein. When wild-type acceptor membranes are coincubated with Lec 8 donor membranes, however, VSV-G can be transported from the medial donor Golgi compartment to the trans compartment of the acceptor, which contains the active translocase. By the inclusion of UDP-[3H]galactose, the amount of protein transported can be quantified by incorporation of [3H]galactose into VSV-G protein.
A similar assay for transport from the trans-Golgi to the TGN (the TGN assay) was employed using donor Golgi isolated from VSV-infected CHO Lec 2 cells that lack CMP– sialic acid translocase activity (15). By the same principle as the trans assay, transport was detected by coincubating Lec 2 donor membranes with wild-type acceptor membranes in the presence of CMP-[3H]sialic acid (48) and measuring incorporation of [3H]sialic acid into VSV-G protein. Unlike the medial assay, where VSV-G protein in the donor ERGIC could acquire GlcNAc upon delivery to the cis acceptor compartment in vitro (51), transport in these new assays is strictly intra-Golgi. This is because incorporation of GlcNAc into VSV-G protein carbohydrates, a Golgi-specific modification, is a prerequisite for the addition of galactose (31) in the assay. Since UDP-GlcNAc is not present in the incubation, only VSV-G protein that has reached the medial-Golgi and acquired GlcNAc in vivo can serve as substrate for addition of galactose in vitro. Likewise, incorporation of sialic acid requires that the VSV-G protein carbohydrates already contain galactose, and that the protein has, therefore, reached the trans-Golgi in vivo.
The requirements for transport to the trans compartment and the TGN demonstrate a fundamental similarity to those for transport to the medial compartment. All assays required ATP and an ATP-regenerating system. In addition, cytosolic protein in the range of 0.5–1.0 mg/ml was necessary to observe optimal transport of VSV-G protein. Some preparations of cytosol were inhibitory at high concentrations in the assays. This was largely due to the suppression of glycosylation of VSV-G protein after transport, rather than inhibition of transport per se (not shown). Although not required for transport, 10 μM palmitoyl- coenzyme A, a purported vesicle fission and fusion agent (44, 45), stimulated transport in all three assays (not shown). As would be expected for processes involving targeted membrane fusion events, transport in all three systems also required the fusion protein NSF (not shown; reference 5). These data suggest that in vitro transport throughout the Golgi complex requires the regulated interaction and eventual fusion of membranes.
The Characteristics of Transport to Each Golgi Compartment Are Distinct
Despite the similarities in general requirements, transport in the medial, trans, and TGN assays exhibits unique characteristics. For example, the rate of overall glycosylation-coupled transport to the trans-Golgi is slower than to the medial compartment, and even slower still to the TGN compartment (Table I). Since glycosylation of VSV-G protein is the rate-limiting step of the medial assay (28), we also measured the rate of transport independent of glycosylation using a two-stage kinetic assay. In the first stage, donor and acceptor membranes were incubated at 37°C with cytosol, ATP, and radiolabeled nucleotide sugar to allow transport and glycosylation to occur. At various times, the membranes were reisolated and incubated in a second stage under conditions that promote glycosylation of VSV-G protein that had been transported, while prohibiting further transport (see Materials and Methods). Analysis of the rates of transport using this two-stage kinetics assay demonstrates that the rate of transport to the trans-Golgi is substantially slower than transport to the medial compartment, and transport to the TGN is slower yet (Table I). Thus, each assay exhibits a characteristic rate of transport, as well as rate of transport-coupled glycosylation.
Table I.
Comparison of In Vitro Transport to Three Distinct Golgi Compartments
| Transfer step | ||||||
|---|---|---|---|---|---|---|
| Cis to medial | Medial to trans | Trans to TGN | ||||
| Donor mutant | Lec1 | Lec 8 | Lec 2 | |||
| Donor defect | GlcNAc transferase | UDP-Galactosyl translocase | CMP-sialic acid translocase | |||
| Substrate | UDP-[3H]GlcNAc | UDP-[3H]Gal | CMP-[3H]SA | |||
| Characteristics of transport | ||||||
| T1/2 transport and glycosylation (min) | 25 | 40 | 50 | |||
| T1/2 transport only (min) | 12 | 20 | 37 | |||
| Donor, IC50 (mM NEM) | 0.2 | 0.8 | 0.9 | |||
| Acceptor, IC50 (mM NEM) | >1.0 | 0.8 | 0.9 | |||
The kinetics of transport-coupled glycosylation were measured by stopping the transport reaction after various lengths of time at 37°C and placing on ice. The kinetics of transport independent of glycosylation were determined using two-stage kinetic assays (see Materials and Methods). The IC50 for inactivation by NEM was determined by pretreating either donor or acceptor membranes with 0–2 mM NEM for 15 min at room temperature, as described in Materials and Methods.
We also tested for asymmetry in the properties of the donor and acceptor membranes in each assay. The donor membranes in the medial assay are known to be more sensitive to irreversible inactivation by NEM than acceptor membranes (1). It has been proposed that this difference reflects the distinct functions of the donor membranes as producers of vesicles and the acceptor membranes as fusion partners for vesicles (1). To determine whether this is a uniform feature of transport throughout the Golgi complex, the sensitivity of each donor and acceptor compartment to pretreatment with NEM was analyzed. As shown in Table I, the donor compartment in the medial assay has a significantly higher sensitivity to irreversible inhibition by NEM than the acceptor membranes, as previously reported (1). In contrast, the donor membranes in the trans and TGN assays are not only less sensitive to inhibition by NEM, but their NEM sensitivity is also indistinguishable from that of the acceptor membranes.
These distinctions between assays could reflect mechanistic differences between transport to early and late Golgi compartments. It is also possible, however, that they are a function of the cell line from which the donor membranes were prepared. To test this hypothesis, we analyzed the characteristics of NSF-dependent transport to the medial-Golgi using donor membranes derived from CHO Lec 8 and Lec 2 cells (see Materials and Methods). In both cases, the NSF-dependent rate of transport, the inhibition of the NSF-dependent transport by GTPγS, and the NEM sensitivity of the donor membranes were identical to Lec 1 donor membranes (data not shown). We also used donor membranes derived from CHO Lec 2 cells to measure NSF-dependent transport to the trans-Golgi. The characteristics of transport matched those of Lec 8 donor membranes (data not shown). We conclude that the differences between the assays are characteristic of the specific intercompartmental transfer step that is measured in each assay, and not the individual donor cell lines or glycosylation reactions.
Transport to trans-Golgi and TGN Is Inhibited by GTPγS at Low Cytosol Concentrations
One possible explanation for the differences between the assays might be that the medial assay measures direct fusion of donor and acceptor compartments, whereas transport in the trans and TGN assays might be coatomer vesicle dependent. If this were true, the lowest concentrations of cytosol (the source of ARF and coatomers) that drive transport to the trans and TGN compartments should be sufficient to observe inhibition of transport by GTPγS since vesicles made with ARF-GTPγS are unable to shed their coats and fuse (54). We therefore examined the cytosol dependence of inhibition by GTPγS in each assay. All three assays were inhibited by GTPγS, but each assay varied in the extent of inhibition and concentration of cytosolic proteins required to cause this inhibition (Fig. 1). As previously reported (35), transport in the medial assay showed a slight stimulation at low levels of cytosol and was incompletely inhibited by GTPγS at the highest concentrations of cytosol that could be tested (Fig. 1 A). The trans assay also exhibited stimulation at low cytosol levels but was more sensitive to GTPγS inhibition at intermediate levels of cytosol than the medial assay (Fig. 1 B). In contrast, the TGN assay was significantly inhibited by GTPγS at cytosol concentrations well below those necessary to drive optimal transport (Fig. 1 C). The IC50 for GTPγS in all three assays was the same (0.3 μM), however, suggesting that the same GTP-binding protein may be the target for inhibition. These data raised the possibility that there is a transition from nonvesicular to vesicular transport as the donor/acceptor compartments are shifted to the trans side of the Golgi stack.
Figure 1.
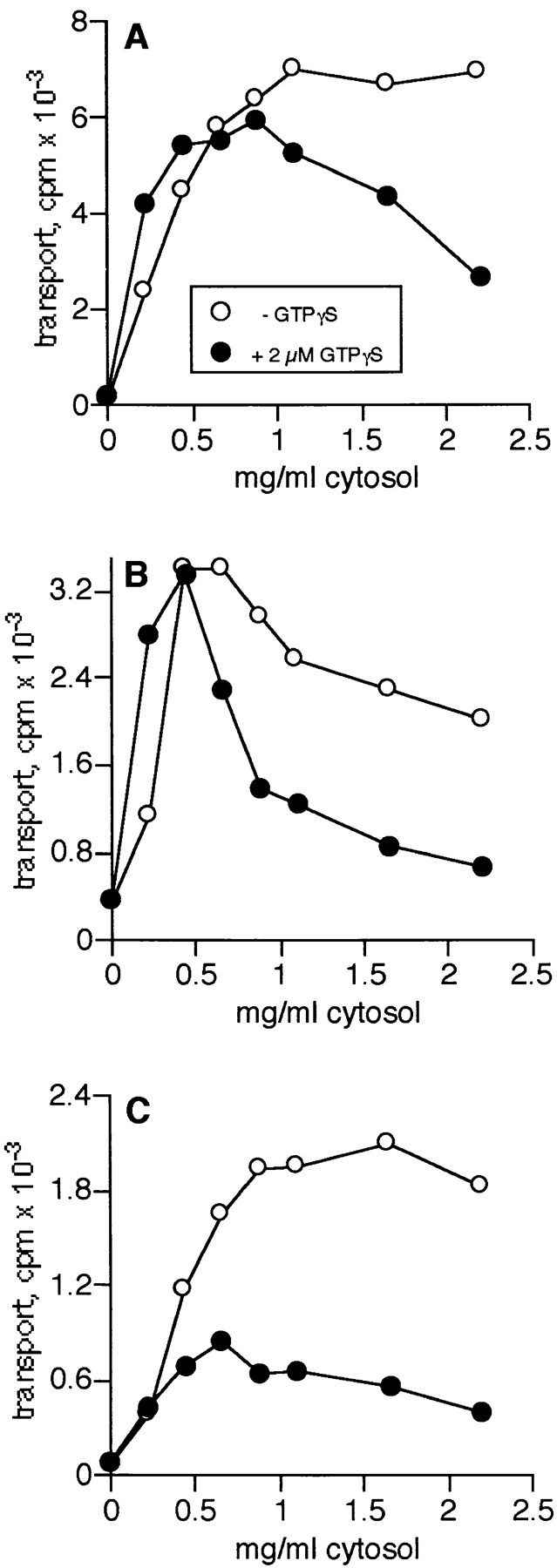
Transport to late Golgi compartments is inhibited by GTPγS at low cytosol concentrations. Inhibition of transport by GTPγS in the medial (A), trans (B), and TGN (C) assays was assessed by titrating CHO cytosol into each assay in the presence of 2 μM GTPγS (closed symbols) or in its absence (open symbols). Data are representative of more than 10 individual experiments in each assay with different cytosol preparations.
ARF-depleted Cytosol Supports In Vitro Transport throughout the Golgi Stack
If transport to the trans-Golgi and/or TGN requires coated vesicles, then ARF should be the primary GTPγS-sensitive component and should also be required for transport in these assays (55). We therefore tested for an ARF requirement using ARF-depleted cytosol. No antibodies currently exist that are capable of immunoprecipitating native ARFs from cell extracts (56). The chromatographic method for preparing ARF-depleted cytosol of Taylor et al. (56) resulted in loss of transport factor(s), other than ARF, that were necessary to reconstitute transport to late Golgi compartments in these assays (data not shown; see Discussion). We therefore developed a modified chromatography protocol that more selectively removed all detectable isoforms of ARF from cytosol, while retaining transport factors needed in all three assays (see Materials and Methods).
Briefly, cytosolic protein was applied to an anion exchange column in 20 mM KCl buffer containing EDTA, eluted first with 65 mM KCl buffer containing Mg2+, and then with 500 mM KCl buffer (Fig. 2 A). The unbound protein and the 500 mM eluate were combined, concentrated, and desalted to produce ARF-depleted cytosol. Recombining this ARF-depleted cytosol with the concentrated ARF-containing 65 mM KCl eluate produced a fully reconstituted cytosol. Western blot analysis revealed that ARF-depleted cytosol prepared by this method is substantially free of ARF (>98%, Fig. 2 B). Recovery of ARFs in the 65 mM KCl eluate was ∼50%, and total protein recovery was 60–80%. As previously described (56), ARFs were virtually undetectable in the Golgi membrane fractions (Fig. 2 C). By comparison with Western blots of purified ARFs (not shown), we estimate that the concentration of ARFs in unfractionated, reconstituted, and ARF-depleted bovine brain cytosols are ∼160, 84, and <3 μg/ml, respectively.
Figure 2.
ARF can be removed from cytosol in one ion-exchange chromatography step. (A) Bovine brain cytosol was fractionated by chromatography on Fast-Flow Q, as described in Materials and Methods. Closed circles indicate the protein elution profile. The dashed line represents the salt concentration of each elution buffer. Additional buffer components are indicated below the protein peaks. (B) Western blot of unfractionated cytosol, the ARF-containing pool, and the ARF-depleted cytosol probed with anti-ARF monoclonal 1D9 antibody, as described in Materials and Methods. Volume equivalents applied to the gel are indicated below each sample. Data are representative of four independent ARF-depleted cytosol preparations. (C) Western blot of isolated donor and acceptor Golgi membranes (0.5 mg/ml) and unfractionated cytosol, probed with anti-ARF monoclonal 1D9. The blot was overexposed to reveal a slight signal at highest level of acceptor Golgi. No signal was observed with the different donor membranes used in each assay. Only the medial donor Golgi is shown.
Fig. 3 shows that reconstituted cytosol (recombination of ARF-depleted and ARF-containing pools; closed circles) is able to fully reconstitute transport compared with unfractionated cytosol (open circles) in all three assays. This demonstrates that transport factors were not lost or inactivated by the chromatographic procedure, although factor(s) that inhibit the glycosylation of VSV-G protein at high cytosol concentrations were frequently lost (compare unfractionated versus reconstituted cytosol). Significantly, ARF-depleted cytosol (open squares) is essentially indistinguishable from reconstituted cytosol in all three assays. The slightly greater volume of reconstituted and ARF-depleted cytosols needed to reach the plateau level of transport observed with unfractionated cytosol is likely to be due to the incomplete recovery of protein after chromatography and concentration. These data demonstrate that maximal transport can be achieved in the absence of ARF in all three assays. We conclude that ARF is not required to observe in vitro transport at any level within the Golgi stack.
Figure 3.
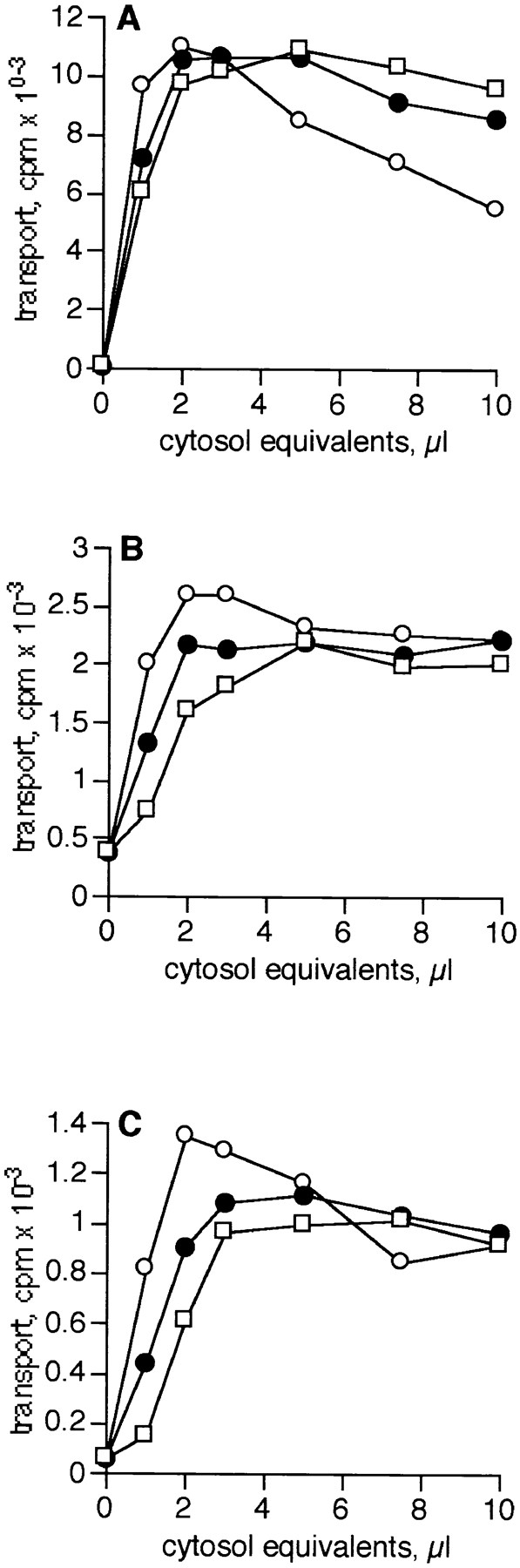
ARF-depleted cytosol fully supports transport in all three in vitro assays. Unfractionated bovine brain cytosol (open circles), reconstituted cytosol (closed circles), and ARF-depleted cytosol (open squares) were titrated into the medial (A), trans (B), and TGN (C) assays. A cytosol equivalent is the volume of sample equal to 1 μl of unfractionated control cytosol after taking into account volume changes after chromatography. The protein concentration of control cytosol was 12.1 mg/ml. Data are representative of two independent ARF- depleted cytosol preparations.
We also determined whether the removal of ARFs rendered the assays insensitive to inhibition by GTPγS. As shown in Fig. 4, transport with reconstituted cytosol (open circles) remained sensitive to inhibition by GTPγS (closed circles) in all three assays, whereas transport with ARF-depleted cytosol (open squares) was insensitive to GTPγS (closed squares), even at high cytosol concentrations. With some preparations of ARF-depleted cytosol, transport in the medial and trans assays was actually slightly stimulated by GTPγS (not shown). A small amount of inhibition by GTPγS was occasionally observed in the TGN assay (Fig. 4 C). This inhibition did not increase with increasing amounts of cytosol, suggesting that the GTPγS-sensitive inhibitory component is membrane associated. Thus, removal of ARFs from cytosol correlates with loss of cytosol-dependent GTPγS inhibition, suggesting that ARFs are responsible for the inhibition observed in all three transport assays.
Figure 4.
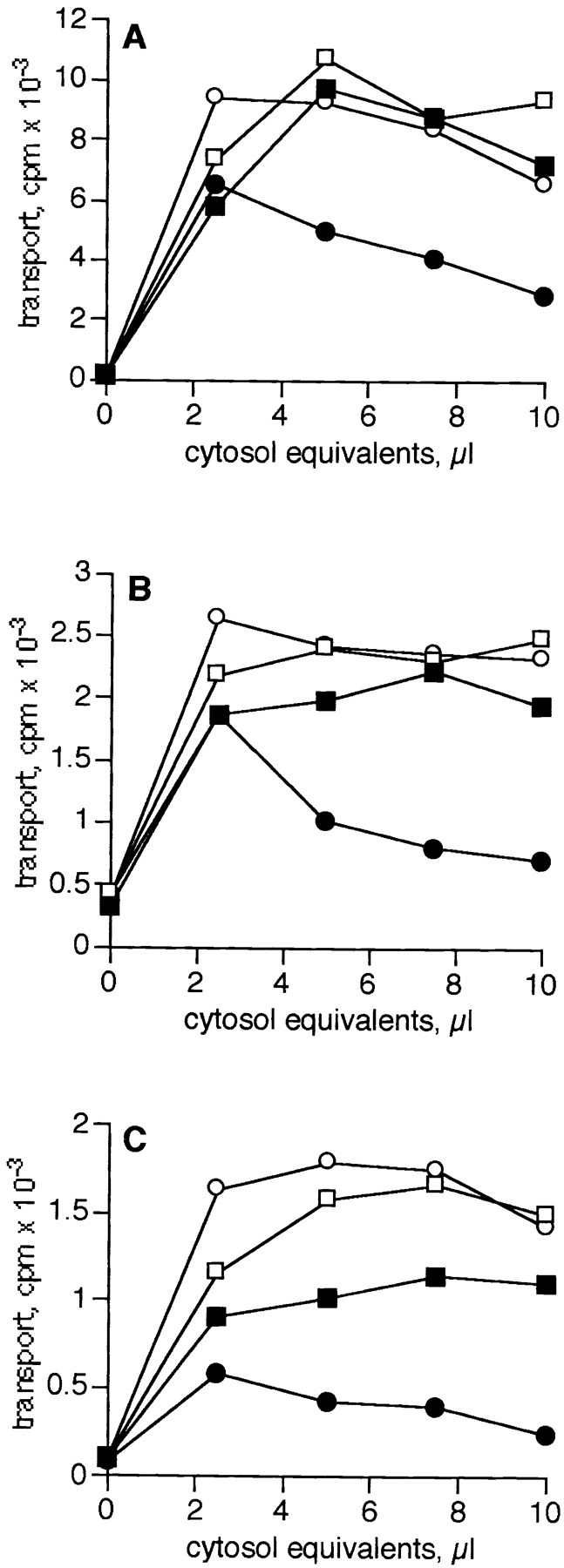
ARF depletion prevents cytosol-dependent inhibition by GTPγS. The ability of GTPγS to inhibit transport with bovine brain reconstituted cytosol (circles) or ARF-depleted cytosol (squares) was determined by titrating increasing cytosol equivalents into the medial (A), trans (B), or TGN (C) assays in the presence of 2 μM GTPγS (closed symbols) or in its absence (open symbols). Data are representative of two independent ARF-depleted cytosol preparations.
To verify this, we examined the GTPγS sensitivity of each assay upon addition of purified recombinant myristoylated ARF1 (myr-rARF1). As shown in Fig. 5, inhibition is restored in all assays by addition of myr-rARF1 to ARF-depleted cytosol in the presence of GTPγS (black bars). Since only 5.7% of the myr-rARF1 is actually myristoylated (Kahn, R., personal communication), the concentrations of myr-rARF1 that cause inhibition are comparable to the concentration of endogenous ARFs in an assay containing 1–4 μl of CHO cytosol (1.6–6.4 ug/ml final at 40 ng ARF per μl of cytosol; reference 56). Addition of non– myr-rARF1 (hatched bar) had no effect, as expected, since myristoylation is required for the biological activities of ARF (6, 20). For a given amount of myr-rARF1, inhibition was consistently least in the medial assay and greatest in the TGN assay, correlating with the differential cytosol dependence of GTPγS inhibition (Fig. 1). These data confirm that the cytosolic GTPγS-sensitive inhibitory component in all three assays is ARF. ARF, therefore, is not directly required to support in vitro Golgi transport, but rather exerts a negative effect when constitutively activated by GTPγS.
Figure 5.
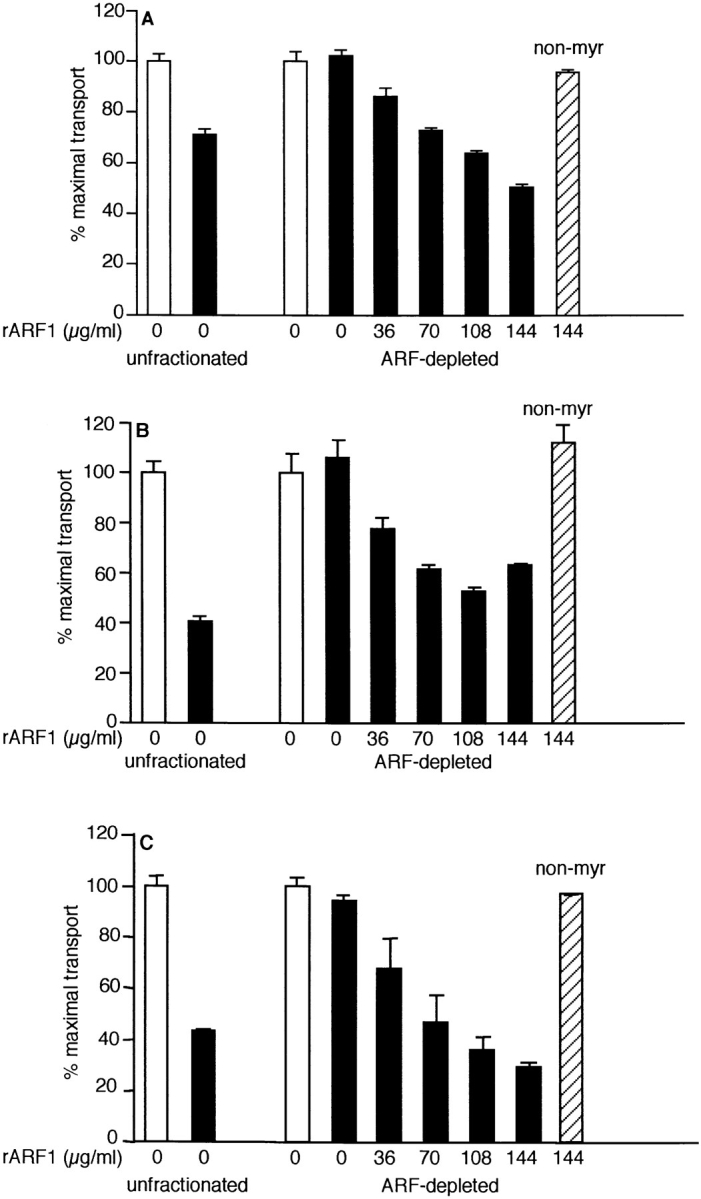
Recombinant ARF1 restores GTPγS sensitivity to ARF-depleted cytosol. Unfractionated bovine brain cytosol (5 μl) and ARF-depleted cytosol (5-μl equivalents) were tested in the medial (A), trans (B), and TGN (C) assays in the presence of 2 μM GTPγS (solid bars) or in its absence (open bars). The indicated amounts of myr-rARF1 (5.7% myristoylated) or non–myr-rARF1 (hatched bar) were titrated into the assay. Data are a combination of two independent experiments. The maximum counts per minute for unfractionated cytosol were 7,764 ± 184 in the medial assay, 4,076 ± 64 and 2,960 ± 203 in the trans assay, 3,030 ± 204 and 3,389 ± 48 in the TGN assay. The maximum counts per minute for ARF-depleted cytosol were 4,866 ± 114 and 8,850 ± 15 in the medial assay, 2,184 ± 323 and 1,881 ± 15 in the trans assay, and 2,981 ± 153 and 2,498 ± 31 in the TGN assay. The acceptor membranes used in these experiments were different from the preparation used in Fig. 4 and exhibit less cytosol-independent inhibition of transport to the TGN by GTPγS (compare Figs. 4 C and 5 C).
ARF-depleted Cytosol Does Not Support Golgi Coated Vesicle Formation
We have previously shown that ARF-depleted cytosol prepared by the method of Taylor et al. does not support coated bud and vesicle formation on Golgi membranes (56). Since we have modified that original procedure to retain additional factors required for transport to late Golgi compartments, we determined whether this ARF-depleted cytosol is also deficient in coated bud formation. High-resolution images from replicas of quick-frozen, freeze-dried Golgi membranes reveal that Golgi incubated in vitro with unfractionated cytosol (Fig. 6 A) or reconstituted cytosol (not shown) exhibit abundant buds and vesicles with a punctate surface coating. In contrast, Golgi incubated with ARF-depleted cytosol exhibit few buds or vesicles, and those few that are observed lack the distinctive punctate surface texture of coated buds (Fig. 6 B). As expected, addition of recombinant myr-rARF1 (Fig. 6 C), but not non– myr-rARF1 (Fig. 6 D), to ARF-depleted cytosol restores formation of coated buds on Golgi cisternae. Quantitative analysis demonstrates that coated bud and vesicle density is reduced 50% in incubations containing reconstituted cytosol (Fig. 7 A, black bars), which contains only half as much ARF as the unfractionated cytosol (Table II). Depletion of ARF reduced the coated bud and vesicle density ∼15-fold relative to unfractionated cytosol (Fig. 7 A, black bars), the same level normally observed with unincubated membranes (58). In contrast, there is a low density of uncoated buds and vesicles in all samples that is independent of the ARF concentration (Fig. 7 A, hatched bars). The tips of tubules extending from cisternae make up the majority of these uncoated buds (>85%). These are likely to have been present at the beginning of the incubation since they are also seen on unincubated membranes (56; not shown).
Figure 6.
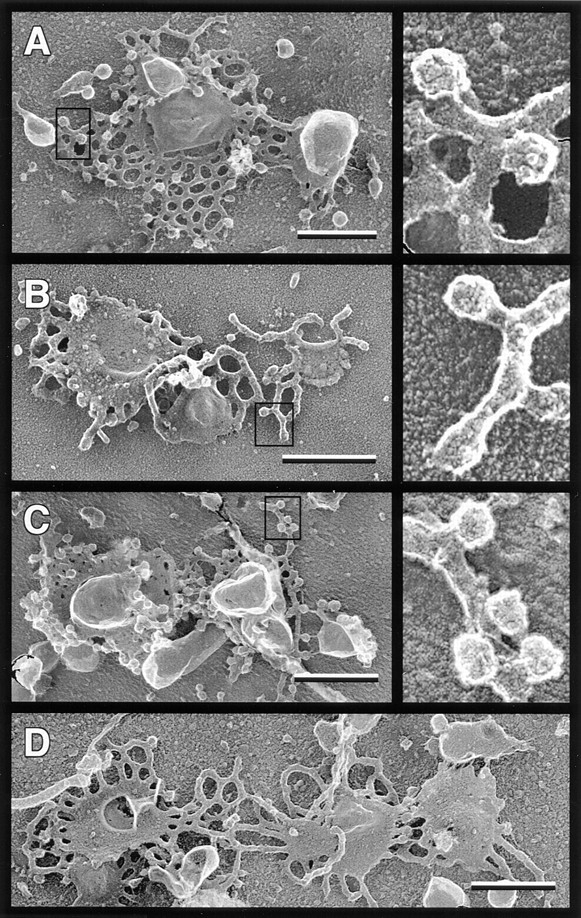
Golgi coated bud formation is blocked by ARF depletion and restored by purified ARF. Replicas of Golgi membranes after a 15-min transport incubation were prepared as described in Materials and Methods. The transport reaction mixtures contained (A) unfractionated bovine brain cytosol (2.4 mg/ml); (B) ARF-depleted bovine brain cytosol (2.4 mg/ml); (C) ARF- depleted cytosol plus myr-rARF1 (120 μg/ml, 5.7% myristorylated); and (D) ARF-depleted cytosol plus non–myr-rARF1 (120 μg/ml). Boxed areas are presented at higher magnification in the panels on the right side of the figure, illustrating the punctate surface coating on Golgi buds in A and C. Buds in B lack this punctate coating and have a granular texture similar to the surrounding tubules. Bars, 0.5 μm.
Figure 7.
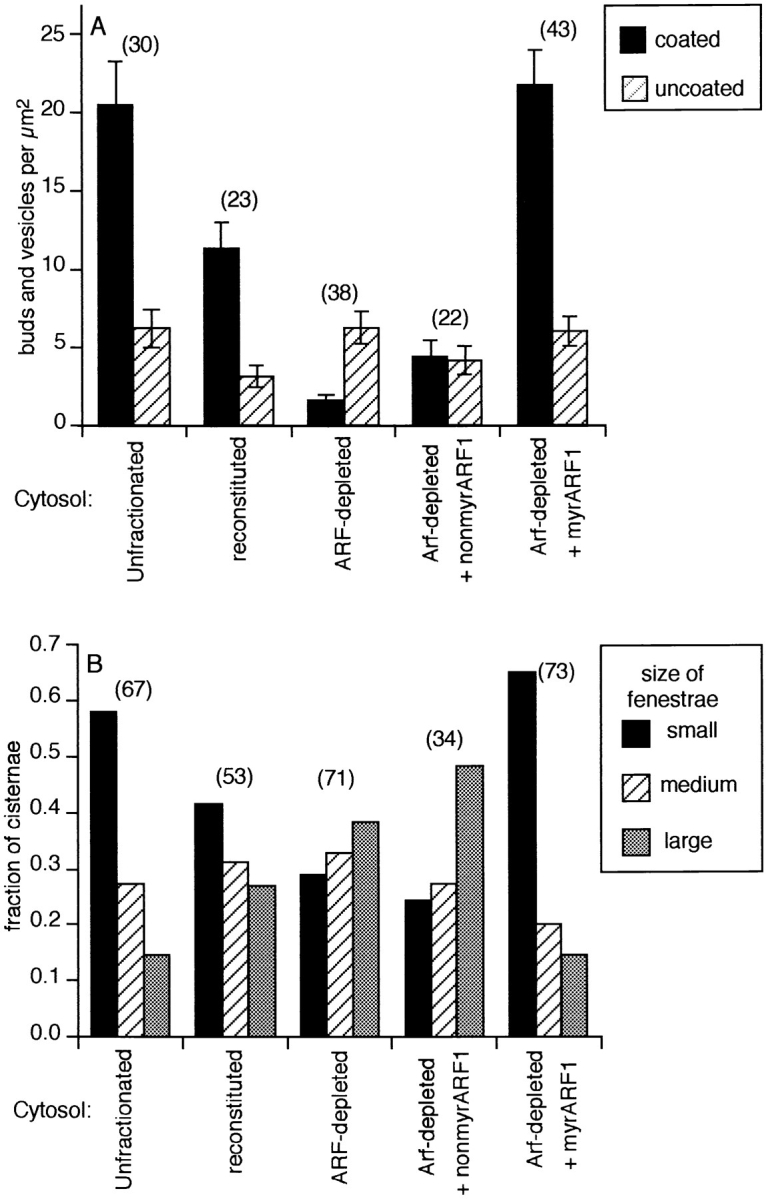
Depletion of ARF decreases the density of coated buds and vesicles and increases the proportion of cisternae with large fenestrae. (A) The densities of buds and vesicles with a smooth surface (uncoated) or a distinctive punctate coating (coated) were determined from electron micrographs as described in Materials and Methods. Blunt-ended tubules made up greater than 85% of the observed uncoated buds and vesicles in all images. Error bars represent the standard error of the mean, and the numbers in parentheses indicate the number of micrographs analyzed. (B) The average size of cisternal fenestrae was estimated from the electron micrographs. Numbers in parentheses above the bars represent the total number of cisternae. Direct measurement of the maximum diameter of the fenestrae on five micrographs representative of each size class revealed that fenestrae scored as small, medium, and large had average diameters (±SD) of 42 ± 25 nm (n = 32), 84 ± 31 nm (n = 36), and 180 ± 80 nm (n = 33), respectively.
Table II.
The General Features of Cisternae after an In Vitro Transport Incubation with or without ARF
| Cytosol | Concentration of ARF | Cisternae per micrograph | Percent with long tubules | Area per cisternae | Fenestrae per cisterna | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| μg/ml | μm2 | |||||||||
| Control | 40 | 2.3 ± 0.3 (30) | 20 | 0.38 ± 0.3 <NOREf>(67) | 7.4 ± 1.6 <NOREf>(67) | |||||
| Reconstituted | 22 | 2.4 ± 0.3 (23) | 19 | 0.39 ± 0.2 (53) | 9.1 ± 2.0 (53) | |||||
| ARF-depleted | <1.4 | 2.0 ± 1.2 (38) | 37 | 0.43 ± 0.2 (71) | 9.6 ± 1.4 (71) | |||||
| ARF-depleted + | <1.4 (120) | 1.5 ± 0.7 (22) | 55 | 0.55 ± 0.6 (34) | 15.4 ± 2.9 (34) | |||||
| non–myr-rARF1 | ||||||||||
| ARF-depleted + | 8.2 <NOREf>(112) | 2.5 ± 1.3 (43) | 12 | 0.39 ± 0.2 (73) | 6.0 ± 0.9 (73) | |||||
| myr-rARF1 |
The concentration of myristoylated ARF in each in vitro incubation was estimated from titrations of the respective cytosols on Western blots, as shown in Fig. 3, where the detection limit was 80 ng ARF. The concentrations of myr-rARF1 and non–myr-rARF1 (in parentheses) were calculated from the protein concentration of the purified recombinant ARFs and the fraction that was myristoylated (5.7%). Cisternae per micrograph, area per cisternae, and fenestrae per cisternae were measured as described in Materials and Methods and are reported as the average plus or minus standard error, with the number of micrographs or cisternae analyzed in parentheses. The number of blunt-ended tubules per cisternae was similar in all samples. The percent with long tubules represents the fraction of cisternae in which these tubules were >100 nm in length.
Membranes incubated with ARF-depleted cytosol prepared by the method of Taylor et al. were previously shown to be morphologically indistinguishable from unincubated membranes (56). This was also the case with many of the cisternae incubated with ARF-depleted cytosol prepared by the new method. However, a subset of the cisternae did exhibit noticeably larger fenestrae and longer blunt-ended tubules extending from the periphery (Fig. 6 B and Table II). Qualitative analysis revealed that the fraction of cisternae exhibiting large fenestrae (>100 nm diameter) increased more than twofold upon depletion of ARF (Fig. 7 B). This tendency was further exacerbated by inclusion of excess non–myr-rARF1 and reversed by inclusion of myr-rARF1. Enlargement of fenestrae was accompanied by an increase in the average area encompassed by each cisterna (Table II). Depletion of ARF alone did not significantly increase the number of fenestrae (Table II) or blunt-ended tubules per cisternae (Fig. 7 A, hatched bars) but did increase the length of the tubules (Table II). This suggests that the enlarged fenestrae arose by growth of tubules enclosing preexisting fenestrae. In contrast, inclusion of excess non–myr-rARF1 increased both the size and number of fenestrae per cisternae but also decreased the number of cisternae per image (Table II). This might indicate that residual endogenous ARF suppresses tubule elongation and that competitive inhibition by non–myr-rARF1 leads to further elongation and membrane destabilization. These results confirm that relatively high levels of myristoylated ARF are needed to sustain coated bud formation on Golgi membranes, but the possibility that a low level of myristoylated ARF may also be necessary to maintain the compactness and integrity of the fenestrated edges of cisternae is also raised.
A Shift in Mechanism Is Not Detected upon Removal of ARF
Since depletion of ARF not only blocks coated vesicle formation but increases the tendency for elongated tubules and fenestrae to form on cisternae, it is possible that the removal of ARF causes the mechanism of transport to shift from vesicular to nonphysiological uncoupled fusion of Golgi membranes, as others have suggested (18). In this case, it would be expected that the distinctive properties of each transport assay would converge on a common value that is characteristic of uncoupled fusion. We therefore examined the effect of removing ARF on the unique properties of each assay, the rate of transport and the sensitivity of the donor membranes to inactivation by NEM. As shown in Fig. 8, when the rate of transport was measured independently of the rate of glycosylation using a two-stage incubation, each assay retained its characteristic kinetics of transport upon depletion of ARF. In addition, the donor membranes in the medial assay remained significantly more sensitive to NEM pretreatment than the acceptor membranes even in the absence of ARF (Fig. 9 A). Moreover, the NEM sensitivity of the donor and acceptor membranes in the trans and TGN assays was also unchanged by removing ARF (Fig. 9, B and C). Thus, the distinctive properties of each assay remain unchanged upon ARF depletion, suggesting that there is no change in the mechanism of transport. It therefore seems likely that vesicles are not required for in vitro transport even when they are formed.
Figure 8.
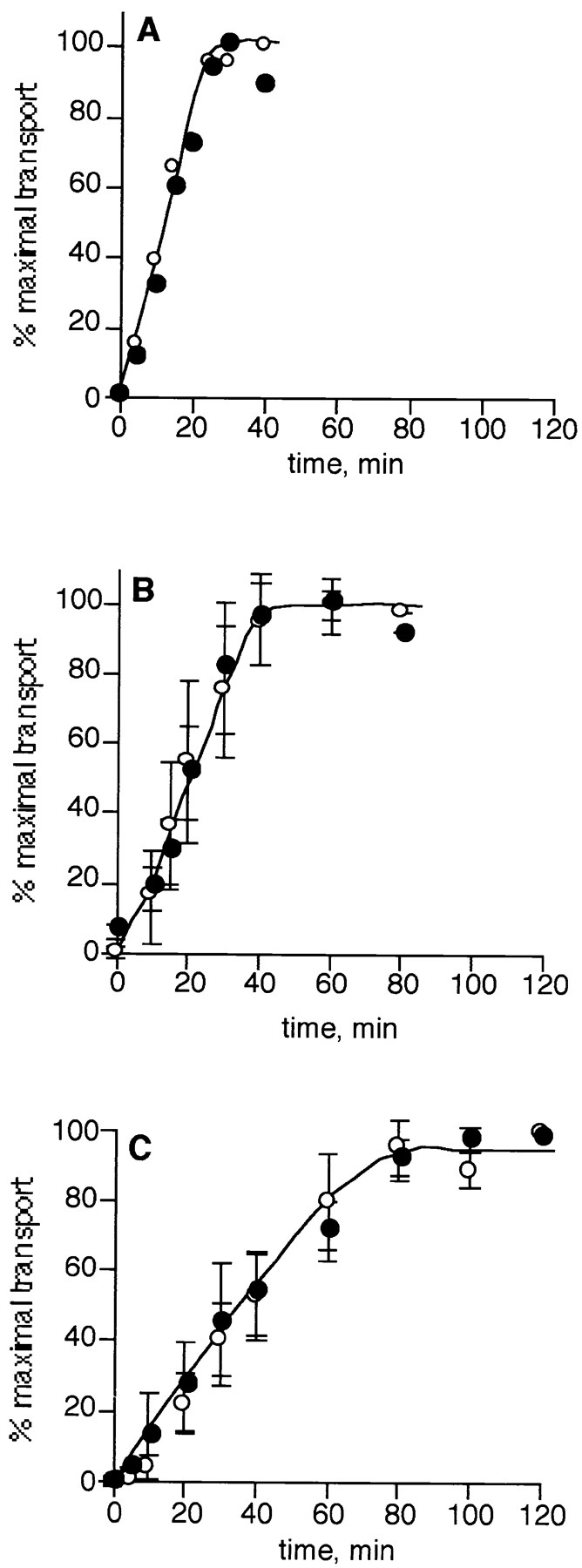
Depletion of ARF does not change the rate of transport. The rate of transport (independent of glycosylation) was assessed in the medial (A), trans (B), and TGN (C) assays in the presence of ARF (unfractionated cytosol, open circles) or the absence of ARF (closed circles) in two-stage assays, as described in Materials and Methods. Each point in B and C represents the mean value of two or five independent experiments, respectively, using 3–7.5-μl equivalents of cytosol per assay. The maximum amounts of 3H incorporated into VSV-G protein were 9,735, 11,682 ± 2,159, and 3,985 ± 980 cpm in for the medial, trans, and TGN assays, respectively.
Figure 9.
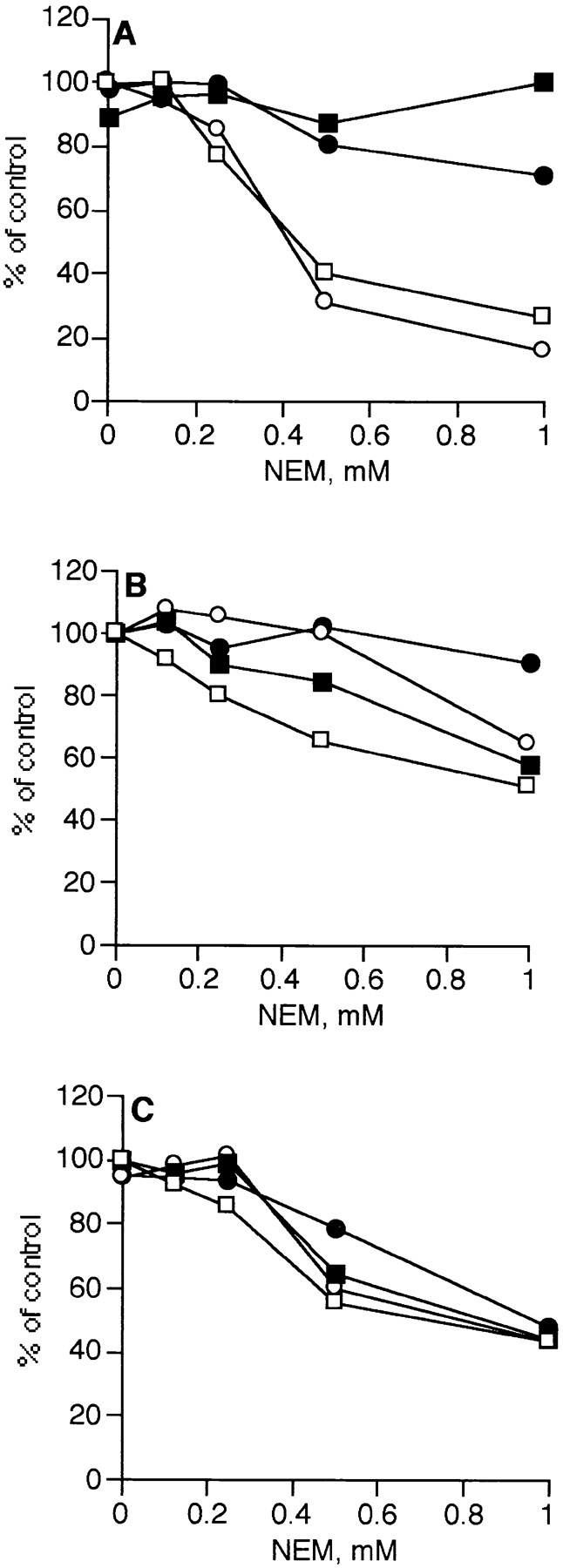
ARF depletion has no effect on the NEM sensitivity of donor and acceptor membranes. Donor (open symbols) or acceptor (closed symbols) membranes were pretreated separately with NEM, as described in Materials and Methods. The medial (A), trans (B), and TGN (C) assays were analyzed with either unfractionated cytosol containing ARF (circles) or ARF-depleted cytosol (squares) at 2.5-μl equivalents per assay. The control values for assays with unfractionated cytosol were 6,724 ± 1, 11,457 ± 923, and 3,461 ± 123 cpm 3H incorporated into VSV-G protein in the medial, trans, and TGN assays, respectively. For the ARF-depleted cytosol, they were 5,433 ± 298, 7,750 ± 354, and 2,562 ± 37 cpm.
Discussion
The molecular details of transport within the Golgi complex have largely come from the analysis of a system that reconstitutes a single transport step between cis and medial compartments of the Golgi complex. Although it has generally been assumed that the mechanism of transport is essentially the same throughout the Golgi, this has never been rigorously examined. Here, we have used a comparative analysis of three different cell-free intra-Golgi transport assays to investigate the role of vesicles in anterograde transport between different compartments of the Golgi complex. The results of our analysis provide novel insights into the complexity of intra-Golgi transport, and evidence in support of the view that, at least in vitro, transport throughout the Golgi complex is vesicle independent.
The In Vitro Assays Reconstitute Transport to Distinct Golgi Compartments
The cell-free assays for transport to the medial-Golgi, trans-Golgi, and TGN exhibit similar overall requirements for optimal reconstitution of transport. This was expected given the fundamental conservation of transport components throughout the secretory pathway (19, 49, 50). Nevertheless, each assay apparently has unique requirements for cytosolic proteins. Cytosol fractionated by the method of Taylor et al. (56) was consistently incapable of reconstituting transport in one and/or the other of the two assays that measure transport to late Golgi compartments, even though this cytosol was functional in the medial assay. This observation is consistent with the finding that sequential transport steps from the ER to the TGN in permeabilized cells exhibit differential requirements for cytosolic factors (13). As further evidence that each assay reconstitutes transport between distinct compartments, our data show that the transport rate, the cytosol dependence of GTPγS inhibition, and the donor membrane NEM sensitivity are also unique for each assay. Moreover, these characteristics are independent of the rate of glycosylation and the source of donor membranes. It thus appears that each in vitro transport assay reconstitutes a distinct compartment-specific transport step within the Golgi complex.
ARF and Vesicles Are Dispensable for In Vitro Transport throughout the Golgi Stack
Using an improved procedure for removing endogenous ARFs from cytosol, we have demonstrated that in vitro transport to the medial-Golgi, trans-Golgi, and TGN can be fully reconstituted with cytosol that is substantially depleted of endogenous ARFs. ARF-depleted cytosol was able to drive transport to nearly the same maximum level attained with reconstituted or unfractionated cytosol, at nearly the same volume equivalents. Nevertheless, ARF-depletion reduced the density of coated vesicles on Golgi membranes 15-fold relative to unfractionated cytosol. Thus, there is no apparent correlation between coated bud and vesicle formation on Golgi membranes and in vitro transport at any level of the stack.
A new finding in this study is that ARF-depletion also resulted in the elongation of fenestrae and blunt-ended tubules on a subset of Golgi cisternae, apparently without significantly increasing their numbers. A similar “tubularization” occurs when Golgi membranes are incubated with ATP in the absence of any cytosol (58). Tubularization in the absence of cytosol is accompanied by the loss of a granular coating normally observed on the periphery of cisternae. We proposed that this granular coating, which we call a type II coat, might function to stabilize curved membrane edges, and that its loss leads to destabilization and rearrangements within the preexisting tubular networks that enclose the fenestrae (58). The tubular cisternae produced during incubations with ARF-depleted cytosol, however, seem to largely retain this peripheral coating (Fig. 7, B and D). Significantly, such tubularization was not observed with ARF-depleted cytosol prepared by the method of Taylor et al. (56). The primary difference between ARF depletion methods is the preservation of additional cytosolic factor(s) in the new protocol that are specifically required for transport to late Golgi compartments. It is thus possible that the tubularization observed here is of a different nature from that occurring in the absence of cytosol and requires specific cytosolic factor(s), in addition to depletion of ARF. A cytosolic protein complex that induces the formation of long Golgi tubules has, in fact, been described (3). The cytosolic factor(s) required for this activity may, however, be distinct from those required for transport in these assays since tubularization had no detectable effect on the characteristics of transport.
Although depletion of ARF promoted growth of fenestrae and tubules on a subset of Golgi cisternae, no biochemical evidence was obtained to support the hypothesis that there is a switch in the mechanism of transport from vesicular to unregulated cisternal fusion at any level of the stack. Since each assay has its own distinctive characteristics, unregulated fusion of membranes in the absence of ARF might be reasonably expected to cause a change, if not a convergence, in these properties. The only biochemical change detected with ARF-depleted cytosol, however, was loss of inhibition by GTPγS. The rate of transport, the maximal extent of transport, and the NEM sensitivity of the membranes were completely unaffected by removing ARF. These findings favor the interpretation that in vitro transport normally occurs by a vesicle-independent mechanism, even when vesicles can be formed.
Constitutive Activation of ARF Differentially Inhibits Each Transport Step
Although not required for in vitro Golgi transport, constitutively activated ARF is clearly an inhibitor of transport at all levels of the Golgi stack. The characteristics of this inhibition, however, are not uniform in each assay. Only partial inhibition is observed in the medial assay and only when the system is saturated with cytosol. In contrast, inhibition of the TGN assay is substantial at the minimal levels of cytosolic protein required for transport. Although it is possible that each assay is preferentially inhibited by different ARF isoforms, the differential sensitivity to GTPγS can be reproduced by recombinant myristoylated ARF1 alone. Each assay might, thus, use compartment-specific factors that have differential affinity for ARF, such as ARF GDP–GTP exchange factors or ARF effector proteins. If inhibition is solely due to sequestration of cisternal membrane into coated vesicles, as we have proposed for the medial assay (58), this differential sensitivity seems to run counter to the observation that coatomer binding is predominantly on the cis rather than trans side of the stack (39). ARF-induced binding of the Golgi-specific adaptor protein, AP-I, could provide a similar block to transport on the trans side of the stack. This seems unlikely, however, since in vitro studies suggest that, if anything, low concentrations of ARF-GTPγS are more effective at recruiting coatomer than AP-1 (52, 57). Studies are underway to determine the mechanisms underlying this differential cytosol dependence of GTPγS-induced inhibition in each assay.
Donor and Acceptor Membrane Asymmetry Is Not Related to Vesicular Transport
It has long been known that the donor and acceptor membranes in the medial assay differ markedly in their stability to various treatments (1). This biochemical asymmetry has been proposed to reflect the need for different sets of proteins for the processes of vesicle formation and vesicle fusion on the donor and acceptor membranes, respectively (1). Our data show that the differential sensitivity of donor and acceptor membranes to inactivation by NEM at 37°C is completely independent of vesicle formation in the medial assay. Moreover, the donor compartments in the trans and TGN assays are considerably less sensitive to NEM-inactivation and have the same sensitivity as the acceptor compartments. Since the donor and acceptor compartments in these assays unambiguously reside within the Golgi complex, it seems likely that their NEM sensitivity is a characteristic of Golgi-specific compartments. Indeed, the IC50 for NEM inhibition in these assays is similar to the EC50 for NEM-induced permeabilization of Golgi membranes (60). The unique NEM sensitivity of donor membranes in the medial assay might thus indicate that the donor in this assay corresponds to a non-Golgi compartment, perhaps elements of the ERGIC (56). These intermediates might be able to fuse with the cis acceptor Golgi compartment, where incorporation of GlcNAc into VSV-G protein can also occur in CHO cells (51). The activity of the ERGIC as donor in this particular assay can now be directly tested.
Possible Mechanisms of ARF-independent In Vitro Golgi Transport
If in vitro Golgi transport does not involve ARF or vesicle carriers, then by what mechanism do proteins move between cisternae in these assays? One possible explanation is that the assays reconstitute only lateral fusion of biosynthetically identical donor and acceptor cisternae (cis–cis, medial–medial, etc.; reference 36), rather than anterograde transport between distinct cisternae. For example, because donor membranes in the medial assay lack GlcNAc transferase I, both the VSV-G protein in the cis and medial compartments of the donor membranes lack GlcNAc and are thus suitable substrates for GlcNAc transferase I in the medial acceptor compartment. Lateral fusion between medial donor and medial acceptor compartments would, therefore, result in addition of [3H]GlcNAc to VSV-G protein carbohydrates. Similarly, lateral fusion in all of these in vitro assays could give rise to a transport signal. In spite of this, substantial NSF-dependent incorporation of [3H]GlcNAc occurred when Lec 8 and Lec 2 donor Golgi were substituted for Lec 1 donor Golgi in the medial assay. If only lateral transport between medial compartments was reconstituted, membrane fusion (i.e., NSF) would not be required to observe glycosylation because Lec 8 and Lec 2 membranes contain active GlcNac transferase I in the medial-Golgi. It thus appears that a significant portion, if not all, of the transport detected in the medial assay is anterograde. Additional studies verifying and extending this conclusion to transport throughout the Golgi stack will be reported elsewhere (Cairns, M., S. Happe, and P. Weidman, manuscript in preparation).
An alternative hypothesis is that transport could occur between biosynthetically distinct donor and acceptor Golgi compartments by fusion of peripheral Golgi tubules (59). For example, the tip of a tubule on a donor cisterna could potentially target to an acceptor cisterna and fuse in an NSF-dependent reaction. Although the de novo formation and/or scission of these Golgi tubules might require ARF and coat proteins (59), the actual fusion event would depend only on the presence of blunt-ended tubules with the requisite components for targeting and fusion. Elongation of fenestrae and tubules might also have no effect on transport, if the number of blunt-ended tubules competent for transport does not change. On the other hand, constitutive activation of ARF might result in irreversible coating of these tubule tips, an increased frequency of tubule scission reactions (59), and/or sequestration of potential fusion sites (58). This could cause inhibition of in vitro transport, even though ARF is not directly required for transport per se.
Consistent with this model, isolated Golgi cisternae exhibit a low but relatively constant number of uncoated buds at the tips of peripheral tubules, and tubular connections between cisternae are commonly seen (Figs. 6 and 7 A). If the uncoated tips of tubules on isolated Golgi are the primary active elements in in vitro transport, then it would be reasonable to expect that the characteristics of transport (i.e., targeting and fusion) would remain constant, independent of whether new coated buds form or tubules elongate. Fusion between distinct compartments could, however, use a subset of compartment-specific targeting and/or fusion components, accounting for the different characteristics of each transport assay. An alternative explanation for the unique characteristics of transport to early and late Golgi compartments might also be that the assays are measuring different phenomena, such as fusion of ERGIC with early Golgi compartments in the medial assay versus intra-Golgi transport by tubules in the trans and TGN assays.
A final possibility that cannot be excluded at present is that transport could involve the targeted fusion of Golgi membrane fragments that are produced during cell homogenization. These fragments might fuse with each other or with cisternae. This might explain the lack of correlation between changes in cisternal morphology and the biochemical properties of transport. If this is the case, our data indicate that compartmental specificity is retained, and that these new in vitro assays will be useful for the identification of compartment specific components in targeting and fusion.
What Is the Relationship between In Vitro and In Vivo Intra-Golgi Transport?
A long standing issue in the use of in vitro systems to study transport processes has been the question of how faithfully the in vitro system reconstitutes the in vivo process. This has been particularly true in the case of transport within the Golgi complex, where neither in vitro nor in vivo studies have been able to unambiguously define the role of ARF and coatomer vesicles in secretory protein transport. The available evidence indicates that ARF and coatomer are essential for maintenance of Golgi integrity (12, 25) and for retrograde transport from the intermediate compartment and Golgi to the ER (11, 22, 27, 33). Golgi enzymes also seem to undergo retrograde transport within the stack (7, 24, 26, 29, 61), although the role of coatomer vesicles in this process has not been established. The possibility that coatomer vesicles are exclusively retrograde carriers has lead to a revival of the cisternal maturation model as an alternative to the classical vesicular model for anterograde intra-Golgi transport (4, 23, 34). In the cisternal maturation model, cargo protein is transported forward in cisternae that “mature” sequentially into cis, medial, and trans cisternae, as a consequence of the retrograde vesicular transport of Golgi enzymes (23). Upon reaching the TGN, the cargo is packaged into vesicles for transport to the cell surface, and the Golgi enzymes recycle in vesicles to the trans cisterna. The possibility that coatomer vesicles mediate both anterograde transport through the Golgi complex, as well as retrograde transport to the ER, has been proposed from the recent finding that retrograde and anterograde cargo within the Golgi segregate into distinct populations of coatomer-coated buds and vesicles (41).
How can these observations be reconciled with the in vitro transport data, where coated vesicles appear to be entirely dispensable for intra-Golgi transport? There is clearly some fidelity in the reconstitution, since there is a requirement for NSF, presumably also SNAPs and SNAREs, and one or more compartment-specific cytosolic factors. Directionality also seems to be preserved. If ARF and coatomer mobilize protein for anterograde transport in vivo (41), either in vesicles or in tubules (37, 38, 59), our data suggest that this function is not reconstituted in these cell-free assays in spite of the fact that coated buds are formed. There is a possibility that only protein mobilized by ARF and coatomer in vivo is competent to be transported in vitro. In this case, the blunt-ended tubules on isolated Golgi, or fragments thereof, are reasonable candidates for such ARF-independent transport intermediates. The segregation of anterograde cargo into coatomer buds in vivo (41) might thus represent mobilization into tubules, rather than vesicles, as previously suggested (59). Alternative approaches will clearly be needed to resolve the potential role(s) of ARF and coatomers in tubule morphogenesis and anterograde transport.
Abbreviations used in this paper
- ARF
ADP-ribosylation factor
- ERGIC
endoplasmic reticulum–Golgi intermediate compartment
- GlcNAc
N-acetylglucosamine
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- MESNA
2-mercaptoethanesulfonic acid
- myr-rARF1
recombinant myristoylated ARF1
- NEM
N-ethylmaleimide
- non-myr-rARF1
recombinant nonmyristoylated ARF1
- NSF
NEM-sensitive fusion protein
- STI
soybean trypsin inhibitor
- VSV-G protein
vesicular stomatitis viral glycoprotein
Footnotes
The authors are indebted to Dr. John Heuser, Robyn Roth, and Mike Morgan for their generous assistance in the electron microscopic portion of these studies. Drs. Paul Melançon, Ben Glick, Maurine Linder, and Steve Scholnick provided helpful comments and suggestions during the preparation of the manuscript. S. Happe also thanks Dr. C. Klein, Dr. J. Corbett, and B. Hunter for their input and encouragement during course of this work. Special thanks go to M. Cairns for preparing many of the materials used in these studies, to R. Kahn for providing ARF antibodies and myr-rARF1, and to S. Berger and P. Melançon for providing non-myr-rARF1.
This research was supported by National Institutes of Health (NIH) grant GM54428 to P. Weidman. S. Happe received one year of support from NIH Research Training Grant HL07050.
Address all correspondence to Peggy Weidman, Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, St. Louis, MO 63104. Tel.: 314-577-8179. Fax: 314-577-8156. E-mail: weidmanp@wpogate.slu.edu
References
- 1.Balch WE, Rothman JE. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985;240:413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 2.Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 3.Banta M, Polizotto RS, Wood SA, De Figueiredo P, Brown WJ. Characterization of a cytosolic activity that induces the formation of Golgi membrane tubules in a cell-free reconstitution system. Biochemistry. 1995;34:13359–13366. doi: 10.1021/bi00041a012. [DOI] [PubMed] [Google Scholar]
- 4.Becker B, Bölinger B, Melkonian M. Anterograde transport of algal scales through the Golgi complex is not mediated by vesicles. Trends Cell Biol. 1995;5:305–306. doi: 10.1016/s0962-8924(00)89047-9. [DOI] [PubMed] [Google Scholar]
- 5.Block MR, Glick BS, Wilcox CA, Wieland RT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci USA. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boman A, Kahn R. Arf proteins: the membrane traffic police. Trends Biochem Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- 7.Bryant NJ, Stevens TH. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J Cell Biol. 1997;136:287–297. doi: 10.1083/jcb.136.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavenagh MM, Whitney JA, Carroll K, Zhang CJ, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells—Arf6 is uniquely localized to the plasma membrane. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- 9.Clary DO, Rothman JE. Purification of three related peripheral membrane proteins needed for vesicular transport. J Biol Chem. 1990;265:10109–10117. [PubMed] [Google Scholar]
- 10.Colombo MI, Gonzalo S, Weidman P, Stahl P. Characterization of trypsin-sensitive factor(s) required for endosome-endosome fusion. J BiolChem. 1991;266:23438–23445. [PubMed] [Google Scholar]
- 11.Cosson P, Domolliore C, Hennecke S, Duden R, Letourneur F. Delta- and zeta-COP, two coatomer subunits homologous to clathrin-associated proteins, are involved in ER retrieval. EMBO (Eur Mol Biol Organ) J. 1996;15:1792–1798. [PMC free article] [PubMed] [Google Scholar]
- 12.Dascher C, Balch WE. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- 13.Davidson HW, Balch WE. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans Golgi network. J Biol Chem. 1993;268:4216–4226. [PubMed] [Google Scholar]
- 14.Deutscher SL, Hirschberg CB. Mechanism of galactosylation in the Golgi apparatus. J Biol Chem. 1986;261:96–100. [PubMed] [Google Scholar]
- 15.Deutscher SL, Nuwayhid N, Stanley P, Briles E, Hirschberg CB. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984;39:295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP- ribosylation factor, a small GTP binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane catalyzed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 18.Elazar Z, Orci L, Ostermann J, Amherdt M, Tanigawa G, Rothman JE. ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J Cell Biol. 1994;124:415–424. doi: 10.1083/jcb.124.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- 20.Franco M, Chardin P, Chabre M, Paris S. Myristoylation-facilitated binding of the G protein ARF1-GDP to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J Biol Chem. 1996;271:1573–1578. doi: 10.1074/jbc.271.3.1573. [DOI] [PubMed] [Google Scholar]
- 21.Fraser IH, Mookerjea S. Studies on the purification and properties of UDP-galactose glycoprotein galactosyltransferase from rat liver and serum. Biochem J. 1976;156:347–355. doi: 10.1042/bj1560347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaynor EC, Emr SD. COPI-independent anterograde transport—cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glick BS, Elston T, Oster G. A cisternal maturation mechanism can explain the asymmetry of the Golgi stack. FEBS Lett. 1997;414:177–181. doi: 10.1016/s0014-5793(97)00984-8. [DOI] [PubMed] [Google Scholar]
- 24.Graham T, Krasnov V. Sorting of yeast α1,3 mannosyltransferase is mediated by a luminal domain interaction and a transmembrane domain signal that can confer clathrin-dependent Golgi localization to a secreted protein. Mol Biol Cell. 1995;6:809–824. doi: 10.1091/mbc.6.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by ε-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris SL, Waters MG. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J Cell Biol. 1996;132:985–998. doi: 10.1083/jcb.132.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harter C, Pavel J, Coccia F, Draken E, Wegehingel S, Tschochner H, Wieland F. Nonclathrin coat protein gamma, a subunit of coatomer, binds to the cytoplasmic dilysine motif of membrane proteins of the early secretory pathway. Proc Natl Acad Sci USA. 1996;93:1902–1906. doi: 10.1073/pnas.93.5.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiebsch RR, Wattenberg BW. Vesicle fusion in protein transport through the Golgi in vitro does not involve long-lived prefusion intermediates. A reassessment of the kinetics of transport as measured by glycosylation. Biochemistry. 1992;31:6111–6118. doi: 10.1021/bi00141a022. [DOI] [PubMed] [Google Scholar]
- 29.Johnston PA, Stieber A, Gonatas NK. A hypothesis on the traffic of MG160, a medialGolgi sialoglycoprotein. J Cell Sci. 1994;107:529–537. doi: 10.1242/jcs.107.3.529. [DOI] [PubMed] [Google Scholar]
- 30.Kahn RA, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman JE. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992;267:13039–13046. [PubMed] [Google Scholar]
- 31.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 32.Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP-ribosylation factor– dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letourneur F, Gaynor EC, Henneke S, Démolière C, Dudden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 34.Lippincott-Schwartz J. Bidirectional membrane traffic between the endoplasmic reticulum and Golgi apparatus. Trends Cell Biol. 1993;3:81–88. doi: 10.1016/0962-8924(93)90078-f. [DOI] [PubMed] [Google Scholar]
- 35.Melançon P, Glick BS, Malhotra V, Weidman PJ, Serafini T, Gleason ML, Orci L, Rothman JE. Involvement of GTP-binding “G” proteins in transport through the Golgi stack. Cell. 1987;51:1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- 36.Mellman I, Simons K. The Golgi complex: in vitro veritas? . Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mironov AA, Weidman P, Luini A. Variation on the intracellular transport theme: maturing cisternae and trafficking tubules. J Cell Biol. 1997;138:481–484. doi: 10.1083/jcb.138.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morré DJ, Keenan TW. Golgi apparatus buds—vesicles or coated ends of tubules. Protoplasma. 1994;179:1–4. [Google Scholar]
- 39.Oprins A, Duden R, Kreis TE, Geuze HJ, Slot JW. β-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orci L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- 41.Orci L, Stamnes M, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 42.Paiement J, Rachubinski RA, Ng NMK Ying Kin, R.A. Sikstrom, and J.M. Bergeron. Membrane fusion and glycosylation in the rat hepatic Golgi apparatus. J Cell Biol. 1982;92:147–154. doi: 10.1083/jcb.92.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer DJ, Helms JB, Beckers C, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- 44.Pfanner N, Orci L, Glick BS, Amherdt M, Arden SR, Malhotra V, Rothman JE. Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell. 1989;59:95–102. doi: 10.1016/0092-8674(89)90872-6. [DOI] [PubMed] [Google Scholar]
- 45.Pfanner N, Glick BS, Arden SA, Rothman JE. Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J Cell Biol. 1990;110:955–961. doi: 10.1083/jcb.110.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth J, Berger EG. Immunocytochemical localization of galactosyltransferase in HeLa. J Cell Biol. 1982;93:223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roth J, Taatjes D, Lucocq J, Weinstein J, Paulson J. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985;43:287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- 48.Rothman J. Transport of the vesicular stomatitis glycoprotein to transGolgi membranes in a cell-free system. J Biol Chem. 1987;262:12502–12510. [PubMed] [Google Scholar]
- 49.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 50.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 51.Schwaninger R, Beckers CJ, Balch WE. Sequential transport of protein between the endoplasmic reticulum and successive Golgi compartments in semi-intact cells. J Biol Chem. 1991;266:13055–13063. [PubMed] [Google Scholar]
- 52.Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- 53.Stanley P, Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. SomCell Gen. 1977;3:391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- 54.Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor TC, Kahn RA, Melançon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- 56.Taylor TC, Kanstein M, Weidman P, Melançon P. Cytosolic ARFs are required for vesicle formation but not for cell-free intra-Golgi transport: evidence for coated vesicle-independent transport. Mol Biol Cell. 1994;5:237–252. doi: 10.1091/mbc.5.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weidman P, Roth R, Heuser J. Golgi membrane dynamics imaged by freeze-etch electron microscopy: views of different membrane coatings involved in tubulation versus vesiculation. Cell. 1993;75:123–133. [PubMed] [Google Scholar]
- 59.Weidman PJ. Anterograde transport through the Golgi complex: do Golgi tubules hold the key? . Trends Cell Biol. 1995;5:302–305. doi: 10.1016/s0962-8924(00)89046-7. [DOI] [PubMed] [Google Scholar]
- 60.Weidman PJ, Winter WM. The G protein–activating peptide, mastoparan, and the synthetic NH2-terminal ARF peptide, ARFp13, inhibit in vitro Golgi transport by irreversibly damaging membranes. J Cell Biol. 1994;127:1815–1827. doi: 10.1083/jcb.127.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan L, Barriocanal JG, Bonifacino JS, Sandoval IV. Two integral membrane proteins in the cis-middle and trans-part of the Golgi system acquire sialylated N-linked carbohydrates and display different turnovers and sensitivity to cAMP-dependent phosphorylation. J Cell Biol. 1987;105:215–227. doi: 10.1083/jcb.105.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]