Abstract
The trafficking of GLUT4, a facilitative glucose transporter, is examined in transfected CHO cells. In previous work, we expressed GLUT4 in neuroendocrine cells and fibroblasts and found that it was targeted to a population of small vesicles slightly larger than synaptic vesicles (Herman, G.A, F. Bonzelius, A.M. Cieutat, and R.B. Kelly. 1994. Proc. Natl. Acad. Sci. USA. 91: 12750–12754.). In this study, we demonstrate that at 37°C, GLUT4-containing small vesicles (GSVs) are detected after cell surface radiolabeling of GLUT4 whereas uptake of radioiodinated human transferrin does not show appreciable accumulation within these small vesicles. Immunofluorescence microscopy experiments show that at 37°C, cell surface–labeled GLUT4 as well as transferrin is internalized into peripheral and perinuclear structures. At 15°C, endocytosis of GLUT4 continues to occur at a slowed rate, but whereas fluorescently labeled GLUT4 is seen to accumulate within large peripheral endosomes, no perinuclear structures are labeled, and no radiolabeled GSVs are detectable. Shifting cells to 37°C after accumulating labeled GLUT4 at 15°C results in the reappearance of GLUT4 in perinuclear structures and GSV reformation. Cytosol acidification or treatment with hypertonic media containing sucrose prevents the exit of GLUT4 from peripheral endosomes as well as GSV formation, suggesting that coat proteins may be involved in the endocytic trafficking of GLUT4. In contrast, at 15°C, transferrin continues to traffic to perinuclear structures and overall labels structures similar in distribution to those observed at 37°C. Furthermore, treatment with hypertonic media has no apparent effect on transferrin trafficking from peripheral endosomes. Double-labeling experiments after the internalization of both transferrin and surface-labeled GLUT4 show that GLUT4 accumulates within peripheral compartments that exclude the transferrin receptor (TfR) at both 15° and 37°C. Thus, GLUT4 is sorted differently from the transferrin receptor as evidenced by the targeting of each protein to distinct early endosomal compartments and by the formation of GSVs. These results suggest that the sorting of GLUT4 from TfR may occur primarily at the level of the plasma membrane into distinct endosomes and that the organization of the endocytic system in CHO cells more closely resembles that of neuroendocrine cells than previously appreciated.
Many cells adjust the composition of their plasma membranes in response to external signals. For example, in fat and muscle cells after exposure to insulin, the facilitative glucose transporter GLUT4 is redistributed from a predominantly intracellular location to the plasma membrane (Cushman and Wardzala, 1980; Suzuki and Kono, 1980; for reviews see James and Piper, 1994; Stephens and Pilch, 1995; Hausdorff et al., 1996; Holman and Cushman, 1996). In the absence of insulin, GLUT4 resides primarily within tubulovesicular elements and small vesicles (Biber and Lienhard, 1986; James et al., 1987; Blok et al., 1988; Slot et al., 1991a ,b; Smith et al., 1991; Rodnick et al., 1992). Different studies have suggested that, in fat cells, the intracellular storage compartment containing the GLUT4 transporter is derived by endocytosis (Slot et al., 1991a ,b; Smith et al., 1991; Czech and Buxton, 1993; Satoh et al., 1993), and that this pathway may represent a specialization of the recycling endocytic pathway analogous to the synaptic vesicle recycling system in neurons and in neuroendocrine cells.
The GLUT4 transporter, a protein of ∼48 kD with 12 transmembrane domains, is internalized in cardiac cells and adipocytes via clathrin coated pits (Slot et al., 1991a ; Robinson et al., 1992). Its endocytosis in fibroblasts, adipocytes, and myocytes is mediated by dileucine and phenylalanine-based motifs (Piper et al., 1993; Corvera et al., 1994; Garippa et al., 1994; Verhey and Birnbaum, 1994; Haney et al., 1995; Martin et al., 1996). GLUT4 has been shown to constitutively recycle to and from the plasma membrane (Jhun et al., 1992; Satoh et al., 1993; Yang and Holman, 1993).
We previously analyzed the intracellular distribution of GLUT4. We found that when expressed in neuroendocrine PC12 cells, GLUT4 was not targeted to synaptic vesicles but accumulated in a population of vesicles with diameters of ∼70 nm, which were distinct from synaptic vesicles. These homogeneously sized vesicles were also seen in transfected 3T3 fibroblasts and CHO cells, and had physical characteristics resembling those seen in primary adipose cells (Herman et al., 1994). In adipose cells, addition of insulin resulted in the redistribution of GLUT4 from the small vesicles to membranes with the sedimentation properties of endosomes and plasma membranes (Herman et al., 1994). In PC12 cells cotransfected with the polymeric immunoglobulin receptor (pIgR),1 the small vesicles excluded markers of synaptic vesicles, as well as markers of the conventional endocytotic pathway (transferrin receptor [TfR]) and the transcytotic pathway (pIgR). These findings suggested that GLUT4 was targeted to a unique class of small vesicles that were present in a variety of cell types and might be involved in transient modification of the cell surface. The accumulation of GLUT4 in intracellular compartments resembling those seen in muscle cells and adipocytes has also been described by other investigators for a variety of transfected cell lines (Haney et al., 1991; Hudson et al., 1992, 1993; Piper et al., 1992; Shibasaki et al., 1992; Thorens and Roth, 1996).
In the present study, a CHO cell line expressing a c-myc epitope–tagged version of GLUT4 was used to determine whether the class of small vesicles containing GLUT4 could be detected by cell surface labeling and, if so, whether the endocytic pathway giving rise to these vesicles differed from the conventional route followed by the transferrin receptor. Antibodies directed against the myc epitope were used to follow the trafficking of GLUT4 labeled at the cell surface. The transferrin receptor was used as a well-accepted marker of the recycling endocytic pathway.
Small vesicles enriched in GLUT4 but not in the TfR were detected after cell surface labeling of these molecules. As in the formation of neuroendocrine synaptic vesicles (also referred to as synaptic-like microvesicles) in PC12 cells (Desnos et al., 1995), formation of GLUT4-containing small vesicles (GSVs) was blocked at low temperature (15°C), consistent with GSVs deriving from an endosomal precursor. Kinetic studies of vesicle formation also suggest an endosomal precursor.
The internalization of both transferrin and antibodies directed against the myc-tagged GLUT4 was also examined by immunofluorescence microscopy. At 15° and at 37°C, peripheral endocytic compartments enriched for GLUT4 and lacking the TfR were observed. Selective traffic from the cell surface to an endosome that excludes the transferrin receptor is not easily reconciled with the traditional model of membrane trafficking in undifferentiated cells (for review see Mellman, 1996) in which proteins are internalized nonselectively from the plasma membrane into a common endosome from which sorting pathways diverge to different intracellular destinations. The data is consistent with a model where the sorting of GLUT4 from the TfR appears to occur at the plasma membrane at a step that precedes the level of small vesicle formation. The endocytic pathway followed by GLUT4 in CHO cells thus appears to be distinct from that followed by the transferrin receptor, and possesses features similar to those found in the endocytic pathways of neuroendocrine cells.
Materials and Methods
Materials and Antibodies
Cell culture reagents were obtained through the University of California (San Francisco, CA) Cell Culture facility. Iodogen reagent was from Pierce Chemical Co. (Rockford, IL). Enhanced chemiluminescence (ECL) reagents were purchased from Amersham Corp. (Arlington Heights, IL). Human apotransferrin was obtained from Sigma Chemical Co. (St. Louis, MO), Texas red–labeled human transferrin from Molecular Probes (Eugene, OR). Miscellaneous chemical reagents were acquired from Sigma Chemical Co. and Fisher Biochemicals (Santa Clara, CA).
Monoclonal antibody 9E10, directed against the c-myc epitope (Schwab et al., 1986) was provided by Dr. J.M. Bishop (University of California, San Francisco, CA). R820, an anti-GLUT4 polyclonal antibody was from East Acres Biologicals (Cambridge, MA). Anti-rab5 monoclonal antibody, anti-rab4 polyclonal sera, and anti-TGN38 polyclonal sera were provided by Drs. I. Mellman, M. Zerial, and A. Wise, respectively. Goat anti–rabbit and goat anti–mouse IgG coupled to HRP or FITC were obtained from Cappel Laboratories (Aurora, OH).
Cell Culture
CHO cells were grown in Ham's F-12 media supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 mg/ml G418 in humidified incubators with 5% CO2 at 37°C. Experiments were performed using cells stably transfected with GLUT4 containing a c-myc epitope tag in the first exofacial domain (Kanai et al., 1993). Some cells were also coexpressing either the human insulin receptor or the mouse PDGF receptor (Kanai et al., 1993; Kamohara et al., 1995). Before experiments, cells were incubated for 1–2 h in serum-free media supplemented with 3% BSA. Under these conditions, no differences could be observed between cells that were coexpressing the insulin or the PDGF receptor, and those that were only expressing GLUT4 myc.
Iodination of Anti-myc Antibodies and Transferrin
Monoclonal antibody 9E10 was purified from serum-free hybridoma supernatant by protein G–Sepharose chromatography. Human apotransferrin was further purified by Sephacryl S-300 gel filtration, and then iron loaded as described (Yamashiro et al., 1984; McGraw et al., 1987). 100-μg aliquots of purified antibody or iron-loaded transferrin were iodinated as described using iodogen-mediated coupling (Grote and Kelly, 1996).
Subcellular Fractionation
Cells were homogenized using a glass–Teflon Potter-Elvehjem homogenizer (Thomas Scientific, Swedesboro, NJ). GSVs were isolated by velocity sedimentation of postnuclear supernatants on glycerol gradients as described previously (Herman et al., 1994) using a modification of the protocol described by Clift-O'Grady et al. (1990). After internalization of radioiodinated antibodies or transferrin (see below), gradients were centrifuged at 60,000 g for 90 min to increase the separation between GSVs and soluble label on top of the gradient. Gradient fractions were analyzed by SDS-PAGE and Western blotting as described previously (Bonzelius et al., 1994), or counted directly in a gamma counter. GLUT4 immunoreactivity was detected using primary polyclonal antibody R820 or mAb 9E10 and HRP-coupled secondary antibodies and visualized using the ECL system.
Endocytosis of Surface-labeled GLUT4
Endocytosis of GLUT4 was measured using a modification of the procedure described by Grote and Kelly (1996). Cells were plated onto poly- d-lysine–coated dishes 2 d before the experiment. On the day of the experiment, they were washed for 15 min with PBS/3% BSA, and then incubated with radioiodinated mAb 9E10 (107 cpm/ml, ∼3.5 μg/ml) in Ham's F-12/3% BSA/10 mM Hepes, pH 7.4, for 1 h at 4°C. After removal of the radioiodinated antibody, cells were washed extensively on ice. Cells were then incubated at 15° or 37°C for various times to allow internalization of antibody, and then returned to 4°C. After extensive washing with ice-cold PBS/3% BSA, cells were scraped from dishes and pelleted at 400 g. Pellets were resuspended in ice-cold PBS and subjected to two additional cycles of washing and pelleting. Antibody remaining at the surface was then removed by resuspending and washing the pellets twice in ice-cold 0.5 N acetic acid/150 mM NaCl for 15 min at 4°C. The supernatant from these washes was counted on a gamma counter. The amount of acid-resistant radioactivity was determined by counting the cell pellets after acid stripping. For each time point, the fraction of the total cell-associated radioactivity that was acid resistant was calculated. Approximately 18% of the bound 125I-9E10 was resistant to acid stripping at time zero before warming of the cells.
Targeting of Surface-labeled GLUT4 to GSVs
Cells were incubated with serum-free nutrient mixture Ham's F-12, 3% BSA, 10 mM Hepes, pH 7.4, containing 107 cpm/ml (∼3.5 μg/ml) of 125I-mAb 9E10 at 15° or 37°C for various times. In parallel, cells were incubated with media containing 125I-transferrin. After incubation, cells were placed on ice and washed extensively with ice-cold PBS/3% BSA. Cells were then scraped in ice-cold buffer A (150 mM NaCl, 1 mM EGTA, 0.1 mM MgCl2, 10 mM Hepes, pH 7.4), and then centrifuged at 400 g for 5 min. Pellets were resuspended in ice-cold buffer A and subjected to three additional cycles of washing and pelleting before homogenization. Postnuclear supernatants were then subjected to velocity sedimentation analysis as described above. For all experiments, equal amounts of protein were loaded onto each gradient.
Pulse-chase experiments were performed by incubating cells with radioiodinated mAb 9E10 for 80 min at 15°C, washing extensively on ice, and then rewarming the cells in media in the absence of labeled antibody for various periods of time before processing as above. Kinetic data were obtained by determining the GSV-associated radioactivity for each time point. The GSV-associated radioactivity was calculated by plotting the gradient profile of 125I-9E10 for each time point and integrating the GSV peaks using NIH Image 1.6 software (National Institutes of Health, Bethesda, MD). Relative units were then calculated to facilitate comparison between independent experiments. An arbitrary value of 1 was assigned to the area of the peak at 30 min (see Fig. 4), 10 min (see Fig. 7), and at time zero (see Fig. 12, A and B).
Figure 4.
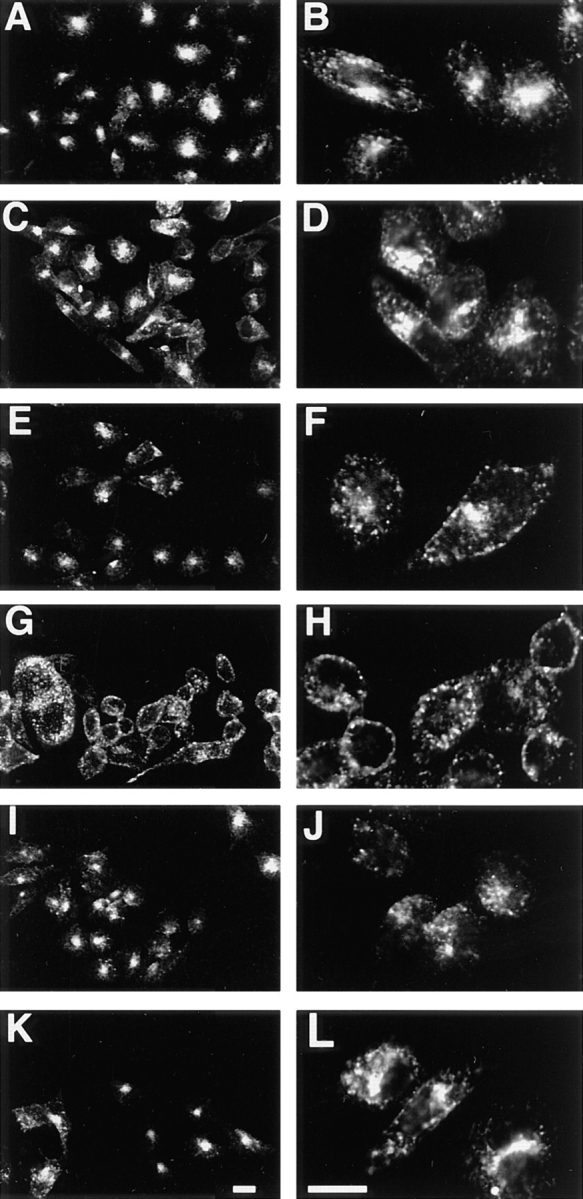
Immunofluorescence microscopy of CHO/G4myc cells after internalization of anti-myc mAb or transferrin. Cells were incubated with either anti-myc mAb or Texas red–coupled human transferrin as indicated below, and fixed with paraformaldehyde. Cells with internalized antibody were permeabilized with saponin and incubated with fluorescently labeled anti–mouse antibodies before processing. Cells with internalized transferrin were processed immediately. Incubation with Texas red–coupled human transferrin was for 30 min at 37°C (A and B), or for 2.5 h at 15°C (E and F), or for 2.5 h at 15°C followed by a chase in transferrin-free media for 10 min at 37°C (I and J). GLUT4-containing compartments were labeled by internalizing anti-myc antibodies for 30 min at 37°C (C and D) or for 2.5 h at 15°C (G and H) or for 2.5 h at 15°C, followed by a chase in antibody-free media for 10 min at 37°C (K and L). Images were obtained by laser scanning confocal microscopy. Low (A, C, E, G, I, and K) and high power (B, D, F, H, J, and L) views are shown. Note that at 37°C, surface-labeled GLUT4 and transferrin both reach peripheral and juxtanuclear structures (A–D). However, at 15°C, GLUT4 is observed primarily in peripheral compartments, with a concomitant loss of labeling of the juxtanuclear structures (G and H), whereas the transferrin pattern is unchanged from that seen at 37°C (E and F). With the 37°C chase, labeling of GLUT4 in juxtanuclear structures is restored (K and L). Bars, 10 μm.
Figure 7.
Formation of GSVs is inhibited by treatment either with hypertonic medium containing sucrose or with cytosol acidification. Cells were incubated with radioiodinated anti-myc mAb for 80 min at 15°C, washed extensively, and warmed in regular media, in media containing 0.45 M sucrose (A: □, control; •, sucrose) or in media acidified with 10 mM acetic acid (B: □, control; •, 10 mM acetic acid). Cells were fractionated by velocity sedimentation as above.
The effect of hypertonic media containing sucrose on GSV formation was assessed as described (Hansen et al., 1993) by placing cells previously labeled at 15°C into media containing 0.45 M sucrose, 37°C, for 10 or 30 min. To study the effect of cytosol acidification, media (DME containing 10 mM Hepes) was acidified to pH 5 as described using 10 mM acetic acid or HCl (Hansen et al., 1993). After cells were labeled with 125I-mAb 9E10 at 15°C and washed, they were incubated in acidified media for 10 min at 15°C and then shifted to 37°C for 10 or 30 min.
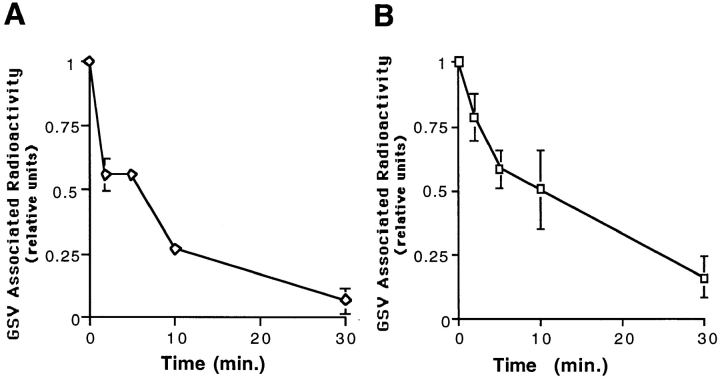
Disappearance of labeled GSVs was assessed by labeling de novo GSVs and measuring the loss of GSV-associated radioactivity under conditions that prevent the formation of additional GSVs. Cells were incubated with 125I-mAb 9E10 for 80 min at 15°C, washed on ice, and then shifted to 37°C for 30 min to allow formation of labeled GSVs. Cells containing the labeled GSVs were then incubated either in regular media at 15°C or in media containing 0.45 M sucrose to block further GSV formation for various times before processing as above.
Antibody-binding Assay for Quantification of Cell Surface GLUT4
GLUT4 present on the cell surface was assayed using a modification of a previously described procedure (Kanai et al., 1993). Cells were incubated for 30 min at either 37°C in the presence or absence of hypertonic media or at 15°C in regular media. Cells were then placed on ice and fixed in 4% paraformaldehyde in PBS for 10 min, followed by 20 min at room temperature. They were then quenched by washing twice with PBS/25 mM glycine and once with PBS. The cells were blocked by incubation with PBS/ 3% BSA for >1 h at room temperature. 125I-mAb 9E10 was bound to the surface by incubating the fixed cells with radioiodinated antibody (107 cpm/ml) for 1 h at room temperature. Cells were then washed extensively with PBS/3% BSA, scraped, and then centrifuged at 400 g for 5 min. Pellets were resuspended in PBS and subjected to two additional cycles of washing and pelleting to remove nonspecifically bound antibody. The amount of antibody bound to the surface was determined by counting the pellets on a gamma counter. Values obtained were normalized to the amount of total protein in each pellet.
Immunofluorescence Microscopy
Cells were analyzed by confocal immunofluorescence microscopy as described (Bonzelius et al., 1994). For single-label immunofluorescence, cells were incubated with either mAb 9E10 (10 μg/ml) or Texas red–coupled human transferrin (40 μg/ml) for 30 min at 37°C, or 2.5 h at 15°C. Pulse-chase experiments were performed by internalizing mAb 9E10 or Texas red–coupled transferrin for 2.5 h at 15°C, washing extensively on ice, and then shifting cells to 37°C in regular media, hypertonic media containing sucrose, or acidified media as above for various times. After experiments with Texas red–coupled transferrin, cells were fixed immediately and prepared for microscopy. After experiments with mAb 9E10, cells were fixed, permeabilized with saponin, and incubated with FITC-coupled goat anti–mouse antibodies before imaging. For double-label immunofluorescence, mAb 9E10 was coupled to fluorescein succinimidyl ester (Molecular Probes, Eugene, OR) following the manufacturer's instructions. Cells were incubated with fluorescein-treated 9E10 (10 μg/ml) and Texas red–coupled human transferrin (40 μg/ml) for the times and conditions indicated, fixed, and then processed for microscopy.
Results
GSVs Contain Cell Surface–labeled GLUT4
To facilitate the study of GLUT4 trafficking, we used a CHO cell line stably expressing a version of GLUT4 containing a c-myc epitope (CHO/G4myc) in the first exofacial domain (Kanai et al., 1993). The GSVs from these cells were isolated by velocity sedimentation as previously described (Herman et al., 1994), using a modification of a procedure for the isolation of synaptic vesicles (Clift-O'Grady et al., 1990). Western blot analysis of fractions from the glycerol velocity gradient revealed that the epitope-tagged version of GLUT4 was present within two membrane populations: a class of slowly sedimenting GSVs and a pool of rapidly sedimenting membranes corresponding to endosomes and plasma membrane (Fig. 1). The sedimentation characteristics of the GSVs containing the epitope-tagged transporter were indistinguishable from those previously described for GSVs containing untagged transporter (Herman et al., 1994).
Figure 1.
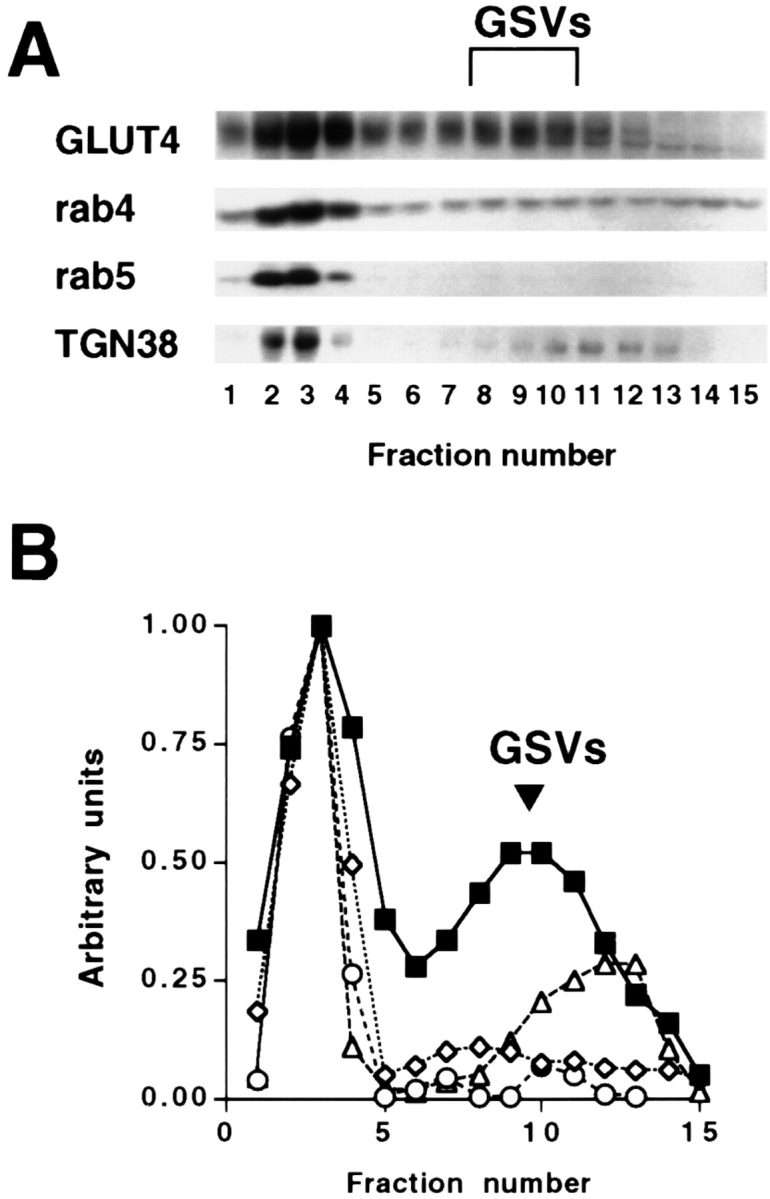
Characterization of GLUT4 small vesicles (GSVs) by velocity sedimentation analysis in CHO cells stably transfected with myc epitope– tagged GLUT4. A postnuclear supernatant from CHO/ G4myc cells was layered onto a 5–25% glycerol gradient over a 50% sucrose pad and centrifuged for 90 min at 60,000 g. Gradient fractions were precipitated with 10% trichloroacetic acid in the presence of 50 μg/ml bovine insulin, subjected to SDS-PAGE and Western blot analysis with anti-myc and anti-rab5 monoclonal antibodies, and anti-rab4 and anti-TGN38 polysera (A). Note that GLUT4 distributes to rapidly sedimenting membranes (endosomes/plasma membrane) at the bottom of the gradient and slowly sedimenting small vesicles (GSVs). (B) Densitometry analysis of A was performed, and for each marker the total signal was calculated by summing the signal from each fraction. The fractional value for each gradient fraction was then calculated. These values were then normalized by reassigning the greatest fractional value for each probe to 1 and the profile was plotted. ▪, GLUT4; ⋄, rab4; ○, rab5; ▵, TGN38.
To further characterize the nature of the GSVs, we analyzed the gradient fractions by Western blotting using anti-rab4, anti-rab5, and anti-TGN38 antibodies (Fig. 1). Neither rab5 nor TGN38 colocalized with GLUT4 in the region of the GSVs, whereas both colocalized with GLUT4 in the region of the rapidly sedimenting membranes. There is a more slowly sedimenting membrane fraction that contains TGN38, but the peak does not coincide with that of the GSVs. Rab4 is found in the region of the GSVs, but is not enriched in those fractions, and is also found colocalizing with GLUT4 in the rapidly sedimenting peak.
To determine if GSVs in CHO cells are formed along the endocytic pathway, we labeled the glucose transporter on the cell surface, allowed internalization of the label, and then analyzed targeting to intracellular compartments. CHO/G4myc cells were incubated for 30 min at 37°C with a radioiodinated monoclonal antibody directed against the c-myc epitope (mAb 9E10). Myc epitope–tagged GLUT4 labeled at the surface was targeted to GSVs as well as to endosomal/plasma membrane fractions (Fig. 2 A) in a manner that is analogous to the distribution of the transporter at steady-state (Fig. 1), suggesting that the binding of exogenous antibody does not detectably alter the distribution of GLUT4. Free ligand was also recovered at the top of the gradient (Fig. 2 A, right). Uptake of the anti-myc antibody into CHO cells not transfected with GLUT4 was negligible, showing that there was no significant internalization of antibody by fluid phase endocytosis (data not shown).
Figure 2.
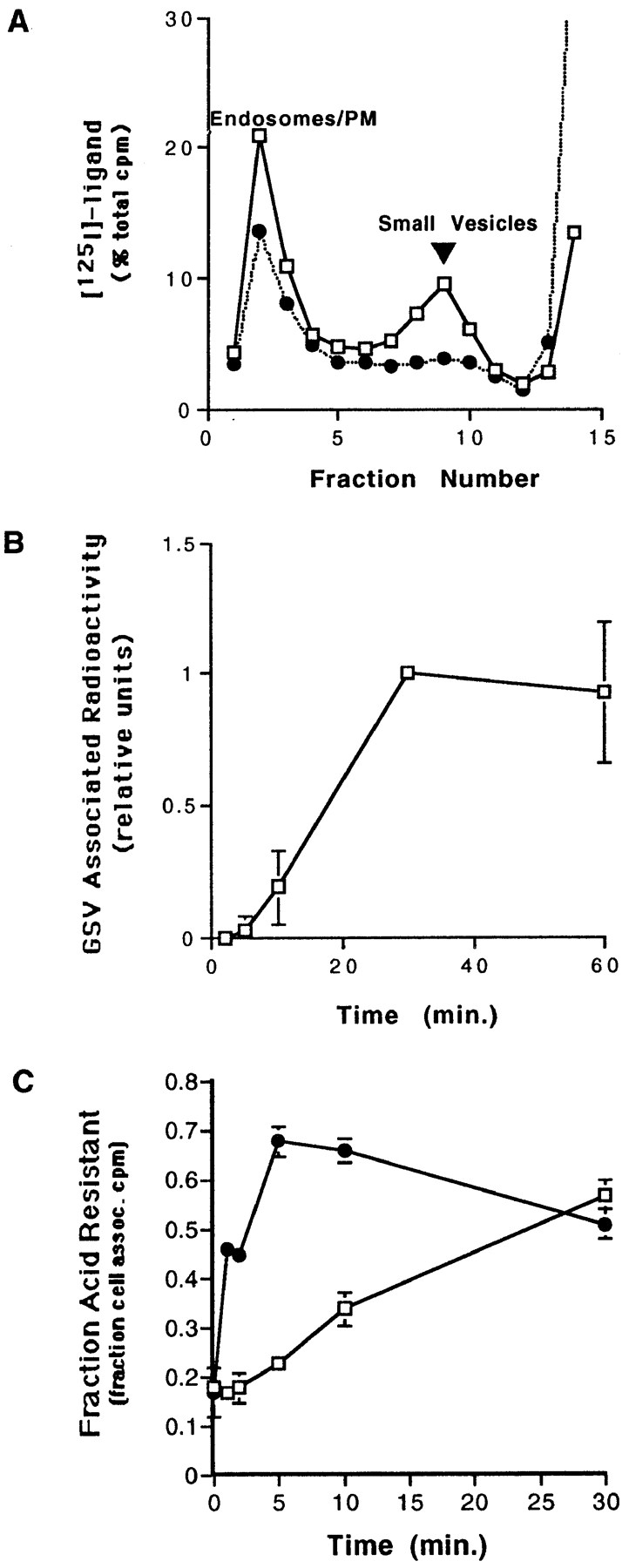
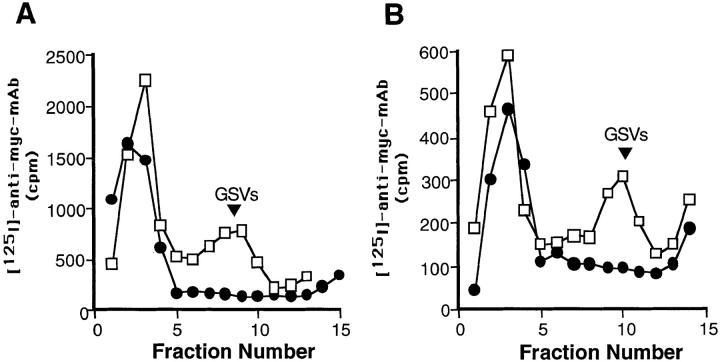
Internalization of surface-labeled myc epitope– tagged GLUT4. (A) CHO/ G4myc cells were incubated with radioiodinated anti-myc mAb 9E10 or human transferrin for 30 min at 37°C. Cells were homogenized and a postnuclear supernatant was fractionated by velocity sedimentation on glycerol gradients. Gradients were centrifuged for 90 min at 60,000 g, and then fractions were collected and analyzed on a gamma counter. Results for each fraction are shown as a percentage of the total cpm on the gradient. Surface-labeled GLUT4, but not transferrin, is targeted to a homogeneously sized population of small vesicles (GSVs). □, 125I–anti-myc mAb (9E10); •, 125I-transferrin. (B) Cells were incubated with radioiodinated anti-myc antibody for the indicated times at 37°C. Cells were then processed as in A, fractionated by velocity sedimentation, and fractions were analyzed on a gamma counter. For each time point, the area under the small vesicle peak was calculated and plotted versus time. Results were determined by averaging three independent experiments. (C) Cells were incubated with radioiodinated anti-myc antibodies for 1 h on ice, washed, and then warmed for various times at either 15° or 37°C followed by acid stripping with 0.5 N acetic acid. The fraction of cell-associated, acid-resistant counts for each time point was then determined. □, 15°C; •, 37°C.
In parallel, CHO/G4myc cells were incubated with radioiodinated human transferrin. The majority of the membrane-bound labeled transferrin was recovered in large endosomal/plasma membrane fractions and did not accumulate within a homogeneous population of small vesicles (Fig. 2 A). Note that these gradient conditions do not resolve sorting from recycling endosomes. In contrast to our observations with PC12 cells (Herman et al., 1994), some iodinated transferrin was distributed at low levels throughout the gradient fractions. When cells were incubated with 50-fold excess of cold transferrin in the presence of radioiodinated transferrin, uptake of 125I was nearly undetectable indicating the specificity of the transferrin internalization (data not shown).
The time-course for the delivery of surface-labeled GLUT4 to GSVs was assessed by incubating cells in the presence of 125I-9E10 for various periods of time and quantifying the GSV-associated radioactivity. After a 5-min lag, surface-labeled GLUT4 began appearing at detectable levels in the GSVs (Fig. 2 B).
Endocytosis of surface-labeled GLUT4 was assayed at both 15° and 37°C (Fig. 2 C). CHO/G4myc cells were incubated with radioiodinated mAb 9E10 on ice, washed, and then shifted to 15° or 37°C for various periods of time. After warming, surface-bound antibody was removed by acid stripping. The fraction of acid-resistant, cell-associated radioactivity was then plotted against time. As expected, endocytosis of GLUT4 into an acid-resistant compartment was slowed at 15°C but not blocked. Morphological analysis by immunofluorescence microscopy confirmed the accumulation of GLUT4 within an acid-resistant endosomal compartment at 15°C (see below).
GSV Formation Is Reversibly Blocked at 15°C
In an effort to dissect the nature of this pathway, we sought to identify whether there were any temperature-sensitive steps in the formation of GSVs. Previous studies have shown that at temperatures <10°C, internalization from the plasma membrane is inhibited (Marsh and Helenius, 1980; Weigel and Oka, 1982; Hopkins and Trowbridge, 1983; Iacopetta and Morgan, 1983; Steinman et al., 1983; Trowbridge et al., 1993). At 15°C, budding of clathrin-coated pits from the plasma membrane takes place (Schmid and Smythe, 1991). At temperatures between 15° and 22°C, endocytosis and recycling is slowed, while transfer to the degradative pathway appears to be blocked (Dunn et al., 1980; Weigel and Oka, 1982; Hopkins and Trowbridge, 1983; Iacopetta and Morgan, 1983; Harding and Unanue, 1990; Trowbridge et al., 1993). Formation of synaptic vesicles from an endosomal precursor in neuroendocrine PC12 cells is completely inhibited at 15°C (Desnos et al., 1995).
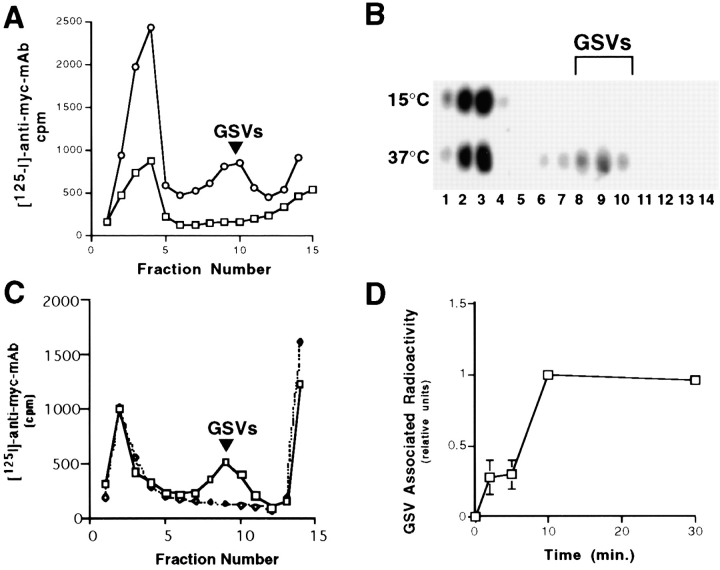
We examined whether GSVs could be formed at 15°C. Surface-labeled GLUT4 was internalized into endosomes but was not detectable in the GSVs (Fig. 3 A). Even after prolonged incubation of up to 3 h at 15°C (data not shown), no labeling of GSVs was detected. Analysis of the steady-state distribution of GLUT4 by immunoblotting demonstrated that incubation at 15°C resulted in the depletion of GSVs as well, suggesting that while formation of GSVs was blocked, fusion with a target compartment could still occur (Fig. 3 B).
Figure 3.
Reversible inhibition of GSV formation at 15°C. Cells were incubated with radioiodinated anti-myc antibodies for 40 min at 37°C or 80 min at 15°C, washed, homogenized, and then fractionated as above. Fractions were counted on a gamma counter (A: ○, 37°C; □, 15°C) or analyzed by SDS-PAGE and Western blotting with polyclonal antibody R820 (B). (In this experiment, repeated washing and pelleting of cells was necessary to lower the background radioactivity. This washing procedure has been observed to cause a preferential loss of GSVs compared with larger GLUT4-containing organelles. [Bonzelius, F., unpublished observations], which may explain the lower yield of GSVs seen here compared with that seen in Fig. 1.) In parallel, cells were incubated with anti-myc antibodies for 80 min at 15°C, and then washed extensively, placed on ice or shifted to 37°C for 30 min in antibody-free media before processing (C: □, 15°C label and then 37°C chase; ⋄, 15°C label without chase). Note that targeting of surface-labeled GLUT4 to GSVs is inhibited at 15°C but is restored upon rewarming to 37°C. (D) Cells were incubated with radioiodinated anti-myc antibody for 80 min at 15°C, washed extensively, rewarmed to 37°C, and harvested at the indicated times. GSV-associated radioactivity was quantitated, analyzed, and plotted as in Fig. 2 B.
The temperature block was reversible since when cells were labeled for 80 min at 15°C, washed to remove free antibody, and then shifted to 37°C, GSV formation was again observed (Fig. 3 C). A time-course of this recovery of GSV formation from the precursor compartment labeled at 15°C showed a half-time of vesicle formation of ∼8 min with an undetectable lag time (Fig. 3 D). These experiments suggest that the GSVs are forming from an endosomal precursor and that exit of GLUT4 out of this endosomal compartment could be blocked at low temperature.
Trafficking of Internalized GLUT4 and Transferrin Receptor Is by Distinct Pathways
Since 125I-transferrin did not accumulate in a homogeneous population of small vesicles, it was difficult to compare the effects of the 15°C block on transferrin receptor trafficking versus GLUT4 trafficking using radioactive labeling techniques. Instead, immunofluorescence microscopy was used to analyze compartments reached by GLUT4 or transferrin after internalization at 37° or 15°C.
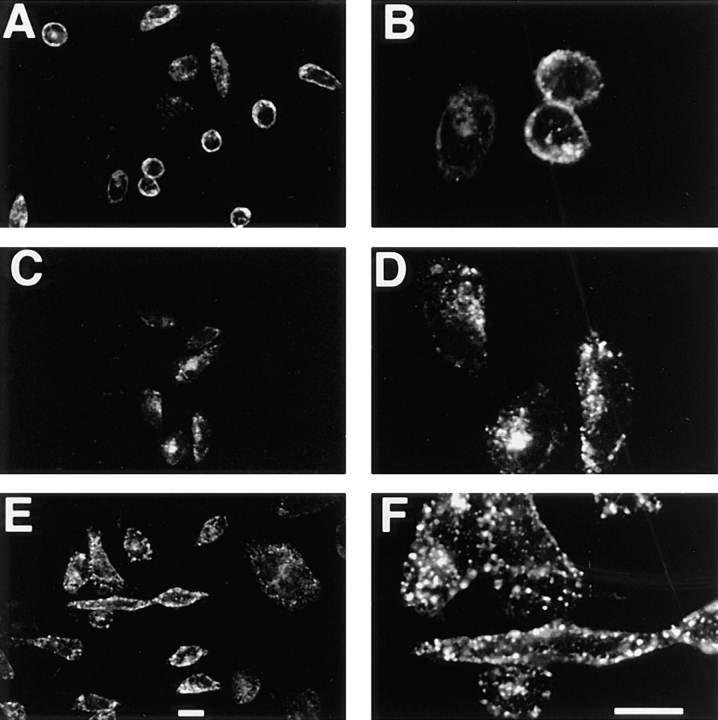
CHO/G4myc cells were incubated with either anti-myc mAb or Texas red–coupled human transferrin at 37° or 15°C and processed for laser scanning confocal immunofluorescence microscopy. After a 30-min incubation at 37°C, transferrin was found to be distributed in punctate structures throughout the cytoplasm and in the juxtanuclear area (Fig. 4, A and B). Most of the label was concentrated in the perinuclear structures consistent with earlier reports (Trowbridge et al., 1993; Gruenberg and Maxfield, 1995) describing the trafficking of transferrin from peripheral sorting endosomes to juxtanuclear recycling endosomes. When cells were incubated with Texas red–coupled transferrin in the presence of a 50-fold excess of unlabeled, iron-loaded transferrin no internalized fluorescein-treated transferrin was detected (data not shown). Furthermore, when cells were subjected to a prolonged incubation (150 min) at 15°C with Texas red–coupled transferrin, no change from the pattern observed at 37°C was seen, and both peripheral and perinuclear structures were again identified (Fig. 4, E and F).
Uptake of anti-myc mAb at 37°C for 30 min resulted in a pattern similar to that of transferrin internalized under the same conditions, demonstrating that GLUT4 trafficked through heterogeneously sized peripheral and perinuclear structures (Fig. 4, C and D). However, when cells internalized anti-myc antibody at 15°C, the labeling of perinuclear structures could no longer be seen. Instead, labeled GLUT4 accumulated in a population of large peripheral endosomes (Fig. 4, G and H). Many of these structures appeared to be localized very close to the plasma membrane. Acid stripping of cells with 0.5 M acetic acid after allowing antibody internalization did not alter the labeling of the peripheral structures, suggesting that they do not communicate with the cell surface. As a control, the steady-state distribution of GLUT4 was determined in cells that were preincubated at 15°C. Large peripheral structures were predominately labeled, with a diminution in labeling of the perinuclear structures (data not shown), indicating that antibody internalization did not significantly perturb GLUT4 trafficking. Thus, although incubation at 15°C appears not to arrest transferrin trafficking, the reduced temperature limits the distribution of GLUT4 to large, peripheral endosomal compartments.
Formation of perinuclear structures containing GLUT4 could be restored by shifting cells, previously fluorescently labeled at 15°, to 37°C. Cells were incubated with either Texas red–coupled transferrin or anti-myc mAb for 150 min at 15°C, washed extensively on ice, and then chased in label-free media at 37°C. After a 10-min chase at 37°C, GLUT4 label was again detected in juxtanuclear structures as well as in heterogeneously sized cytoplasmic structures (Fig. 4, K and L). By 30 min, most of the label was localized to juxtanuclear structures (data not shown), and resembled the pattern seen after uptake of antibody at 37°C alone. Large, peripheral endosomes could no longer be easily identified. In the case of transferrin, after 10 min at 37°C, most of the internalized transferrin was localized to juxtanuclear structures (Fig. 4, I and J). By 30 min, staining was almost undetectable suggesting that most of the transferrin had recycled back to the surface and was released into the media (data not shown), as has been demonstrated earlier.
These experiments suggest that GLUT4 traffics between two separate populations of endosomes: large peripheral endosomes and perinuclear endosomes. GLUT4 movement between these two compartments appears to be directional, with traffic from peripheral to perinuclear endosomes blocked at 15°C.
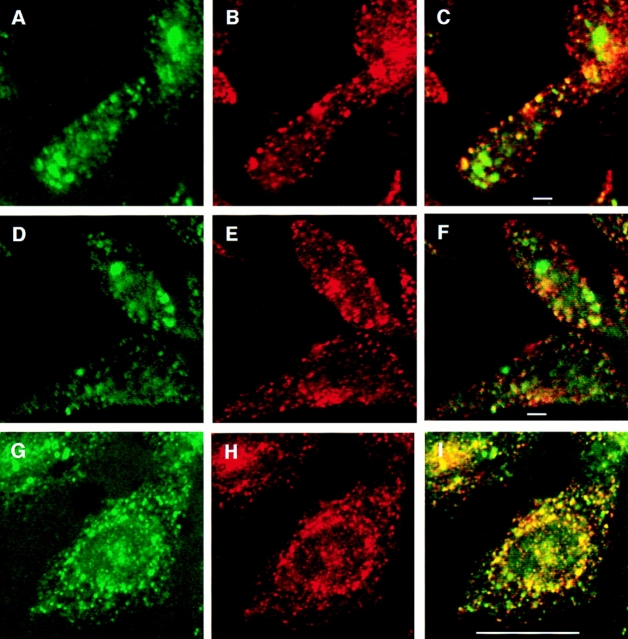
The large peripheral GLUT4-containing compartment observed at 15°C was further characterized by double- labeling immunofluorescence assays. Cells were incubated at 15°C for 2.5 h with both Texas red–coupled transferrin and fluoresceinated anti-myc antibody. Cells were then either processed immediately (Fig. 5, A–F) for confocal microscopy or shifted to 37°C for 10 min in label-free media (Fig. 5, G–I), and then processed. At 15°C, large peripheral compartments containing GLUT4 but not transferrin were identified. The perinuclear structures containing transferrin were less apparent in these images because of the plane of sectioning, which was chosen to optimize the visualization of the GLUT4 structures. After shifting to 37°C, GLUT4 and TfR did appear to be colocalized to some extent in the perinuclear region, although the possibility of distinct GLUT4- and transferrin-containing compartments in very close apposition is not eliminated. In the periphery, many structures enriched for GLUT4 but not for the TfR were apparent, suggesting that GLUT4 sorts away from the TfR at 37°C as well. This latter pattern (Fig. 5, G–I) is identical to that seen in cells which have been incubated with both labels at 37°C for 30 min, without a prior incubation at 15°C (data not shown).
Figure 5.
Double-label immunofluorescence with internalized GLUT4 and transferrin. Cells were incubated with both Texas red–coupled transferrin and fluoresceinated 9E10 at 15°C for 2.5 h, and then either processed immediately for microscopy (A–F) or shifted to 37°C for 10 min followed by processing for microscopy (G–I). A, D, and G, FITC-9E10; B, E, and H: Texas red–transferrin; and C, F, and I: merged images. Areas of overlap are indicated in yellow. Bars: (A–F) 2 μm; (G–I) 10 μm.
Exit of GLUT4 from Peripheral Endosomes and Formation of GSVs Are Inhibited by Hypertonic Sucrose or Cytosol Acidification
The above experiments suggested that sorting mechanisms are involved in the traffic of GLUT4 and TfR in CHO cells. Previous studies have noted the presence of clathrin and COP-related coat proteins on endosomal membranes (Gruenberg and Maxfield, 1995; Whitney et al., 1995; Aniento et al., 1996; Stoorvogel et al., 1996), and suggest these proteins may play a role in the sorting of membrane proteins. In addition, subjecting cells to either incubation in media containing sucrose (Daukas and Zigmond, 1985) or cytosol acidification with acetic acid (Davoust et al., 1987; Sandvig et al., 1987) has been shown to inhibit clathrin-mediated endocytosis by interfering with clathrin– adaptor interactions (Hansen et al., 1993), or by altering the structure of clathrin itself (Heuser, 1989; Heuser and Anderson, 1989; Hansen et al., 1993). We tested the effect of sucrose and cytosol acidification on GLUT4 trafficking from peripheral endosomes and on GSV formation.
Trafficking of GLUT4 out of the peripheral endosomes was again assessed by immunofluorescence microscopy. Peripheral endosomes were labeled by incubating cells with anti-myc mAb for 150 min at 15°C. Cells were then chilled on ice, washed extensively, and rewarmed in the presence or absence of 0.45 M sucrose for 10 or 30 min at 37°C. In the absence of sucrose, GLUT4 is detected in juxtanuclear structures as well as peripheral endosomes as noted above (Fig. 4, K and L). However, after 10 (Fig. 6, A and B) or 30 min (data not shown) in the presence of sucrose, no labeling of juxtanuclear endosomes was detected. Instead, labeled GLUT4 was restricted to the large peripheral endosomes and the plasma membrane. When TfR was analyzed under the same conditions, no obvious differences between the sucrose-treated and untreated cells could be detected (Figs. 4, I and J; and 6, C and D). By 30 min, nearly all of the internalized transferrin had exited the cells despite the presence of sucrose (data not shown), indicating that the presence of sucrose had not nonspecifically blocked all vesicular traffic.
Figure 6.
Effect of hypertonic sucrose and cytosol acidification on the endocytic trafficking of GLUT4 and transferrin. Cells were incubated with either anti-myc mAb 9E10 (A, B, E, and F) or Texas red–coupled transferrin (C and D) for 2.5 h at 15°C, and then shifted to 37°C for 10 min in either hypertonic medium containing 0.45 M sucrose (A–D) or in regular medium acidified with acetic acid (E and F). Processing for laser confocal microscopy was as in Fig. 4. Note that in the presence of sucrose and acetic acid, GLUT4 labeling of the juxtanuclear structures is lacking, whereas the distribution of transferrin in sucrose is unaffected. Bars, 10 μm.
Parallel experiments were performed to test the effect of cytosol acidification on GLUT4 distribution. Peripheral endosomes were labeled as above at 15°C, cells were then incubated with acidified media containing 10 mM acetic acid, pH 5.0, to acidify the cytosol, and then shifted to 37°C for 10 or 30 min in the acidified media. As with sucrose, labeling of juxtanuclear structures was not evident (Fig. 6, E and F). Most of the label appeared to be restricted to the large peripheral endosomes.
If radioactively labeled GSVs form from the peripheral endosomes identified by the internalization of fluorescent label at 15°C, treatment with either hypertonic medium or cytosol acidification should also inhibit the biogenesis of GSVs. GSV formation was assayed using velocity sedimentation, after cells were labeled with radioiodinated anti-myc mAb for 80 min at 15°C, washed to remove free antibody, and then shifted to 37°C in the presence or absence of sucrose or acetic acid. Both sucrose (Fig. 7 A) and acetic acid (Fig. 7 B) completely prevented the appearance of GSVs upon rewarming the cells. Since reducing the pH can have pleiotropic effects on the cell, for a control experiment we tested the ability of GSVs to form in the presence of HCl, pH 5, which does not alter the cytosolic pH (Hansen et al., 1993), and observed that GSV formation occurred at levels comparable to those seen in untreated cells (data not shown).
Newly Formed GSVs Are Dynamic
We next examined the fate of newly formed GSVs. To do so, we labeled GSVs, then measured their rate of disappearance under conditions that prevented additional GSV formation. CHO/G4myc cells were incubated with radioiodinated anti-myc mAb for 80 min at 15°C to label precursor endosomes, washed to remove free antibody, and then warmed for 30 min at 37°C to allow formation of labeled GSVs. Cells were then shifted to conditions that would block further GSV formation: to 15°C or to media containing hypertonic sucrose at 37°C. The amount of GSV-associated radioactivity was determined over time. In the presence of hypertonic medium containing sucrose, the GSVs rapidly disappeared (Fig. 8 A). As a control, when cells containing labeled GSVs were incubated in media without sucrose for 30 min at 37°C, the amount of GSV-associated radioactivity was stable and unchanged (data not shown). Vesicle disappearance was also apparent during the 15°C incubation (Fig. 8 B). When the steady-state distribution of GLUT4 was analyzed by Western blotting after incubating the cells either at 15°C (Fig. 3 B), or with hypertonic medium (data not shown), disappearance of GSVs was also observed.
Figure 8.
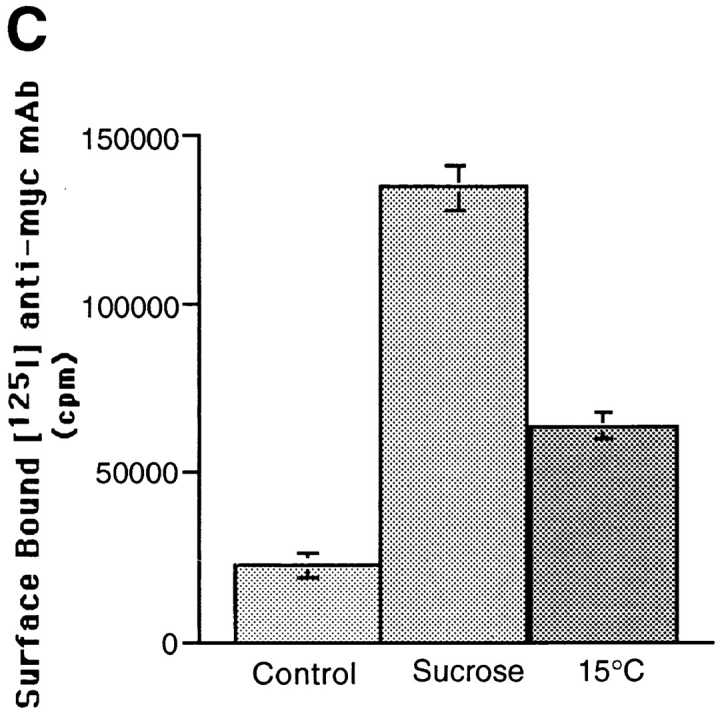
Redistribution of GLUT4 from GSVs to plasma membrane after inhibition of GSV formation. GSVs were labeled by incubating cells with radioiodinated anti-myc mAb for 80 min at 15°C, and then shifting cells to 37°C for 30 min. The rate of disappearance of GSVs was then assayed by incubating cells at 37°C in media containing 0.45 M sucrose (A) or at 15°C in regular media (B). GSV-associated radioactivity was determined for each time point and plotted as in Fig. 2 B. In parallel, the relative amount of GLUT4 on the cell surface under each of these conditions was also determined (C). Cells were incubated with hypertonic sucrose or incubated at 15°C in regular media for 30 min, fixed, and then incubated with radioiodinated 9E10 antibody for 1 h at room temperature, and washed extensively. Cell-associated radioactivity was then determined by counting cell pellets in a gamma counter. With the disappearance of GSVs in the presence of sucrose or with incubation at 15°C, there is a concurrent increase of GLUT4 on the cell surface.
We then examined the distribution of GLUT4 under these conditions that inhibit formation of GSVs but allow for their disappearance. Specifically, we compared cell surface levels of GLUT4 on control versus treated cells (Fig. 8 C). Cells were incubated for 30 min either at 37°C in the presence or absence of hypertonic media, or at 15°C in regular media. Control and treated cells were then fixed and incubated with radioiodinated anti-myc antibody to determine the level of antibody binding to the cell surface. After 30 min in hypertonic media, the level of cell surface GLUT4 was increased more than fivefold as compared to untreated cells. After 30 min at 15°C, a threefold increase in cell surface GLUT4 was observed. These results are consistent with a redistribution of GLUT4 from GSVs to the plasma membrane. The large increase in cell surface GLUT4 seen with sucrose treatment suggests that endocytosis of GLUT4 from the surface is also efficiently blocked by sucrose. Less pronounced redistribution is seen at 15°C, consistent with our observation that at 15°C, GLUT4 can be reinternalized, albeit at a slowed rate, into peripheral endosomes. These experiments do not address whether GSVs fuse directly with the plasma membrane or must first fuse with an endosomal intermediate that subsequently delivers GLUT4 to the plasma membrane.
Discussion
We have previously demonstrated (Herman et al., 1994) that in fat cells, a subset of the intracellular GLUT4 storage pool is in small uniformly sedimenting vesicles (GSVs). We also showed that GSVs can be found in fibroblasts, PC12, and CHO cells transfected with the GLUT4 cDNA, thus raising the possibility that the intracellular storage of selected proteins such as GLUT4 can be a general property of cells.
In this study, we further characterize the GSVs in CHO cells and present two types of data comparing the trafficking of GLUT4 with that of the TfR. In the first set of data, biochemical experiments using radioloabeling and velocity sedimentation analysis demonstrate that GSVs, enriched in GLUT4 but not the TfR, can be detected by internalization of surface-labeled GLUT4. In the second set of data, morphological studies using confocal immunofluorescence microscopy demonstrate that internalized GLUT4 and transferrin label distinct endocytic structures.
The GSVs found at steady state were characterized. The distribution of the small GTP-binding proteins, rab4 and rab5, which are associated with early endosomes and are proposed to have a role in the regulation of endocytosis (Mellman, 1996), were examined. It was found that rab5 did not colocalize with the GSVs, consistent with findings in 3T3-L1 adipocytes (Cormont et al., 1996; Livingstone et al., 1996), and suggesting that the GSVs are not produced by fragmentation of the endosomes during the cell homogenization process. Rab4, which has been reported to be associated with GLUT4-containing membranes (Cormont et al., 1993, 1996; Sherman et al., 1996), was seen on the gradient in the region of the GSVs but was not enriched within GSV gradient fractions. Definitive colocalization requires immunoisolation of the GSVs. Another marker, TGN38 (which labels the trans-Golgi network), also did not significantly colocalize with the GSVs. This is in agreement with compartment ablation studies done in 3T3-L1 adipocytes (Martin et al., 1994; Livingstone et al., 1996) and further suggests that the GSVs are not formed by fragmentation of the trans-Golgi reticulum during cell homogenization.
The likelihood that GSVs arise by fragmentation of tubular endosomes or of Golgi stacks is further reduced by the observation that GLUT4-containing vesicles with the same sedimentation properties as GSVs are detected in the homogenates of multiple cell types (Herman et al., 1994), and when different homogenization conditions are used (Bonzelius, F., and G.A. Herman, unpublished observations). Furthermore, GSVs can also be generated in vitro from precursor membranes under conditions in which no shearing forces are applied (Scully, R.M., and G.A. Herman, unpublished observations).
Antibody binding to surface GLUT4 did not appear to significantly perturb its intracellular targeting since the distribution of surface-labeled GLUT4 seen on gradients was identical to that observed for GLUT4 by Western blotting. Kinetic and temperature studies (Fig. 2 B versus Fig. 3, D and A) suggest that GLUT4 internalized from the cell surface traffics initially through the 15°C compartment before being targeted to the GSVs, i.e., that the GSVs may be formed from an endosomal precursor.
This study demonstrates that GLUT4 is sorted from the TfR during its endocytic processing. The currently favored model for membrane trafficking proposes that internalized membrane proteins enter a common early endosome and are then sorted to pathways leading to other cellular compartments such as the plasma membrane, the lysosome, the trans-Golgi network, and to storage vesicles such as synaptic vesicles. In this study, however, GLUT4 is shown by confocal immunofluorescence microscopy to accumulate in peripheral endocytic compartments distinct from those containing the TfR, at both 15° and 37°C (Fig. 5). To explain this result according to the above model, in which GLUT4 and TfR are sorted from one another at a common early endosome, it is necessary to hypothesize that the TfR traffics through the peripheral endosomes labeled by GLUT4 at 15°C at a rapid rate and at low receptor number, precluding detectable accumulation of transferrin ligand. Alternatively, the data are consistent with a model whereby most of the sorting of GLUT4 from TfR occurs at the level of the plasma membrane.
Recent work by Schmidt et al. (1997) is also consistent with a model in which some sorting of recycling membrane proteins occurs at the level of the plasma membrane. The synaptic vesicle protein, synaptophysin, is shown in PC12 cells to be segregated from the TfR at the level of the plasma membrane, both at 18° and 37°C, with internalization of biotinylated synaptophysin into peripheral compartments distinct from those containing the TfR. These data and ours suggest that the conventional model of a single endosome generated by fusion of vesicles derived from the plasma membrane may need to be refined to one in which endosomes of different composition that subserve different functions can be generated in parallel fashion from the plasma membrane.
Also consistent with our results are studies in neuroendocrine cells and neurons that demonstrate the existence of separate populations of early endosomes based on the topographical separation of internalized ligands to different regions of the cells (Parton et al., 1992; Mundigl et al., 1993; Bonzelius et al., 1994). Endocytic trafficking in neuroendocrine cells has also previously been shown to display temperature sensitivity that resembles that reported here for GLUT4 in CHO cells. The formation of synaptic vesicles from an endosomal precursor is inhibited at 15°C in undifferentiated PC12 cells (Desnos et al., 1995). Similarly, we observed that formation of GSVs as well as the exit of GLUT4 from peripheral endosomes was inhibited at 15°C. Thus, the endocytic trafficking pathway described here for GLUT4 has characteristics similar to pathways for synaptic membrane proteins in neuroendocrine cells: segregation from transferrin, temperature sensitivity, and sorting to homogeneously sized vesicles.
Wilson and Colton (1997) recently described evidence in fibroblasts for an early endosomal compartment that has characteristics of an apical or axonal endosomal compartment and that largely excludes transferrin. Data from Yoshimori et al. (1996) suggest that apical and basolateral transport routes to the plasma membrane along the biosynthetic pathway also exist in CHO cells. Recent work also suggests that fibroblasts are capable of some level of regulated exocytosis (Kanai et al., 1993; Morimoto et al., 1995; Chavez et al., 1996; Coorssen et al., 1996; Ninomiya et al., 1996). Collectively, these recent data suggest that specialized membrane trafficking pathways may arise by adaptation and differentiation of preexisting pathways found in all cell types.
What would be the function of multiple populations of early endosomes and of postendocytic organelles such as the GSV in CHO cells? The function may be to provide a reserve pool of selected plasma membrane proteins that can be rapidly mobilized to the cell surface in the appropriate physiological context thereby controlling the cellular distribution of some plasma membrane proteins independently of others. Such regulation may be as important in undifferentiated cells as it is in such cell types such as fat and muscle cells. Validation or disproof of this concept may require characterizing the protein composition of GSVs, identifying the cellular compartment with which they fuse, determining if fusion mechanisms are regulated, and comparing GSV biogenesis with that of storage vesicles in fat and muscle cells.
Acknowledgments
We thank Drs. K. Mostov, V. Faundez, J. Roos, J.-T. Horng, (all UCSF) L. Nagy (Case Western Reserve, Cleveland, OH), and S. Marullo (Hôpital Cochin, Paris, France) for critical reading of the manuscript, and L. Spector (UCSF) for excellent administrative support. We thank Drs. Y. Ebina and H. Hayashi (University of Tokushima, Japan) for their generous gift of the CHO cell lines transfected with the myc epitope–tagged GLUT4.
This work was supported by the National Institutes of Health (NIH) grants NS09878, BS15927, DA10154 (to R.B. Kelly); K11 DK02163 and P30 HD28825, and University of California Individual Investigator and Research Evaluation and Allocation Committee (to G. Herman); NIH Training Grant AR07175 and the Howard Hughes Medical Institute Postdoctoral Research Fellowship (to M. Wei); and Deutsche Forschungsgemeinschaft BO1065/2-1 (to F. Bonzelius).
Abbreviations used in this paper
- COP
coatomer-associated proteins
- GSV
GLUT4-containing small vesicles
- pIgR
polymeric immunoglobulin receptor
- TfR
transferrin receptor
Footnotes
Address all correspondence to Gary Herman, Hormone Research Institute, Box 0534, University of California, San Francisco, CA 94143-0534. Tel: (415) 476-4095. Fax: (415) 731-3612. E-mail: Herman@cgl.ucsf.edu
F. Bonzelius' present address is Zoologisches Institut Biozentrum der J.W. Goethe-Universität, Marie-Curie-Strasse 9, D-60439 Frankfurt, Germany.
References
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal β COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber JW, Lienhard GE. Isolation of vesicles containing insulin- responsive, intracellular glucose transporters from 3T3-L1 adipocytes. J Biol Chem. 1986;261:16180–16184. [PubMed] [Google Scholar]
- Blok J, Gibbs EM, Lienhard GE, Slot JW, Geuze HJ. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988;106:69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzelius F, Herman GA, Cardone MH, Mostov KE, Kelly RB. The polymeric immunoglobulin receptor accumulates in specialized endosomes but not synaptic vesicles within the neurites of transfected neuroendocrine PC12 cells. J Cell Biol. 1994;127:1603–1616. doi: 10.1083/jcb.127.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez RA, Miller SG, Moore H-P. A biosynthetic regulated secretory pathway in constitutive secretory cells. J Cell Biol. 1996;133:1177–1191. doi: 10.1083/jcb.133.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift-O'Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorssen JR, Schmitt H, Almers W. Ca2+triggers massive exocytosis in Chinese hamster ovary cells. EMBO (Eur Mol Biol Organ) J. 1996;15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- Cormont M, Tanti J-F, Zahraoui A, Obberghen EV, Tavitian A, le Marchand-Brustel Y. Insulin and okadaic acid induce rab4 redistribution in adipocytes. J Biol Chem. 1993;268:19491–19497. [PubMed] [Google Scholar]
- Cormont M, Obberghen EV, Zerial M, le Marchand-Brustel Y. Insulin induces a change in rab5 subcellular localization in adipocytes independently of phosphatidylinositol 3-kinase activation. Endocrinology. 1996;137:3408–3415. doi: 10.1210/endo.137.8.8754768. [DOI] [PubMed] [Google Scholar]
- Corvera S, Chawla A, Chakrabarti R, Joly M, Buxton J, Czech MP. A double leucine within the GLUT4 glucose transporter COOH-terminal domain functions as an endocytosis signal. J Cell Biol. 1994;126:979–989. doi: 10.1083/jcb.126.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- Czech MP, Buxton JM. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- Daukas G, Zigmond SH. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985;101:1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoust J, Gruenberg J, Howell KE. Two threshold values of low pH block endocytosis at different stages. EMBO (Eur Mol Biol Organ) J. 1987;6:3601–3609. doi: 10.1002/j.1460-2075.1987.tb02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos C, Clift-O'Grady L, Kelly RB. Biogenesis of synaptic vesicles in vitro. J Cell Biol. 1995;130:1041–1049. doi: 10.1083/jcb.130.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WA, Hubbard AL, Aronson NN., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980;255:5971–5978. [PubMed] [Google Scholar]
- Garippa RJ, Judge TW, James DE, McGraw TE. The amino terminus of GLUT4 functions as an internalization motif but not an intracellular retention signal when substituted for the transferrin receptor cytoplasmic domain. J Cell Biol. 1994;124:705–715. doi: 10.1083/jcb.124.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Kelly RB. Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–549. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Haney PM, Slot JW, Piper RC, James DE, Mueckler M. Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J Cell Biol. 1991;114:689–699. doi: 10.1083/jcb.114.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney PM, Levy MA, Strube MS, Mueckler M. Insulin-sensitive targeting of the GLUT4 glucose transporter in L6 myoblasts is conferred by its COOH-terminal cytoplasmic tail. J Cell Biol. 1995;129:641–658. doi: 10.1083/jcb.129.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: Effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CV, Unanue ER. Low-temperature inhibition of antigen processing and iron uptake from transferrin: deficits in endosome functions at 18°C. Eur J Immunol. 1990;20:323–329. doi: 10.1002/eji.1830200214. [DOI] [PubMed] [Google Scholar]
- Hausdorff SF, Fingar DC, Birnbaum MJ. Signalling pathways mediating insulin-activated glucose transport. Semin Cell Dev Biol. 1996;7:239–247. [Google Scholar]
- Herman GA, Bonzelius F, Cieutat AM, Kelly RB. A distinct class of intracellular storage vesicles, identified by expression of the glucose transporter GLUT4. Proc Natl Acad Sci USA. 1994;91:12750–12754. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989;108:401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Anderson RG. Hypertonic media inhibit receptor- mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman GD, Cushman SW. Subcellular trafficking of GLUT4 in insulin target cells. Semin Cell Dev Biol. 1996;7:259–268. [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AW, Ruiz M, Birnbaum MJ. Isoform-specific subcellular targeting of glucose transporters in mouse fibroblasts. J Cell Biol. 1992;116:785–797. doi: 10.1083/jcb.116.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AW, Fingar DC, Seidner GA, Griffiths G, Burke B, Birnbaum MJ. Targeting of the “insulin-responsive” glucose transporter (GLUT4) to the regulated secretory pathway in PC12 cells. J Cell Biol. 1993;122:579–588. doi: 10.1083/jcb.122.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopetta BJ, Morgan EH. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983;258:9108–9115. [PubMed] [Google Scholar]
- James DE, Piper RC. Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol. 1994;126:1123–1126. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DE, Lederman L, Pilch PF. Purification of insulin-dependent exocytic vesicles containing the glucose transporter. J Biol Chem. 1987;262:11817–11824. [PubMed] [Google Scholar]
- Jhun BH, Rampal AL, Liu H, Lachaal M, Jung CY. Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J Biol Chem. 1992;267:17710–17715. [PubMed] [Google Scholar]
- Kamohara S, Hayashi H, Todaka M, Kanai F, Ishii K, Imanaka T, Escobedo JA, Williams LT, Ebina Y. Platelet-derived growth factor triggers translocation of the insulin-regulatable glucose transporter (type 4) predominantly through phosphatidylinositol 3-kinase binding sites on the receptor. Proc Natl Acad Sci USA. 1995;92:1077–1081. doi: 10.1073/pnas.92.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F, Nishioka Y, Hayashi H, Kamohara S, Todaka M, Ebina Y. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J Biol Chem. 1993;268:14523–14526. [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980;142:439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Martin S, Reaves B, Banting G, Gould GW. Analysis of the co- localization of the insulin-responsive glucose transporter (GLUT4) and the transGolgi network marker TGN38 within 3T3-L1 adipocytes. Biochem J. 1994;300:743–749. doi: 10.1042/bj3000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Popov S, Buckley KM, Poo MM. Calcium-dependent transmitter secretion from fibroblasts: modulation by synaptotagmin I. Neuron. 1995;15:689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- Mundigl O, Matteoli M, Daniell L, Thomas-Reetz A, Metcalf A, Jahn R, de Camilli P. Synaptic vesicle proteins and early endosomes in cultured hippocampal neurons: Differential effects of brefeldin A in axon and dendrites. J Cell Biol. 1993;122:1207–1221. doi: 10.1083/jcb.122.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Kishimoto T, Miyashita Y, Kasai H. Ca2+-dependent exocytotic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+compound. J Biol Chem. 1996;271:17751–17754. doi: 10.1074/jbc.271.30.17751. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;109:3259–3272. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Tai C, Slot JW, Hahn CS, Rice CM, Huang H, James DE. The efficient intracellular sequestration of the insulin-regulatable glucose transporter (GLUT-4) is conferred by the NH2terminus. J Cell Biol. 1992;117:729–743. doi: 10.1083/jcb.117.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Tai C, Kulesza P, Pang S, Warnock D, Baenziger J, Slot JW, Geuze HJ, Puri C, James DE. GLUT-4 NH2terminus contains a phenylalanine-based targeting motif that regulates intracellular sequestration. J Cell Biol. 1993;121:1221–1232. doi: 10.1083/jcb.121.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Pang S, Harris DS, Heuser J, James DE. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTPγS and localization of GLUT4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnick KJ, Slot JW, Studelska DR, Hanpeter DE, Robinson LJ, Geuze HJ, James DE. Immunocytochemical and biochemical studies of GLUT4 in rat skeletal muscle. J Biol Chem. 1992;267:6278–6285. [PubMed] [Google Scholar]
- Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- Schmid SL, Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol. 1991;114:869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Klempnauer KH, Alitalo K, Varmus H, Bishop JM. Rearrangement at the 5′ end of amplified c-myc in human COLO 320 cells is associated with abnormal transcription. Mol Cell Biol. 1986;6:2752–2755. doi: 10.1128/mcb.6.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LA, Hirshman MF, Cormont M, Marchand-Brustel YL, Goodyear LJ. Differential effects of insulin and exercise on rab4 distribution in rat skeletal muscle. Endocrinology. 1996;137:266–272. doi: 10.1210/endo.137.1.8536622. [DOI] [PubMed] [Google Scholar]
- Shibasaki Y, Asano T, Lin JL, Tsukuda K, Katagiri H, Ishihara H, Yazaki Y, Oka Y. Two glucose transporter isoforms are sorted differentially and are expressed in distinct cellular compartments. Biochem J. 1992;281:829–834. doi: 10.1042/bj2810829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci USA. 1991a;88:7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991b;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Charron MJ, Shah N, Lodish HF, Jarett L. Immunoelectron microscopic demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxyl-terminal epitope of intracellular GLUT4. Proc Natl Acad Sci USA. 1991;88:6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JM, Pilch PF. The metabolic regulation and vesicular transport of GLUT4, the major insulin-responsive glucose transporter. Endocrine Rev. 1995;16:529–546. doi: 10.1210/edrv-16-4-529. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Roth J. Intracellular targeting of GLUT4 in transfected insulinoma cells: evidence for association with constitutively recycling vesicles distinct from synaptophysin and insulin vesicles. J Cell Sci. 1996;109:1311–1323. doi: 10.1242/jcs.109.6.1311. [DOI] [PubMed] [Google Scholar]
- Trowbridge, I.S., Collawn, J.F., and C.R. Hopkins. 1993. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9:129–161. [DOI] [PubMed]
- Verhey KJ, Birnbaum MJ. A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. J Biol Chem. 1994;269:2353–2356. [PubMed] [Google Scholar]
- Weigel PH, Oka JA. Endocytosis and degradation mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. J Biol Chem. 1982;257:1201–1207. [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Colton TL. Targeting of an intestinal apical endosomal protein to endosomes in nonpolarized cells. J Cell Biol. 1997;136:319–330. doi: 10.1083/jcb.136.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Holman GD. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J Biol Chem. 1993;268:4600–4603. [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]