Abstract
Procathepsin D (pCD), the precursor form of lysosomal aspartic protease, is overexpressed and secreted by various carcinomas. The fact that secreted pCD plays an essential role in progression of cancer has been established. In this study, we describe substantial secretion of pCD by the human keratinocyte cell line HaCaT, under serum-free conditions. Moreover, exogenous addition of purified pCD enhanced the proliferation of HaCaT cells. The proliferative effect of pCD was inhibited by a monoclonal antibody against the activation peptide (AP) of pCD. Treatment of HaCaT cells with pCD or AP led to the secretion of a set of cytokines that might promote the growth of cells in a paracrine manner. The role of secreted pCD and its mechanism of action were studied in a scratch wound model and the presence of pCD and AP enhanced regeneration, while this effect was reversed by the addition of anti-AP antibody. Expression and secretion of pCD was upregulated in HaCaT cells exposed to various stress conditions. Taken together, our results strongly suggest that the secretion of pCD is not only linked to cancer cells but also plays a role in normal physiological conditions like wound healing and tissue remodeling.
Keywords: Keratinocytes, Procathepsin D, Stress, Wound healing
Introduction
The epidermis, the barrier between the body and the external environment, is constantly exposed to various environmental and physical stresses. Keratinocytes are elemental cells forming the epidermis and are crucial for normal regeneration and healing of the epidermis. Skin healing is dependent upon several processes that comprise inflammation, protein synthesis, matrix deposition, migration and subsequent proliferation of keratinocytes (Kanzler et al., 1986; Clark, 1993). Keratinocytes are known to secrete numerous proteins that include proteolytic enzymes such as matrix metalloproteinases (Stamenkovic, 2003), interstitial collagenase (Saarialho-Kere et al., 1995) and cathepsin B (Buth et al., 2004). It is presumed that during the wound healing process, these proteolytic enzymes play a role in motility of keratinocytes by remodeling the extracellular matrix for migration of keratinocytes to peripheral layers of the epidermis.
Katz and Taichmann (1999) have described approximately 20 proteins secreted by cultured human epidermal keratinocytes that include cathepsin D (CD). CD is an aspartic protease, ubiquitously expressed in mammalian cells. The major function of CD involves the digestion of proteins and peptides within the acidic compartment of lysosomes (Dean, 1975). In addition, it participates in the processing of antigens (Mohamadzadeh et al., 2004), hormones and neuropeptides (Orlowski, 1983). The role of CD in other physiological functions such as tissue remodeling (Safting et al., 1995) and programmed cell death – apoptosis (Deiss et al., 1996; Bidere et al., 2003) has been suggested. CD is synthesized and translocated into the endoplasmic reticulum as an inactive proenzyme (52 kDa), which is then processed into an enzymatically active, intermediate form (48 kDa) and finally gets converted into the mature form of 33 kDa and 14 kDa two-chain protein in lysosomes (Fujita et al., 1991).
Increased levels of procathepsin D (pCD), proform of CD, and/or CD are correlated with tumor cell invasion and metastasis in breast carcinoma (Rochefort et al., 2000). It is now well established that the secreted pCD promotes growth of cancer cells (Stewart et al., 1994; Vetvicka et al., 1994, 1997). Our earlier studies established that pCD exerts mitogenic activity through interaction of its activation peptide (AP, propeptide) with an unknown receptor on cancer cells (Fusek and Vetvicka, 1994). Recently, we have shown that the interaction of pCD with cancer cell lines led to secretion of a defined set of cytokines that promotes the growth of these cells. A similar effect was also observed in fibroblasts (Fusek et al., 2007).
In skin, increased levels of the mature form of CD have been shown in squamous cell carcinoma (Maurizi et al., 1996; Kawada et al., 1997) and also in basal keratinocytes during hyperproliferative skin disorders such as psoriasis (Chen et al., 2000). The involvement of different isoforms of CD in epidermal cell differentiation was also suggested. The presence of the 52-kDa form was shown in the spinous layer and activation occurred in the granular layer (48 kDa and 33 kDa form). These two active forms were present in the stratum corneum, where they played a role in epidermal desquamation (Horikoshi et al., 1998; Igarashi et al., 2004). Although, the role of CD in epidermal differentiation has been defined, the presence of pCD at different stages of differentiation is still unclear. Moreover, most of these studies were performed using cell lysates where all the isoforms are present and so the possible role of individual isoforms remains ambiguous.
In the current study, we have found substantial secretion of pCD from HaCaT cells which indicates that this secretion is not only linked to cancer tissues but may be a normal physiological feature in skin. We have also studied the mechanism of the interaction of secreted pCD with HaCaT cells and studied the influence of pCD on secretion of cytokines. Further, the influence of secreted pCD was examined in these cells under mechanical scratch-wound and stress conditions.
Materials and methods
Cell culture
The human immortalized non-tumorigenic keratinocyte cell line HaCaT was obtained from Dr. Ulrichova (Institute of Medical Chemistry, Faculty of Medicine, Olomouc) and maintained in RPMI-1640 (Sigma Chemical Co., St. Louis, MO) containing HEPES (Sigma) buffer supplemented with 10% heat-inactivated fetal calf serum (FCS) (Hyclone Lab., Logan, UT), 100 U/ml penicillin (Sigma) and 100 μg/ml streptomycin (Sigma), in plastic disposable tissue culture flasks at 37° C in a 5% CO2/95% air incubator. Breast cancer cell line ZR-75-1 was obtained from Dr. R. Ceriani (John Muir Cancer and Aging Research Institute, Walnut Creek, CA) and maintained in the above mentioned medium. Cell viability was routinely assayed by standard trypan blue staining.
Determination of protein content, SDS-PAGE and immunoblotting
To check for the level of pCD secretion, HaCaT and ZR-75-1 cells were seeded in normal growth medium and after 24 h the medium was replaced with RPMI without serum and the cells were allowed to grow for an additional 48 h. Conditioned medium was collected, centrifuged to remove dead cells and concentrated (10×) (Centricon; Pall Life Sciences, Ann Arbor, MI). The cell pellet was dissolved in cold lysis buffer (10 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl, 1% Triton-X 100, pH 7.4 containing protease inhibitor cocktail; Sigma). Lysis was performed on ice for 30 min.
Protein lysates were then cleared by centrifugation at 14,000 rpm for 20 min. Protein contents of conditioned media and lysates were determined using a BCA protein assay kit (Pierce, Rockford, IL). Lysates were normalized to equal amounts of protein and boiled in sample buffer consisting of 10 mM Tris-HCl (pH 7.6), 0.5% (w/v) SDS, 2% (v/v) β-merceptoethanol, 10% (w/v) glycerol and 25 μg/ml bromophenol blue. As molecular mass markers, prestained protein standards were used (Bio-Rad Laboratories Inc., Hercules, CA). Proteins were separated by SDS-PAGE (Laemmli, 1970), blotted onto nitrocellulose and blocked with 3% bovine serum albumin (BSA) in Tris-buffered saline (TBS, 10 mM Tris-HCl, 100 mM NaCl, pH 7.5) plus 0.1% (v/v) Tween-20. The membrane was then immunolabeled by incubating with anti-CD (Calbiochem, San Diego, CA; 1:750 dilution) or anti-AP antibodies (Vetvicka et al., 1999; 1:200 dilution) in TBS-0.1% Tween-20 for 1 h at room temperature. The membrane was then washed thrice for 5 min with TBS-0.1% Tween 20 followed by incubation for 1 h with anti-mouse or anti-rabbit IgG-alkaline phosphatase conjugate secondary antibody (Sigma; 1:10,000 in TBS-0.1% Tween 20). After washing the membrane with TBS-0.1% Tween 20 (2×) and TBS (2×), pCD-antibody complexes were detected using an NBT-BCIP alkaline phosphatase substrate kit (Biorad).
ELISA
Micro-ELISA plates (Immunlon, Dynatech, Alexandria, VA) were coated at 4 °C with anti-AP monoclonal antibody (Vetvicka et al., 1999), using 200 μl/well of antibody diluted to 5 μg/ml in 0.1 M sodium carbonate/bicarbonate buffer. After washing and blocking steps, the plates were incubated with 200 μl/well of tissue culture supernatant. The bound pCD was detected by sequential incubations, first for 2 h with 200 μl/well of rabbit anti-CD antibody (Calbiochem), next for 2 h with 200 μl/well of anti-rabbit Ig conjugated with alkaline phosphatase (diluted 1:5000 in phosphate-buffered saline (PBS)/1 % Tween 20 (Sigma, St. Louis), and then for 30 min with 200 μl/well of p-nitrophenylphosphate substrate diluted according to the manufacturer’s instructions (Sigma). Absorbance was measured using an SLT ELISA reader (Tecan, Research Triangle Park, NC) at 410 nm. The levels of pCD in culture supernatant were calculated from the calibration curve prepared with synthetic AP.
RNA isolation and reverse transcriptase-PCR
Total cellular RNA was extracted from control and treated HaCaT cells using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and extensively treated with RNase-free DNase. One microgram RNA was reverse transcribed in a volume of 20 μl using the Superscript™ first strand synthesis system (Invitrogen). Reverse transcription (RT) was performed using 50 ng random hexamer primer and 10 U Moloney murine leukemia virus reverse transcriptase. An aliquot of RT reactions was PCR-amplified using pCD primers 5′CCAGTACTACGGGGAGATTG3′ and 5′CCATAGTGTGGATGTCAAACGA3′ and the β-actin primers 5′TGCTATCCAGGCTGTGCTAT3′ and 5′TTCCAGTTTTTAAATCCTGAGTC3′ to check for pCD expression and equal amount of RNA, respectively. PCR was carried out in 50 μl with 1.5 U Taq DNA polymerase for 25 cycles at 94°C for 30 s, 62°C for 45 s for pCD (52°C for β-actin), 72°C for 90 s with final extension at 72°C for 7 min. The PCR products were resolved on 1.5% agarose gels and visualized after staining with ethidium bromide.
Real-time RT-PCR
Total RNA was isolated from control and treated HaCaT cells using TRIzol reagent (Invitrogen). The cDNA was synthesized using 250 ng total RNA and TaqMan reverse transcription teagents (Applied Biosystems, Foster City, CA) in a 25-μl RT reaction. Real-time PCR was then carried out in a total of 20 μl reaction mixture using 2 μl cDNA per reaction, 5 μM of each cat D primers (forward: 5′ggacatcgcttgctggat3′ and reverse: 5′cttggctgcgatgaaggt3′) or β-actin primers (forward: 5′cactggcatcgtgatgga3′ and reverse: 5′ ggccatctcttgctcgaa 3′) and 10 μl 2× SYBR Green PCR core reagents on an ABI Prism sequence cetection system 7300 (Applied Biosystems). The PCR program was initiated by 10 min at 95°C before 40 thermal cycles, each for 15 s at 95°C and 1 min at 60°C. At the end of the PCR cycle, a dissociation curve was generated to ensure the amplification of a single product and the threshold cycle time (Ct values) for each gene was determined. Relative mRNA levels were calculated based on the Ct values normalized to β-actin.
Isolation of pCD, synthesis of AP and preparation of anti-AP antibodies
Human pCD was isolated from the culture supernatants of human breast cancer cell line ZR-75-1 as described earlier (Koelsch et al., 1995). Briefly, a two-step procedure was used. First, the culture supernatant was subjected to immunoaffinity chromatography using anti-AP antibodies attached to protein A Sepharose followed by a second FPLC chromatography using a Mono-Q column.
The 44-amino-acid peptide corresponding to AP of pCD was synthesized in the Institute of Organic Chemistry and Biochemistry, Czech Republic. The purity of AP was checked using HPLC, amino-acid analysis and mass spectrometry.
A monoclonal antibody (IgG1) against AP was prepared in our laboratory as described earlier (Vetvicka et al., 1999) and isolated from ascites fluid by 50% ammonium precipitation followed by Mono-Q anion exchange chromatography.
In vitro cell proliferation assay
For growth experiments, cells were harvested by centrifugation and washed three times in Iscove’s modified Dulbecco’s medium (Sigma) with HEPES buffer supplemented with glutamine, antibiotics and 10 μg/ml human transferrin (Sigma). Cells were seeded in 96-well tissue culture plates at a density of 5 × 104 cells/ml (150 μl/well) in the absence or presence of HaCaT concentrated conditioned medium, and in a separate experiment in the presence of 1 μg/ml of pCD, synthetic peptide corresponding to AP, or anti-AP antibody in triplicate wells. After 5 days in culture, the proliferation was evaluated using the Biotrak cell proliferation ELISA system measuring incorporation of BrdU in actively dividing cells (Amersham Pharmacia Biotech, Piscataway, NJ). Cells were incubated in BrdU-containing medium at 37°C for 2 h, then fixed and incubated with blocking solution for 30 min. Peroxidase-labeled anti-BrdU was added and incubated for 90 min. Wells were washed three times and chromogenic peroxidase substrate was added to each well and incubated for 30 min. The optical density was measured at 450 nm using an SLT ELISA reader (Tecan, Research Triangle Park, NC).
Cytokine array
Human cytokines were measured in tissue culture supernatants by Allied Biotech Inc. (Ijamsville, MD). HaCaT cells were incubated with either pCD or AP in serum-free conditions. The supernatant was collected, filtered through a 0.22-μm filter and stored in a −80°C freezer. For the cytokine analysis, we used protein microarray services provided by Allied Biotech. In brief, the services used a sandwich antibody-based protein detection multiplex assay. A streptavidin-Cy5 conjugate was used for assay detection. The assay was done in quadruplicate with positive and negative controls spotted on each microarray. The assay detects the following cytokines: IL-2, IFN-γ, TNF-α, IL-8, IL-12 P70, IL-12 P40, IL-4, IL-6, IL-10, IL-5, IP-10, MIP-1β, IL-13 and IL-1β.
Mechanical wounding of confluent HaCaT cells: scratch-wound assay
HaCaT cells were grown to a confluent monolayer on a 5-cm Petri dish. The in vitro wound assay was performed after washing the cells with PBS. Three scratches per dish were performed in a rectangular pattern with the tip of a 5-ml pipette. Cells were then washed three times with PBS to remove cellular debris before incubation with serum-free medium for 24 h at 37°C (Buth et al., 2004). Purified pCD, synthetic AP, CD (Sigma), or anti-AP antibody were used at a concentration of 1 μg/ml in serum-free medium. As a control, non-scratched cells were treated identically as described for the scratched cells. Multiple photographs of the wound were obtained using a phase-contrast microscope (Nikon Inverted microscope eclipse TE300), and the percentage of the cellular recovery area was analyzed using image analysis software (MetaMorph 6.2r6, Universal Imaging, West Chester, PA).
Exposure to stresses
HaCaT cells (3 × 105) were seeded in 12-well plates and after 24 h the normal growth medium was replaced with serum-free medium for 6 h. Then, cells were exposed to heat shock (45°C, 1 h), oxidative stress (0.4 mM H2O2, 1 h), apoptotic inducer (0.4 mg/ml sodium butyrate (Sigma), 1 h) or scratch-wound (1 h) in serum-free medium. At the end of the treatment, supernatants of the control and treated cells were collected, centrifuged to remove dead cells and TCA precipitated, followed by Western blot analysis. The cell pellets were collected and processed for protein and RT-PCR analysis as described above.
Statistical analysis
Student’s unpaired t-test was used to calculate statistical differences between data sets. Differences were considered significant at p<0.05.
Results
HaCaT keratinocytes secrete pCD
HaCaT cells were grown in serum-free medium and secretion of pCD was checked in conditioned medium by immunoblotting. The blot revealed that HaCaT cells secreted large amounts of pCD and the level was comparable to pCD secreted by ZR-75-1 breast cancer cells (Fig. 1A). Further, we checked the intracellular levels of pCD and CD in cell lysates of both cell lines by immunoblotting and the levels were found to be similar (Fig. 1B). The concentration of pCD in the HaCaT-conditioned medium was also measured by ELISA and found to be 153 ± 25 ng/ml and 177 ± 12 ng/ml after 24 h and 48 h, respectively.
Fig. 1.
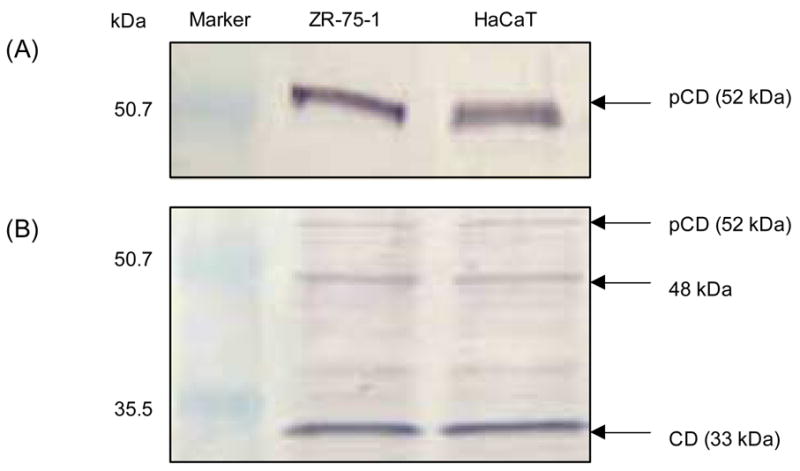
Analysis of pCD secretion by HaCaT and ZR-75-1 cells. HaCaT and ZR-75-1 cells were seeded in normal growth medium followed by replacing to serum-free media for additional 48 h. Conditioned media were collected and concentrated (10×). The cell pellets were lysed in lysis buffer. Protein contents of conditioned media and lysates were determined and equal amounts of total protein were analyzed by SDS-PAGE followed by immunoblotting. pCD protein in conditioned media was detected using anti-AP antibody (A); pCD and CD levels were detected in cell lysates using anti-CD antibody (B).
pCD acts as an autocrine mitogen on keratinocytes
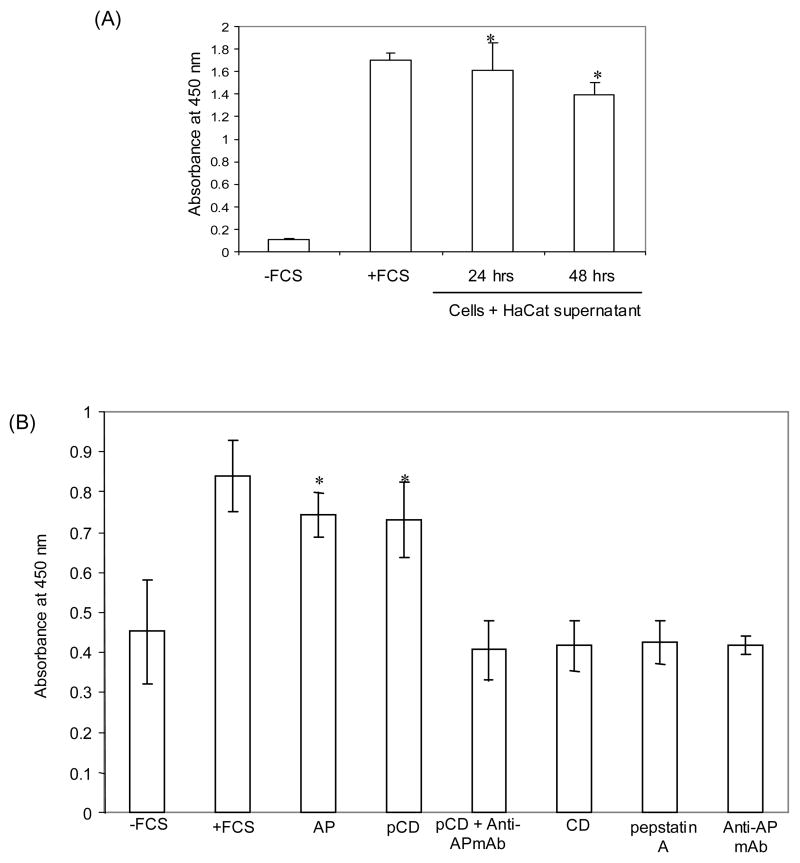
The proliferation of HaCaT cells was checked after stimulation with HaCaT-conditioned medium and the results showed a significant increase in proliferation as compared to the negative control (Fig. 2A). Further, we tested the effect of pCD and AP on proliferation of HaCaT keratinocytes in serum-free condition (Gamady et al., 2003; Grossman et al., 2004; Pozzi et al., 2004). The growth of cells in 10% FCS was taken as positive control and cell growth in medium without FCS served as a negative control. The effects of exogenously added pCD, AP and anti-AP antibody (1 μg/ml) were tested. The concentration of different proteins used was based on the dose-dependent proliferative activity assay performed with different human breast cancer cell lines (Vetvicka et al., 1999). The addition of pCD and AP showed significant increase in proliferation of HaCaT cells that was similar to the positive control (Fig. 2B). This proliferative effect was blocked by the addition of a monoclonal antibody against the AP region. The monoclonal antibody (anti-AP) alone had no effect on proliferation of HaCaT cells. Also, the addition of pepstatin A (inhibitor of CD activity; 1 μM) showed no effect on proliferation of cells. Moreover, no effect was observed with addition of pepstatin A to pCD- or AP-treated cells (data not shown). This suggested that the catalytic activity of mature CD was not involved.
Fig. 2.
Influence of exogenous addition of pCD and AP on the proliferation of HaCaT cells. Cells were seeded in 96-well tissue culture plates in the presence of either (A) concentrated HaCaT-conditioned medium, or (B) pCD, synthetic AP, anti-AP antibody, CD, or pepstatin A in serum-free medium. Cells were evaluated for proliferation at the fifth day using the Biotrak Cell proliferation ELISA system according to the manufacturer’s instructions. The growth of cells in FCS-containing medium was taken as positive control and in medium without FCS as negative control. The growth of cells is shown as absorbance at 450 nm and represents mean values ± SD of three independent experiments performed in triplicates. * p<0.05 versus −FCS, AP+Anti-APmAb and mAb (Student’s t-test).
Influence of pCD on the secretion of cytokines by keratinocytes
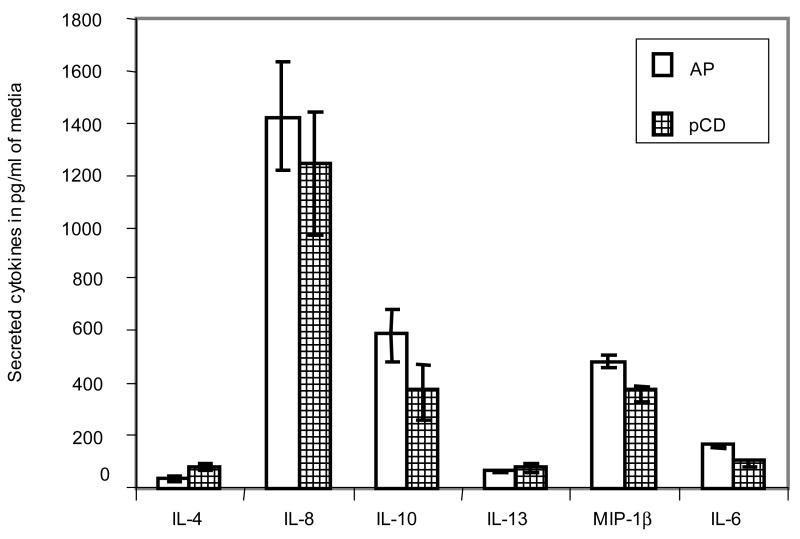
Recently, we have shown that the addition of pCD and AP to breast cancer and fibroblast cell lines led to secretion of a defined set of cytokines into the culture medium of these cells (Fusek et al., 2007). To investigate the influence of pCD and AP on the secretion of cytokines by cultured keratinocytes, the culture supernatant of pCD- and AP-treated HaCaT cells was analyzed by protein microarray services provided by Allied Biotech and the result revealed that from the panel of monitored cytokines (IL-2, IFN-γ, TNF-α, IL-8, IL-12 P70, IL-12 P40, IL-4, IL-6, IL-10, IL-5, IP-10, MIP-1β, IL-13, IL-1β), only IL-8, IL-10, MIP-1β, IL-6, IL-13, and IL-4 were detected. The concentration of these cytokines in our experiment was highest for IL-8 followed by IL-10, MIP-1β, IL-6, IL-13, and IL-4 (Fig. 3). Control cells grown in the presence and absence of FCS showed no secretion of cytokine (without FCS) or very low levels of IL-8 (52.8 ± 14.1) and IL-10 (31.5 ± 5.5) in the presence of FCS.
Fig. 3.
Effect of pCD and AP on secretion of cytokines by HaCaT cells. HaCaT cells were incubated with either pCD or AP in serum-free conditions and subjected to a cytokine array using the sandwich antibody-based protein detection multiplex assay provided by Allied Biotech. The assay was done in quadruplicate with positive and negative controls spotted on each microarray. Each bar represents respective cytokines in pg/ml medium (mean ± SD).
pCD enhances the regeneration from scratch wounding
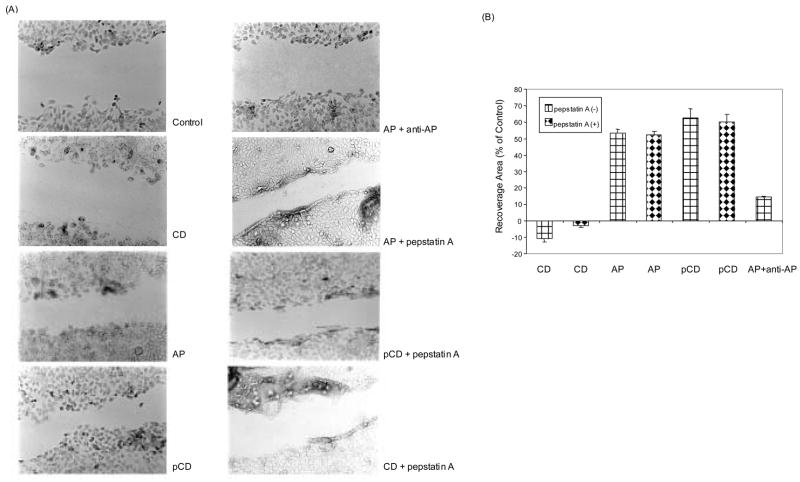
As the above results suggested that pCD influences the proliferative activity of HaCaT cells, we examined its impact on normal wound healing. The confluently grown HaCaT monolayers were scratch-wounded and further incubated in the presence of CD, AP, pCD, and anti-AP antibody (1 μg/ml) in serum-free medium to test the ability of these cells to regenerate a monolayer. Twenty-four hours post wounding, the treatment of HaCaT cells with pCD and AP increased the percentage of cellular recovery area compared to control, while the addition of CD had no effect on the cellular recovery (Fig. 4). The addition of AP plus anti-AP antibody decreased the recovery area compared to AP alone. The addition of monoclonal antibody to AP considerably slowed down the process of reconstitution of a monolayer while the process was enhanced in the presence of pCD and AP. In contrast, addition of pepstatin A was not able to reverse the effects of AP or pCD thus suggesting that the proteolytic activity was not essential for the reconstitution of the HaCat cell monolayer.
Fig. 4.
Effect of pCD on regeneration from scratch wounding of HaCaT cells. Confluent HaCaT cells were scratch-wounded, washed and allowed to regenerate in serum-free medium only (control) or in the presence of 1 μg/ml CD, AP, pCD, or pCD with anti-AP antibody with or without pepstatin A for 24 h and photographed (A). Multiple photographs of the wound were obtained and the percentage of cellular recovery areas was determined using image analysis software. The percentage of cellular recovery area compared to that of control was measured. The combined result of three independent experiments is shown (B).
Increased expression of pCD during stresses
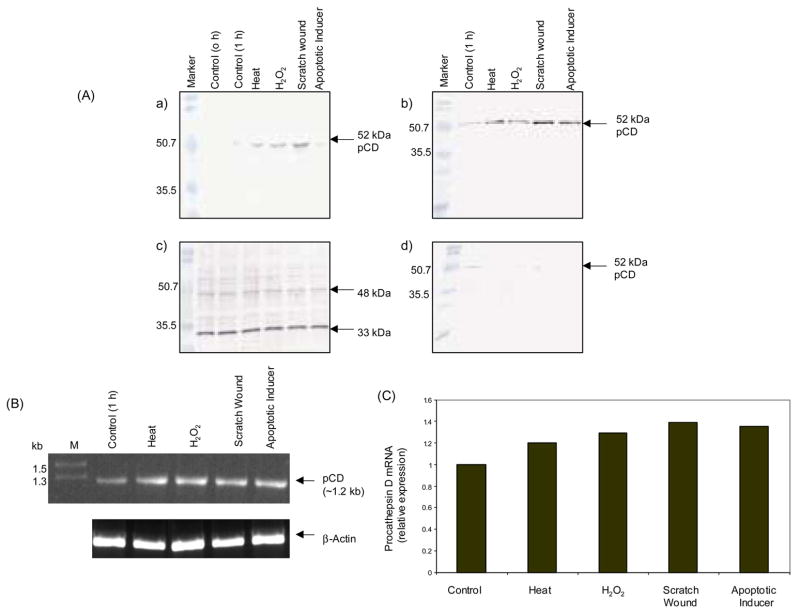
Confluently grown HaCaT cells were exposed to heat shock, oxidative stress, apoptotic inducer and scratch wounding in serum-free medium. Analysis of the culture supernatant by immunoblotting showed that the secretion of pCD increased in all conditions as compared to control [Fig. 5A (a and b)]. On the contrary, analysis of the cell lysates revealed that there was no difference in the intracellular CD levels [Fig. 5A (c)], however, there was decrease in intracellular pCD in cell lysates as compared to control [Fig. 5A (d)]. The analysis of pCD mRNA by RT-PCR and real-time PCR revealed a slight upregulation upon exposure to these stresses (Fig. 5B, C). The increased secretion of pCD might exert its protection by enhancing cellular pathways responsible for cell survival.
Fig. 5.
Expression of pCD in HaCaT cells during stress conditions. HaCaT cells were seeded in normal growth medium and after 24 h replaced to serum-free medium for 6 h before exposure to the following stresses: heat shock (45°C, 1 h), hydrogen peroxide (0.4 mM, 1 h), apoptotic inducer (0.4 mg/ml sodium butyrate, 1 h) or scratch wounding (1 h). Conditioned media of the control and treated cells was TCA precipitated and subjected to Western blot analysis using anti-CD antibody [A (a)] and anti-AP antibody [A (b)]. Protein contents of extracts from control and treated cells were determined and lysates were normalized to equal amounts of protein and immunoblotted using anti-CD antibody [A (c)] and anti-AP antibody [A (d)]. Amplification of pCD-specific sequence was performed using the reverse-transcribed mRNA from control and treated cells as indicated, and β-actin was used as an internal control (B). Expression of pCD mRNA was quantified by real-time PCR and the result is depicted as relative expression of pCD mRNA normalized to expression of β-actin (C). The data represent one of the three independent experiments.
Discussion
Regeneration of epidermis is a complex process that requires the proliferation of keratinocytes (Kanzler et al., 1986). The molecular mechanisms that regulate normal epidermal repair are not fully known, but protein-type growth factors – mitogens and cytokines – are central to the process of wound healing in the orchestration of tissue regeneration (McKay and Leigh, 1991; Kiritsy et al., 1993; Martin, 1997). Keratinocytes release a variety of cytokines, e.g. transforming growth factor-α (TGF-α), tumor necrosis factor-α (TNF-α), epidermal growth factor (EGF), fibroblast growth factors (FGFs), interleukin-1 α and β (IL-1 α and β), interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemoattractant protein (MCP-1), and macrophage inflammatory protein (MIP-1 α) expression increase during wound healing and thus play a role in localized as well as systemic inflammatory and immunologic effects (Ansel et al., 1990; Gharaee-Kermani and Phan, 2001). The presence of different CD isoforms has been reported at various stages of epidermal differentiation (Horikoshi et al., 1998). The importance of pCD or CD was established in CD-deficient mice that showed an impaired stratum corneum (Egberts et al., 2004). In this paper, we describe for the first time that keratinocytes also secrete pCD (inactive proform of CD) which influences secretion of cytokines and plays a role in the wound healing process.
Secretion of pCD has been studied mainly in cancer cell lines and is often considered a consequence of pathological condition. The determination of CD and pCD in tissue and serum as an independent cancer prognostic marker has been suggested (Tandon et al., 1990; Brouillet et al., 1997). In the current study, we have confirmed secretion of pCD by HaCaT cells, cultured in serum-free media. Secretion of pCD by human keratinocytes suggests that pCD is not only related to pathological conditions but may play a role in the normal physiological functions of the skin. The viability of cells during the experiment was always found to be more than 95% (tested by trypan blue assay), suggesting that the extracellular pCD resulted from active secretion.
The proliferative effect of purified pCD and synthetic peptide corresponding to AP has been established in cancer cell lines (Vetvicka et al., 1994). Here, we observed a significant increase in proliferation of keratinocytes by addition of exogenous pCD. A similar effect on proliferation was obtained in the presence of synthetic AP, whereas simultaneous addition of anti-AP antibody inhibited this effect. Thus, in HaCaT cells, pCD exerts its effect through its AP as seen earlier in case of cancer cells (Fusek and Vetvicka, 1994).
We have recently shown that exogenously added pCD influences the secretion of a defined group of cytokines (IL-4, IL-8, IL-10, IL-13 and MIP-1β) by various cancer cell lines. In our experiments we have shown that these cytokines have proliferative effects on parental and surrounding cells (Fusek et al., 2007). In the present paper we tested the secretion of cytokines by HaCaT cells after stimulation with pCD in serum-free medium. The group of cytokines secreted includes IL- 4, IL-6, IL-8, IL-10, IL-13, and MIP-1β. The expression of IL-4, 6, 8, 10, and 13 has been observed in keratinocytes under pathological conditions as an inflammatory response (Shiraki et al., 2006). The increased expression of IL-6 and IL-8 has been shown in a murine wound healing model (Bryan et al., 2005). Also, induction of IL-10 has been reported in human keratinocytes upon ultraviolet B irradiation (Enk et al., 1995) and in cutaneous wound healing as a regulator of inflammatory response (Sato et al., 1999). The rapid and strong expression of MIP-1 within the wound site in a mouse model suggested its role in the repair process (Jackman et al., 2000).
To allow investigation of the effect of secreted pCD on the wound healing process, a simple scratch-wound model was used. We have seen a remarkable influence of the presence of pCD and AP on monolayer regeneration while this effect was reversed by addition of a monoclonal antibody against AP. These data suggested that pCD enhances the regeneration process of wound healing both by its autocrine proliferative effect and by stimulation of the cytokines.
Epidermal keratinocytes are routinely exposed to various stresses that include dehydration, change in ambient temperature and inflammation. Keratinocytes respond to these stimuli by activation of different signaling pathways of programmed cell death and cellular survival, and the balance between the two decides the ultimate fate of the cell. In order to test the effect of various stress conditions on pCD expression and secretion, HaCaT keratinocytes were subjected to an array of stresses: heat shock, oxidative stress, apoptotic inducer, and scratch wound. To detect the cellular response, the conditions and duration used were mild enough to avoid killing of the cells. All the stresses slightly increased the expression of pCD at the mRNA level. However, the increased secretion of pCD could not be explained by higher expression levels alone. Increased secretion might be due to a change in protein sorting that helps the cell to overcome the stress. The increase in pCD secretion in oxidative stress is in accordance with an earlier report where high expression of pCD has been shown as an anoxic response protein in healing wounds (Anderson et al., 1995).
In conclusion, our results demonstrate that human keratinocytes secrete significant amount of pCD. The secreted pCD affects the proliferation of HaCaT cells and also induces secretion of cytokines that help in normal physiological functions such as overcoming stresses and enhancing the regeneration process of wound healing.
Acknowledgments
The authors are thankful to Prof. Norbert E. Fusenig (German Cancer Research Center, Heidelberg, Germany) for providing the HaCaT cell line to Dr. Uirichova. We also thank Dr. Venkatakrishna R. Jala (James Brown Center, University of Louisville, Louisville, KY) for help with the photographs and use of software for wound healing experiments at the real-time microscope facility (James Brown Center). This study was supported by a grant from National Institutes of Health NIH-RO1 CA82159-01A2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GR, Volpe CM, Russo CA, Stoler DL, Miloro SM. The anoxic fibroblast response is an early-stage wound healing program. J Surg Res. 1995;59:666–674. doi: 10.1006/jsre.1995.1221. [DOI] [PubMed] [Google Scholar]
- Ansel J, Perry P, Brown J, Damm D, Phan T, Hart C, Luger T, Hefeneider S. Cytokine modulation of keratinocyte cytokines. J Invest Dermatol. 1990;4:101S–107S. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- Bidere N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, Senik A. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–31411. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- Brouillet JP, Dufour F, Lemamy G, Garcia M, Schlup N, Grenier J, Mani JC, Rochefort H. Increased cathepsin D level in the serum of patients with metastatic breast carcinoma detected with a specific pro-cathepsin D immunoassay. Cancer. 1997;79:2132–2136. [PubMed] [Google Scholar]
- Bryan D, Walker KB, Ferguson M, Thorpe R. Cytokine gene expression in a murine wound healing model. Cytokine. 2005;31:429–438. doi: 10.1016/j.cyto.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Buth H, Wolters B, Hartwig B, Meier-Bornheim R, Veith H, Hansen M, Sommerhoff CP, Schaschke N, Machleidt W, Fusenig NE, Boukamp P, Brix K. HaCaT keratinocytes secrete lysosomal cysteine proteinases during migration. Eur J Cell Biol. 2004;83:781–795. doi: 10.1078/0171-9335-00428. [DOI] [PubMed] [Google Scholar]
- Chen SH, Arany I, Apisarnthanarax N, Rajaraman S, Tyring SK, Horikoshi T, Brysk H, Brysk MM. Response of keratinocytes from normal and psoriatic epidermis to interferon-gamma differs in the expression of zinc-alpha(2)-glycoprotein and cathepsin D. FASEB J. 2000;14:565–571. doi: 10.1096/fasebj.14.3.565. [DOI] [PubMed] [Google Scholar]
- Clark RA. Biology of dermal wound repair. Dermatol Clin. 1993;11:647–666. [PubMed] [Google Scholar]
- Dean RT. Lysosomal enzymes as agents of turnover of soluble cytoplasmic proteins. Eur J Biochem. 1975;58:9–14. doi: 10.1111/j.1432-1033.1975.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- Egberts F, Heinrich M, Jensen JM, Winoto-Morbach S, Pfeiffer S, Wickel M, Schunck M, Steude J, Saftig P, Proksch E, Schutze S. Cathepsin D is involved in the regulation of transglutaminase 1 and epidermal differentiation. J Cell Sci. 2004;117:2295–2307. doi: 10.1242/jcs.01075. [DOI] [PubMed] [Google Scholar]
- Enk CD, Sredni D, Blauvelt A, Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154:4851–4856. [PubMed] [Google Scholar]
- Fujita H, Tanaka Y, Noguchi Y, Kono A, Himeno M, Kato K. Isolation and sequencing of a cDNA clone encoding rat liver lysosomal cathepsin D and the structure of three forms of mature enzymes. Biochem Biophys Res Commun. 1991;179:190–196. doi: 10.1016/0006-291x(91)91353-e. [DOI] [PubMed] [Google Scholar]
- Fusek M, Vetvicka V. Mitogenic function of human procathepsin D – the role of the propeptide. Biochem J. 1994;303:775–780. doi: 10.1042/bj3030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusek M, Vetvickova J, Vetvicka V. Secretion of cytokines in breast cancer cells – the molecular mechanism of procathepsin D proliferative effects. J Interferon Cytokine Res. 2007;27:191–199. doi: 10.1089/jir.2006.0105. [DOI] [PubMed] [Google Scholar]
- Gamady A, Koren R, Ron D, Liberman UA, Ravid A. Vitamin D enhances mitogenesis mediated by keratinocyte growth factor receptor in keratinocytes. J Cell Biochem. 2003;89:440–449. doi: 10.1002/jcb.10508. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7:1083–1103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- Grossman N, Binyamin LA, Bodner L. Effect of rat salivary glands extracts on the proliferation of cultured skin cells – a wound healing model. Cell Tissue Bank. 2004;5:205–212. doi: 10.1007/s10561-005-4367-2. [DOI] [PubMed] [Google Scholar]
- Horikoshi T, Arany I, Rajaraman S, Chen SH, Brysk H, Lei G, Tyring SK, Brysk MM. Isoforms of cathepsin D and human epidermal differentiation. Biochimie. 1998;80:605–612. doi: 10.1016/s0300-9084(98)80013-8. [DOI] [PubMed] [Google Scholar]
- Igarashi S, Takizawa T, Takizawa T, Yasuda Y, Uchiwa H, Hayashi S, Brysk H, Robinson JM, Yamamoto K, Brysk MM, Horikoshi T. Cathepsin D, but not cathepsin E, degrades desmosomes during epidermal desquamation. Br J Dermatol. 2004;151:355–361. doi: 10.1111/j.1365-2133.2004.06061.x. [DOI] [PubMed] [Google Scholar]
- Jackman SH, Yoak MB, Keerthy S, Beaver BL. Differential expression of chemokines in a mouse model of wound healing. Ann Clin Lab Sci. 2000;30:201–207. [PubMed] [Google Scholar]
- Kanzler MH, Gorsulowsky DC, Swanson NA. Basic mechanisms in the healing cutaneous wound. J Dermatol Surg Oncol. 1986;12:1156–1164. doi: 10.1111/j.1524-4725.1986.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Katz AB, Taichman LB. A partial catalog of proteins secreted by epidermal keratinocytes in culture. J Invest Dermatol. 1999;112:818–821. doi: 10.1046/j.1523-1747.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Kawada A, Hara K, Kominami E, Hiruma M, Akiyama M, Ishibashi A, Abe H, Ichikawa E, Nakamura Y, Watanabe S, Yamamoto T, Umeda T, Nishioka K. Expression of cathepsin D and B in invasion and metastasis of squamous cell carcinoma. Br J Dermatol. 1997;137:361–366. [PubMed] [Google Scholar]
- Kiritsy CP, Lynch AB, Lynch SE. Role of growth factors in cutaneous wound healing: a review. Crit Rev Oral Biol Med. 1993;4:729–760. doi: 10.1177/10454411930040050401. [DOI] [PubMed] [Google Scholar]
- Koelsch G, Metcalf P, Vetvicka V, Fusek M. Human procathepsin D: three-dimensional model and isolation. Adv Exp Med Biol. 1995;362:273–278. doi: 10.1007/978-1-4615-1871-6_31. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing – Aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Maurizi M, Almadori G, Cadoni G, Scambia G, Ottaviani F, Ferrandina G, Paludetti G, D’Abramo G, Mancuso S. Cathepsin D concentration in primary laryngeal cancer: correlation with clinico-pathological parameters, EGFR status and prognosis. Int J Cancer. 1996;69:105–109. doi: 10.1002/(SICI)1097-0215(19960422)69:2<105::AID-IJC6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- McKay IA, Leigh IM. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991;124:513–518. doi: 10.1111/j.1365-2133.1991.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Mohamadzadeh H, Brammer M, Sestak K, Luftig RB. Identification of proteases employed by dendritic cells in the processing of protein purified derivative (PPD) J Immune Based Ther Vaccines. 2004;2:8. doi: 10.1186/1476-8518-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M. Pituitary endopeptidases. Mol Cell Biochem. 1983;52:49–74. doi: 10.1007/BF00230588. [DOI] [PubMed] [Google Scholar]
- Pozzi G, Guidi M, Laudicina F, Marazzi M, Falcone L, Betti R, Crosti C, Muller EE, DiMattia GE, Locatelli V, Torsello A. IGF-I stimulates proliferation of spontaneously immortalized human keratinocytes (HaCaT) by autocrine/paracrine mechanisms. J Endocrinol Invest. 2004;27:142–149. doi: 10.1007/BF03346259. [DOI] [PubMed] [Google Scholar]
- Rochefort H, Garcia M, Glondu M, Laurent V, Liaudet E, Rey J, Roger P. Cathepsin D in breast cancer: mechanisms and clinical applications: a 1999 overview. Clin Chim Acta. 2000;291:157–170. doi: 10.1016/s0009-8981(99)00226-0. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Vaalamo M, Airola K, Niemi KM, Oikarinen AI, Parks WC. Interstitial collagenase is expressed by keratinocytes that are actively involved in reepithelialization in blistering skin disease. J Invest Dermatol. 1995;104:982–988. doi: 10.1111/1523-1747.ep12606231. [DOI] [PubMed] [Google Scholar]
- Safting P, Hetman M, Schmahl W. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun. 1999;265:194–199. doi: 10.1006/bbrc.1999.1455. [DOI] [PubMed] [Google Scholar]
- Shiraki Y, Ishibashi Y, Hiruma M, Nishikawa A, Ikeda S. Cytokine secretion profiles of human keratinocytes during Trichophyton tonsurans and Arthroderma benhamiae infections. J Med Microbiol. 2006;55:1175–1185. doi: 10.1099/jmm.0.46632-0. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Piggott NH, May FEB, Westley BR. Mitogenic activity of procathepsin D purified from conditioned medium of breast-cancer cells by affinity chromatography on pepstatinyl agarose. Int J Cancer. 1994;57:715–718. doi: 10.1002/ijc.2910570518. [DOI] [PubMed] [Google Scholar]
- Tandon AK, Clark GM, Chamness GC, Chirgwin JM, McGuirre WL. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990;322:297–302. doi: 10.1056/NEJM199002013220504. [DOI] [PubMed] [Google Scholar]
- Vetvicka V, Vetvickova J, Fusek M. Effect of human procathepsin D on proliferation of human cell lines. Cancer Lett. 1994;79:131–135. doi: 10.1016/0304-3835(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Vetvicka V, Vetvickova J, Hilgert I, Voburka Z, Fusek M. Analysis of the interaction of procathepsin D activation peptide with breast cancer cells. Int J Cancer. 1997;73:403–409. doi: 10.1002/(sici)1097-0215(19971104)73:3<403::aid-ijc15>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Vetvicka V, Vetvickova J, Fusek M. Anti-human procathepsin D activation peptide antibodies inhibit breast cancer development. Breast Cancer Res Treat. 1999;57:261–269. doi: 10.1023/a:1006238003772. [DOI] [PubMed] [Google Scholar]