Abstract
Hypoxia-Inducible Factor (HIF)-1 is a dimeric protein complex that plays an integral role in the body's response to low oxygen concentrations, or hypoxia. HIF-1 is among the primary genes involved in the homeostatic process, which can increase vascularization in hypoxic areas such as localized ischemia and tumors. It is a transcription factor for dozens of target genes; HIF-1 is also essential for immunological responses and is a crucial physiological regulator of homeostasis, vascularization, and anaerobic metabolism. Furthermore, HIF-1 is increasingly studied because of its perceived therapeutic potential. As it causes angiogenesis, enhancement of this gene within ischemic patients could promote the vessel proliferation needed for oxygenation. In contrast, as HIF-1 allows for survival and proliferation of cancerous cells due to its angiogenic properties, inhibition potentially could prevent the spread of cancer. With a growing understanding of the HIF-1 pathway, the inhibition and stimulation of its transcriptional activity via small molecules is now an attractive goal. Gene therapy to achieve both vessel proliferation and tumor regression has been demonstrated in animal studies but requires significant improvement and modification before becoming commercially available. This review focuses on the potential of the HIF-1 pathway in therapeutic intervention for the treatment of diseases such as cancer and ischemia.
Oxygen Metabolism in Mammals
Oxygen is required by the cells of most organisms to produce adequate amounts of ATP necessary for metabolic activities. Hypoxia, or oxygen deprivation, occurs within human tissues and cells due to a variety of conditions, including disorders of the heart and lungs, anemia, and circulatory problems. Depending on the severity, permanent damage to tissues and cells may occur [1].
However, hypoxia also can play an important and beneficial role in human physiology and development. It is integral for proper embryonic development. Although the exact mechanisms are unknown, oxygen tension is related to closure of the neural tube, mediation of apoptosis, and proper morphological development during gestation. Such findings indicate that in addition to genetic cues, environmental conditions such as hypoxia serve as signals in embryonic development [2,3,4].
Many organisms have evolved adaptive mechanisms for hypoxic conditions. Changing oxygen levels can result in activation or repression of certain homeostatic regulatory genes, allowing for the survival of tissues and cells despite fluctuating environmental conditions. Genes such as HIF-1, whose activation is prompted by hypoxic conditions, can interact with enzymes and other transcription factors in order to control vascularization and tissue growth. While microenvironments surrounding cancerous tumors are extremely hypoxic, proliferation of such masses often is made possible by HIF-1 activation, which leads to increased angiogenesis and, thus, an increased oxygen supply to the area [5,6].
Given its prominent function, manipulation of HIF-1 activity within areas of ischemia and tumor masses has become a focus in the effort to develop noninvasive, pharmaceutical treatment options for cancer and heart disease patients. Although no such human protein has been successfully regulated by scientific means, control of HIF-1 activity is increasingly feasible as details of its structure, function, and genetic pathway are elucidated.
HIF-1 Domain Structure
HIF-1 is a heterodimeric transcription factor consisting of a constitutively expressed β-subunit and an oxygen-regulated α-subunit. The HIF-1α and HIF-1β proteins both contain basic helix-loop-helix motifs that bind DNA and cause subunit dimerization [7,8,9]. Both subunits also have a Per-ARNT-Sim (PAS) domain, with similar functions. In the α-subunit, there is an oxygen-dependent degradation (ODD) domain, which is hydroxylated by proline-hydroxylase-2 (PHD-2), rendering the α-subunit vulnerable to proteasomal degradation under normoxic cellular conditions [10]. The structure of HIF-1α and HIF-1β is depicted in Figure 1.
Figure 1.
The structure of the HIF-1α and HIF-1β genes. These genes contain a basic helix-loop-helix (bHLH) motif and Per-ARNT-Sim (PAS) domain, both of which aid in dimerization and the binding of the subunits to DNA. The carboxy-terminal transactivation domains of these genes serve as regulatory and transactivation regions. Transcriptional activators CBP and p300 bind to the TAD-C region of the HIF-1α gene. Also depicted on the diagram of HIF-1α gene is the inhibitory domain, an important regulatory region.
The HIF-1α subunit also contains two transactivation domains (TAD), which regulate HIF-1 target genes. CREB binding protein (CBP) and p300, two transcriptional co-activators of HIF-1, interact with the carboxy-terminal transactivation domain (C-TAD) of HIF-1α.
Both activators are essential for HIF-1 transcription and are therefore targets in the effort to regulate HIF-1 expression; inhibition of HIF-1α C-TAD interactions by proline hydroxylation inhibits HIF-1 gene expression, preventing normal transcription and translation [11]. HIF-1β contains only one such analogous region, which is unnecessary for HIF-1 complex function [7,10,12]. Recent reports show that HIF-1β is identical to the previously discovered vertebrate protein, aryl hydrocarbon receptor nuclear translocator (ARNT) [12].
HIF-1 Regulates Oxygen Homeostasis
HIF-1 is a major regulator of oxygen homeostasis within cells. As a transcription factor, it affects and regulates the expression of dozens of genes involved in maintaining homeostasis as oxygen concentrations change [13]. HIF-1 further mediates cellular responses to hypoxia by regulating glucose uptake and anaerobic respiration in oxygen-depleted environments [5,2].
Transcriptional Regulation Controls Angiogenesis in Hypoxia
One important HIF-1 function is to promote angiogenesis; HIF-1 directs migration of mature endothelial cells toward a hypoxic environment [2,5]. This is done via HIF-1 regulation of vascular endothelial growth factor (VEGF) transcription. VEGF is a major regulator of angiogenesis, which promotes endothelial cell migration toward a hypoxic area. During hypoxia, HIF-1 binds the regulatory region of the VEGF gene, inducing its transcription and initiating its expression [12,15,16]. Such endothelial cells ultimately help to form new blood vessels, supplying the given area with oxygenated blood [14].
HIF-1 Regulates Shift to Anaerobic Metabolism
HIF-1 also can regulate anaerobic metabolism. When oxygen is available, most cells produce ATP via oxidative phosphorylation. However, in hypoxic environments, there is a shift to anaerobic metabolism for cellular energy production. HIF-1 is among the principal genes to coordinate this shift, by inducing a variety of glycolytic enzymes and glucose transporters such as aldolase A and pyruvate kinase M, which help cells efficiently produce energy in hypoxic environments [5,16]. In addition to increasing the expression of these enzymes, HIF-1 decreases mitochondrial oxygen consumption by activating pyruvate dehydrogenase kinase I and halting the citric acid cycle [17].
Cancer, Inflammation, and Hypoxia
Environments surrounding metastasizing tumor masses are often hypoxic. HIF-1 is a crucial protein in such masses; it enables tumor progression by inducing alternative metabolic pathways within cancer cells, as discussed above in the context of physiologic hypoxia.
Tumor Proliferation
Due to its role in hypoxia, HIF-1 plays a critical part in tumor proliferation [18]. As a tumor develops and grows, a hypoxic environment is created because of the extreme energy demands of the numerous, rapidly dividing cells. Angiogenesis is often induced by such cellular masses to meet the needs for increased oxygen, energy, and blood supplies [5,16]. Concurrently, HIF-1 contributes to the shift to anaerobic metabolism. The importance of this transcription factor in tumor cell survival is reflected in the finding that the levels of HIF-1α in glioma tumor cells increase proportionally with the grade of the tumor [19].
The mechanisms of HIF-1 mediated tumor survival have been partially revealed by the work of Semenza et al. on VHL-lacking renal carcinoma cells. HIF-1 was found to decrease oxygen consumption in these cells by inhibiting C-MYC, a transcription factor that regulates mitochondrial mass and oxygen consumption and is known to be down-regulated in a variety of human cancers. Semenza et al. report that HIF-1 decreases levels of C-MYC by increasing the transcription of MXI1, a repressor of C-MYC, and by increasing the rates of proteosome degradation of the C-MYC protein. The decreased levels of C-MYC in these cancer cells were found ultimately to lead to increased glycolysis and decreased mitochondrial respiration, crucial characteristics of cancer cells that survive and proliferate in the hypoxic conditions of the tumor micro-environment [20].
Overexpression of HIF-1 Causes Apoptosis
A multitude of studies currently are being conducted on the role of HIF-1 in hypoxia-induced apoptosis of various cell types. For example, Krick et al. recently reported that overexpression of HIF-1 in alveolar epithelial cells resulted in increased apoptosis [21]. Although the exact pathways and mechanisms involved in this process remain unclear, data suggest that in hypoxic conditions the tumor suppressor p53 is activated. Via interaction with the HIF-1 protein, p53 is stabilized and begins to activate genes such as p21, which, in turn, cause cell death [5,21].
HIF-1 Supports Inflammatory Responses and Hypoxic Recovery
In addition to its other roles in adaptation to hypoxia, HIF-1 has been shown to play a role in inflammation. Cramer and coworkers demonstrated that HIF-1 is necessary for metabolism within myeloid cells [22]. Overexpression of HIF-1 in vivo resulted in increased localized inflammation, while loss of the HIF-1 gene decreased the ability of myeloid cells to aggregate, migrate, and promote bactericidal responses. This dependence of myeloid cells on HIF-1 may be related to their reliance on anaerobic respiration as a means of energy production. Myeloid cells lacking this gene are unable to efficiently produce ATP, effectively migrate to injured tissues, or destroy foreign invaders [22]. In addition, HIF-1α expression plays a role in the differentiation of myeloid cells into monocytes and macrophages [23].
In contrast, HIF-1 can function to prevent tissue and cardiac damage caused by ischemia, which may result in a variety of long-term cardiac problems. Overexpression of HIF-1 in such tissues can cause angiogenesis and thus increase oxygenation of the area [24,25]. This serves as the basis for the current efforts to find pharmaceutical and other non-invasive treatments for ischemia and related diseases.
Activation and Suppression Pathways
Normoxia Causes Degradation; Hypoxia Allows Activation
Under normoxic conditions, HIF-1α is degraded by proteasomes. The HIF-1α subunit is "marked" for such degradation by proline-hydroxylase-2 (PHD-2) and by von-Hippel-Lindau (VHL)-ubiquitin ligase complexes. Therefore, HIF-1 does not function in the presence of sufficient oxygen [10,26]. Also contributing to HIF-1 inactivation in normoxic conditions is Factor Inhibiting HIF-1 (FIH) protein, which hydroxylates HIF-1, preventing interaction of this subunit with the co-activators p300 and CBP. Expression and stabilization of the HIF-1 complex is regulated via feedback inhibition, as PHD-2 itself is activated by HIF-1 [12].
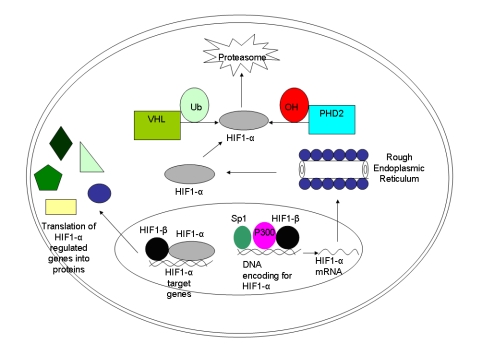
However, in hypoxic conditions, HIF-1 protein is stable and active as hydroxylases, VHL proteins, and FIH are all inhibited by a lack of oxygen. Then HIF-1 is able to interact with its co-activators and can dimerize with its constitutively expressed β-subunit. Once stabilized, the HIF-1 protein can bind to the regulatory regions of its target genes, inducing their expression [7,10,27] [Figure 2].
Figure 2.
The hypoxia-inducible factor (HIF)-1 pathway. The HIF-1α gene is transcribed in the nucleus with the help of specificity protein (Sp) 1, P300, and HIF-1β. Once translated in the cytoplasm, the HIF-1α protein can either become hydroxylated and ubiquinated, in which case it will be degraded by proteasomes (under normal oxygen conditions). In the setting of hypoxia, it can re-enter the nucleus and form a transcription complex with the HIF-1β subunit. If successfully stabilized with the latter subunit, the final complex ultimately will function to regulate target genes such as vascular endothelial growth factor and cathepsin D. Possible therapeutic intervention points are: the hydroxylation that leads to degradation of HIF-1α, the binding of HIF-1α to its coactivators, and the modulation of HIF-1α activity. Additionally, gene therapy approaches have been used to induce the overexpression of HIF or the disruption of the HIF pathway with antisense oligonucleotides. Abbreviations: PHD: proline-hydroxylase domain containing molecules; Ub: ubiquitin; VHL: von Hippel-Lindau protein.
Oxygen-Independent Stimuli
A variety of HIF-1 stimuli function independently of oxygen concentration. These stimuli are primarily proteins, which regulate HIF-1 translation, contrasting sharply with the hypoxic stimuli of this gene, which act upon the already expressed α-subunit. Protein kinase C (PKC) increases the rate of HIF-1α transcription of and functions in conjunction with the phosphatidylinositol 3-kinase (PI3K) pathway, which also enhances HIF-1α translation. The PKC pathway activates expression of the S6 ribosomal protein, which specifically recognizes mRNA transcripts such as HIF-1α. Via phosphorylation of the S6 protein in normoxic conditions, the rates of HIF-1α mRNA translation can be greatly increased, effectively countering the effects of the proteasome degradation of this subunit and increasing levels of the HIF-1 complex within the cell. The PI3K pathway has been identified as the primary means by which various mediators, such as lipopolysaccharides, affect activation of HIF-1α in vascular smooth muscle cells and macrophages [12,27].
Therapeutic Targets in the HIF-1 Pathway: Ischemia
Overexpression
In the case of ischemia treatments, HIF-1α upregulation may stimulate angiogenesis and increase blood flow. Many genes involved in angiogenesis, such as VEGF, matrix metalloproteinase 2 (MMP2), cathepsin D (CATHD), and keratin (KRT), are targets of the HIF-1 transcription complex. It is believed increased HIF-1 levels lead to proportional increases in these proteins [12,28]. In several recent studies, mice injected with HIF-1α DNA lacking an ODDD displayed increased blood supply to wounded or ischemic areas, suggesting increases in HIF-1α levels can aid in supply of blood, oxygen, and nutrients to areas of focal ischemia [29,30].
Introduction of a constitutively stable HIF-1α hybrid to rat cardiomyocytes resulted in decreased ischemic damage. This hybrid consisted of the DNA-binding and dimerization domains from HIF-1α and the transactivation domain of HSV VP16 protein [31]. HIF-1α overexpression in mouse myocardial infarction models reduces infarct size, thereby preserving cardiac function [32]. Increasing HIF-1 expression may prove to be a successful pharmaceutical treatment for ischemic patients on whom surgery cannot be performed.
Direct HIF-1 Modifications
Direct phosphorylation of the HIF-1α subunit itself can increase HIF-1 activity, presumably by impeding proteasome/VHL recognition. Although very little is known about phosphorylation of HIF-1α, p42/p44 mitogen activated protein kinases phosphorylate this protein in vitro. In vivo, such phosphorylation is necessary for HIF-1 function. Activation of the p42/p44 pathway results in increased levels of HIF-1α transcription. This phosphorylation may be an optimal step in the HIF-1 pathway to induce overexpression [33].
The HIF-1 hydroxylases consist of several related molecules, including Factor Inhibiting HIF (FIH) and Prolyl Hydroxylase Domain (PHD) proteins. As VHL mediates proteasome degradation of hydroxylated HIF-1α, the levels of HIF-1α can be increased by silencing HIF-1alpha-prolyl-4 hydroxylase-2 (PHD2). Inhibition of PHD2 via siRNA also results in a decrease in the cardiac infarction size in mice. These pathways may be amenable to pharmacological approaches [34].
Small Molecule Inhibitors
Several small molecules, such as dimethyloxalylglycine, a prolyl hydroxylase inhibitor, activate HIF-1. Hydroxylase activity can be rescued by mutating specific regions or by adding cobalt ions to the cell, which presumably compete for iron binding sites [35]. Some hydroxylases in the prolyl family can be selectively inhibited by adriamycin in vitro [36]. Cobalt (II) and Nickel (II) ions in cells increase HIF-1 activity, presumably as such ions displace the iron from the active sites of 2OG hydroxylases.
Small molecule therapy may be useful not only in HIF-1 suppression, but also in its activation for the treatment of ischemic diseases [7]. Hormones such as angiotensin II and platelet-derived growth factor stimulate the HIF pathway by increasing HIF-1α protein levels via production of reactive oxygen species (ROS) within the cell. Although the exact mechanism is unclear, it appears to be entirely distinct from the hypoxia pathways. Thrombin and other growth factors increase angiogenesis via HIF-1α protein agonist mechanisms [14,33]. Insulin likewise activates HIF-1α by activating multiple protein kinases necessary for expression and function [37].
In another study on HIF-1 activation, homozygous deletion of the p53 gene led to HIF-1 activation [38]. Therefore, p53, responsible for promoting ubiquitination of HIF-1α, may be another possible target.
Gene therapy eventually may be used to increase HIF-1 levels and relieve complications of ischemia. For example, delivery of a stabilized, recombinant form of HIF-1α via adeno-associated virus (AAV) in order to overexpress HIF-1 in skeletal muscle resulted in significantly increased capillary numbers [38,39]. While gene therapy approaches aimed at the process and effects of angiogenesis continue to be developed and studied, higher levels of success in preclinical trials currently are being sought before clinical applications are pursued. Among the most prominent of the remaining obstacles in gene therapy is the mode of delivery [38]. The search for the most efficient delivery vector continues.
Therapeutic Targets in the HIF-1 Pathway: Cancer
With a clearer understanding of the HIF-1 pathway, efforts are directed at manipulation of this complex genetic process in order to ultimately increase or decrease cellular HIF-1 levels. Although a human transcription factor has yet to be successfully controlled through external manipulation, such a goal remains attractive as it may lead to non-invasive treatment methods for diseases such as cancer and ischemia. Optimal points in the HIF-1 pathway for such therapeutic intervention are currently under investigation.
The nature of cancer growth and metastasis provides multiple phases at which therapeutic intervention is possible. As HIF-1 has been found to regulate the shift within the tumor cells to anaerobic metabolism and to activate VEGF and angiogenesis, downregulation of the HIF-1 complex may suppress cancer progression. Among the methods considered for HIF-1 downregulation is the activation of hydroxylases, which target the HIF-1 complex for eventual degradation. Such hydroxylases are members of the 2-oxoglutarate (2OG)-dependent oxygenase superfamily and require a ferrous ion at the active site. Although the exact mechanism remains unknown, HIF-1α degradation is increased by treating cells with iron and ascorbate, which most likely decrease levels of HIF-1 protein and of HIF-target genes by activating the hydroxylases [40].
Specific Therapeutic Targets: Proteins
HIF-1α binding to its co-activators has also been a target for HIF-1 inhibition. The CAD of HIF-1 must bind to the calponin homology 1 (CH1) domains of the co-activators CBP and p300 proteins to initiate transcription [41,42,43]. When excess HIF-1α-CAD polypeptides are transfected into cells, the expression of hypoxia-inducible reporters decreases [41,42]. These peptides compete with HIF-1α for the CPB site, disrupting the interaction, which is crucial for downstream effects.
Stephen et al. have shown that p300/CBP interaction with HIF-1α can be prevented via stimulation of the CITED4 protein. CITED4 is a transcription factor that binds to the CH1 domain of the p300/CBP co-activators, thus competing with co-activators for HIF-1α. In this study, it was demonstrated that HIF-1α levels decrease with stimulation of CITED4 expression and tumor cells are capable of decreasing the levels of CITED4, leading to an increase in the amount of HIF-1α [33].
Alteration of specific HIF-1α amino acid residue oxidation-reduction status can suppress HIF-1 expression. Pleurotin and PX-12 inhibit thioredoxin-1, which reduces a residue in the C-TAD of HIF-1α, in turn increasing the effectiveness of HIF-1 as a transcription factor. Such inhibitors simultaneously decrease levels of VEGF within the cell, as well as angiogenesis in tumors by a mechanism unrelated to HIF-1 inhibition [28]. The underlying mechanisms of these effects remain unclear [44].
Specific Therapeutic Targets: Small Molecules/RNA
Several small molecule inhibitors of HIF-1 activity have been identified and are currently being studied. Topotecan, a topoisomerase inhibitor, decreases cellular accumulation of HIF-1α [31]; it may alter the ribosome entry site on the HIF-1α mRNA molecule, preventing translation [44,45]. 103D5R is another small molecule that recently has been found to have HIF-1α inhibiting properties. The molecular mechanisms and pathways behind the repression of HIF-1α by 103D5R are also unknown, but it may have an action similar to topotecan [42]. However, only in vitro studies of 103D5R have as yet been completed.
HIF-1-HIF-1α inhibition via gene therapy has been investigated using both siRNA and antisense RNA in various cell types. Jensen et al. transfected glioma cells with HIF-1α siRNA, which decreased cell growth both in vivo and in vitro [46]. Similar results were obtained by Sun et al.; plasmid delivery of antisense HIF-1α led to decreased microvessel densities in EL-4 tumors in mice [47]. Chang et al. recently demonstrated that transfection of BxPc-3 cells with antisense HIF-1α results in decreased progression and metastasis of pancreatic cancer [48]. Although such results have yet to be replicated in humans, they have significant implications for the future of cancer treatment. While less studied, the VHL protein, which marks HIF-1α for degradation, is another potential therapeutic target. The VHL tumor suppressor gene becomes inactivated in many types of carcinomas, which causes increased HIF-1α levels and allows for survival in hypoxia. As the promoter regions in VHL in which transcription factors bind now have been identified, silencing may one day be possible [49].
Conclusions
As more is learned about functions, target genes, and activation pathways of HIF-1, novel treatments of diseases such as cancer and ischemia can be developed. Despite limited success in mouse ischemic models and human clinical trials, significant advances in manipulating HIF-1 expression continue to be made [39]. Such results further stress the need to target the HIF-1 pathway directly, as opposed to merely its effects, to truly develop efficient pharmaceutical therapies. In this respect, advances in gene therapy may allow for a greater ability to directly influence intracellular HIF-1 levels.
In the search to find a non-invasive treatment for ischemia, it appears inhibition of 2OG hydroxylases via small molecule therapy is the most promising way to stimulate HIF-1 activity, assuming that selective suppression of such hydroxylases ultimately can be achieved. Selective inhibition of collagen prolyl hydroxylases [7,12] and in clinical trials of a 2OG-related enzyme, cyclooxygenase II, have been successful [50]. Suppression of HIF-1 in the treatment of cancer is also a focus, as the significant role of this transcription complex in tumor progression is now recognized [15,16,19].
The entire spectrum of HIF-1 function is not yet understood. Accordingly, regulation of transcription factor activation in humans has not yet been achieved. That the various HIF-1α hydroxylases affect and regulate levels of this transcription factor differently only recently was described. Much remains to learn about the selectivity and properties of factors influencing the HIF-1 pathway [51]. Although current efforts to manipulate the HIF-1 pathway have been principally centered on the need for treatments of cancer and ischemia, it has been suggested that HIF-1α may play a role in the onset of pulmonary fibrosis [52]. As the molecular mechanisms leading to the onset of this disease remain unknown, HIF-1α inactivation in respiratory cells may be a fruitful line of inquiry. Despite such gray areas in the knowledge of HIF-1, control of this transcription factor does in fact remain a high-priority target. Modulation of the HIF-1 pathway promises to have significant effects on cancer and cardiac treatments, potentially affecting the lives of millions who are afflicted with such diseases.
Acknowledgments
Dr. Jovin's work was supported by NIH grant 5F32HL076016.
Abbreviations
- HIF-1
Hypoxia-Inducible Factor
- PAS
Per-ARNT-Sim
- ODD
oxygen-dependent degradation
- PHD-2
proline-hydroxylase-2
- TAD
transactivation domains
- CBP
CREB binding protein
- C-TAD
carboxy-terminal transactivation domain
- ARNT
aryl hydrocarbon receptor nuclear translocator
- VEGF
vascular endothelial growth factor
- FIH
factor Inhibiting HIF-1
- VHL
von-Hippel-Lindau
- PKC
protein kinase C
- PI3K
phosphatidylinositol 3-kinase
- MMP2
matrix metalloproteinase 2
- CATHD
cathepsin D
- KRT
keratin
- AAV
adeno-associated virus
- 2OG
2-oxoglutarate; CH1
- CH1
calponin homology 1
References
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Chen EY, Fujinaga M, Giaccia AJ. Hypoxic microenvironment within an embryo induces apoptosis and is essential for proper morphological development. Teratology. 1999;60:215–225. doi: 10.1002/(SICI)1096-9926(199910)60:4<215::AID-TERA6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Iver NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert J, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, et al. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang B, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang L, Erbel PJ, Gardner KH, Ding K, Garcia JA, Bruick RK. Functions of the Per/ARNT/Sim domains of the hypoxia-inducible factor. J Biol Chem. 2005;280:36047–36054. doi: 10.1074/jbc.M501755200. [DOI] [PubMed] [Google Scholar]
- Chapman-Smith A, Lutwyche JK, Whitelaw ML. Contribution of the Per/Arnt/Sim (PAS) domains to DNA binding by the basic helix-loop-helix PAS transcriptional regulators. J Biol Chem. 2004;279:5353–5362. doi: 10.1074/jbc.M310041200. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependant degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Bio. 2004;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Donghoon Y, Pastore YD, Vladimir D, et al. HIF-1α-deficiency results in dysregulated EPO signaling and iron homeostasis in mouse development. JBC Papers in Press. 2006 doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- Hewitson KS, Schofield CJ. The HIF pathway as a therapeutic target. Drug Discov Today. 2004;9:704–711. doi: 10.1016/S1359-6446(04)03202-7. [DOI] [PubMed] [Google Scholar]
- Vaupel P. The Role of Hypoxia-Induced Factors in Tumor Progression. Oncologist. 2004;9:10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:150–151. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Shi YH, Fang WG. Hypoxia-inducible factor-1 in tumour angiogenesis. World J Gastroenterol. 2004;10:1082–1087. doi: 10.3748/wjg.v10.i8.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1α in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- Zhang H, Gao P, Fukuda R, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. Role of hypoxia-inducible factor-1α in hypoxia induced apoptosis of primary alveolar epithelial type II cells. Am J Resp Cell Molec Bio. 2005;32:395–403. doi: 10.1165/rcmb.2004-0314OC. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Hirota K, Nishi K, et al. Activation of hypoxia-inducible factor 1 during macrophage differentiation. Physiol Cell Physiol. 2006;291:C104–C113. doi: 10.1152/ajpcell.00614.2005. [DOI] [PubMed] [Google Scholar]
- Ho TK, Rajkumar V, Ponticos M, et al. Increased endogenous angiogenic response and hypoxia-inducible factor-1α in human critical limb ischemia. J Vasc Surg. 2006;43:125–133. doi: 10.1016/j.jvs.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Rebar EJ. Development of pro-angiogenic engineered transcription factors for the treatment of cardiovascular disease. Expert Opin Investig Drugs. 2004;13:829–839. doi: 10.1517/13543784.13.7.829. [DOI] [PubMed] [Google Scholar]
- Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing. IUBMB Life. 2001;52:43–47. doi: 10.1080/15216540252774757. [DOI] [PubMed] [Google Scholar]
- Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising cancer reagent. Pharmacotherapy. 2003;23:165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Shyu KG, Luo Y, et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 Hybrid Transcription Factor. Circulation. 2000;102:2255–2261. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- Trentin D, Hall H, Wechsler S, Hubbell JA. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1 alpha variant for local induction of angiogenesis. Proc Natl Acad Sci USA. 2006;103:2506–2511. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T, Mochizuki S, Belanger AJ, et al. Expression of constitutively stable hybrid hypoxia-inducible factor-1α protects cultured rat cardiomyocytes against simulated ischemia-reperfusion injury. Am J Physiol. 2005;288:C314–C320. doi: 10.1152/ajpcell.00374.2004. [DOI] [PubMed] [Google Scholar]
- Kido M, Du L, Sullivan CC, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2006;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Fox SB, Braganca J, Turley H, et al. CITED4 inhibits hypoxia-activated transcription in Cancer Cells, and its cytoplasmic location in breast cancer is associated with elevated expression of tumor cell hypoxia-inducible factor 1α. Cancer Res. 2004;64:6075–6081. doi: 10.1158/0008-5472.CAN-04-0708. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA. Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Ivan M, Haberberger T, Gervasi DC, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronzo G, Russo I, Mattiello L, Rignati C, Anfossi G, Trovati M. Insulin activates hypoxia-inducible factor-1α in human and rat vascular smooth muscle cells via phosphatidylinositol-3 kinase and mitogen-activated protein kinase pathways: impairment in insulin resistance owing to defects in insulin signaling. Diabetologia. 2006;9:1049–1063. doi: 10.1007/s00125-006-0156-0. [DOI] [PubMed] [Google Scholar]
- Pajusola K, Kunnapuu J, Vuorikoski S, et al. Stabilized HIF-1α is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. FASEB J. 2005;19:1365–1367. doi: 10.1096/fj.05-3720fje. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Takeshita S, Chen D, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- Tan C, Noronha RG, Roecker AJ, et al. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005;65:605–612. [PubMed] [Google Scholar]
- Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. Embo J. 2005;24:3846–3858. doi: 10.1038/sj.emboj.7600846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belozerov VE, Van Meir EG. Hypoxia inducible factor-1: a novel target for cancer therapy. Anti-Cancer Drugs. 2005;16:901–909. doi: 10.1097/01.cad.0000180116.85912.69. [DOI] [PubMed] [Google Scholar]
- Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–1482. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78:233–247. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 2001;8:638–645. doi: 10.1038/sj.gt.3301388. [DOI] [PubMed] [Google Scholar]
- Chang Q, Qin JR, Huang T, Gao J, Feng Y. Effect of antisense hypoxia-inducible factor 1alpha on progression, metastasis, and chemosensitivity of pancreatic cancer. Pancreas. 2006;32:297–305. doi: 10.1097/00006676-200604000-00010. [DOI] [PubMed] [Google Scholar]
- Zatyka M, Morrissey C, Kuzmin I, Lerman MI, Latif F, Richards FM, Maher ER. Genetic and functional analysis of the von Hippel-Lindau (VHL) tumour suppressor gene promoter. J Med Genet. 2002;39:463–472. doi: 10.1136/jmg.39.7.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan R, McLeod H, Mantravadi P, et al. Cisplatin, fluorouracil, celecoxib, and RT in resectable esophageal cancer: preliminary results. Oncology. 2004;18:18–21. [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Klei LR, Barchowsky A. Nickel requires hypoxia-inducible factor-1α, not redox signaling, to induce plasminogen activator inhibitor-1. Am J Physiol Lung Cell Mol Physiol. 2001;281:L607–L615. doi: 10.1152/ajplung.2001.281.3.L607. [DOI] [PubMed] [Google Scholar]