Abstract
The activity of integrins on leukocytes is kept under tight control to avoid inappropriate adhesion while these cells are circulating in blood or migrating through tissues. Using lymphocyte function-associated antigen-1 (LFA-1) on T cells as a model, we have investigated adhesion to ligand intercellular adhesion molecule-1 induced by the Ca2+ mobilizers, ionomycin, 2,5-di-t-butylhydroquinone, and thapsigargin, and the well studied stimulators such as phorbol ester and cross-linking of the antigen-specific T cell receptor (TCR)– CD3 complex. We report here that after exposure of T cells to these agonists, integrin is released from cytoskeletal control by the Ca2+-induced activation of a calpain-like enzyme, and adhesive contact between cells is strengthened by means of the clustering of mobilized LFA-1 on the membrane. We propose that methods of leukocyte stimulation that cause Ca2+ fluxes induce LFA-1 adhesion by regulation of calpain activity. These findings suggest a mechanism whereby engagement of the TCR could promote adhesion strengthening at an early stage of interaction with an antigen-presenting cell.
Lymphocytes have a dual function that requires they must circulate in nonadherent form through blood and lymph, but become adherent to allow transmigration across the vasculature or after contact with an antigen-presenting cell in a lymph node. Regulation of adhesion is achieved by controlling the activity of receptors such as the integrins on the cell surface. The major integrin on T cells is termed lymphocyte function-associated antigen-1 (LFA-1).1 This integrin is a heterodimeric transmembrane receptor composed of a unique α subunit (αL; CD11a) and a β2 subunit (CD18) that is common to a subset of leukocyte integrins. Leukocyte integrins such as LFA-1 are not constitutively adhesive, but become firmly able to engage their ligands after stimuli received through other cell membrane receptors such as the antigen-specific T cell receptor (TCR) (Brown and Hogg, 1996; Shaw and Dustin, 1997). How the signal transduction pathways involved in this “inside out” signaling alter the adhesive state of integrin on the membrane, and whether these alterations are similar for all integrins, needs clarification. Integrin-mediated adhesion can occur through avidity changes (Jakubowski et al., 1995; Stewart et al., 1996), but there is also evidence that some naturally occurring agonists cause an increase in the intrinsic affinity of the integrin (Faull and Ginsberg, 1995). Treatment with divalent cations Mn2+ or Mg2+/EGTA is an alternative method for inducing active integrin with increased affinity (Stewart and Hogg, 1996). This form of integrin activation is considered to alter the ectodomain directly, bypassing the requirement for intracellular signaling events (Kassner et al., 1994; Stewart et al., 1996).
The cytoplasmic domains of integrins are essential for control of their function. Mutation or deletion of specific cytoplasmic sequences causes integrins to be constitutively active and has also revealed links with the cytoskeleton (Hughes et al., 1995; Peter and O'Toole, 1995; Lu and Springer, 1997). Interaction with the cytoskeleton is regulated during the course of adhesion and may be involved at more than one stage of the adhesion process. For example, LFA-1 is reported to associate with the cytoskeleton after TCR–CD3 cross-linking (Pardi et al., 1992), but low doses of the cytoskeletal disrupting agent cytochalasin facilitates adhesion by Mac-1 and LFA-1 (Elemer and Edgington, 1994; Kucik et al., 1996; Lub et al., 1997).
Increases in cytoplasmic Ca2+ ([Ca2+]i) accompany triggering through the TCR and are also an important component of other adhesion-inducing mechanisms. [Ca2+]i activates enzymes and mediators such as protein kinase C (PKC), calcineurin, calmodulin, calreticulin, myosin light chain kinase (MLCK), and calpain, many of which have been implicated in integrin function. For example, Ca2+-activated calcineurin functions in the recycling of integrin αvβ3 on the moving neutrophil (Hendey et al., 1992; Lawson and Maxfield, 1995). Phorbol ester-induced T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is Ca2+-dependent (Rothlein and Springer, 1986; Stewart et al., 1996), and Ca2+ ionophores such as ionomycin can cause integrin-mediated adhesion (Altieri et al., 1988; Hartfield et al., 1993; van Kooyk et al., 1993). Ca2+ fluxes also activate actin-binding proteins causing actin dissociation and cytoskeletal rearrangements (Stossel, 1989).
In this study, we have used three Ca2+ mobilizing agents, ionomycin, thapsigargin, and 2,5-di-t-butylhydroquinone (dBHQ) as tools to gain further understanding of the role of Ca2+ in the activation of adhesion by the integrin LFA-1. By inducing Ca2+ fluxing, the objective was to short circuit the early phases of signaling to dissect the events leading more immediately to adhesion. We show that these three reagents, as well as cross-linking the TCR– CD3 complex (XL-TCR–CD3) and phorbol ester treatment, induce LFA-1–mediated adhesion through a mechanism involving activation of calpain. The presented results suggest that Ca2+-mediated activation of calpain releases LFA-1 from cytoskeletal control allowing integrin clustering.
Materials and Methods
Reagents
Ionomycin, thapsigargin, phorbol 12,13 dibutyrate (PdBu), and the membrane soluble calpain inhibitor calpeptin were obtained from Calbiochem/ Novabiochem Corp. (La Jolla, CA); dBHQ, and the calpain inhibitor, CBZ-LVG, were purchased from Sigma Chemical Co. (Dorset, UK). Jasplakinolide was obtained from Molecular Probes, Inc. (Eugene, OR). SK&F 96365 was purchased from BiomoL Feinchemikalien GmbH (Hamburg, Germany). With the exception of SK&F 96365, which was directly soluble in H2O, stock concentrations of inhibitors were prepared in DMSO and in every relevant experiment an equivolume of DMSO was added in the control sample. The above reagents had no effect on the viability of the T cells at the levels used in the reported experiments.
Flow Cytometry, mAbs, and Assessment of Soluble ICAM-1Fc Binding
A dimeric form of an ICAM-1Fc chimaeric protein consisting of the five extracellular domains of ICAM-1 fused to the Fc fragment of human IgG1 was prepared as previously described (Berendt et al., 1992). Flow cytometry and measurement of the binding of soluble recombinant ICAM-1Fc to T cells was carried out as previously described (Stewart et al., 1996). mAbs used in this study were the LFA-1 α subunit (CD11a) mAbs 38 (Dransfield and Hogg, 1989), F110.22 (Schmidt, 1989), and G25.2 (Becton and Dickinson Co., Mountain View, CA). The anti-CD3 mAbs G19.4 and UCHT1 were obtained from Bristol Myers Squibb (Seattle, WA) and P. Beverley (University College, London), respectively. The secondary FITC-conjugated antibodies goat anti–mouse IgG Fc and goat anti–human IgG Fc were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, CA).
T Cell Adhesion to ICAM-1Fc
T lymphoblastoid cells were expanded from unstimulated peripheral blood mononuclear cells by culture for 1–2 wk in RPMI-1640 medium containing recombinant IL-2 (20 ng/ml; Cetus Corp., Berkeley, CA) with details as previously described (Dransfield et al., 1992). Cells were used between days 10 and 14. The method for quantifying T cell adhesion to ICAM-1Fc protein has been previously described (Stewart et al., 1996), with the exception that the assay buffer used was RPMI 1640 unless controlled cation conditions were being analyzed. For these experiments T cells were treated with 5 mM Mg2+/1 mM EGTA in Hepes/NaCl buffer. The ICAM-1Fc protein was coated at 0.24 μg/well onto 96-well Immulon 1 plates (Dynatech, Chantilly, VA).
Confocal Microscopy
For immunofluorescence analysis by confocal microscopy, 13-mm-round glass coverslips were precoated with a 0.01% solution of poly-L-lysine (Sigma Chemical Co.) for 10 min at room temperature, washed twice in RPMI 1640, and then left to air dry. T cells were washed three times in RPMI 1640 buffer before addition onto coverslips (5 × 105 cells/coverslip), in the presence of stimulants and CD11a mAbs at 10 μg/ml. Coverslips were spun at 40 g, and then incubated for 30 min at 37°C. Unbound cells were removed by gentle washing in warm RPMI 1640 buffer. To prevent antibody-induced clusters, cells were fixed with 1% formaldehyde in PBS-A for 10 min at room temperature before a second incubation with 10 μg/ml FITC-conjugated goat anti–mouse IgG Fc (Jackson ImmunoResearch Laboratories) for 25 min at 4°C. Cells stimulated via CD3 triggering were incubated with CD3 mAbs UCHT1 or G19.4 for 30 min at 37°C and fixed as above.
Cells were mounted for confocal microscopy which was carried out using a MRC-600 Confocal Laser Scanning System (Bio-Rad Laboratories Ltd, Hertfordshire, UK). The regions of the confocal microscopy images with the highest fluorescence intensity (i.e., with the pixel color intensity in the 150–255 range) were highlighted in red using the segmentation utility of the IP Lab Spectrum Version 3.1 software (Signal Analytics Co., Vienna, VA).
This software was also used to quantify the levels of fluorescence on the images. Background fluorescence was estimated by measuring the signal strength in areas visibly devoid of specific staining but in close proximity to cells. The average of these values was then subtracted from the original image to give a corrected image. The areas of interest (e.g., free membrane or membrane at cell–cell contact points) on the corrected images were selected and the average signal strength calculated automatically by the computer software. A statistical assessment of the differences between the treatment groups and the untreated resting T cells was performed using the one-way ANOVA test.
Results
Agents That Mobilize Intracellular Ca2+ Induce LFA-1 Adhesion
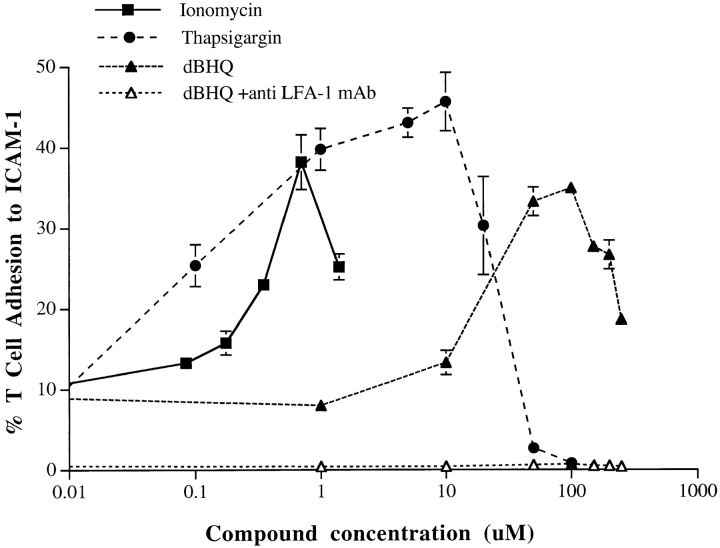
To gain insight into the role of Ca2+ in LFA-1 adhesion, we have analyzed the effect on leukocyte adhesion to ICAM-1 of the Ca2+ ionophore ionomycin and two other Ca2+ mobilizers, thapsigargin, and dBHQ. The latter two compounds act by inhibiting the ATPase pumps on Ca2+ storage organelles, which maintain homeostasis by pumping Ca2+ from the cytosol into these organelles and the endoplasmic reticulum (Thomas and Hanley, 1994). Inhibition of pump action causes emptying of intracellular Ca2+ stores and a resultant Ca2+ influx across the plasma membrane via capacitative entry (Thastrup et al., 1990). The three agents showed a dose-dependent stimulation of T cell LFA-1–mediated adhesion to immobilized ICAM-1 (Fig. 1). The peak adhesion with ionomycin, thapsigargin, and dBHQ occurred at 0.7, ∼5, and ∼50 μM, respectively. The higher optimal concentration of dBHQ may be due to its comparatively poor ability to penetrate the plasma membrane (Thomas and Hanley, 1994). The reason for the phase of decline in the bell-shaped response curves was not due to loss of cell viability, but was not further pursued. Therefore, mobilization of [Ca2+]i by three distinct agents can cause LFA-1–mediated T cell adhesion, highlighting a role for [Ca2+]i in the LFA-1 activation pathway.
Figure 1.
Ca2+ mobilizers induce adhesion of T cell LFA-1 to ICAM-1Fc. T cells were treated with ionomycin, thapsigargin, and dBHQ at the indicated concentrations and incubated on ICAM-1Fc–coated 96-well plates for 30 min at 37°C. The proportion of adhering cells was calculated as a percentage of total cells added per well. For all stimulants, adhesion was reduced to background levels by means of anti–LFA-1 mAb used at 10 μg/ml. An experiment representative of five similar experiments is presented.
LFA-1–mediated Adhesion after Ca2+ Mobilization Requires Extracellular Ca2+
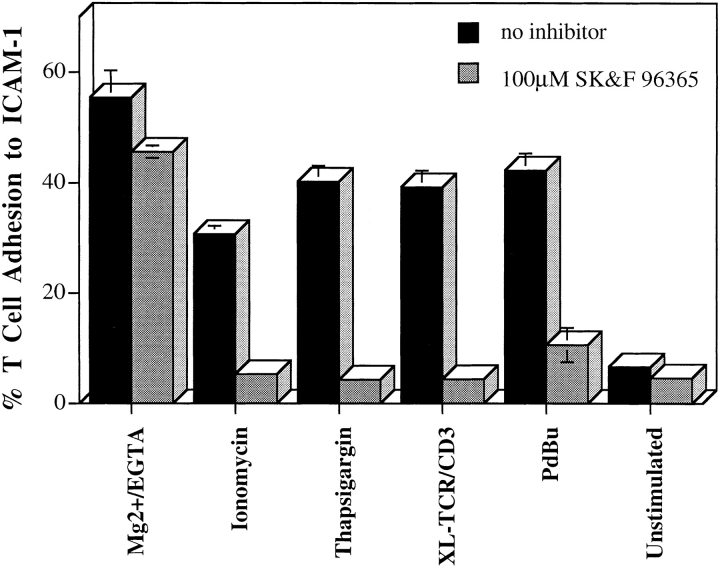
The tested pharmacological agents raise [Ca2+]i levels not only by inducing release from intracellular stores, but also by capacitative fluxing in through the plasma membrane (Putney and Bird, 1993; Breittmayer et al., 1994; Wenzel-Seifert et al., 1996). To define the source of Ca2+ that facilitates T cell adhesion, we made use of the imidazole compound SK&F 96365, which blocks Ca2+ channels on leukocytes that open as a result of depletion of Ca2+ stores (Wenzel-Seifert et al., 1996). Adhesion stimulated by ionomycin, thapsigargin, through the TCR–CD3 complex, and by PdBu was inhibited by SK&F 96365 (Fig. 2). As expected, T cell adhesion to ICAM-1 induced by Mg2+/EGTA was not altered by the Ca2+ channel blocker (Stewart et al., 1996). Further evidence that extracellular Ca2+ has an intracellular function came from the use of the intracellular Ca2+ chelator BAPTA–AM, which at 20–30 μM reduced LFA-1 adhesion by 50–80% for all inducers in this study with the exception of Mg2+/EGTA (data not shown). Therefore, adhesion stimulated in several ways by “inside out” signaling is dependent upon an extracellular source of Ca2+.
Figure 2.
Thapsigargin and ionomycin-induced adhesion is dependent on extracellular Ca2+. T cells were preincubated with 100 μM SK&F 96365 in RPMI for 30 min at 37°C, and then incubated with thapsigargin (5 μM) and ionomycin (0.7 μM) on ICAM-1Fc–coated 96-well plates for a further 30 min at 37°C. The proportion of adhering cells was calculated as a percentage of total cells added per well. For all stimulants, adhesion was reduced to background levels by anti–LFA-1 mAb used at 10 μg/ ml. An experiment representative of three is presented.
Measurements of Soluble ICAM-1 Binding
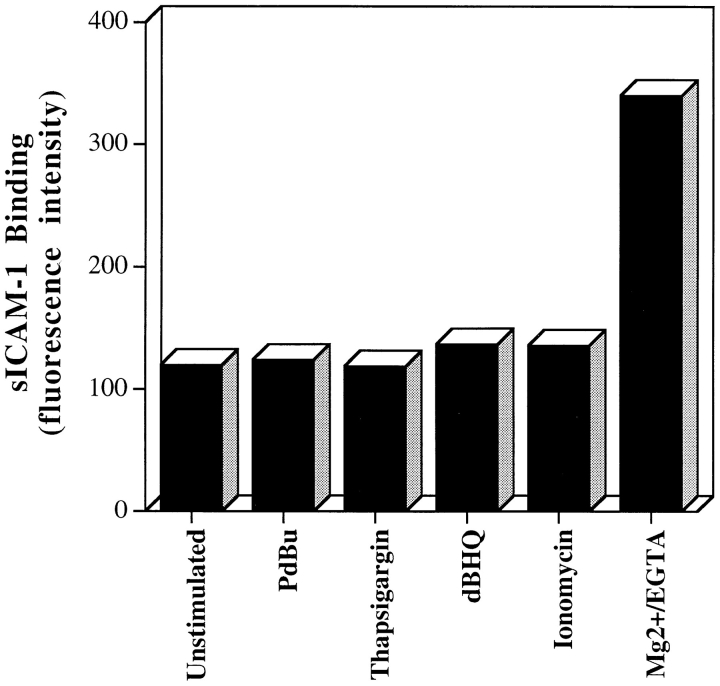
To investigate the mechanism of Ca2+ action, the characteristics of LFA-1–mediated adhesion caused by the Ca2+ mobilizers was examined. One question of interest was whether the Ca2+-mediated signaling increased the ability of LFA-1 to bind soluble ICAM-1 (sICAM-1). In a previous study, sICAM-1 binding distinguished high affinity Mg2+-stimulated LFA-1 from low affinity LFA-1 on phorbol ester-stimulated and XL-TCR–CD3 T cells (Stewart et al., 1996). None of the Ca2+ mobilizers showed any induction of sICAM-1 binding even at sICAM-1 levels of 1 mg/ml (4.5 μM), in contrast to the enhanced ability of Mg2+-stimulated T cells to bind sICAM-1, which served as a positive control (Fig. 3). It can be concluded that the Ca2+ mobilizers resemble the phorbol ester or TCR–CD3 model of adhesion by failing to promote an increase in the ability of LFA-1 to bind to soluble ICAM-1.
Figure 3.
Thapsigargin, ionomcyin, and dBHQ do not induce binding of soluble ICAM-1 to LFA-1. Soluble ICAM-1Fc at 1 mg/ml (or 4.5 μM) was incubated with thapsigargin (5 μM), ionomcyin (0.7 μM), dBHQ (50 μM), PdBu (50 nM), and Mg2+/ EGTA (5 mM/1 mM)–stimulated cells for 30 min at 37°C (Stewart et al., 1996). Bound sICAM-1 was detected with FITC-conjugated goat anti–human (Fc specific) Ab and analyzed by flow cytometry. Results are expressed as median fluorescence intensity. An experiment representative of three is presented.
Distribution of LFA-1 on T Cells after Exposure to Ca2+-mobilizing Agents
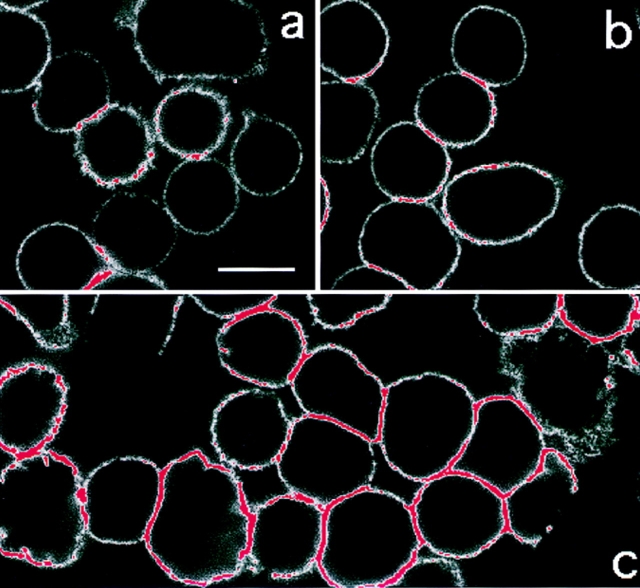
As the Ca2+ mobilizers did not cause a detectable increase in the affinity of LFA-1, we used confocal microscopy to investigate whether the membrane distribution of LFA-1 was altered in a manner facilitating adhesion. To analyze LFA-1 distribution, we highlighted the confocal microscopy images such that the membrane regions with the highest LFA-1 fluorescence (i.e., in the pixel intensity range 150–250, where 250 is the highest possible value) are depicted in red and all others in white (Fig. 4). Unstimulated cells (Fig. 4 a) and Mg2+/EGTA–stimulated cells (Fig. 4 b) have very little high intensity LFA-1 fluorescence (red) in comparison to that of thapsigargin-stimulated cells (Fig. 4 c). These observations were confirmed by quantifying (see Materials and Methods) the levels of fluorescence for each sample on regions of the membrane where there was no contact between cells (Table I, Free membrane). Statistical analysis (one way ANOVA) of these measurements confirmed that the level of fluorescence on the thapsigargin-stimulated cells, but not on the Mg2+/EGTA–stimulated T cells, was significantly increased compared to the level on the resting T cells (Table I, Significance levels). This increase in the intensity of LFA-1 fluorescence upon thapsigargin stimulation was detected with three distinct CD11a mAbs, 38, F110.22, and G25.2 (data not shown), and therefore does not represent the exposure of a particular epitope. These results suggested that thapsigargin might stimulate an increase in the cell surface expression of LFA-1. When measured by flow cytometry, however, results revealed that the level of LFA-1 expression after thapsigargin stimulation did not differ from LFA-1 expression on the resting or Mg2+/EGTA–treated cells (Table I, Flow cytometry). This suggested that the increase in signal strength observed by confocal microscopy upon thapsigargin stimulation reflects increased clustering that creates a higher LFA-1 fluorescence intensity. Similar results were obtained with the other Ca2+-mobilizing agents, with PdBu, and by XL-TCR–CD3 (data not shown).
Figure 4.
Thapsigargin stimulates LFA-1 clustering. T cells adhered onto poly-L-lysine were (a) unstimulated; (b) treated with 5 mM Mg2+/1 mM EGTA; or (c) treated with 5 μM thapsigargin for 30 min at 37°C. LFA-1 was detected with FITC-conjugated LFA-1–specific mAb and analyzed by confocal microscopy. Red color indicates fluorescence intensity exceeding a preset pixel value (see Materials and Methods). This setting remained constant between samples. Bar, 10 μm.
Table I.
Detection of T Cell LFA-1 by Either Confocal Microscopy or Flow Cytometry after Activating Treatments
| Confocal microscopy | Flow cytometry | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Significance levels (compared to resting T cells) | Contact zone (fluorescence) | |||||||||||||
| Free membrane (fluorescence) | F1,103 | P | Contact/free | Fluorescence | ||||||||||
| fluorescence units ± SEM | fluorescence units ± SEM | MFI | ||||||||||||
| Resting T cells | 58.2 ± 6.1 | n = 23 | — | — | 112.4 ± 10.4 | 1.93 | 858.6 | |||||||
| Mg2+/EGTA | 60.5 ± 4.8 | n = 33 | 0.09 | >0.1 | 131.8 ± 6.5 | 2.18 | 897.9 | |||||||
| Thapsigargin | 102.3 ± 4.8 | n = 33 | 31.90 | <0.0001 | 185.8 ± 5.7 | 1.82 | 827.4 | |||||||
| Thapsigargin and | 57.8 ± 7.6 | n = 18 | 0.00 | >0.1 | 109.6 ± 14.1 | 1.90 | 909.6 | |||||||
| calpeptin | ||||||||||||||
On T cells visualized by confocal microscopy, the level of LFA-1 was quantified (see Materials and Methods) on the membrane where there was no cell contact (Free membrane) or at T cell contact points (Contact zone). When the fluorescence units (± SEM) were compared between treatment groups, only the thapsigargin-treated samples differed significantly from the resting T cell sample (Significance levels). To ask whether LFA-1 redistributes to cell–cell contact zones, a ratio of contact zone fluorescence and free membrane fluorescence was calculated (Contact/free). In no sample was the ratio significantly different from 2.0, indicating that the level of LFA-1 at cell–cell contact zones was the sum of the fluorescence of the two associated membranes and therefore LFA-1 did not preferentially migrate to regions of the membrane where cells are in contact. In the same experiments, the samples were measured by flow cytometry and the level of LFA-1 (MFI) demonstrated not to differ significantly between samples (Flow cytometry).
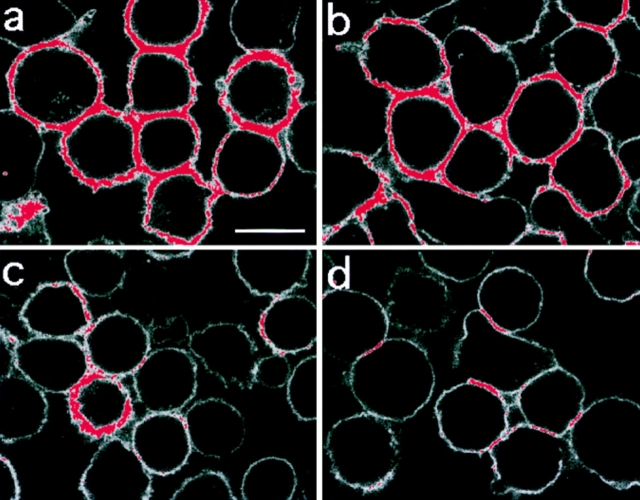
It was observed that some of the brightest LFA-1 fluorescence was found where cells were in contact with each other, and we therefore quantified the level of fluorescence in these regions (Table I, Contact zone) to determine whether this high fluorescence reflected a redistribution of LFA-1 to cell–cell contacts or was merely the additive value of the two cell membrane measurements. As the ratio of the average fluorescence intensity at cell contact areas to that of free membrane was ∼2 (Table I, Contact/Free), we concluded that there is no large scale redistribution of LFA-1 to points of cell contact upon thapsigargin treatment. In summary, the confocal results revealed that upon thapsigargin stimulation LFA-1 becomes clustered but does not change its distribution to regions of the cell membrane in contact with other cells. Similar results were obtained with the other Ca2+-mobilizing agents as well as PdBu (data not shown), and by cross-linking the TCR/CD3 (see Fig. 7) suggesting that local LFA-1 clustering may be a general feature of several T cell adhesion- activating protocols.
Figure 7.
LFA-1 clustering induced by thapsigargin and anti-TCR–CD3 triggering is inhibited by calpeptin. Clustering of LFA-1 induced by 5 μM thapsigargin (a) and 10 μg/ml anti-CD3 mAb (b) was prevented by preincubation with 100 μg/ml calpeptin, (c and d, respectively), for 30 min at 37°C. Bar, 10 μm.
Effect of Jasplakinolide on T Cell Adhesion to ICAM-1 and on LFA-1 Distribution
The implication of the above confocal experiments was that the Ca2+ mobilizers promoted LFA-1 clustering. This clustering might occur as a result of breakdown of cytoskeletal tethering (Elemer and Edgington, 1994; Kucik et al., 1996; Lub et al., 1997) or, conversely, might follow from the formation of new cytoskeletal connections (Pardi et al., 1992). To address these alternative possibilities, we investigated the effects on LFA-1 adhesion and membrane distribution of jasplakinolide, a compound that stabilizes pre-existing actin filaments, promotes actin polymerization, and prevents actin depolymerization (Bubb et al., 1994). Jasplakinolide caused dose-dependent inhibition of LFA-1–mediated adhesion to ICAM-1 with complete inhibition between 1 to 2 μM (data not shown). Therefore, the general inhibitory effects of jasplakinolide on agonists that indirectly stimulate LFA-1–mediated adhesion is consistent with a requirement for disassembly of the actin cytoskeleton.
We next used confocal microscopy to ask whether jasplakinolide affected the distribution of LFA-1 after pretreatment of thapsigargin-stimulated T cells. As seen in Fig. 5, jasplakinolide diminished the fluorescence levels of LFA-1 detected by confocal microscopy (i.e., there is less fluorescence highlighted [red] in Fig. 5 b than in a). Identical levels of LFA-1 expression were detected by flow cytometry before and after jasplakinolide treatment (data not shown), indicating that the inhibitor interfered with LFA-1 clustering rather than causing receptor loss. Similar results were obtained after T cell stimulation with phorbol ester and through CD3–TCR (data not shown). Therefore, clustering of LFA-1 on the membrane is dependent on the disassembly of the actin fibers, suggesting that adhesion through LFA-1 clustering is promoted by active release from restraints imposed by the cytoskeleton.
Figure 5.

Thapsigargin stimulates LFA-1 clustering through a cytoskeletal release mechanism. T cells adhered onto poly-L-lysine were either (a) treated with 5 μM thapsigargin for 30 min at 37°C, or (b) preincubated with 1 μM jasplakinolide for 30 min at 37°C, before treatment as in a. LFA-1 was detected with FITC-conjugated LFA-1 specific mAb and analyzed by confocal microscopy as in Fig. 4. Bar, 10 μm.
Analysis of the Intracellular Molecules Controlling LFA-1–mediated Adhesion: Involvement of Calpain in Ca2+-induced Effects
In an attempt to understand the molecular mechanism by which Ca2+ induces LFA-1 clustering, we investigated the effect of inhibitors and mAbs specific for several major Ca2+-using enzymes that have a link with adhesion. After stimulation with ionomycin or thapsigargin, we failed to find a role for PKC, calcineurin, or calmodulin using the specific inhibitors Ro 31-8220 (1–5 μM), FK506 (0–7 ng/ml), and trifluoperazine (0.5–10 μM), respectively. Similarly, immunoprecipitation and blotting with specific antibodies failed to show an association of the 208-kD MLCK (Gallagher et al., 1995) or calreticulin (Coppolino et al., 1995) with stimulated LFA-1 (data not shown).
Another enzyme activated by Ca2+ is calpain, a multifunctional protease that is located in the cytosol (Sorimachi et al., 1994). We monitored LFA-1 adhesion stimulated by thapsigargin, PdBu, XL-TCR–CD3, and Mg2+/EGTA after preincubation with the membrane permeable calpain inhibitor, calpeptin (Tsujinaka et al., 1988; Kwak et al., 1993). This agent caused maximal inhibition of T cell adhesion at 100 μg/ml (280 μM) after stimulation by thapsigargin, PdBu, and XL-TCR–CD3, but had no effect on Mg2+-induced adhesion (Fig. 6). A further calpain inhibitor, CBZ-LVG, also blocked adhesion at similar concentrations (data not shown). There was no alteration in cell viability at concentrations at which these inhibitors were maximally active. We next found that the levels of clustered LFA-1 detected after treatment with thapsigargin (Fig. 7 a), XL-CD3 (Fig. 7 b), and PdBu (data not shown) were diminished by calpeptin treatment (i.e., there is less fluorescence highlighted [red] in Fig. 7 c than in a and in Fig. 7 d than in b) to levels similar to those on resting T cells (Table I, Free membrane).
Figure 6.
Calpain inhibitors block LFA-1–mediated adhesion induced by agonists acting intracellularly. Cells were preincubated with or without 280 μM (100 μg/ml) calpeptin for 30 min at 37°C before analysis in a T cell adhesion assay where stimulants were 50 nM PdBu, 5 μM thapsigargin, 10 μg/ml anti-CD3 mAb G19.4, 5 mM Mg2+/1 mM EGTA.
The calpain inhibitors used in this study are not entirely specific for calpain but also inhibit lysosomal cathepsins and proteasome activity. To rule out the involvement of the proteasome, we investigated the effect of the highly specific proteasome inhibitor lactacystin on T cell adhesion to ICAM-1. Although antigen presentation was inhibited by 20 μM lactacystin (Correa, I., and J. Trowsdale, personal communication) T cell adhesion was unaffected by concentrations as high as 200 μM (data not shown). Furthermore, T cells lack lysosomal cathepsins that are also susceptible to the calpain inhibitors. In summary, these results indicate that a calpain-like enzyme is pivotal in causing the clustering of LFA-1 and by this means adhesion to ICAM-1 is facilitated.
Discussion
In this study, we have examined a role for Ca2+ in the induction of integrin-mediated adhesion, using the leukocyte integrin LFA-1 on T cells as the model. Three agents that cause Ca2+ mobilization trigger LFA-1–mediated adhesion to ICAM-1 to the same extent as other inside out inducers of adhesion such as XL-TCR–CD3 and phorbol ester. Furthermore, all three forms of stimulation cause LFA-1 clustering on T cells. The following points can be made about the Ca2+-dependent mechanism by which adhesion is brought about: (a) LFA-1 clusters in the cell membrane thereby strengthen adhesion by increasing the integrin avidity; (b) the adhesion is dependent upon Ca2+ influx; (c) the overall affinity of LFA-1 for ligand does not alter as no increase in the ability to bind soluble ICAM-1 can be detected; (d) both adhesion and LFA-1 redistribution are prevented by inhibiting cytoskeletal disassembly, implying a role for actin reorganization; (e) calpain has a central role in the clustering of LFA-1; (f) the above features are not shared by Mg2+/EGTA–treated LFA-1, which binds ICAM-1 with high affinity and mediates adhesion without dependence on the intracellular events described here.
Stimulation of T cells by the Ca2+ mobilizers, through the TCR–CD3 complex and by phorbol ester, results in an increase in integrin avidity by clustering of LFA-1 in the membrane. These results are in agreement with previous reports of integrin clustering after phorbol ester stimulation (Burn et al., 1988; Kupfer and Singer, 1989). Both phorbol esters and the other Ca2+-dependent adhesion inducers used in this study cause LFA-1 clustering over the entire cell membrane. In a more physiological setting, when the TCR is triggered upon T cell–APC contact Ca2+ fluxes occur locally (Zweifach and Lewis, 1993; Hall et al., 1993; Donnadieu et al., 1994; Negulescu et al., 1996). The subsequent effect on LFA-1 would be confined to the immediate points of cell contact. Support for this prediction comes from the observation that LFA-1 segregates away from the “TCR clusters” after TCR signaling, and therefore is no longer uniformly distributed (Dustin et al., 1996). Whether clustering involves direct lateral association between integrin subunits or is driven by ligand (Singer, 1992) requires further investigation. The expression of the epitopes of both nonfunction blocking (G25.2) and function blocking (38, F110.22) anti–LFA-1 α-subunit mAb epitopes is comparable when detected by confocal microscopy, implying that the clustering in this study is probably not driven by ligand (data not shown). The work of van Kooyk et al. (1994) suggests that Ca2+ may also have an extracellular role in integrin multimerization. Finally, a recent analysis of α4 integrin made use of the confocal microscope to investigate integrin clustering in the absence of an alteration in α4 integrin cell membrane expression (Yauch et al., 1997) in a similar manner to the approach described in our study.
Calpain is implicated in the clustering and adhesive activity of LFA-1 by the ability of selective inhibitors to diminish these processes. Calpains are Ca2+-dependent neutral cysteine proteases that are activated by local Ca2+ fluxes and are widely expressed in mammalian cells (Suzuki et al., 1987; Saido et al., 1994; Sorimachi et al., 1994). Calpain is highly expressed in T cells and is increased at both mRNA and protein levels by phorbol ester, calcium ionophore, and anti-CD3 treatment, all agents that can induce LFA-1 adhesion (Deshpande et al., 1995). In platelets Ca2+ ionophore will directly activate calpain, but when platelets are stimulated by a physiological agonist, such as thrombin, calpain activation is then dependent upon interaction of integrin αIIbβ3 with ligand (Fox et al., 1993). However, for T cells, the evidence suggests that the Ca2+ flux is not dependent on ligand binding by LFA-1, but, as we argue here, is directly responsible for LFA-1 clustering, with ligand binding a secondary event. Firstly, anti-CD3 stimulation of T cells causes activation of calpain proteolytic activity (Selliah et al., 1996). Secondly, the Ca2+ flux in T cells stimulated in this manner is not affected by function blocking CD11a and CD18 mAbs (Monard, 1991). Finally, there has been no indication that LFA-1 is physically associated with Ca2+ channel activity as has been suggested for αIIbβ3 (Rybak et al., 1988; Fujimoto et al., 1991). Therefore, the evidence suggests that calpain activity is regulated differently in these two cell types, but this does not preclude further action of calpain after the ICAM-1 binding phase of T cells.
There are a number of possibilities to explain how calpain might be acting. Calpain may be activated to cleave a key protein, physically releasing LFA-1 from its cytoskeletal restraint and allowing movement in the membrane as observed by single particle tracking (Kucik et al., 1996) and resonance energy transfer studies (Poo et al., 1994). Proteins that have been identified as calpain targets include talin (Inomata et al., 1996), filamin (Collier and Wang, 1982), and α-actinin (Selliah et al., 1996). In resting PBMC, both filamin and α-actinin are associated with CD18 (Pavalko and LaRoche, 1993; Sharma et al., 1995), and in T cells calpain cleaves α-actinin after TCR–CD3 triggering (Selliah et al., 1996) with kinetics similar to that of LFA-1–mediated adhesion (Dransfield et al., 1992; Dustin and Springer, 1989). Another possibility is that calpain cleaves a signaling protein. Potential candidates include focal adhesion kinase (Cooray et al., 1996) and phosphotyrosine phosphatase kinase 1B (Frangioni et al., 1993). It will be of interest to test the sensitivity to calpain of LFA-1–binding proteins such as cytohesin-1 (Kolanus et al., 1996). There is evidence that calpain can cleave the β3 subunit (Du et al., 1995). Whether the β2 subunit can also be cleaved by calpain remains to be investigated, and it is uncertain whether a β2 integrin cleaved at the sites homologous to the β3 calpain sensitive sites would be able to attach to the cytoskeleton or to bind ligand.
The cytoskeleton is intimately involved in the adhesive process. Use of jasplakinolide, an agent that inhibits actin disassembly (Bubb et al., 1994) prevents LFA-1 clustering and T cell adhesion. Alternatively, low levels of cytochalasin D promote integrin-mediated adhesion (Elemer and Edgington, 1994; Kucik et al., 1996; Lub et al., 1997; Yauch et al., 1997) and allow movement of the integrin in the membrane (Kucik et al., 1996). Put together, these findings indicate that in the nonactive state, LFA-1 is tethered to the cytoskeleton and release from this constraint allows motility leading to LFA-1 clustering and adhesion to ICAM-1. LFA-1 has also been reported to associate with cytoskeletal elements after activation (Pardi et al., 1992), and it is possible that clustered integrin, either before or after contact with ligand, might renew interaction with the cytoskeleton. The role of calpain in cytoskeletal rearrangements remains unresolved. Cytoskeletal reorganization may be necessary for the exposure of the proteolytic target of calpain or, conversely, calpain activation may lead to alterations in the cytoskeleton itself. Phorbol ester and other stimulants also cause coincident PKC and calpain translocation to the membrane (Pontremoli et al., 1989; Hong et al., 1995). In platelets activating agonists are reported to cause calpain to move from a generalized distribution in cells to a peripheral location (Fox et al., 1993), and this could be brought about by cytoskeletal reorganization.
Here we have provided evidence that the protease calpain plays a role in the regulation of LFA-1 adhesion induced by Ca2+ flux. This process involves an increase in ligand-binding avidity of the T cell through clustering of LFA-1 but no detectable increase in LFA-1 affinity. In many aspects this route to LFA-1 adhesion differs from that of Mg2+/EGTA treatment, which requires neither Ca2+ nor calpain but activates from the “outside” causing an affinity increase in LFA-1, indicating direct integrin conformational change (Stewart et al., 1996). Thus, there are two alternative pathways to LFA-1–mediated adhesion with different mechanisms. Migrating cells need adhesive contacts that can be rapidly made and easily broken. We suggest here that such contacts may be regulated through proteolysis and cytoskeletal control. On the other hand, once a leukocyte has arrived at an inflammatory site, migration is halted and stable cell–cell contacts form, which may then allow the bi-directional signaling necessary for other aspects of T cell function (Shaw and Dustin, 1997). High affinity adhesion would be an efficient means of facilitating these interactions and therefore it is of interest that the Mg2+ concentration of wound fluids is in favorable balance with Ca2+ and considerably higher than in normal plasma (Grzesiak and Pierschbacher, 1995). There is also evidence that this higher affinity LFA-1 may succeed the primary low affinity interaction of LFA-1 as a result of conformational change brought about by contact with ligand (Cabañas and Hogg, 1993).
In summary, our findings show that the Ca2+-regulated activation of calpain causes the clustering of LFA-1, possibly by cleaving a key cytoskeletal component. In vivo this Ca2+-induced clustering would be speculated to be confined to local sites of receptor triggering. Recent studies have outlined the phases of contact between T cells with antigen-presenting cells and highlight the importance of local Ca2+ fluxes in converting the motile “scanning” T cell into an immobile cell, stably engaged with its partner (Donnadieu et al., 1994; Negulescu et al., 1996). Our observations lead to the prediction that any receptor trigger which stimulates a Ca2+ flux in T cells would induce LFA-1 adhesion. It will be interesting to discover whether this mechanism is unique to control of LFA-1 adhesion or part of the adhesion mechanism for integrins found on other cells.
Acknowledgments
We gratefully acknowledge the help of Imperial Cancer Research Fund colleagues Rainer Pepperkok, Peter Jordan, Alex Stokes, and Andrew Edwards (Digital Imaging Microscopy Department) and Henry Potts (Medical Statistics Group) for performing the statistical analysis. We thank K.K. Wang (Parke Davis, Ann Arbor, MI) for discussion about calpain and A. Koffer (University College of London, London) and G. Nash (University of Birmingham, Birmingham, UK) for discussion about jasplakinolide. We thank T. Plesner (University of Copenhagen, Copenhagen, Denmark) for LFA-1 mAb F110.22, F. Pavalko (Indiana School of Medicine, Indianapolis, IN) for sharing unpublished data and for the kind gift of embMLCK-specific mAb, S. Dedhar (Terry Fox Institute, Vancouver, British Columbia, Canada) for antisera specific for calreticulin, and N. Clipstone (Stanford, Palo Alto, CA) for FK506. We thank colleagues Matthew Robinson, Birgit Leitinger, Joanna Porter, and Rebecca Newton for their helpful comments about the manuscript.
This work was supported by the Imperial Cancer Research Fund.
Abbreviations used in this paper
- dBHQ
2,5-di-t-butylhydroquinone
- ICAM-1
intercellular adhesion molecule-1
- LFA-1
lymphocyte function-associate antigen-1
- MLCK
myosin light chain kinase
- Pdbu
phorbol 12,13 dibutyrate
- PKC
protein kinase C
- sICAM-1
soluble intercellular adhesion molecule-1
- TCR
T cell receptor
Footnotes
Address all correspondence to Nancy Hogg, Leukocyte Adhesion Laboratory, Imperial Cancer Research Fund, Lincoln's Inn Fields, London WC2A 3PX, United Kingdom. Tel.: 44 171 269 3255. Fax: 44 171 269 3093. E-mail: hogg@icrf.icnet.uk
M.P. Stewart and A. McDowall contributed equally to this work.
References
- Altieri DC, Bader R, Mannucci PM, Edgington TS. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J Cell Biol. 1988;107:1893–1900. doi: 10.1083/jcb.107.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt AR, McDowall A, Craig AG, Bates PA, Sternberg MJE, Marsh K, Newbold CI, Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell. 1992;68:71–81. doi: 10.1016/0092-8674(92)90207-s. [DOI] [PubMed] [Google Scholar]
- Breittmayer J-P, Bernard A, Aussel C. Regulation by sphingomyelinase and Sphingosine of Ca2+signals elicited by CD3 monoclonal antibody, thapsigargin, or ionomycin in the Jurkat T cell line. J Biol Chem. 1994;269:5054–5058. [PubMed] [Google Scholar]
- Brown E, Hogg N. Where the outside meets the inside: integrins as activators and targets of signal transduction cascades. Immunol Lett. 1996;54:189–193. doi: 10.1016/s0165-2478(96)02671-5. [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AMJ, Sausville EA, Duncan KLK, Korn ED. Jasplakinlide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Burn P, Kupfer A, Singer SJ. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivoafter phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1988;85:497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabañas C, Hogg N. Ligand intercellular adhesion molecule 1 has a necessary role in the activation of integrin lymphocyte function-associated molecule 1. Proc Natl Acad Sci USA. 1993;90:5838–5842. doi: 10.1073/pnas.90.12.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier NC, Wang K. Purification and properties of human platelet P235. A high molecular weight protein substrate of endogenous calcium- activated protease(s) J Biol Chem. 1982;257:6937–6943. [PubMed] [Google Scholar]
- Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem J. 1996;318:41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino M, Leung-Hagesteijn C, Dedhar S, Wilkins J. Inducible interaction of integrin α2β1 with calreticulin. J Biol Chem. 1995;270:23132–23138. doi: 10.1074/jbc.270.39.23132. [DOI] [PubMed] [Google Scholar]
- Deshpande RV, Goust J-M, Chakrabarti AK, Barbosa E, Hogan EL, Banik NL. Calpain expression in lymphoid cells, increased mRNA and protein levels after cell activation. J Biol Chem. 1995;270:2497–2505. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- Donnadieu E, Bismuth G, Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr Biol. 1994;4:584–595. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Dransfield I, Cabañas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I, Hogg N. Regulated expression of Mg2+binding epitope on leukocyte integrin α subunits. EMBO (Eur Mol Biol Organ) J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Saido TC, Tsubuki S, Indig FE, Williams MJ, Ginsberg MH. Calpain cleavage of the cytoplasmic domain of the integrin β3subunit. J Biol Chem. 1995;270:26146–26151. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Miller JM, Raganath S, Vignali DAA, Viner NJ, Nelson CA, Unanue ER. TCR-mediated adhesion of T cell hybridomas to planar bilayers containing purified MHC class II/peptide complexes and receptor shedding during detachment. J Immunol. 1996;157:2014–2021. [PubMed] [Google Scholar]
- Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Elemer GS, Edgington TS. Microfilament reorganisation is associated with functional activation of αMβ2 on monocytic cells. J Biol Chem. 1994;269:3159–3166. [PubMed] [Google Scholar]
- Faull RJ, Ginsberg MH. Dynamic regulation of integrins. Stem Cells. 1995;13:38–46. doi: 10.1002/stem.5530130106. [DOI] [PubMed] [Google Scholar]
- Fox JEB, Taylor RG, Taffarel M, Boyles JK, Goll DE. Evidence that activation of platelet calpain is induced as a consequence of binding of adhesive ligand to the integrin, glycoprotein IIb-IIIa. J Cell Biol. 1993;120:1501–1507. doi: 10.1083/jcb.120.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni JV, Oda A, Smith M, Salzman EW, Neel BG. Calpain-catalyzed cleavage and subcellular relocation of protein phosphatase 1B (PTB-1B) in human platelets. EMBO (Eur Mol Biol Organ) J. 1993;12:4843–4856. doi: 10.1002/j.1460-2075.1993.tb06174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T, Fujimura K, Kuramoto A. Electrophysiological evidence that glycoprotein IIb-IIIa complex is involved in calcium channel activation on human platelet plasma membrane. J Biol Chem. 1991;266:16370–16375. [PubMed] [Google Scholar]
- Gallagher PJ, Garcia JGN, Herring BP. Expression of a novel myosin light chain kinase in embryonic tissues and cultured cells. J Biol Chem. 1995;270:29090–29095. doi: 10.1074/jbc.270.49.29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Pierschbacher MD. Shifts in the concentrations of magnesium and calcium in early porcine and rat wound fluids activate the cell migratory response. J Clin Invest. 1995;95:227–233. doi: 10.1172/JCI117644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CG, Sancho J, Terhorst C. Reconstitution of T cell receptor ζ-mediated calcium mobilization in nonlymphoid cells. Science. 1993;261:915–917. doi: 10.1126/science.8346442. [DOI] [PubMed] [Google Scholar]
- Hartfield PJ, Greaves MW, Camp RDR. β1 integrin-mediated T cell adhesion is regulated by calcium ionophores and endoplasmic reticulum Ca2+-ATPase inhibitors. Biochem Biophys Res Commun. 1993;196:1183–1187. doi: 10.1006/bbrc.1993.2376. [DOI] [PubMed] [Google Scholar]
- Hendey B, Klee CB, Maxfield FR. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992;258:296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- Hong D-H, Huan J, Ou B-R, Yeh J-Y, Saido TC, Cheeke PR, Forsberg NE. Protein kinase C isoforms in muscle cells and their regulation by phorbol ester and calpain. Biochim Biophys Acta. 1995;1267:45–54. doi: 10.1016/0167-4889(95)00024-m. [DOI] [PubMed] [Google Scholar]
- Hughes PE, O'Toole TE, Ylanne J, Shattil SJ, Ginsberg MH. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J Biol Chem. 1995;270:12411–12417. doi: 10.1074/jbc.270.21.12411. [DOI] [PubMed] [Google Scholar]
- Inomata M, Hayashi M, Ohno-Iwashita Y, Tsubuki S, Saido TC, Kawashima S. Involvement of calpain in integrin-mediated signal transduction. Arch Biochem Biophys. 1996;328:129–134. doi: 10.1006/abbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- Jakubowski A, Rosa MD, Bixler S, Lobb R, Burkly LC. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes Commun. 1995;3:131–142. doi: 10.3109/15419069509081282. [DOI] [PubMed] [Google Scholar]
- Kassner PD, Kawaguchi S, Hemler ME. Minimum α chain cytoplasmic tail sequence needed to support integrin-mediated adhesion. J Biol Chem. 1994;269:19859–19867. [PubMed] [Google Scholar]
- Kolanus W, Nagel W, Schiller B, Zeitmann L, Godar S, Stockinger H, Seed B. αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion- activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989;170:1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak KB, Kambayashi J, Kang MS, Ha DB, Chung CH. Cell-penetrating inhibitors of calpain block both membrane fusion and filamin cleavage in chick embryonic myoblasts. FEBS Lett. 1993;323:151–154. doi: 10.1016/0014-5793(93)81468-f. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Lu C-F, Springer TA. The α subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin Lymphocyte Function-Associated Antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- Lub M, van Kooyk Y, van Vliet SJ, Figdor CG. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin Lymphocyte Function-associated Molecule-1. Mol Biol Cell. 1997;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monard, S.P. 1991. The role of LFA-1 in generating calcium fluxes during T cell receptor mediated T cell activation. In Immunology. Kings College, University of London, London. 27–32.
- Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- Pardi R, Inverardi L, Bender JR. Regulatory mechanisms in leukocyte adhesion: flexible receptors for sophisticated travelers. Immunol Today. 1992;13:224–230. doi: 10.1016/0167-5699(92)90159-5. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, LaRoche SM. Activation of human neutrophils induces an interaction between the integrin β2-subunit (CD18) and the actin binding protein α-actinin. J Immunol. 1993;151:3795–3807. [PubMed] [Google Scholar]
- Peter K, O'Toole TE. Modulation of cell adhesion by changes in αLβ2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J Exp Med. 1995;181:315–326. doi: 10.1084/jem.181.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S, Melloni E, Salamino F, Patrone M, Michetti M, Horecker BL. Activation of neutrophil calpain following its translocation to the plasma membrane induced by phorbol ester or fMet-Leu-Phe. Biochem Biophys Res Commun. 1989;160:737–743. doi: 10.1016/0006-291x(89)92495-9. [DOI] [PubMed] [Google Scholar]
- Poo H, Fox BA, Petty HR. Ligation of CD3 triggers transmembrane proximity between LFA-1 and cortical microfilaments in a cytotoxic T cell clone derived from tumour infiltrating lymphocytes: a quantitative resonance energy transfer microscopy study. J Cell Physiol. 1994;159:176–180. doi: 10.1002/jcp.1041590121. [DOI] [PubMed] [Google Scholar]
- Putney JW, Bird GSJ. The signal for capacitative calcium entry. Cell. 1993;75:199–201. doi: 10.1016/0092-8674(93)80061-i. [DOI] [PubMed] [Google Scholar]
- Rothlein R, Springer TA. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986;163:1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak ME, Renzulli LA, Bruns MJ, Cahaly DP. Platelet glycoproteins IIb and IIIa as a calcium channel in liposomes. Blood. 1988;72:714–720. [PubMed] [Google Scholar]
- Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB (Fed Am Soc Exp Biol) J. 1994;8:814–822. [PubMed] [Google Scholar]
- Schmidt, R.E. 1989. Non-lineage/natural killer section report: new and previously defined clusters. In Leukocyte Typing IV: White Cell Differentiation Antigens. W. Knapp, B. Dorken, W.R. Gilks, E.P. Rieber, R.E. Schmidt, H. Stein, and A.E.G. von dem Borne, editors. Oxford University Press, Oxford. 517–574.
- Selliah N, Brooks WH, Roszman TL. Proteolytic cleavage of α-actinin by calpain in T cells stimulated with anti-CD3 monoclonal antibody. J Immunol. 1996;156:3215–3221. [PubMed] [Google Scholar]
- Sharma CP, Ezzell RM, Arnaout MA. Direct interaction of filamin (ABP-280) with the β2-integrin subunit CD18. J Immunol. 1995;154:3461–3470. [PubMed] [Google Scholar]
- Shaw AS, Dustin ML. Making the T cell receptor go the distance: review a topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Singer SJ. Intercellular communication and cell-cell adhesion. Science. 1992;255:1671–1677. doi: 10.1126/science.1313187. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, Saido TC, Suzuki K. New era of calpain research — discovery of tissue-specific calpains. FEBS Lett. 1994;343:1–5. doi: 10.1016/0014-5793(94)80595-4. [DOI] [PubMed] [Google Scholar]
- Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Stewart MP, Cabañas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- Stossel TP. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989;264:18261–18264. [PubMed] [Google Scholar]
- Suzuki K, Imajoh S, Emori Y, Kawasaki H, Minami Y, Ohno S. Calcium-activated neutral protease and its endogenous inhibitor. FEBS Lett. 1987;220:271–277. doi: 10.1016/0014-5793(87)80828-1. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumour promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Hanley MR. Pharmacological tools for perturbing intracellular calcium storage. Methods Cell Biol. 1994;40:65–89. doi: 10.1016/s0091-679x(08)61110-3. [DOI] [PubMed] [Google Scholar]
- Tsujinaka T, Kajiwara Y, Kambayashi J, Sakon M, Higuchi N, Tanaka T, Mori T. Synthesis of a new cell penetrating calpain inhibitor (calpeptin) Biochem Biophys Res Commun. 1988;153:1201–1208. doi: 10.1016/s0006-291x(88)81355-x. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Weder P, Heije K, Figdor CG. Extracellular Ca2+modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J Cell Biol. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y, Weder P, Keije K, Malefijt RDW, Figdor CG. Role of intracellular Ca2+levels in the regulation of CD11a/CD18 mediated cell adhesion. Cell Adhes Commun. 1993;1:21–32. doi: 10.3109/15419069309095679. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Krautwurst D, Musgrave I, Seifert R. Thapsigargin activates univalent- and bivalent-cation entry in human neutrophils by a SK&F 96365- and Gd3+-sensitive pathway and is a partial secretagogue: involvement of pertussis-toxin-sensitive G-proteins and protein phosphatases 1/2A and 2B in the signal transduction pathway. Biochem J. 1996;314:679–686. doi: 10.1042/bj3140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Felsenfeld DP, Kraeft S-K, Chen LB, Sheetz MP, Hemler ME. Mutational evidence for control of adhesion through diffusion/clustering, independent of ligand binding. J Exp Med. 1997;186:1347–1355. doi: 10.1084/jem.186.8.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]