Abstract
DnaJ homologues function in cooperation with hsp70 family members in various cellular processes including intracellular protein trafficking and folding. Three human DnaJ homologues present in the cytosol have been identified: dj1 (hsp40/hdj-1), dj2 (HSDJ/hdj-2), and neuronal tissue-specific hsj1. dj1 is thought to be engaged in folding of nascent polypeptides, whereas functions of the other DnaJ homologues remain to be elucidated. To investigate roles of dj2 and dj1, we developed a system of chaperone depletion from and readdition to rabbit reticulocyte lysates. Using this system, we found that heat shock cognate 70 protein (hsc70) and dj2, but not dj1, are involved in mitochondrial import of preornithine transcarbamylase. Bacterial DnaJ could replace mammalian dj2 in mitochondrial protein import. We also tested the effects of these DnaJ homologues on folding of guanidine-denatured firefly luciferase. Unexpectedly, dj2, but not dj1, together with hsc70 refolded the protein efficiently. We propose that dj2 is the functional partner DnaJ homologue of hsc70 in the mammalian cytosol. Bacterial DnaJ protein could replace mammalian dj2 in the refolding of luciferase. Thus, the cytosolic chaperone system for mitochondrial protein import and for protein folding is highly conserved, involving DnaK and DnaJ in bacteria, Ssa1–4p and Ydj1p in yeast, and hsc70 and dj2 in mammals.
The 70K heat shock protein (hsp70)1 family is a group of molecular chaperones which mediates protein folding and targeting (reviewed in Bukau et al., 1996; Hartl, 1996; Rassow et al., 1997). Members of the hsp70 family usually require partner proteins for specifying their functions in distinct cellular compartments of eukaryotic cells (Rassow et al., 1995). An essential and ubiquitous group of these partner proteins for hsp70 family is the DnaJ family (Cyr et al., 1994).
Three DnaJ homologues have been identified in human cytosol: dj1 (hsp40/hdj-1) (Ohtsuka, 1993; Raabe and Manley, 1991), dj2 (HSDJ/hdj-2) (Chellaiah et al., 1993; Oh et al., 1993), and hsj1 (Cheetham et al., 1992). The structure of dj2 has the closest similarity to those of bacterial DnaJ and yeast Ydj1p, Mdj1p, and Scj1p (Cyr et al., 1994). These members of the DnaJ subfamily have the J-domain, G/F-domain, and the cysteine-rich region. The cysteine-rich region of DnaJ coordinates two zinc atoms and is important for binding to chemically denatured luciferase (Szabo et al., 1996). Ydj1p and dj2 also have the CaaX prenylation motif at their COOH termini and undergo farnesyl modification, posttranslationally (Caplan et al., 1992a ; Kanazawa et al., 1997). Although the functional significance of this modification of dj2 is unknown, studies on Ydj1p revealed temperature-sensitive defects in protein import into mitochondria and endoplasmic reticulum with the lack of farnesylation (Caplan et al., 1992b ).
Both dj1 and hsj1 contain a J-domain and G/F-domain, but lack a cysteine-rich region and a farnesylation motif. These structural features are similar to those of yeast Sis1p, another member of the DnaJ family (Caplan and Douglas, 1991; Luke et al., 1991). Sis1p is localized mainly in the cytosol and partially in the nucleus and is essential for viability (Luke et al., 1991). It also associates with ribosomes and promotes the initiation of translation (Zhong and Arndt, 1993). The intracellular localization of human dj1 is similar to that of yeast Sis1p (Hattori et al., 1993). dj1 also associates with ribosomes and is engaged in folding of nascent polypeptides (Frydman et al., 1994). A protein refolding activity of dj1 was also reported (Freeman and Morimoto, 1996; Freeman et al., 1995). hsj1 is preferentially expressed in neuronal tissues (Cheetham et al., 1992), and inhibits heat shock cognate 70 protein (hsc70)-catalyzed clathrin uncoating (Cheetham et al., 1996).
To investigate the roles of hsc70 and the two cytosolic DnaJ homologues dj1 and dj2 of mammals, we developed systems of depletion–readdition of these chaperones. We found that both hsc70 and dj2 are required for efficient mitochondrial import of preornithine transcarbamylase (pOTC) during its synthesis. We also tested our depletion– readdition systems to study possible differential roles of these DnaJ homologues in folding of chemically denatured luciferase. Unexpectedly, dj2, but not dj1, facilitated productive folding in cooperation with hsc70. DnaJ could replace dj2 both in mitochondrial protein import and in protein folding. Thus, the cytosolic chaperone system is highly conserved: DnaK-DnaJ-GrpE in bacteria, Ssa1– 4p-Ydj1p in yeast, and hsc70-dj2 in mammals.
Materials and Methods
Materials
The nuclease-treated rabbit reticulocyte lysate and the luciferase assay system were purchased from Promega Corp. (Madison, WI). [35S]Pro-mix™ (>37 TBq/mmol [35S]methionine) was purchased from Amersham Corp. (Arlington Heights, IL). Firefly luciferase was purchased from Sigma Chemical Co. (St. Louis, MO).
Purification of Chaperones
Mouse hsc70 was purified from Ehrlich ascites fluid by ATP-agarose column chromatography and Superdex gel filtration column chromatography. Human dj2 was expressed using the insect/baculovirus system (BaculoGold Transfection kit; PharMingen, San Diego, CA). The DNA fragment of histidine-tagged human dj2 was excised from a plasmid encoding the MBP-His-dj2 fusion protein (Kanazawa et al., 1997), inserted into a pVL1392 transfer vector, as specified by the manufacturer. Sf9 cells in suspension were infected with the recombinant virus and cultured for 72 h. Farnesylation of His-dj2 was enhanced by exogenously adding a mevalonate precursor, mevalonolactone (Sigma), to the medium, as described for the expression of small G proteins (Takai et al., 1995). His-dj2–expressing Sf9 cells were lysed and His-dj2 was purified with a nickel chelate affinity column. Details of the expression and purification will be described elsewhere. Histidine-tagged human dj1 was expressed in Escherichia coli and purified as described (Minami et al., 1996). In brief, pQE-9/Hsp40 plasmid was transformed into M15[pREP4] cells and grown at 30°C. After 4-h induction with 0.1 mM IPTG, cells were lysed and loaded onto Ni2+-NTA Sepharose (Pharmacia LKB Biotechnology, Piscataway, NJ) column. The column was washed with 60 mM imidazole and then proteins were eluted with 1 M imidazole. Peak fractions of His-dj1 was dialyzed against 100 mM potassium phosphate buffer (pH 7.6) and loaded onto hydroxyapatite HTP column (Bio-Rad Labs., Richmond, CA). His-dj1 was eluted with a linear gradient of 100–500 mM potassium phosphate buffer (pH 7.6). The purified His-dj1 was concentrated by ultrafiltration.
Antibodies
Rat 1B5 mAb was purified from rat ascites fluid as described in Terada et al. (1995). Anti-human dj2 antibody was that described previously (Kanazawa et al., 1997). Anti-dj1 antibody was raised in a rabbit by injecting purified histidine-tagged human dj1.
Immunoblot Analysis
Proteins were separated by 8% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Clear blot-P; Atto Co., Tokyo, Japan). The membranes were probed with 1B5 mAb (2 μg IgG/ml) for detection of hsc70, anti-human dj2 antiserum (1/2,500 dilution) for detection of dj2, and anti-human dj1 antiserum (1/2,500 dilution) for detection of dj1 and the chaperone polypeptides were identified using the biotin/avidin system (ABC kit; Vector Laboratories, Burlingame, CA) and a chemiluminescence (ECL; Amersham).
Depletion of Chaperones from Rabbit Reticulocyte Lysate
Depletion of hsc70 from rabbit reticulocyte lysates was done by treatment with 1B5 antibody-resin (Terada et al., 1995, 1996). Depletion of other chaperones was done with protein A–Sepharose 4FF beads (Pharmacia) cross-linked to IgG from either nonimmune serum (mock-depleted), anti-dj2 serum (dj2-depleted) or anti-dj1 serum (dj1-depleted) by dimethylpimelimidate (Pierce Chemical Co., Rockford, IL) as described (Harlow and Lane, 1988). The antibody-resins were equilibrated with 20 mM Hepes-KOH and 120 mM potassium acetate (pH 7.6) and an equal volume of reticulocyte lysate was added to the pelleted resins. The mixtures were incubated at 4°C for 60 min with occasional agitation, and the resins were removed by centrifugation.
Import of In Vitro-synthesized pOTC into Isolated Mitochondria
Translation of rat pOTC mRNA was performed in vitro in a rabbit reticulocyte lysate depleted for each chaperone. Where indicated, purified chaperones were added to the depleted lysates before translation. The import mixture (50 μl) containing 5.0 μl of the lysate and 35S-labeled pOTC (2–23 KBq) was incubated with isolated rat liver mitochondria (100 μg of protein) at 25°C, as described (Terada et al., 1996). The reaction was stopped by adding ice-cold mitochondria isolation buffer containing 0.1 mM dinitrophenol (Terada et al., 1995). The mitochondria were reisolated and subjected to SDS-PAGE. The radioactive polypeptides were visualized by fluorography and quantitated using an imaging plate analyzer (FUJIX BAS2000; Fuji Photo Film Co., Tokyo, Japan).
Refolding of Chemically Denatured Luciferase
Firefly luciferase (0.5 mg/ml, 8.1 μM) was denatured in buffer A (25 mM Hepes-KOH, pH 7.2, 50 mM potassium acetate, 5 mM dithiothreitol) containing 6 M guanidine hydrochloride. The solution was incubated at 25°C for 60 min. The denatured luciferase was placed on ice and diluted 1:40 in buffer A. Then, 2.0 μl of diluted luciferase was added to 48 μl of refolding buffer (28 mM Hepes-KOH, pH 7.6, 120 mM potassium acetate, 1.2 mM magnesium acetate, 2.2 mM dithiothreitol, 1 mM ATP, 8.8 mM creatine phosphate, 2 μg creatine kinase, 50 μM antipain, and 50 μM leupeptin) containing 15 μl of lysate and/or chaperones where indicated. Refolding was started by incubating at 25°C. At indicated times, 1.0 μl was withdrawn from the reaction and added to 50 μl of a luciferase assay solution (Promega), and light production was immediately monitored for 12 s in a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA). In all experiments, the enzyme activity was expressed as a percent of that of the native enzyme measured after dilution with buffer A containing 1 mg/ml bovine serum albumin.
Results
Intracellular Concentration of Cytosolic Chaperones
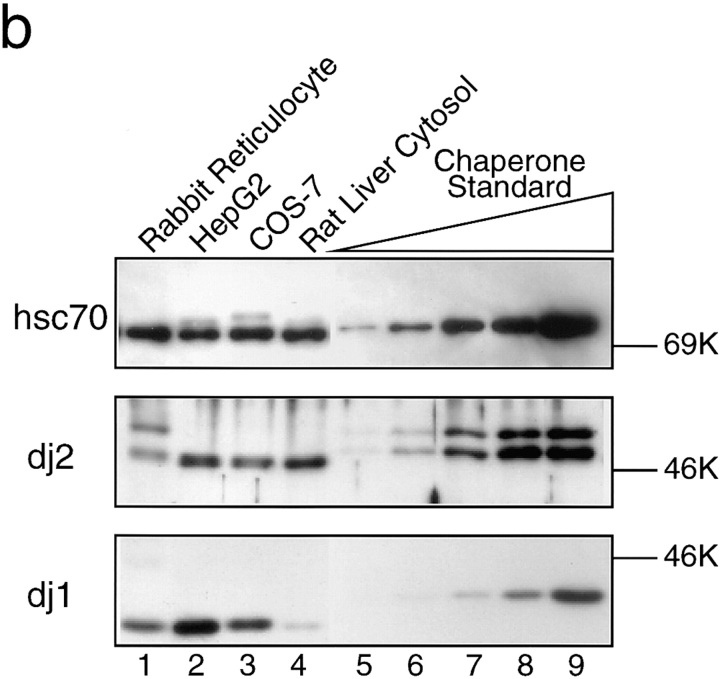
We purified chaperones including mouse hsc70, human recombinant histidine-tagged dj2 (His-dj2) and dj1 (His-dj1), and Escherichia coli DnaJ (Fig. 1 a). hsc70 migrated as a protein of 73K in SDS–PAGE (lane 1). His-dj2 expressed in Sf9 insect cells gave 49K and 47K polypeptides (lane 2). We have shown that human dj2 is subject to farnesylation at Cys-394 and that the farnesylated form migrates faster than the unfarnesylated form on SDS–polyacrylamide gel (Kanazawa et al., 1997). These two polypeptides also proved to be dj2 by immunoblot analysis. The ratio of unfarnesylated form to farnesylated one was ∼1:2 (Fig. 1 b, middle, lanes 5–9), but this ratio varied from one preparation to another. When His-dj2 expressing insect cells were cultured in the absence of a mevalonate precursor, farnesylation was much decreased (data not shown). On the other hand, human dj1 contains no prenylation motif, and a single 42K polypeptide was observed (Fig. 1 a, lane 3). E. coli DnaJ migrated as a 41K polypeptide (lane 4).
Figure 1.
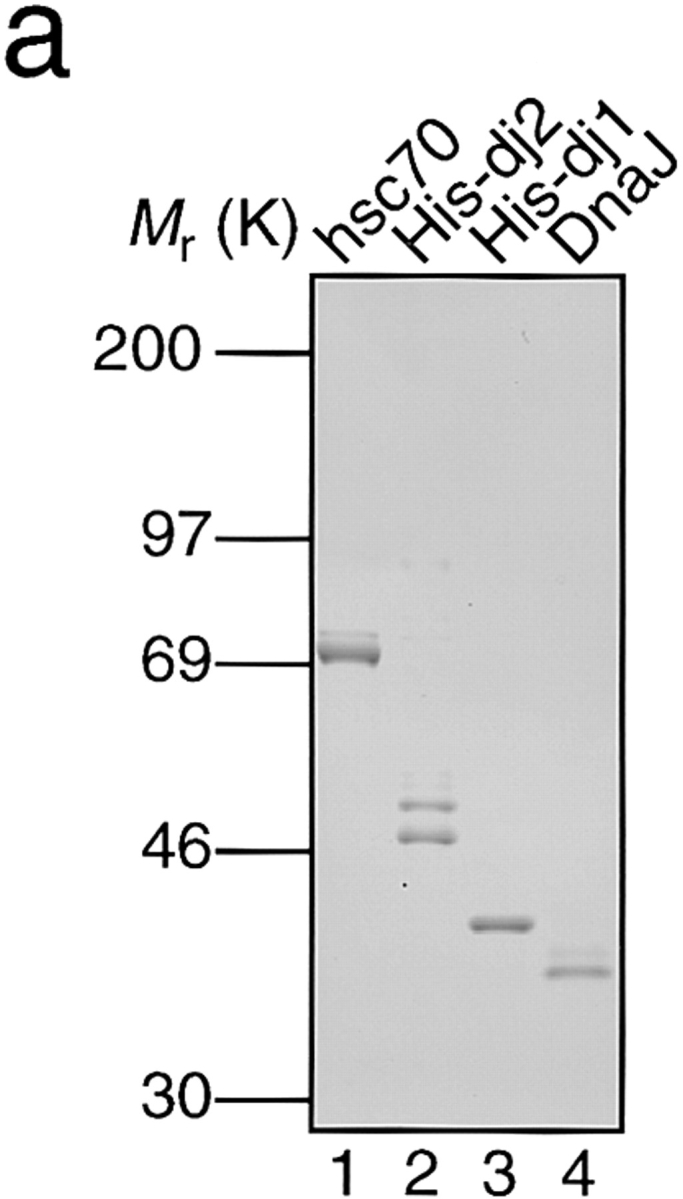
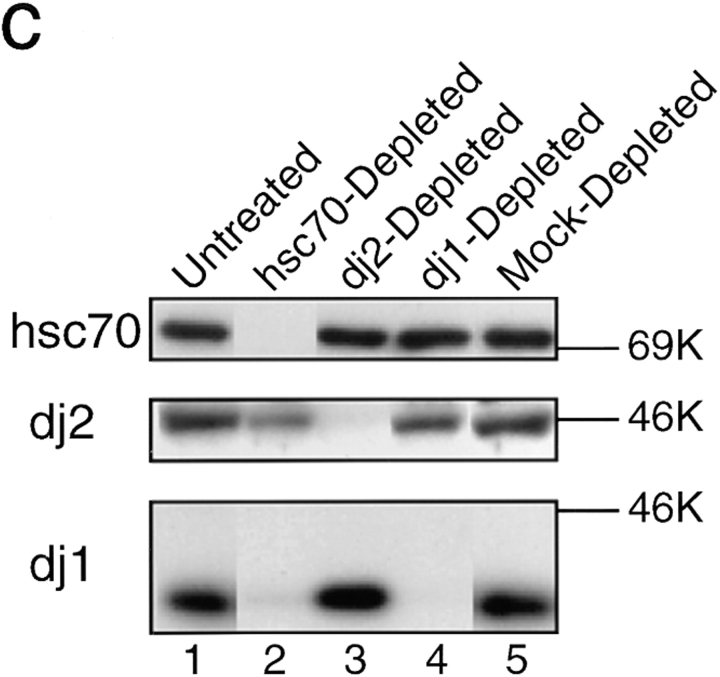
Intracellular concentrations of chaperones and depletion for chaperones of rabbit reticulocyte lysate. (a) Purified chaperones (0.5 μg each) were analyzed by SDS-PAGE. Lane 1, hsc70; lane 2, His-dj2; lane 3, His-dj1; lane 4, DnaJ. (b) Immunoblot analysis of hsc70 (top), dj2 (middle), and dj1 (bottom) in rabbit reticulocyte lysate (lane 1, 20 μg protein), HepG2 cell extract (lane 2, 2 μg protein), COS-7 cell extract (lane 3, 2 μg protein), and rat liver cytosol (lane 4, 2 μg protein). Purified chaperones were used as standards (lanes 5–9): top, mouse hsc70 (8, 16, 31, 63 and 130 ng); middle, baculovirus- expressed human His-dj2 (0.63, 1.3, 2.5, 5.0, and 10 ng); bottom, E. coli-expressed human His-dj1 (0.060, 0.13, 0.25, 0.50, and 1.0 ng). Note that standard dj2 and dj1 had histidine tags and migrated more slowly than the endogenous chaperones in 8% SDS-PAGE. The reason for the presence of immunoreactive 49K band in rabbit reticulocyte lysate is unknown (middle, lane 1). Human recombinant hsc70 (a gift from N. Imamoto and Y. Yoneda) and mouse hsc70 gave signals of similar intensities. (c) Immunodepletion was performed with antibody-coupled Sepharose resins as described in Materials and Methods. Extent of the depletion for the endogenous chaperones was assessed by immunoblot analysis of the reticulocyte lysates (0.5 μl each, ∼50 μg protein). Protein molecular mass markers (rainbow-colored markers; Amersham) are myosin (200K), phosphorylase b (97K), serum albumin (69K), ovalbumin (46K), and carbonic anhydrase (30K).
We then examined intracellular concentrations of the three cytosolic chaperones in rabbit reticulocytes, human hepatoma HepG2 cells, monkey kidney COS-7 cells, and rat liver (Fig. 1 b). Assuming that the cytosolic protein concentration is 200 mg/ml, hsc70 concentration in HepG2 cells was estimated to be about 2.0 mg/ml of cytosol (30 μM, 1.0% of total cytosolic protein). Assuming that this antibody against Chinese hamster hsc70 reacted equally with the human, monkey, mouse, rat, and rabbit hsc70s, the concentrations in COS-7 cells and rat liver were similar to that in HepG2 cells, whereas that in rabbit reticulocytes was much lower (0.31 mg/ml, 4.2 μM, 0.15% of total protein). These events may reflect the fact that reticulocytes are highly specialized cells containing high contents of hemoglobin, that is ∼90% of total protein in reticulocyte lysates. When an anti-mouse hsp70 antiserum that is highly cross-reactive with hsp70s of other mammals was used for immunodetection, extracts of HepG2 and COS-7 cells gave weak signals of 72K, whereas rabbit reticulocyte lysate and rat liver cytosol gave no signal (data not shown).
The intracellular concentrations of dj2s in HepG2 cells, COS-7 cells and rat liver were estimated to be 0.20–0.25 mg/ml (4.3–5.4 μM, 0.08–0.10% of total protein) and that in the reticulocyte lysate was estimated to be 10 μg/ml (0.22 μM, 0.05% of total protein). In addition to the dj2-immunoreactive polypeptide of 46K, another immunoreactive polypeptide of 49K was observed in the reticulocyte lysate, the nature of which is unknown.
Endogenous dj1s of the tested tissue and cells were detected as 40K proteins. The concentration was highest in HepG2 cells (0.20 mg/ml, 5.0 μM, 0.10% of total protein), that in the COS-7 cells was 80 μg/ml (2.0 μM, 0.04% of total protein), that in rat liver cytosol was 20 μg/ml (0.50 μM, 0.01% of total protein), and that in rabbit reticulocyte lysate was 6.0 μg/ml (0.15 μM, 0.03% of total protein). These values agree well with documented data (Frydman et al., 1994). The molar ratio of hsc70/dj2/dj1 in HepG2 human cell line is 30:4:5 μM.
Development of Chaperone Depletion–Readdition Systems
To investigate the effects of these chaperones on the import of pOTC into mammalian mitochondria, we developed a system of chaperone depletion from the reticulocyte lysate. We also tested this system to examine the roles of chaperone(s) in the folding of a model protein luciferase. Antibody-coupled resins were prepared for each of the chaperones. Mock-treated lysate prepared with nonimmune rabbit IgG-coupled resin or untreated lysate (two times diluted) was used as controls for all experiments. Depletion of the chaperones was monitored by immunodetection (Fig. 1 c).
Depletion of hsc70 from the lysate was efficient and always exceeded 90% (Fig. 1 c, lane 2). The procedure of hsc70 depletion led to a partial reduction of dj2 (by ∼50%), whereas it caused a marked reduction of dj1 (by ∼85%). These results indicate that most dj1, but not dj2, is tightly associated with hsc70 in rabbit reticulocyte lysate. These findings agree with those of Yamane et al. (1995) that anti-hsp40 (dj1) antibody coprecipitated hsc70. When the lysate was treated with the anti-dj2–coupled resin, more than 85% of dj2 was removed (lane 3). Similarly, when the lysate was treated with the anti-dj1–coupled resin, more than 85% of dj1 was removed (lane 4). However, little decrease of hsc70 was observed in the dj1- depleted lysate. Intracellular concentration of hsc70 is much higher than that of dj1 (Fig. 1 b). Thus, a portion of hsc70 is indeed tightly associated with dj1, while the remaining portion of hsc70 is not. Depletion of either one of these two DnaJ homologues caused little reduction of the remaining two chaperones.
Effect of dj2 Depletion on pOTC Import into Mitochondria
To investigate the roles of cytosolic chaperones in mitochondrial protein import, we synthesized rat pOTC in the untreated or the chaperone(s)-depleted reticulocyte lysate and import assays were done. When hsc70 (and concomitantly dj1) was depleted from the lysate, a decrease in pOTC synthesis was observed (Terada et al., 1995, 1996). The reason for the decrease is unknown. Import of pOTC synthesized in the hsc70-depleted lysate was about one-third that of the precursor synthesized in the untreated lysate (Fig. 2 a). When purified mouse hsc70 (130 μg/ml, 1.8 μM) was readded to the hsc70-depleted lysate before translation, the decreased pOTC import was almost completely recovered. Addition of hsc70 after translation and before import assay was not effective (Terada et al., 1995). Immunoblot analysis ruled out dj1 contamination in our hsc70 preparation (data not shown). These findings indicate a role for hsc70, but not dj1, in pOTC import into mitochondria.
Figure 2.
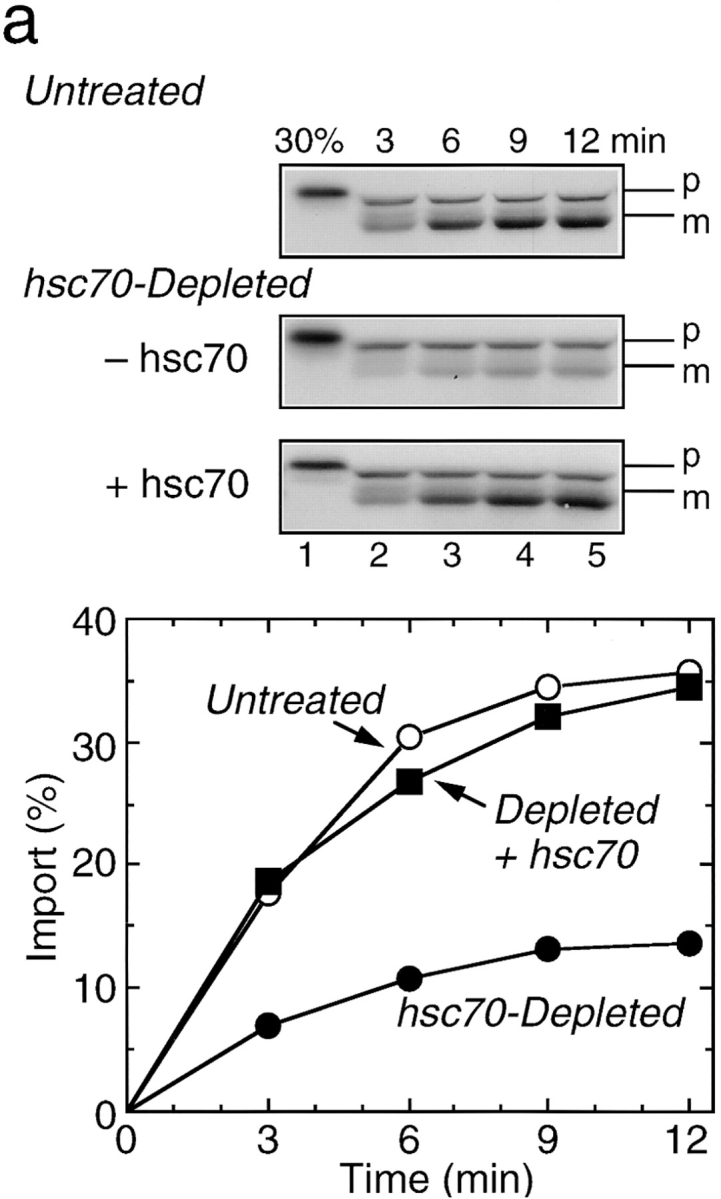
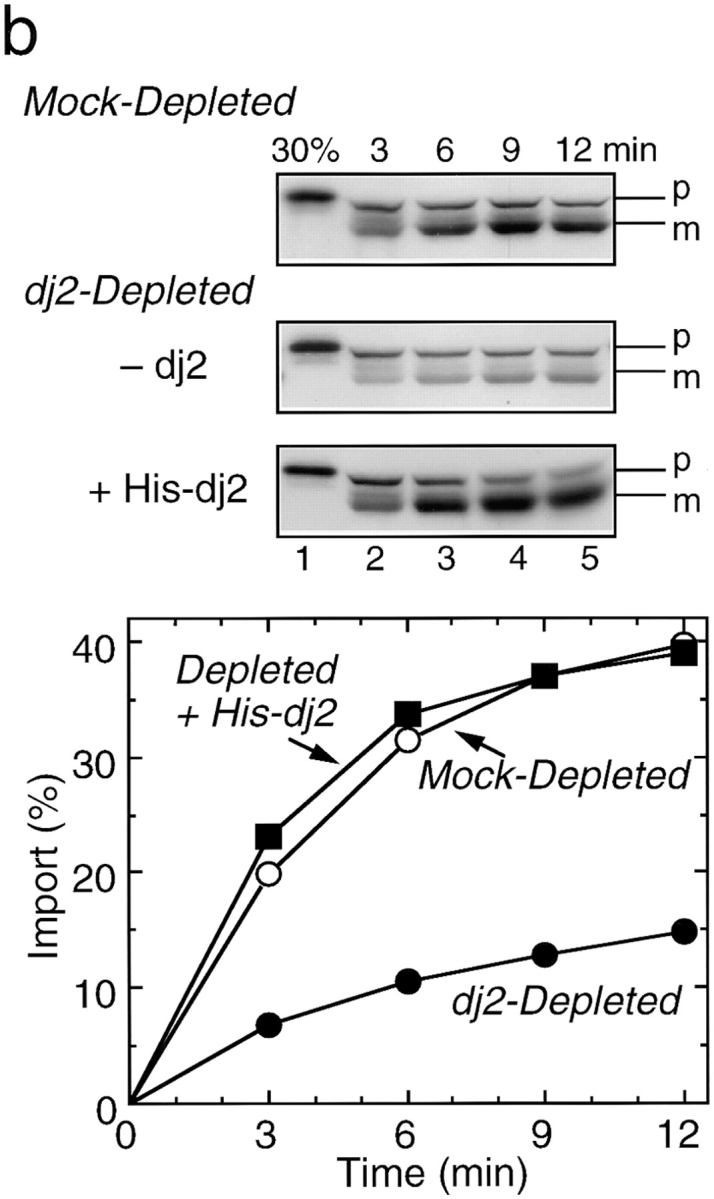
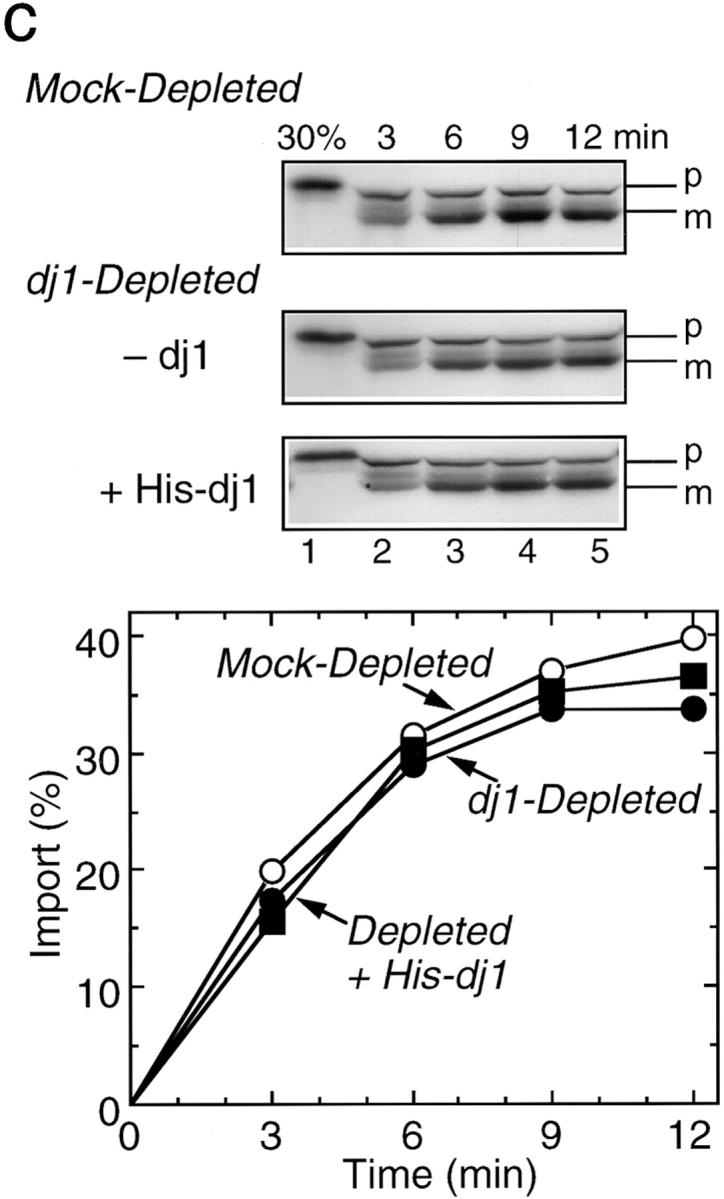
Effect of depletion and readdition of chaperones on import of pOTC into rat liver mitochondria. (a) Rat pOTC synthesized in the untreated rabbit reticulocyte lysate (Untreated, 15 KBq) or in the hsc70-depleted lysate without readdition (Depleted, 2.0 KBq) or with readdition (Depleted + Hsc70, 2.0 KBq) of 1.8 μM mouse hsc70 before translation, was subjected to import assay, as described in Materials and Methods. (b) Rat pOTC synthesized in the mock-depleted lysate (Mock-Depleted, 23 KBq) or in the dj2-depleted lysate without readdition (Depleted, 20 KBq) or with readdition (Depleted + His-dj2, 16 KBq) of 0.4 μM His-dj2 before translation was subjected to import assay. (c) Rat pOTC synthesized in the mock-depleted lysate (Mock-Depleted, 23 KBq) or in the dj1-depleted lysate without readdition (Depleted, 14 KBq) or with readdition (Depleted + His-dj1, 16 KBq) of 0.5 μM His-dj1 before translation was subjected to import assay. Portions of the fluorograms are shown in the upper part of each panel. p, precursor form of OTC; m, mature form of OTC; 30%, 30% of the input pOTC. The results were quantitated by imaging plate analysis using FUJIX BAS2000 analyzer and are shown in the lower part.
When dj1-depleted lysate was tested for pOTC import, little decrease was observed (Fig. 2 c). Readdition of His-dj1 to the dj1-depleted lysate had little effect on import. These results are consistent with those obtained for hsc70-depletion where dj1-depletion occurred concomitantly and the import restoration was achieved by the readdition of only hsc70. Efficiency of pOTC translation in the dj1- depleted lysate was slightly lower than that in the mock-depleted lysate.
We next examined the effects of dj2-depletion. Contrary to the effect of hsc70-depletion on translation efficiency, dj2-depletion led to only a slight decrease. Mitochondrial import of pOTC synthesized in the dj2-depleted lysate decreased to one-third that of the precursor synthesized in the mock-depleted lysate (Fig. 2 b). This finding is in accord with our previous inhibition studies using anti-dj2 antibody (Kanazawa et al., 1997). Since posttranslational addition of the antibody had no effect on mitochondrial import, dj2 is apparently required during pOTC synthesis but not during import, the results being similar to those for hsc70 (Terada et al., 1995). Requirement of dj2 was further confirmed by the addition of baculovirus-expressed His-dj2 to the dj2-depleted lysate. The pOTC import was almost completely recovered when His-dj2 was added before translation. The restoration of pOTC import depended on the amount of added His-dj2, and a nearly complete restoration was achieved with 0.4 μM (data not shown). This concentration was about double that in the reticulocyte lysate and was about one-tenth of that estimated for cultured cells and rat liver (see above). Thus, dj2, not dj1, is the partner DnaJ homologue of hsc70 facilitating mitochondrial import of pOTC.
Bacterial DnaJ is highly homologous to dj2, but lacks farnesyl modification in its COOH terminus. We asked whether DnaJ could replace dj2 in pOTC import into mitochondria. The pOTC import was nearly completely recovered when DnaJ was readded to the dj2-depleted lysate (data not shown). Thus, farnesyl modification is not essential for the members of cytosolic DnaJ family in mitochondrial protein import, though it may be required for functionally active dj2 in the context of its structural basis.
Facilitation of Refolding of Chemically Denatured Luciferase by hsc70 and dj2
We next examined the roles of DnaJ homologues in folding of cytosolic proteins. dj1 (hdj-1) has been reported to be the DnaJ homologue promoting folding of chemically denatured luciferase (Freeman et al., 1995). We speculated that dj2 participates in stabilization of precursor proteins targeted to the mitochondria in an import-competent state, whereas dj1 facilitates productive folding of proteins in the cytosol. Thus, these two DnaJ homologues have distinct roles depending on the final destination of their protein substrates. To test this hypothesis, we investigated chaperones required for productive folding of luciferase, using our depletion–readdition system.
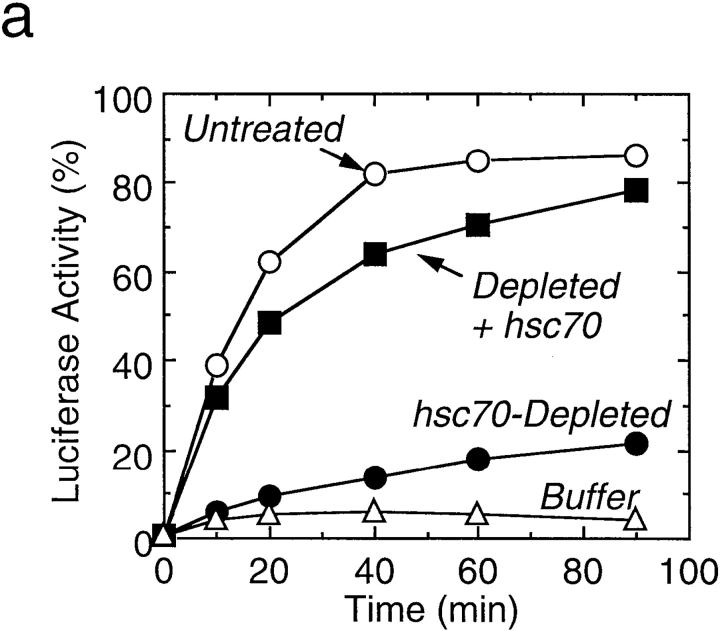
Chemically denatured luciferase when incubated alone at 25°C was not efficiently refolded into the enzymatically active form (Fig. 3 a). On the other hand, it was efficiently refolded in the untreated reticulocyte lysate. Refolding of luciferase proceeded with time and reached a plateau within 60 min. 87% of the enzyme activity was recovered in 90 min. These results are in good accord with the documented data (Nimmesgern and Hartl, 1993). When the reaction was conducted in the hsc70-depleted lysate, refolding was markedly decreased. The residual low refolding activity in the hsc70-depleted lysate may be attributed to remaining chaperones other than hsc70 and dj1. When purified hsc70 (1.8 μM) was supplemented to the hsc70- depleted lysate before the refolding assay, the refolding activity recovered almost completely. It is to be noted that this recovery was independent of dj1 that was also removed from the lysate by the hsc70-depletion procedure (see above).
Figure 3.
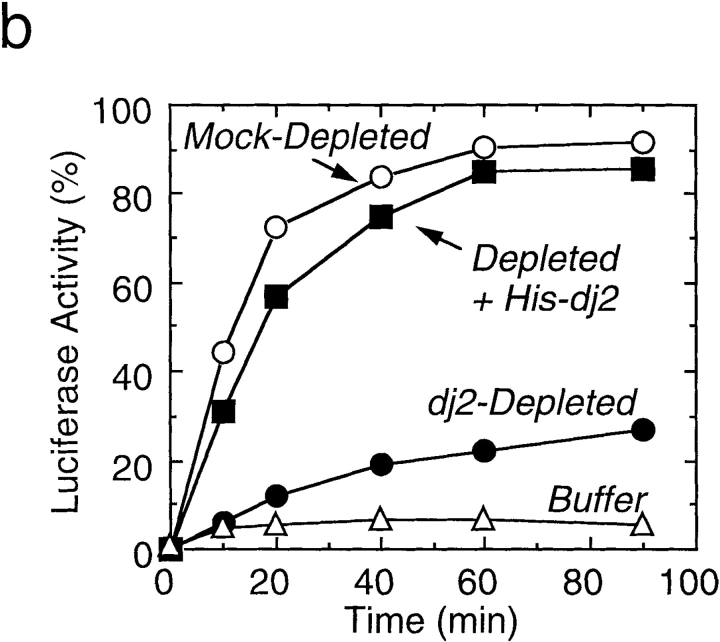
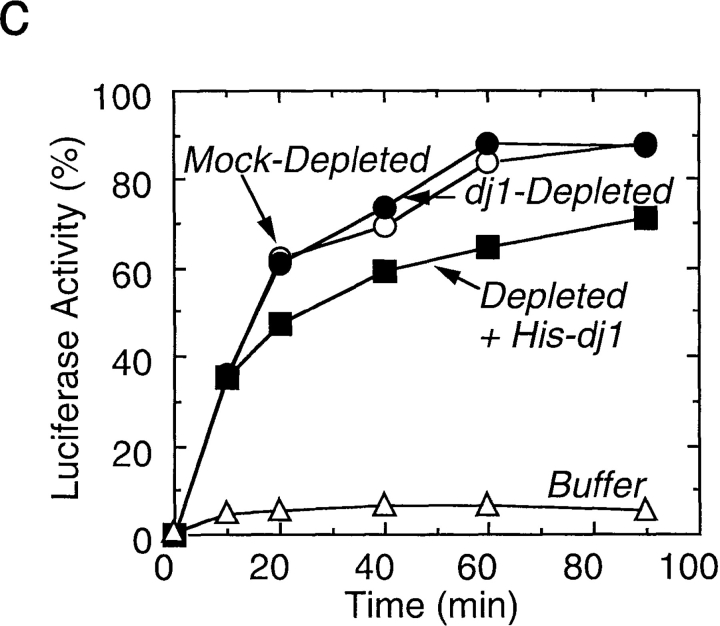
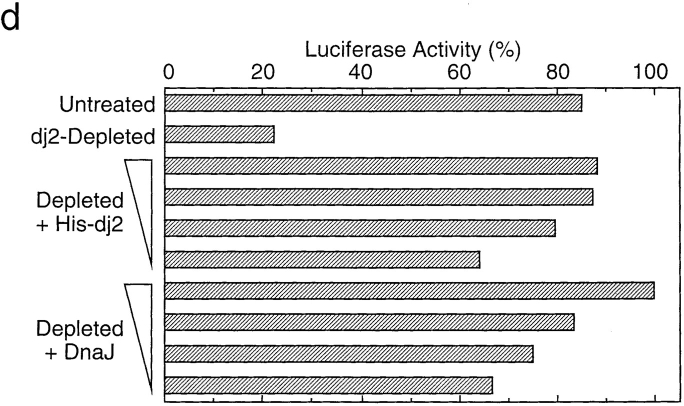
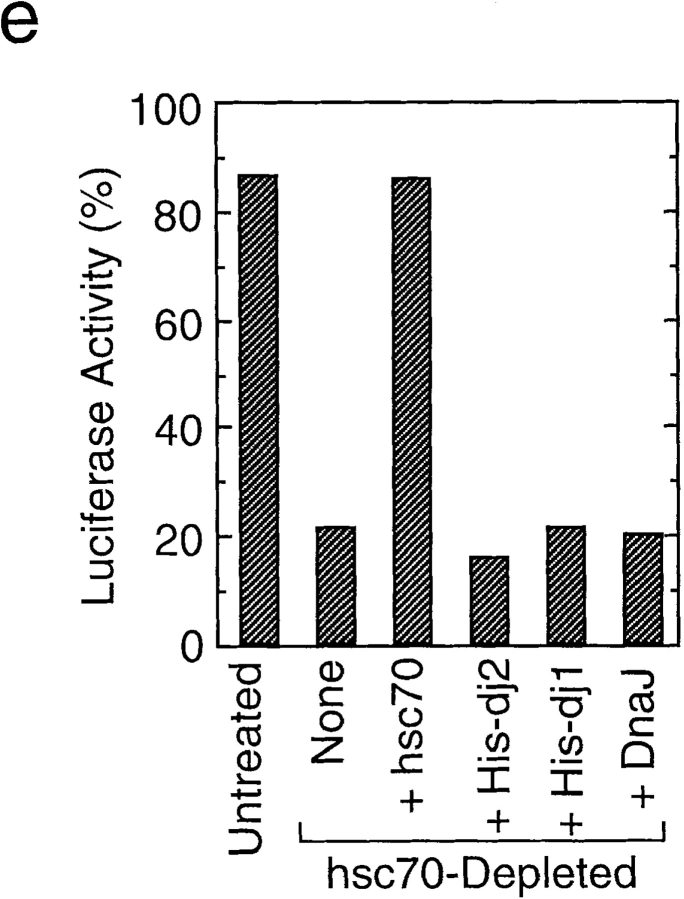
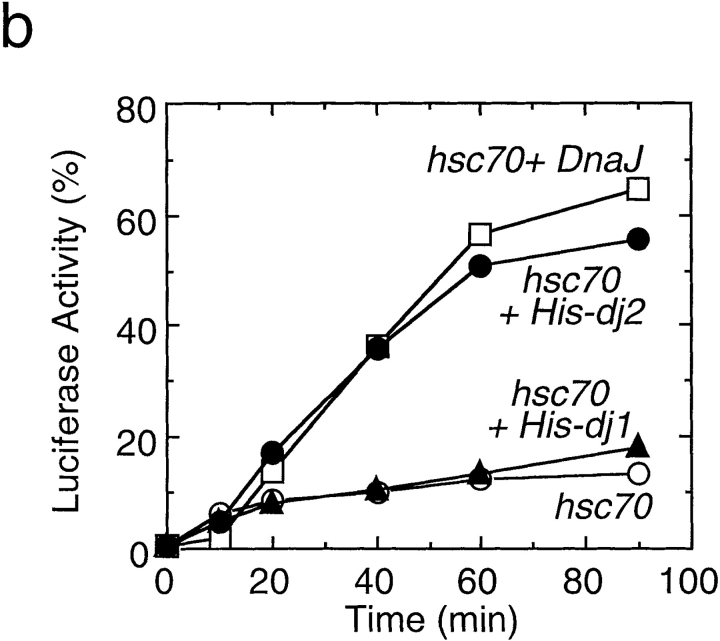
Effect of depletion and readdition of chaperones on refolding of chemically denatured luciferase. (a) Chemically denatured luciferase was renatured in the untreated rabbit reticulocyte lysate (Untreated) or in the hsc70-depleted lysate without readdition (Depleted) or with readdition (Depleted + Hsc70) of 1.8 μM mouse hsc70. For comparison, luciferase was renatured in the absence of the lysate (Buffer). (b) Denatured luciferase was renatured in the mock-depleted lysate (Mock-Depleted) or in the dj2-depleted lysate without readdition (Depleted) or with readdition (Depleted + His-dj2) of 0.2 μM His-dj2. Luciferase was also renatured in the absence of the lysate (Buffer). (c) Denatured luciferase was renatured in the mock-depleted lysate (Mock-Depleted) or in the dj1-depleted lysate without readdition (Depleted) or with readdition (Depleted + His-dj1) of 0.4 μM His-dj1. Luciferase was also renatured in the absence of the lysate (Buffer). (d) Refolding of luciferase was conducted for 60 min at 25°C in the dj2-depleted lysate supplemented with decreasing amounts of His-dj2 (0.4, 0.2, 0.1, and 0.03 μM) or E. coli DnaJ (0.4, 0.2, 0.1, and 0.03 μM). (e) Refolding of luciferase was conducted for 90 min in the hsc70-depleted lysate supplemented with mouse hsc70 (1.8 μM), His-dj2 (0.4 μM), His-dj1 (0.5 μM), or DnaJ (0.5 μM).
We next examined the effect of dj2-depletion on refolding activity of the lysate. Surprisingly, dj2-depletion caused a dramatic decrease in refolding (Fig. 3 b). Requirement of dj2 was further confirmed in readdition experiments. When 0.2 μM His-dj2 was readded to the dj2-depleted lysate, an almost complete recovery of refolding was observed. Restoration of the refolding activity depended on the amount of added His-dj2 (Fig. 3 d). Thus, dj2 is required for productive folding of denatured luciferase. The concentration of His-dj2 required for the maximal restoration was similar to that in the reticulocyte lysate and was much lower than those in tissues and cultured cells.
To verify the effect of dj1, refolding of luciferase was monitored in the dj1-depleted lysate. However, no significant effect was observed regardless of whether or not dj1 was present. When His-dj1 was readded to the dj1-depleted lysate, a slight reduction in the refolding reaction occurred. Thus, it is dj2, and not dj1, that cooperates with hsc70 to promote productive folding of chemically denatured luciferase.
Effect of DnaJ on Luciferase Refolding
Since bacterial DnaJ could replace dj2 in mitochondrial protein import, we asked if DnaJ could replace dj2 in luciferase refolding. In combination with yeast cytosolic hsp70 Ssa1p, DnaJ was shown to refold luciferase efficiently (Levy et al., 1995). Although the cytosolic hsp70 system of yeast is complicated (Craig et al., 1995) and differs from that of mammals, it may be that the combination of mammalian hsc70 and bacterial DnaJ will refold luciferase. As shown in Fig. 3 d, bacterial DnaJ could replace dj2 when added to the dj2-depleted lysate. The amount of DnaJ required for the refolding of luciferase was similar to that of His-dj2. At the highest concentration tested, the refolding activity of DnaJ exceeded that of His-dj2. DnaJ was not effective in luciferase refolding, when added to the hsc70-depleted lysate (Fig. 3 e). Thus, the activity of DnaJ depends on the presence of hsc70.
Refolding of Luciferase by Purified Chaperones
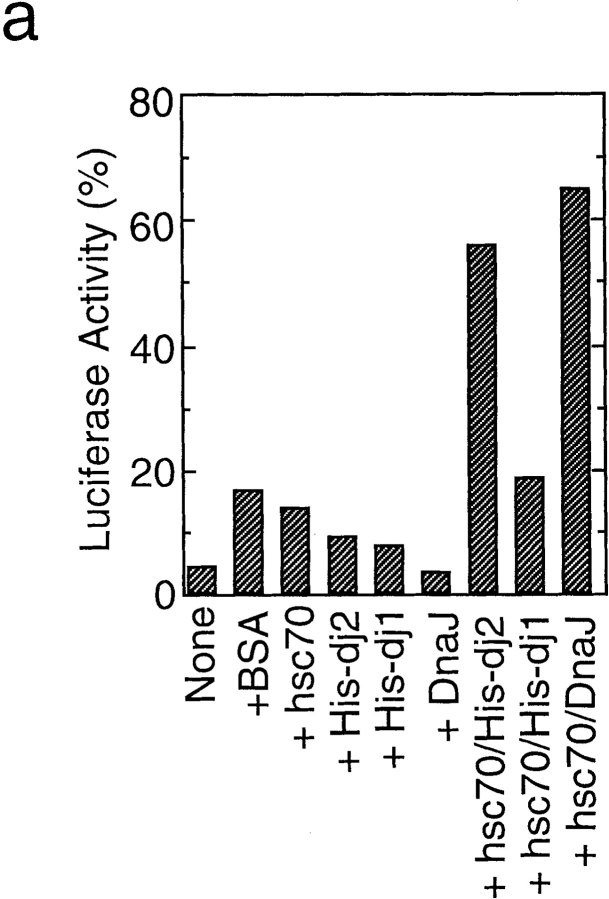
Refolding of luciferase by these chaperones was studied in greater detail using purified components. As shown in Fig. 4 a, luciferase refolding was somewhat facilitated by hsc70, His-dj2, and His-dj1. However, bovine serum albumin at a similar concentration was also effective. Therefore, the facilitation effects by these chaperones do not appear to be due to their specific chaperone activities. On the other hand, luciferase activity could be recovered by the addition of either His-dj2 or DnaJ together with hsc70. The addition of His-dj1 together with hsc70 had no significant effect.
Figure 4.
Refolding of luciferase by purified chaperones. (a) Chemically denatured luciferase was renatured for 90 min at 25°C in the presence of each chaperone or their combinations. Concentrations of proteins added were: hsc70, 1.8 μM; His-dj2, 0.4 μM; His-dj1, 0.5 μM; DnaJ, 0.5 μM; bovine serum albumin, 1.8 μM. (b) Refolding was performed for indicated periods in the presence of indicated chaperone(s).
Kinetics of luciferase refolding by His-dj2 or DnaJ plus hsc70 differed from that in the lysate (Fig. 4 b, see also Fig. 3 a). Refolding of luciferase was slow for the first 10 min and then proceeded linearly up to 60 min. The reason for the difference in refolding kinetics is unknown. However, it is possible that other component(s) in the lysate supported folding activity of the hsc70-dj2 chaperone system. This component may be another chaperone system(s) that maintains denatured proteins in a folding-competent state(s) and/or a regulatory element(s) that modulates folding activity of the hsc70-dj2 system.
Thus, it is obvious that hsc70 and dj2 constitute a mammalian chaperone system promoting mitochondrial import of pOTC and the productive folding of luciferase. dj1 was not effective in our studies, but it may play a role under special conditions such as heat-stress. Minami et al. (1996) reported that hsc70-dj1 prevented thermally denatured luciferase from insoluble aggregates, though no restoration of the enzyme activity was observed. It seems likely that the hsc70-dj1 chaperone system maintains thermally denatured luciferase in a refolding-competent state. Interestingly, they also reported the presence of a component(s) in the reticulocyte lysate that is required for productive folding of luciferase. dj2 may well be a candidate.
Discussion
We found that dj2 among the mammalian DnaJ homologues participates in mitochondrial protein import and in productive protein folding as an indispensable partner chaperone of hsc70. Alignment of the sequences of DnaJ, Ydj1p, and dj2 also suggest their functional similarity. Furthermore, functional replacement of mammalian dj2 (this study) or yeast Ydj1p (Levy et al., 1995) with bacterial DnaJ in luciferase folding indicates the universal importance of these limited members of DnaJ family in living cells.
We tested possible chaperoning activity of dj1 in mitochondrial protein import and in protein folding. However, immunodepletion of dj1 from the rabbit reticulocyte lysate did not affect refolding of denatured luciferase. Furthermore, purified His-dj1 in combination of hsc70 could not support luciferase refolding. One reported refolding of luciferase by hdj-1(dj1)–hsc70 (Freeman et al., 1995), while the other reported hsp40 (dj1)–hsc70 only prevented thermally denatured luciferase from insoluble aggregate without recovery of the enzyme activity (Minami et al., 1996). In the former report, hdj-1 (dj1) was prepared from E. coli expressing human dj1, according to procedures for DnaJ preparation, and the endogenous DnaJ might have been present in their preparation. In fact, a large amount of the hdj-1 preparation (1.6 μM) was needed for the refolding of chemically denatured proteins (Freeman and Morimoto, 1996; Freeman et al., 1995). The molar ratio of hdj-1 to hsc70 used in their study for refolding was 2:1, a ratio much higher than that in our studies (∼1:5, see below) and that in living cells (Fig. 1 b).
We previously showed that the requirement of hsc70 for mitochondrial protein import varies among precursor proteins (Terada et al., 1996). We also tested effects of dj2- depletion on mitochondrial import of several precursor proteins. The requirement of dj2 correlated well with that of hsc70 (Terada, K., and M. Mori, unpublished observations). The differences in hsc70-dj2 dependency may be due to different tendencies of the precursor proteins to fold, misfold, or aggregate. A high hsc70 and dj2 dependency of pOTC for mitochondrial import may be reflected by the high tendency of purified recombinant pOTC to aggregate (Murakami et al., 1990). We speculate cotranslational interaction of hsc70 and dj2 exists. We reported that pOTC synthesized in the lysate rapidly looses its import competence though there are large amounts of hsc70 and dj2 (Terada et al., 1995). Neither addition of antibodies against hsc70 and dj2 nor readdition of these chaperones to the depleted lysate before import reaction affect import of pOTC synthesized in vitro (Terada et al., 1995; Kanazawa et al. 1997). Thus at least these chaperones are not required at the step of translocation.
It is striking that hsc70 and dj2 promote both folding of a denatured protein and import of a protein into mitochondria. A similar enigma was presented in yeast and E. coli. In yeast, Ssa1–2p-Ydj1p promotes both protein folding and protein import into mitochondria and endoplasmic reticulum (Cyr et al., 1994). In E. coli, DnaK-DnaJ-GrpE promotes not only folding but also export of proteins in a SecB-deficient strain (Wild et al., 1992, 1996). A common functional mechanism of hsp70-DnaJ family systems may be to stabilize unfolded proteins and to protect them from unproductive folding and aggregation. However, cytosolic proteins must be folded into their final conformations whereas mitochondrial proteins must be maintained in an import-competent unfolded conformation (Schatz and Dobberstein, 1996). We reported that sedimentation coefficients of import-competent forms of mitochondrial precursor proteins differ from those of their folded mature forms (Terada et al., 1996). In the case of 3-oxoacyl-CoA thiolase, the native form in the mitochondrial matrix is a tetramer, whereas the import-competent form apparently behaves as a monomer (Terada et al., 1996). Thus, mitochondrial precursor proteins must bypass the cytosolic folding pathway that is composed of hsc70 and probably dj2 and TriC complex. This may be achieved by the presence of NH2-terminal presequences and the presequence-specific factors (PBF or MSF) that may prevent further folding of the precursor proteins in the cytosol (Mihara and Omura, 1996). MSF was reported to depolymerize and unfold aggregated precursor proteins in the presence of ATP and to maintain import competence of precursor proteins (Hachiya et al., 1993). Cooperation of these factors with the hsc70-dj2 system in mitochondrial protein import remains to be elucidated.
Ydj1p was initially identified as a component necessary for post-translational protein import into mitochondria and endoplasmic reticulum (Caplan and Douglas, 1991; Atencio and Yaffe, 1992), then it was shown to be a partner chaperone of Ssa1p (Cyr and Douglas, 1994; Becker et al., 1996). Ydj1p was also shown to be involved in signal transduction pathways mediated by steroid receptors and v-Src tyrosine kinase (Caplan et al., 1995; Kimura et al., 1995; Dey et al., 1996; Chang et al., 1997) and in a ubiquitin- dependent protein degradation pathway (Lee et al., 1996; Yaglom et al., 1996). In mammals, these functions may possibly be maintained by dj1 and/or dj2.
Acknowledgments
We thank K. Ohtsuka of Aichi Cancer Center Research Institute and Y. Minami of Oita Medical College for the generous gift of the His-hsp40 expression system and N. Imamoto and Y. Yoneda of Osaka University for human recombinant hsc70. We also thank T. Ogura, M. Takiguchi, and other colleagues of Kumamoto University for suggestions and technical advice and M. Ohara for comments on the manuscript.
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (09276103 and 08457040 to M. Mori and 09780658 to K. Terada).
Footnotes
1. Abbreviations used in this paper: dj1, mammalian counterpart of hsp40/ hdj-1; dj2, mammalian counterpart of HSDJ/hdj-2; His-dj1 and His-dj2, hexahistidine-tagged dj1 or dj2; hsc70, 70K heat shock cognate protein; pOTC, preornithine transcarbamylase.
Please address all correspondence to M. Mori, Department of Molecular Genetics, Kumamoto University School of Medicine, Kuhonji 4-24-1, Kumamoto 862, Japan. Tel.: (81) 96-373-5140. Fax: (81) 96-373-5145. e-mail: masa@gpo.kumamoto-u.ac.jp
The current address of M. Kanazawa is Department of Pediatrics, Chiba University School of Medicine, Chiba 280, Japan.
References
- Atencio DP, Yaffe MP. MAS5, a yeast homologue of DnaJ involved in mitochondrial protein import. Mol Cell Biol. 1992;12:283–291. doi: 10.1128/mcb.12.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Walter W, Yan W, Craig EA. Functional interaction of cytosolic hsp70 and DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol Cell Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Hesterkamp T, Luirink J. Growing up in a dangerous environment: a network of multiple targeting and folding pathways for nascent polypeptides in the cytosol. Trends Cell Biol. 1996;6:480–486. doi: 10.1016/0962-8924(96)84946-4. [DOI] [PubMed] [Google Scholar]
- Caplan AJ, Douglas MG. Characterization of YDJ1: a yeast homologue of the bacterial dnaJ protein. J Cell Biol. 1991;114:609–621. doi: 10.1083/jcb.114.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Tsai J, Casey PJ, Douglas MG. Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. . J Biol Chem. 1992a;267:18890–18895. [PubMed] [Google Scholar]
- Caplan AJ, Cyr DM, Douglas MG. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992b;71:1143–1155. doi: 10.1016/s0092-8674(05)80063-7. [DOI] [PubMed] [Google Scholar]
- Caplan AJ, Langley E, Wilson EM, Vidal J. Hormone-dependent transactivation by the human androgen receptor is regulated by a dnaJ protein. J Biol Chem. 1995;270:5251–5257. doi: 10.1074/jbc.270.10.5251. [DOI] [PubMed] [Google Scholar]
- Chang HC, Nathan DF, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham ME, Anderton BH, Jackson AP. Inhibition of hsc70-catalysed clathrin uncoating by HSJ1 proteins. Biochem J. 1996;319:103–108. doi: 10.1042/bj3190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham ME, Brion JP, Anderton BH. Human homologues of the bacterial heat-shock protein DnaJ are preferentially expressed in neurons. Biochem J. 1992;284:469–476. doi: 10.1042/bj2840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah A, Davis A, Mohanakumar T. Cloning of a unique human homologue of the Escherichia coliDNAJ heat shock protein. Biochim Biophys Acta. 1993;1174:111–113. doi: 10.1016/0167-4781(93)90103-k. [DOI] [PubMed] [Google Scholar]
- Craig E, Ziegellhoffer T, Nelson J, Laloraya S, Halladay J. Complex multigene family of functionally distinct Hsp70s of yeast. Cold Spring Harbor Symp Quant Biol. 1995;60:441–449. doi: 10.1101/sqb.1995.060.01.049. [DOI] [PubMed] [Google Scholar]
- Cyr DM, Douglas MG. Differential regulation of Hsp70 subfamilies by the eukaryotic DnaJ homologue YDJ1. J Biol Chem. 1994;269:9798–9804. [PubMed] [Google Scholar]
- Cyr DM, Langer T, Douglas MG. DnaJ-like proteins—molecular chaperones and specific regulators of hsp70. Trends Biochem Sci. 1994;19:176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Dey B, Caplan AJ, Boschelli F. The Ydj1 molecular chaperone facilitates formation of active p60v-src in yeast. Mol Biol Cell. 1996;7:91–100. doi: 10.1091/mbc.7.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Morimoto RI. The human cytosolic molecular chaperone hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO (Eur Mol Biol Organ) J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO (Eur Mol Biol Organ) J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Hachiya N, Alam R, Sakasegawa Y, Sakaguchi M, Mihara K, Omura T. A mitochondrial import factor purified from rat liver cytosol is an ATP-dependent conformational modulator for precursor proteins. EMBO (Eur Mol Biol Organ) J. 1993;12:1579–1586. doi: 10.1002/j.1460-2075.1993.tb05802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York. 726 pp.
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hattori H, Kaneda T, Lokeshwar B, Laszlo A, Ohtsuka K. A stress-inducible 40 kD protein (hsp40) J Cell Sci. 1993;104:629–638. doi: 10.1242/jcs.104.3.629. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Terada K, Kato S, Mori M. HSDJ, a human homolog of DnaJ, is farnesylated and is involved in protein import into mitochondria. J Biochem (Tokyo) 1997;121:890–895. doi: 10.1093/oxfordjournals.jbchem.a021670. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yahara I, Lindquist S. Role of the protein chaperone YDJ1 in establishing Hsp90-mediated signal transduction pathways. Science. 1995;268:1362–1365. doi: 10.1126/science.7761857. [DOI] [PubMed] [Google Scholar]
- Lee DH, Sherman MY, Goldberg AL. Involvement of the molecular chaperone Ydj1 in the ubiquitin-dependent degradation of short-lived and abnormal proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4773–4781. doi: 10.1128/mcb.16.9.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy EJ, McCarthy J, Bukau B, Chirico WJ. Conserved ATPase and luciferase refolding activities between bacteria and yeast Hsp70 chaperones and modulators. FEBS Lett. 1995;368:435–440. doi: 10.1016/0014-5793(95)00704-d. [DOI] [PubMed] [Google Scholar]
- Luke MM, Sutton A, Arndt KT. Characterization of SIS1, a Saccharomyces cerevisiae homologue of bacterial dnaJ proteins. J Cell Biol. 1991;114:623–638. doi: 10.1083/jcb.114.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Omura T. Cytoplasmic chaperones in precursor targeting to mitochondria: the role of MSF and hsp70. Trends Cell Biol. 1996;6:104–108. doi: 10.1016/0962-8924(96)81000-2. [DOI] [PubMed] [Google Scholar]
- Minami Y, Hohfeld J, Ohtsuka K, Hartl FU. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- Murakami K, Tokunaga F, Iwanaga S, Mori M. Presequence does not prevent folding of a purified mitochondrial precursor protein and is essential for association with a reticulocyte cytosolic factor(s) J Biochem (Tokyo) 1990;108:207–214. doi: 10.1093/oxfordjournals.jbchem.a123182. [DOI] [PubMed] [Google Scholar]
- Nimmesgern E, Hartl FU. ATP-dependent protein refolding activity in reticulocyte. Evidence for the participation of different chaperone components. FEBS Lett. 1993;331:25–30. doi: 10.1016/0014-5793(93)80290-b. [DOI] [PubMed] [Google Scholar]
- Oh S, Iwahori A, Kato S. Human cDNA encoding DnaJ protein homologue. Biochim Biophys Acta. 1993;1174:114–116. doi: 10.1016/0167-4781(93)90104-l. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K. Cloning of a cDNA for heat-shock protein hsp40, a human homologue of bacterial DnaJ. Biochem Biophys Res Commun. 1993;197:235–240. doi: 10.1006/bbrc.1993.2466. [DOI] [PubMed] [Google Scholar]
- Raabe T, Manley JL. A human homologue of the Escherichia coli DnaJ heat-shock protein. Nucleic Acids Res. 1991;19:6645. doi: 10.1093/nar/19.23.6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J, von Ahsen O, Bömer U, Pfanner N. Molecular chaperones: towards a characterization of the heat-shock protein 70 family. Trends Cell Biol. 1997;7:129–133. doi: 10.1016/S0962-8924(96)10056-8. [DOI] [PubMed] [Google Scholar]
- Rassow J, Voos W, Pfanner N. Partner proteins determine multiple functions of Hsp70. Trends Cell Biol. 1995;5:207–212. doi: 10.1016/s0962-8924(00)89001-7. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Szabo A, Korszun R, Hartl FU, Flanagan J. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. EMBO (Eur Mol Biol Organ) J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Kaibuchi K, Kikuchi A, Sasaki T. Effects of prenyl modifications on interactions of small G proteins with regulators. Methods Enzymol. 1995;250:122–133. doi: 10.1016/0076-6879(95)50067-7. [DOI] [PubMed] [Google Scholar]
- Terada K, Ohtsuka K, Imamoto N, Yoneda Y, Mori M. Role of heat shock cognate 70 protein in import of ornithine transcarbamylase precursor into mammalian mitochondria. Mol Cell Biol. 1995;15:3708–3713. doi: 10.1128/mcb.15.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Ueda I, Ohtsuka K, Oda T, Ichiyama A, Mori M. The requirement of heat shock cognate 70 protein for mitochondrial import varies among precursor proteins and depends on precursor length. Mol Cell Biol. 1996;16:6103–6109. doi: 10.1128/mcb.16.11.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J, Altman E, Yura T, Gross CA. DnaK and DnaJ heat shock proteins participate in protein export in Escherichia coli. Genes Dev. 1992;6:1165–1172. doi: 10.1101/gad.6.7.1165. [DOI] [PubMed] [Google Scholar]
- Wild J, Rossmeissl P, Walter WA, Gross CA. Involvement of the DnaK-DnaJ-GrpE chaperone team in protein secretion in Escherichia coli. J Bacteriol. 1996;178:3608–3613. doi: 10.1128/jb.178.12.3608-3613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, Goldberg AL, Finley D, Sherman MY. The molecular chaperone Ydj1 is required for the p34CDC28-dependent phosphorylation of the cyclin Cln3 that signals its degradation. Mol Cell Biol. 1996;16:3679–3684. doi: 10.1128/mcb.16.7.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane M, Hattori H, Sugito K, Hayashi Y, Tohnai I, Ueda M, Nishizawa K, Ohtsuka K. Cotranslocation and colocalization of hsp40 (DnaJ) with hsp70 (DnaK) in mammalian cells. Cell Struct Funct. 1995;20:157–166. doi: 10.1247/csf.20.157. [DOI] [PubMed] [Google Scholar]
- Zhong T, Arndt KT. The yeast SIS1 protein, a DnaJ homolg, is required for the initiation of translation. Cell. 1993;73:1175–1186. doi: 10.1016/0092-8674(93)90646-8. [DOI] [PubMed] [Google Scholar]