Abstract
In the present paper, we show that transport from early to late endosomes is inhibited at the restrictive temperature in a mutant CHO cell line (ldlF) with a ts-defect in ε coatomer protein (εCOP), although internalization and recycling continue. Early endosomes then appear like clusters of thin tubules devoid of the typical multivesicular regions, which are normally destined to become vesicular intermediates during transport to late endosomes. We also find that the in vitro formation of these vesicles from BHK donor endosomes is inhibited in cytosol prepared from ldlF cells incubated at the restrictive temperature. Although εCOP is rapidly degraded in ldlF cells at the restrictive temperature, cellular amounts of the other COP-I subunits are not affected. Despite the absence of εCOP, we find that a subcomplex of β, β′, and ζCOP is still recruited onto BHK endosomes in vitro, and this binding exhibits the characteristic properties of endosomal COPs with respect to stimulation by GTPγS and sensitivity to the endosomal pH. Previous studies showed that γ and δCOP are not found on endosomes. However, αCOP, which is normally present on endosomes, is no longer recruited when εCOP is missing. In contrast, all COP subunits, except obviously εCOP itself, still bind BHK biosynthetic membranes in a pH-independent manner in vitro. Our observations thus indicate that the biogenesis of multivesicular endosomes is coupled to early endosome organization and depends on COP-I proteins. Our data also show that membrane association and function of endosomal COPs can be dissected: whereas β, β′, and ζCOP retain the capacity to bind endosomal membranes, COP function in transport appears to depend on the presence of α and/or εCOP.
After internalization, cell surface proteins and lipids, as well as solutes, first appear in peripheral early endosomes. Depending on their fate, internalized molecules can then either be recycled back to the cell surface for reutilization or transported to late endosomes and then lysosomes for degradation (Gruenberg and Maxfield, 1995; Mellman, 1996). These two pathways exhibit major differences with respect to membrane organization and dynamics. In contrast to the recycling route, transport to late endosomes is highly selective, accounting for the bulk of downregulated receptors but only a minor fraction (⩽10%) of total internalized protein and lipid (Koval and Pagano, 1989; Trowbridge et al., 1993). Endosomes along these two pathways also exhibit marked ultrastructural differences. Whereas elements of the recycling pathway consist of very thin tubules (50–60 nm in diameter and up to several microns in length), endosomes at all stages of the degradation pathway exhibit a typical multivesicular appearance caused by the accumulation of internal membranes within their lumen, hence the name multivesicular body (MVB)1 (Dunn et al., 1986; Tooze and Hollinshead, 1991; Parton et al., 1992; van Deurs et al., 1993; Futter et al., 1996). Finally, these two pathways differ in their acidification properties. The lumenal pH decreases from 6.2 in early endosomes to ∼5.5 in endosomes of the degradation pathway but increases to ∼6.4 in recycling endosomes (Yamashiro et al., 1984; Mellman et al., 1986; Sipe and Murphy, 1987). It is intriguing how early endosomal membranes can give rise to elements that differ so widely in their organization, internal milieu, and protein composition.
In previous in vivo and in vitro studies, we have identified and characterized intermediates (endosomal carrier vesicles [ECVs]), which mediate transport from early to late endosomes (Gruenberg et al., 1989; Bomsel et al., 1990; Aniento et al., 1993, 1996; Clague et al., 1994; Robinson et al., 1997). These vesicles exhibit a typical multivesicular ultrastructure and will be referred to as ECV/MVBs in this study. We found that the formation of ECV/MVBs from early endosomes depends on β coatomer protein (βCOP) (Aniento et al., 1996), a subunit of the COP-I coat previously shown to mediate anterograde and/or retrograde transport at early stages of the biosynthetic pathway (Orci et al., 1986; Ostermann et al., 1993; Pepperkok et al., 1993; Letourneur et al., 1994). Studies comparing endosomal and biosynthetic COPs, however, revealed that these exhibit, at least in part, different properties. Two subunits of the biosynthetic COP-I coat, γ and δ, are not present on endosomes (Whitney et al., 1995; Aniento et al., 1996), suggesting that the composition of the endosomal coat is simpler, or that the endosomal homologues of γ and δ have not been identified. In addition, we have observed that ECV/MVB formation from early endosomes depends on the acidic lumenal pH and that βCOP binding to early endosomes is itself pH dependent (Clague et al., 1994; Aniento et al., 1996). In contrast, COPs in the biosynthetic pathway are recruited onto the membranes of nonacidic organelles.
Candidates responsible for COP-I binding to biosynthetic membranes include proteins normally retained in or retrieved to the endoplasmic reticulum via a KKXX motif (Cosson and Letourneur, 1994), as well as members of a novel family of biosynthetic membrane proteins in the ∼20–25-kD range (Fiedler et al., 1996; Sohn et al., 1996). Recent studies have also provided some information about the possible role of individual COP-I subunits during binding to biosynthetic membranes. Two COP-I subcomplexes, consisting of the γ/ζ and α/β′/ε subunits, could be dissociated in vitro, and the α/β′/ε COP subcomplex was shown to interact with membranes and with cytoplasmic KKXX motifs (Lowe and Kreis, 1995). However, studies using peptides derived from the cytoplasmic domains of different 20–25-kD proteins suggest that an -FF- motive typical for this family is required for binding, but also that different COP-I subunits may bind to different members of this protein family (Fiedler et al., 1996). The physiological significance of these different mechanisms remains to be elucidated. In fact, very little is known about the role of individual COP-I subunits, in particular in the process of driving transport itself. In the endocytic pathway, essentially nothing is known about the function of any subunit.
In the present paper, we have studied the role of COP proteins in ECV/MVB biogenesis, using the ldlF mutant CHO cell line with a ts mutation in the gene encoding for εCOP. At the restrictive temperature, εCOP is rapidly degraded (Guo et al., 1994, 1996; Hobbie et al., 1994). Our data show that ECV/MVB biogenesis, hence transport to late endosomes, is coupled to the maintenance of early endosome organization. In the absence of functional COPs, accumulation of internal membranes and ECV/MVB formation no longer occur on early endosomal membranes. Early endosomes, then, become clusters of thin tubules. We also find that εCOP degradation does not affect the amounts of other subunits to any significant extent and that β, β′, and ζCOP, but not αCOP, are still recruited onto endosomes in a pH-sensitive and GTPγS-dependent manner. Our data show that β, β′, and ζCOP retain the full capacity to mediate membrane association but are not sufficient to drive ECV/MVB biogenesis in vivo and in vitro, and thus that α and/or εCOP may confer coat activity in transport.
Materials and Methods
Cell Culture and Immunological Reagents
Monolayers of baby hamster kidney cell line (BHK-21) were grown and maintained as described (Gruenberg et al., 1989). For large scale endosome preparation, 24 × 24 cm dishes were used. Wide-type CHO and mutant ldlF cell line were obtained from M. Krieger (Massachusetts Institute of Technology, Cambridge, MA). CHO and ldlF cells were grown and maintained as described (Guo et al., 1994), using F-12 medium (Nutrient mixture F-12 HAM; Sigma, Buchs, Switzerland) containing 5% FCS (Sera-Tech, St. Salvator, Germany).
The M3A5 and maD monoclonal antibodies against βCOP peptide were a gift of T. Kreis (University of Geneva, Geneva, Switzerland). The antibodies against all other COP-I subunits were a gift of F. Wieland (Ruprecht Karl University, Heidelberg, Germany). Human serum against EEA1 was a gift of B.H. Toh (Monash Medical School, Victoria, Australia). The polyclonal antibody against the mannose-6-phosphate receptor (Man6P-R) was obtained from B. Hoflack (Institut Pasteur, Lilles, France), and the polyclonal antibody against calnexin from Ari Helenius (Yale University, New Haven, CT). The antibody against BHKp23 was raised after injection of the RLEDLSESIVNFAY lumenal peptide of BHKp23 into rabbits (LP2; Rojo et al., 1997). The antibodies against rab5 and rab7 were raised after injection of the corresponding COOH-terminal peptides into rabbits, as in Chavrier et al. (1990). FITC-labeled secondary antibodies were from Dianova (Hamburg, Germany).
Internalization and Recycling of Endocytosed Markers in ldlF Cells
Cells were grown onto 10-cm dishes at the permissive temperature (34°C) for 3 d and then subsequently incubated for 6 h at the same temperature or at the restrictive temperature (40°C). To measure continuous HRP uptake, cells were washed twice with PBS and incubated in internalization medium (MEM, 25 mM glucose, 10 mM Hepes, pH 7.4) containing 3 mg/ ml HRP and 2 mg/ml BSA for 5, 15, 30, 60, or 120 min at either temperature. Cells were extensively washed for 10 min six times with PBS containing 5 mg/ml BSA (PBS-BSA) on ice, scraped off the dish, and collected by centrifugation at 450 g. The cell pellet was solubilized for 30 min in 400 μl homogenization buffer (HB; 250 mM sucrose, 3 mM imidazole) containing 0.2% Triton X-100, and both HRP activity and protein content were quantified. To quantify HRP recycling, cells were incubated as above with internalization medium containing 0.5 mg/ml HRP for 5 min at either temperature and then washed on ice as above. Then, cells were reincubated in 10 ml internalization medium containing 2 mg/ml BSA for 10, 20, 30, or 40 min at the corresponding temperature. The medium was collected, cells were processed as above, and HRP activity of cell extract and medium was measured. In some experiments, ldlF cells were transiently transfected with the cDNA encoding for the human transferrin receptor (Zerial et al., 1987; Harder and Gerke, 1993). Transfected cells were then incubated at the permissive or restrictive temperature for 6 h and then reincubated in internalization medium containing 50 μg/ml rhodamine-transferrin (Sigma) for 5 min at the corresponding temperature. Cells were extensively washed with PBS-BSA, fixed in 3% paraformaldehyde at room temperature for 20 min, and analyzed by fluorescence microscopy with 100× objective.
Subcellular Fractionation of ldlF Cells
Cells were grown onto 12 × 10 cm dishes at the permissive temperature (34°C) for 3 d. When needed, six dishes were subsequently incubated at the restrictive temperature (40°C) for 6 h. In either case, cells were rinsed in PBS and incubated 5 min with 4 mg/ml HRP in internalization medium to label early endosomes. To label late endosomes, cells were washed three times for 10 min on ice in PBS containing 5 mg/ml BSA and then reincubated for 40 min at the desired temperature (34 or 40°C) in internalization medium containing 2 mg/ml BSA. In all cases, cells were then washed twice with PBS, homogenized in HB containing 1 mM EDTA, and centrifuged at 1,300 g for 10 min to prepare a postnuclear supernatant (PNS). The PNS was brought to 30% sucrose, 3 mM imidazole, 1 mM EDTA up to a volume of 2 ml, loaded on top of a 1-ml cushion of 40.6% sucrose, 3 mM imidazole, 1 mM EDTA in a SW60 tube (Beckman Instruments, Fullerton, CA), and overlaid with 1.5 ml of HB containing 1 mM EDTA. The gradient was then centrifuged at 35,000 rpm for 90 min, and fractions were collected. Early and late endosomal fractions were recovered from the 30% sucrose/HB and 40.6%/30% sucrose interfaces, respectively. To ensure efficient recoveries, the 30% cushion was brought back to 34% sucrose in the same buffer and subjected to a second round of centrifugation, as used for the PNS. Early and late endosomal fractions from both centrifugation rounds were pooled, and HRP content of the fractions were quantified.
Cytosol Preparation
CHO and ldlF cells were grown onto 24 × 24 cm dishes at 37 and 34°C, respectively. Then, ldlF cells, as well as control CHO cells, were further incubated at the restrictive temperature (40°C) for 12 h. Cells were washed twice with PBS on ice and homogenized. A PNS was then prepared and centrifuged at 55,000 rpm for 35 min in a rotor (model TLS 55; Beckman Instruments, Fullerton, CA). The supernatant containing the cytosol was collected, aliquoted, frozen in liquid N2, and stored at −80°C.
Fluorescence Microscopy
Monolayers of ldlF cells were grown on glass coverslips for 2 d at 34°C to 80% confluency and then reincubated at 34 or 40°C for 6 h. The following protocols were used: (a) cells were fixed in 2% paraformaldehyde for 20 min at room temperature, permeabilized with 1% Triton X-100, and then labeled with human antiserum against EEA1 diluted 1:400; (b) cells were fixed and permeabilized using MeOH at −20°C and then labeled with a rabbit antiserum against Man6P-R diluted 1:200, the maD monoclonal antibody against βCOP diluted 1:3,000, or the rabbit antiserum against calnexin diluted 1:500; (c) cells were first permeabilized with 0.004% digitonin for 5 min, fixed with 2% paraformaldehyde for 20 min at room temperature, blocked with 2% fish skin gelatin, and then labeled with a rabbit antiserum against rab7 diluted 1:100. In all cases, the bound antibodies were revealed using FITC-conjugated secondary antibodies. Samples were processed as described (Kreis, 1986; Mu et al., 1994) and viewed using an inverted fluorescence light microscope (model Axiovert 135 TV; Carl Zeiss, Inc., Thornwood, NY) and a 100× objective.
We used acridine orange (Calbiochem, La Jolla, CA) that had been stored at a concentration of 20 mM in DMSO at −20°C. We also used two forms of LysoSensor (Molecular Probes, Eugene, OR), which detect pH values in the 4.5–6.0 (DND-189) and 6.5–8.0 (DND-153) ranges, respectively, and were stored and used according to the manufacturer's instructions. Cells were washed twice with PBS++ (1 mM CaCl2, 1 mM MgCl2) at room temperature, incubated for 10 min in PBS++ containing 5 μM of the dye and 5 mM glucose, and then observed by fluorescence microscopy using a 100× objective.
Phase Contrast Light Microscopy and Electron Microscopy of ldlF Cells
Early and late endosomes were labeled with HRP as described above, except that 10 mg/ml high activity HRP (Serva, Heidelberg, Germany) was used to ensure proper detection of the marker. The cells were then fixed in LG-fix (0.5% glutaraldehyde in 100 mM cacodylate, pH 7.35) for 60 min at room temperature and washed six times for 10 min with 100 mM cacodylate, pH 7.35. The distribution of internalized HRP was revealed using a cytochemical reaction with diaminobenzidine (Sigma) and H2O2 as substrates. Fixed cells were treated in the dark with 1 mg/ml diaminobenzidine in 200 mM cacodylate for 5 min and then with the same solution but also containing 0.0012% H2O2 for 30 min. Samples were then washed four times for 5 min with 100 mM cacodylate and observed by phase contrast light microscopy using a 100× objective. For electron microscopy, the cells were postfixed with 2% osmium tetroxide for 1 h at room temperature, and processed for Epon embedding as described (Parton et al., 1992 a).
COP Binding Assay
Cytosol from ldlF cells was prepared as described above. Membranes were obtained from BHK cells incubated with or without 20 μg/μl brefeldin A (Sigma) for 1 h to allow disassembly of the Golgi complex to occur (Rojo et al., 1997). Cells were then washed, and endosomes were prepared using a step flotation gradient, as described (Gruenberg and Gorvel, 1992). Briefly, cells were grown onto four 24 × 24 cm dishes and homogenized to prepare a PNS. The PNS was adjusted to 40.6% sucrose, 3 mM imidazole, pH 7.4, and loaded at the bottom of six SW40 tubes (Beckman Instruments). Each one was then overlaid sequentially with 4.5 ml of 35% sucrose, 3 ml of 25% sucrose in 3 mM imidazole, pH 7.4, and 3 ml of HB (250 mM sucrose, 3 mM imidazole, pH 7.4). The gradients were centrifuged for 90 min at 35,000 rpm using an SW40 rotor (Beckman Instruments). Early endosomes and biosynthetic membranes were then collected at the 35%/25% and 40.6%/35% interfaces, respectively. In the binding assay, 500 μg cytosol was mixed on ice with 50 μg of each membrane fraction and complemented with 12.5 mM HEPES, 1.5 mM MgOAc2, 1 mM DTT, 65 mM KCl, and 15 μl of an ATP-regenerating system (Gruenberg and Howell, 1986) in a total volume of 350 μl. The mixture was then incubated at 37°C for 15 min. When early endosomes were being analyzed, the whole reaction mixture was adjusted to 40.6% sucrose, 3 mM imidazole and loaded on the bottom of a TLS 55 tube, overlaid with 500 μl of 35% sucrose, 3 mM imidazole, and HB, and centrifuged at 45,000 rpm for 45 min. Early endosomal membranes were collected at the 35%/HB interface. For the analysis of biosynthetic membranes, the reaction mixture was loaded on top of a step gradient formed by 200 μl of 50% sucrose, 3 mM imidazole, and 500 μl of 20% sucrose, 3 mM imidazole in a TLS 55 tube. After centrifugation at 45,000 rpm for 45 min, biosynthetic membranes were recovered at the 20%/50% sucrose interface. In all cases, proteins were then precipitated with CHCl3/methanol, solubilized in SDS gel sample buffer (2% SDS, 10% glycerol, 100 mM DTT, 60 mM Tris, pH 6.8, 0.001% bromophenol blue) and separated by gel electrophoresis. After transfer to nitrocellulose, analysis of COP binding was then carried out by Western blotting using antibodies against each COPI subunit.
In Vitro Formation of ECV/MVBs from Early Endosomes
ECV/MVB formation from donor early endosomal membranes was measured exactly as described in Aniento et al. (1996). Briefly, BHK cells were incubated for 5 min with 5 mg/ml HRP to provide a marker of the early endosomal content, and then cells were homogenized and a PNS was prepared. The PNS was fractionated as described above. Early endosomes were the collected from the 35%/25% interface, well separated from ECVs and late endosomes (25%/HB interface; Aniento et al., 1993). In the assay, 300–500 μg of early endosomal protein in a final volume of 1.4-2.3 ml was incubated for 30 min at 37°C in 12.5 mM Hepes, pH 7.0, 1 mM DTT, 1.5 mM MgOAc, 60 mM KCl, and supplemented with an ATP-regenerating system and 4 mg/ml cytosol. The cytosol was prepared from wild-type (WT) CHO cells or from ldlF cells, as above. Then, the mixture containing both donor early endosomes and vesicles formed in vitro was brought to 25% sucrose, 3 mM imidazole, pH 7.4, loaded at the bottom of an SW60 tube and overlaid with HB. After 1 h of centrifugation at 35,000 rpm, donor early endosomes and budded vesicles were recovered from the pellet and the 25% sucrose/HB interface, respectively. Both fractions were recentrifuged for 30 min at 100,000 g to sediment membranes, and the HRP activity was quantified in the pellets.
Other Methods
Quantification of protein was carried out using the procedure of Bradford (1976) or with bicinchoninic acid (Pierce Chemical Co., Rockford, IL). SDS-PAGE was performed according to Laemmli (1970). HRP activity was measured as in Gruenberg and Gorvel (1992). Western blot analysis was carried out using peroxidase-conjugated secondary antibodies (Bio-Rad Labs, Hercules, CA) and detected by chemiluminescence using the SuperSignal™ reagent (Pierce Chemical Co.). Blot exposure times were always within the linear range of detection.
Results
It has been previously shown that ts-εCOP is rapidly degraded in ldlF cells at the restrictive temperature (Guo et al., 1996) and that already at the permissive temperature, amounts of εCOP are reduced when compared with WT CHO cells (Guo et al., 1996; Fig. 1). The other COP subunits (β, β′, γ, δ, and ζCOP) were not affected by incubation at the restrictive temperature, except for an approximately twofold reduction in αCOP. Amounts of each subunit were, in fact, comparable to those found in WT CHO cells cultured at 37 or 40°C (Fig. 1), indicating that COP-I subunits or subcomplexes were stable despite the complete degradation of one subunit.
Figure 1.
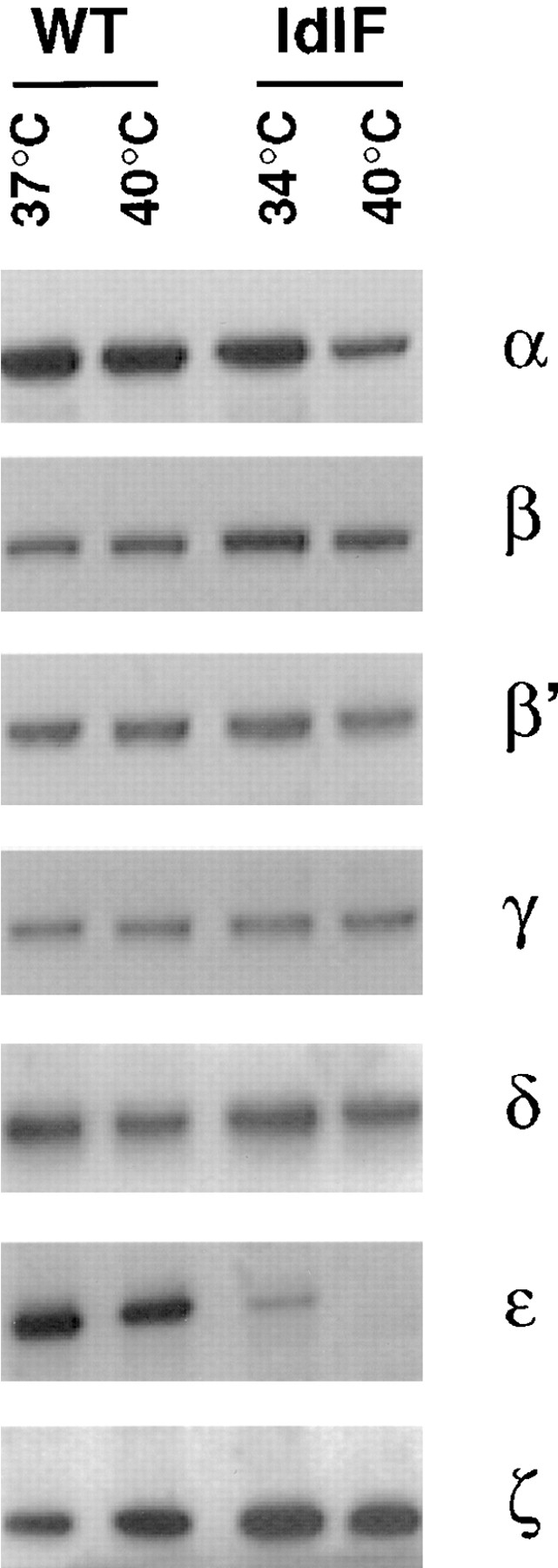
Distribution of COP-I subunits in CHO and ldlF cells. Cytosols were prepared from ldlF cells incubated at the permissive (34°C) or restrictive (40°C) temperature. For comparison, cytosols were also prepared from WT CHO cells incubated at 37 or 40°C. The COP-I composition of each cytosol was analyzed by SDS-PAGE followed by Western blotting using antibodies against each of the COP-I components. 20 μg protein was loaded per lane.
Endocytosis in ldlF Cells at the Restrictive Temperature
As a first step, we investigated whether early steps of the endocytic pathway were affected at the restrictive temperature. Cells were transiently transfected with the cDNA encoding for the human transferrin receptor (Zerial et al., 1987; Harder and Gerke, 1993), a well-established marker of clathrin-dependent endocytosis (Pearse and Robinson, 1990; Trowbridge et al., 1993) and then incubated at the desired temperature for 6 h. At this time, εCOP was fully degraded (Guo et al., 1996; data not shown), yet all other COP-I subunits were present (see Fig. 1). Then, the cells were incubated with human rhodamine-transferrin for only 5 min at the corresponding temperature, to allow one wave of receptor-mediated endocytosis to occur. As shown in Fig. 2 A, transferrin appeared to be internalized as efficiently at the restrictive or permissive temperature. Similarly CD4, which is endocytosed through clathrin-coated pits in T cell lines and transfected HeLa cells, was internalized at similar rates in stably transfected ldlF cells incubated at permissive or restrictive temperature (Bowers, K., and M. Marsh personal communication). These data indicate that clathrin-dependent endocytosis continued in the absence of εCOP.
Figure 2.
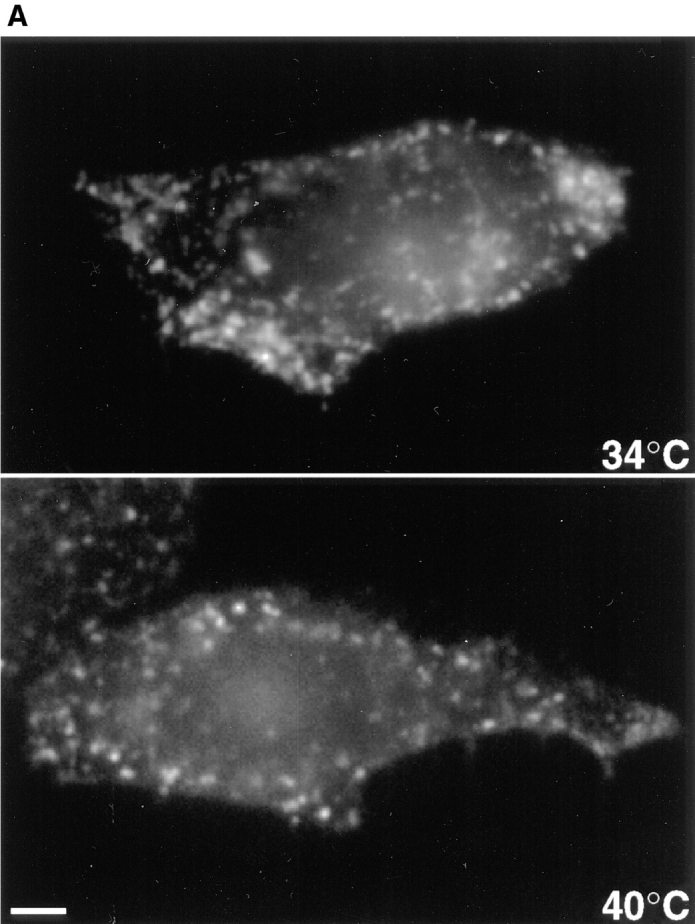
Endocytosis in ldlF cells. Cells were incubated at the permissive (34°C) or restrictive (40°C) temperature for 6 h. (A) Transferrin internalization. Cells had been transiently transfected with the cDNA encoding for the human transferrin receptor before incubation at 34 or 40°C. Transferrin internalization was visualized by fluorescence microscopy after 5 min incubation with 50 μg/ml rhodamine-transferrin at the corresponding temperature. (B) Continuous internalization of HRP. Cells were incubated with 3 mg/ml HRP at the corresponding temperature for 5, 15, 30, 60, or 120 min. The amounts of endocytosed HRP were quantified and expressed as OD U/min/mg cellular protein. (C) Recycling of internalized HRP. Cells were incubated with 0.5 mg/ ml HRP for 5 min at the corresponding temperature, washed, and then reincubated for 10, 20, 30, or 40 min. At each time point, cells and the media were collected. At each time point, HRP remaining associated to the monolayer is expressed as a percentage of the total (cell-associated and regurgitated). In B and C, each panel shows the mean of two representative series of experiments. Bar, 5 μm.
We found that internalization into endosomes and recycling back to the plasma membrane of the lipid bodipy-sphingomyelin (Koval and Pagano, 1989; Pagano et al., 1991) was not changed by the temperature shift (Kobayashi, T., F. Gu, K. Bowers, M. Marsh, and J. Gruenberg, unpublished observations). We then measured whether fluid phase endocytosis of HRP was affected at the restrictive temperature. Cells were incubated with HRP for increasing time periods, and the amounts of cell-associated HRP were quantified. At the permissive temperature, HRP accumulated intracellularly with time, as expected (Fig. 2 B). At the restrictive temperature, HRP was clearly taken up, but the process exhibited a classical saturation profile. Uptake was somewhat reduced after short times (∼60 –70% of the unshifted control) and reached a plateau after longer times, indicating that intracellular accumulation did not occur. Recycling back to the cell surface was then measured by following regurgitation of HRP, which had been preinternalized into early endosomes for a short (5 min) period of time. At the permissive temperature, recycling was rapid, ∼60% of HRP being regurgitated within 10 min (Fig. 2 C), in good agreement with previous observations (Besterman et al., 1981; Parton et al., 1992 b). At the restrictive temperature, however, ∼80% of preinternalized HRP was regurgitated within 10 min. These observations suggest that when intracellular HRP accumulation was impaired, the endosomal content was recycled back into the medium.
Inhibition of Early to Late Endosome Transport at the Restrictive Temperature
Our previous data showed that βCOP is present on early endosomes and is required for the formation of vesicles that mediate transport from early to late endosomes in vitro (Aniento et al., 1996). We therefore investigated whether early to late endosome transport still occurred in ldlF cells incubated at the restrictive temperature for 6 h in vivo. Early endosomes were labeled with HRP internalized for 5 min from the medium. To label late endosomes, HRP was subsequently chased for 30 min in marker-free medium (Gruenberg and Howell, 1989; Aniento et al., 1993). The subcellular distribution of HRP was analyzed by subcellular fractionation (Fig. 4), as well as by light (Fig. 3) and electron (Figs. 7 and 8) microscopy.
Figure 4.
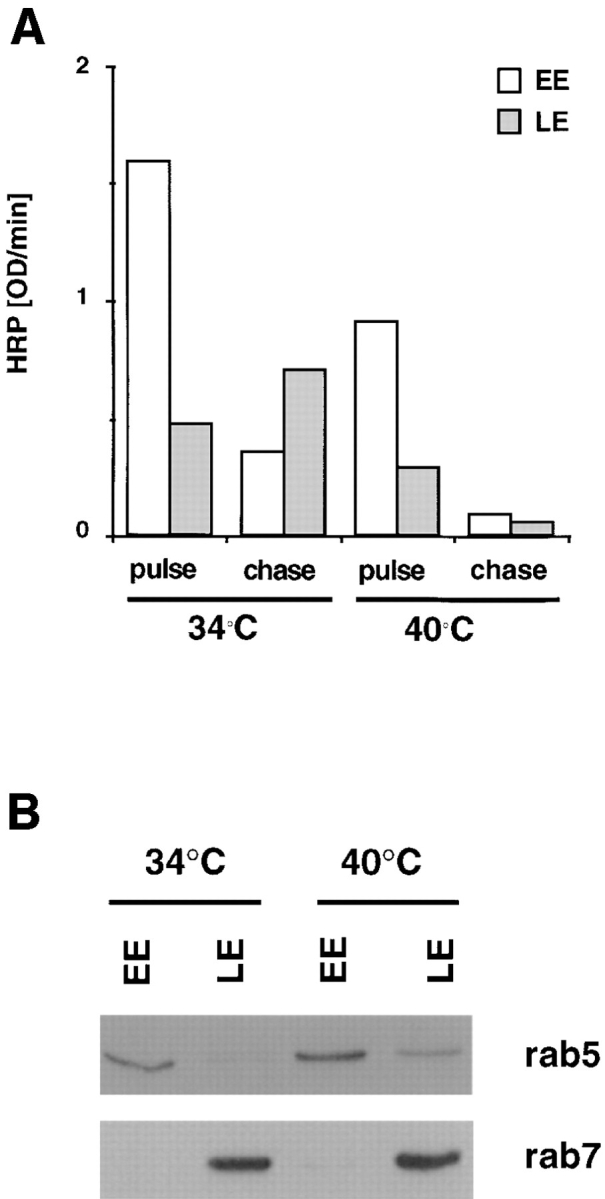
Subcellular fractionation of ldlF endosomes. (A) Cells prepared as in Fig. 3 were incubated at the permissive (34°C) or restrictive (40°C) temperature with medium containing 4 mg/ml HRP for 5 min (pulse) or subsequently reincubated for 40 min in the absence of marker (chase). Early (EE) and late (LE) endosomes were then separated by subcellular fractionation. The total HRP content of the fractions was quantified using a colorimetric reaction and is expressed as OD U/min. HRP recovery in the fractions corresponded to ∼40% of the total, latent cell-associated activity (∼50% lost to the nuclear pellet after gentle homogenization) and HRP latency after homogenization was always >70%. (B) The recovery of rab5 and rab7 in fractions (EE or LE at each temperature) prepared as in A was analyzed by SDS gel electrophoresis, followed by Western blotting with indicated antibodies. Each lane contained 20% of the total protein in the corresponding fraction.
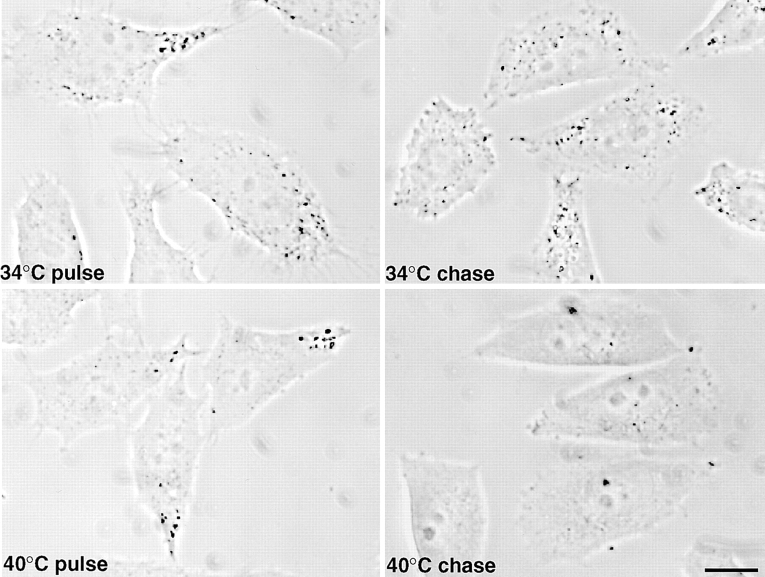
Figure 3.
Distribution of endocytosed HRP in ldlF cells. Cells were maintained at the permissive (34°C) or restrictive (40°C) temperature for 6 h. Then, they were incubated at the corresponding temperature in the presence of 10 mg/ml high activity HRP for 5 min to label early endosomes (pulse). Late endosomes were labeled after reincubation of the cells for 30 min in the absence of HRP, at the corresponding temperature (chase). After cell fixation, intracellular HRP distribution was revealed using a cytochemical reaction and analyzed by phase contrast light microscopy. Bar, 10 μm.
Figure 7.
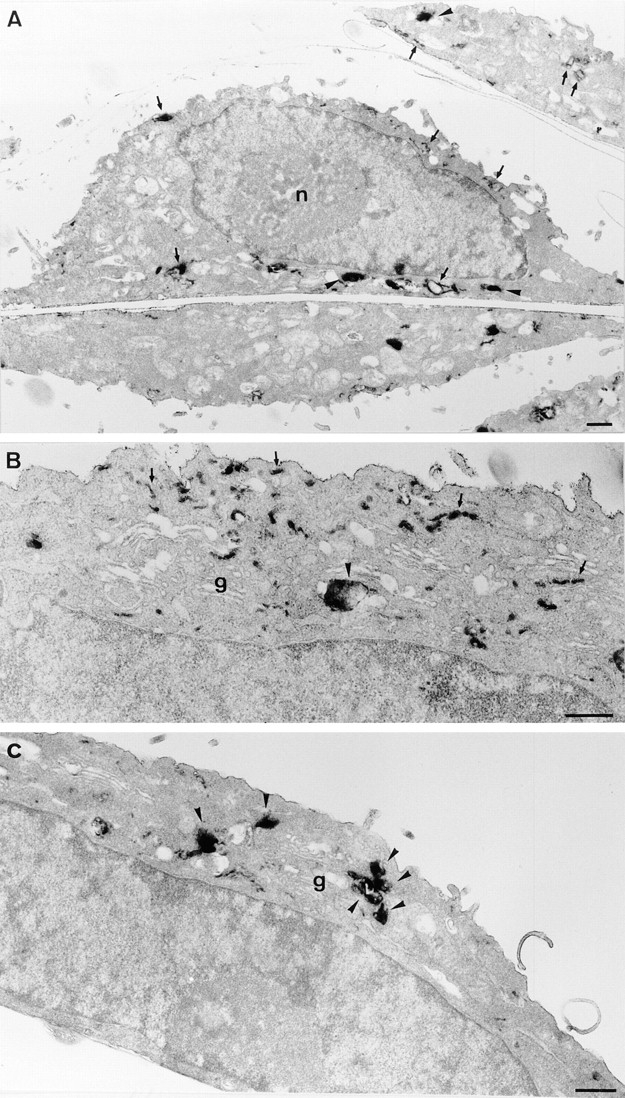
Ultrastructure of ldlF endosomes at the permissive temperature. Cells cultured at 34°C were incubated with HRP for 5 min to label early endosomes and then either fixed immediately (A and B) or further incubated at 34°C for 30 min to label late endosomes (C). Cells were then processed for plastic sections, and semithick (∼150 nm; A and C) or ultrathin sections (50 nm; B) were prepared. Early endosomal compartments (A and B) are comprised of tubular and cisternal regions (arrows) and vesicular domains (arrowheads), as in other cells. B shows a higher magnification view of the Golgi (g) area. After further incubation for 30 min (C), HRP was rarely observed within tubular domains. As expected, it was distributed within larger multivesicular elements concentrated in the Golgi area (arrowheads), which presumably correspond to late endosomes. Bars, 0.5 μm.
Figure 8.
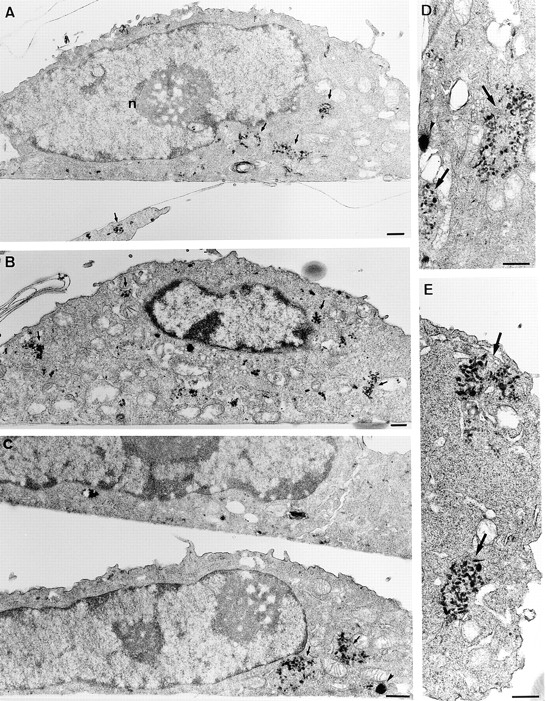
Ultrastructure of ldlF endosomes at the restrictive temperature. Cells cultured at 40°C for 6 h were incubated with HRP for 5 min and then either fixed immediately (A and B) or further incubated at 40°C for 30 min (C–E). Semithick (A and C) or ultrathin (B, D, and E) sections of the cell pellet were prepared as in Fig. 8. Early endosomal compartments (A and B) were composed of small tubular and vesicular elements that were predominantly in discrete clusters (arrows). Few large vesicular profiles were evident (compare with Fig. 7, A and B). After further incubation for 30 min, little HRP remained in the cells (see Figs. 2–4). However, when detected (C–E), the bulk of HRP was still observed within clusters of tubular and vesicular elements (arrows), which appeared identical to those labeled after the 10 min pulse. Few vesicular elements were labeled (arrowhead). Note the clear difference when compared with cells incubated for the same time at the permissive temperature (Fig. 7 C). As shown at higher magnification (D and E), labeled elements comprise vesicles and short tubules, which appear discontinuous from the analysis of both semithick (C) and thin (D and E) sections. Bars, 0.5 μm.
After the 5-min pulse, HRP was internalized within elements with the typical peripheral location of the early endosome (Fig. 3), both at the permissive and restrictive temperatures. After the chase at the permissive temperature, HRP redistributed, as expected, to structures that were often clustered in the perinuclear region, corresponding to late endosomes (see Fig. 7). In contrast, little marker was found in the cells after chase at the restrictive temperature (Fig. 3). These observations were confirmed by fractionation on a step sucrose gradient. Both at the permissive and restrictive temperatures, HRP, which had been internalized for 5 min (Fig. 4 A), cofractionated with rab5 (Fig. 4 B), an early endosomal marker (Chavrier et al., 1990). Consistent with our HRP uptake experiments (Fig. 2 B), cells incubated at the restrictive temperature contained ∼60– 70% of the HRP internalized at the restrictive temperature. After the chase at the permissive temperature, the bulk of HRP (Fig. 4 A) then cofractionated with rab7 (Fig. 4 B), a late endosomal marker (Chavrier et al., 1990), as expected. However, after the chase at the restrictive temperature, HRP was reduced both in the total cell extracts and in the late endosomal fractions (Fig. 4 A) containing rab7 (Fig. 4 B). These data indicate that the physical properties of early and late endosomes were not affected by the temperature shift. These observations also show that HRP, which was internalized at the restrictive temperature, was not transported to late endosomes.
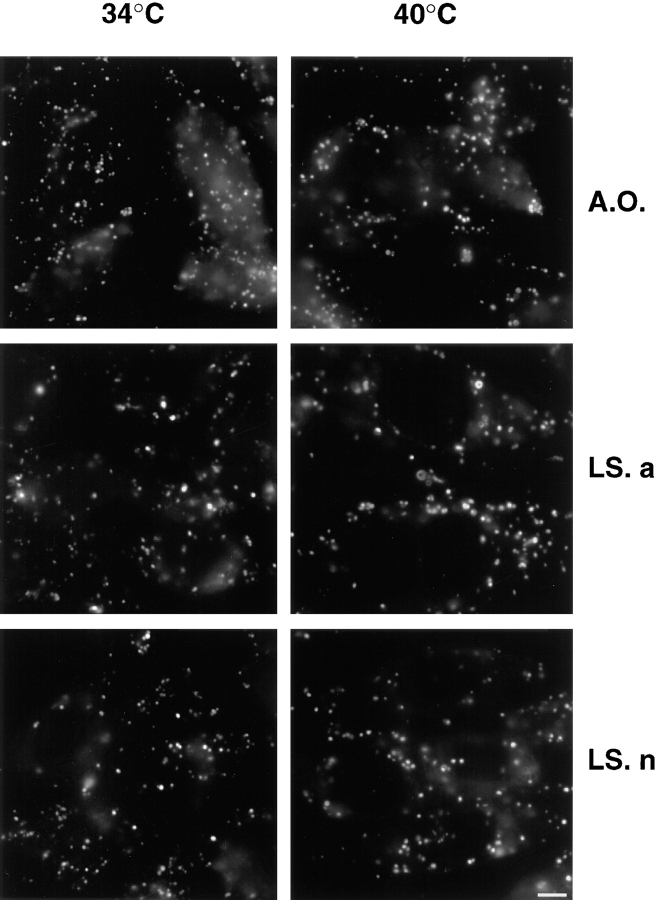
In our previous studies, we had observed that neutralization of the endosomal pH also caused inhibition of early to late endosome transport (Aniento et al., 1996; Clague et al., 1994). We thus investigated whether ldlF cells exhibited an acidification defect at the restrictive temperature, using the pH-sensitive dyes acridine orange and two forms of LysoSensor, which detect pH values in the 4.5– 6.0 and 6.5–8.0 ranges, respectively. As shown in Fig. 5, no difference could be observed between cells incubated at the permissive or restrictive temperature for 6 h. In fact, we did not observe any difference with anyone of the three dyes when cells were incubated at permissive or restrictive temperature over a time period ranging from 1 to 12 h (not shown). These experiments show that, after εCOP degradation, major differences in the acidification properties of endosomes and lysosomes could not be detected using these dyes. Altogether, our biochemical and morphological data indicate that transport from early to late endosomes is inhibited in vivo, at the restrictive temperature.
Figure 5.
Acidification in ldlF cells. Cells prepared as in Fig. 3 at the permissive or restrictive temperature were treated with acridine orange (A.O.), LysoSensor acidic (detection range pH 4.5–6; LS. a) and LysoSensor neutral (detection range pH 6.5–8; LS. n) for 10 min to reveal acidic compartments. The intrinsic fluorescence of each dye after accumulation within acidic endosomes and lysosomes was observed by fluorescence microscopy. Bar, 5 μm.
Disruption of Early Endosome Organization at the Restrictive Temperature
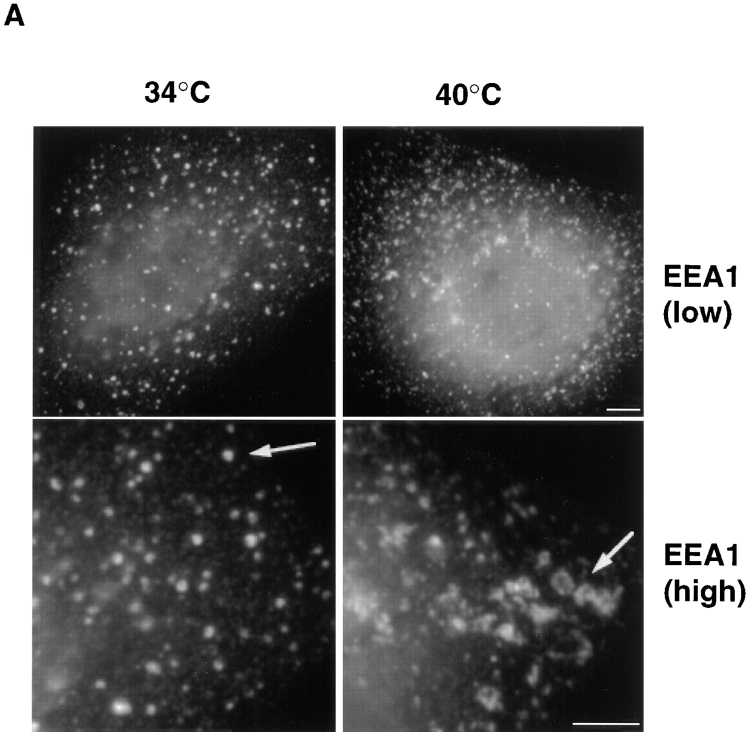
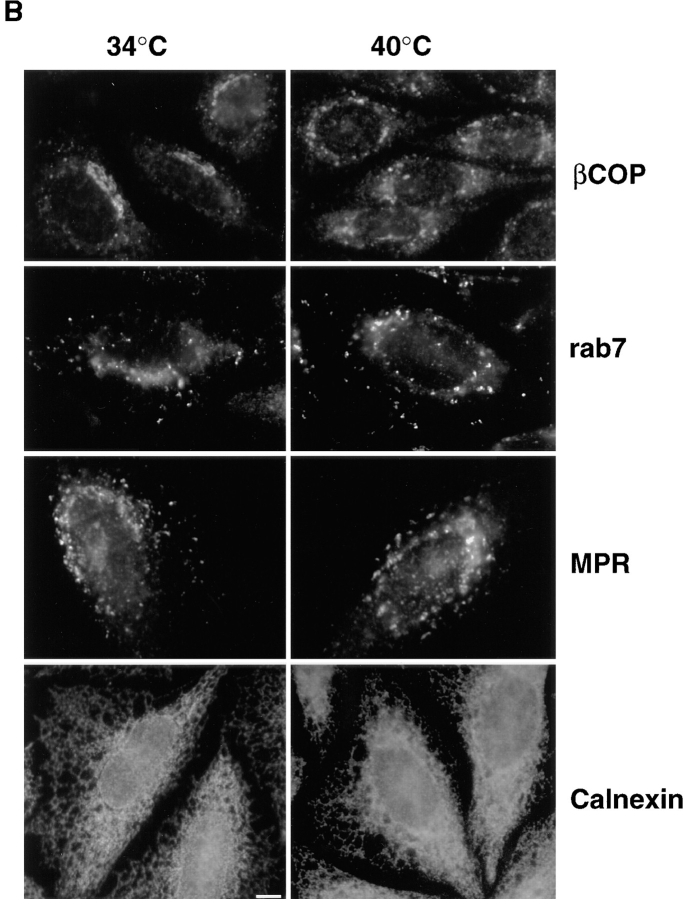
We then investigated whether the organization of endosomes was affected in the absence of εCOP. First, we used antibodies against EEA1, a recently discovered protein that localizes to early endosomal vesicles (Mu et al., 1994). At the permissive temperature, EEA1-positive elements exhibited a highly punctate distribution (Fig. 6 A), which is characteristic for this protein. Although the overall topology was similar at the restrictive temperatures, EEA1- labeled elements then appeared more clustered or tubular even at the light microscopy level, as illustrated in higher magnification images (Fig. 6 A). In contrast, the distribution of a late endosomal marker, the small GTPase rab7 (Chavrier et al., 1990), remained unchanged after incubation at the restrictive temperature for 6 h (Fig. 6 B), as was the distribution of the cation-independent mannose-6-phosphate receptor, which localizes primarily to late endosomes and TGN (Brown et al., 1986; Griffiths et al., 1988), and calnexin, a marker of the endoplasmic reticulum (Wada et al., 1991). As reported by Guo et al. (1994), we also observed some redistribution of βCOP at the restrictive temperature, using an antibody that recognizes primarily biosynthetic βCOP by immunofluorescence.
Figure 6.
Distribution of different markers in ldlF cells. (A) Cells prepared as in Fig. 3 were processed for immunofluorescence microscopy using human antiserum against EEA1. Arrows point at changes in the appearance of early endosomal elements at 40°C, when compared with 34°C. low, low magnification; high, high magnification. (B) Cells were processed for immunofluorescence microscopy as in A using the maD antibody against βCOP or a rabbit antiserum against either the Man6P-R, rab7, or calnexin. The maD antibody does not label endosomal βCOP to any significant extent under these conditions. Bars, 5 μm.
To further investigate the effects of εCOP degradation on early endosome ultrastructure, cells containing internalized HRP were analyzed by electron microscopy. At the permissive temperature (Fig. 7), HRP distributed within early endosomes after 5 min and reached structures with the typical appearance and topology of late endosomes after the chase, as expected. At the restrictive temperature (Fig. 8), however, the structure of early endosomes containing HRP endocytosed for 5 min was dramatically changed into characteristic clusters of thin 50-60 nm tubules lacking multivesicular domains. These appeared essentially identical to early endosomes observed after neutralization of the endosomal pH (Clague et al., 1994). In addition, little, if any, HRP could be detected in late endosomes (and elsewhere in the cells) after the chase, in agreement with our biochemical and light microscopy data. However, in the rare cases where some HRP could still be detected intracellularly, the marker had remained primarily within tubular clusters (Fig. 8 C-E), identical to those labeled after the pulse. Altogether these data show that degradation of εCOP at the restrictive temperature has little influence on bulk internalization into and recycling from early endosomes, but inhibits early to late endosome transport, and causes a dramatic change in the general organization of early endosomal elements.
Inhibition of ECV/MVB Formation in the Absence of εCOP
We have previously described an in vitro assay that measures the formation of ECV/MVBs from donor early endosomal membranes prepared from BHK cells (Aniento et al., 1996). Using this assay, we found that vesicles formed in vitro excluded early endosomal markers and that vesicle formation depended on βCOP. Here, we used the same assay to test whether cytosol prepared from ldlF cells incubated at the restrictive temperature supported ECV/MVB formation in vitro. Early endosomes were prepared from BHK cells to ensure that donor membranes were fully transport-competent (Aniento et al., 1996). BHK cells were incubated for 5 min at 37°C in the presence of HRP to label the early endosomal content. Early endosomes were then separated from the lighter ECV/MVBs and late endosomes by flotation on a step sucrose gradient (Aniento et al., 1993, 1996). In the assay, early endosomes were incubated at 37°C in the presence of ATP and cytosol, and then vesicles that may have formed in vitro were separated from the denser donor membranes on a similar flotation gradient. The percentage of the total early endosomal content entrapped within vesicles formed in the assay was quantified by measuring the HRP activity in both fractions.
As shown in Fig. 9 A, cytosol prepared from ldlF cells incubated at the permissive temperature (34°C) or from WT CHO cells incubated at 37°C supported ECV/MVB formation from donor membranes, and the process was ATP-dependent as observed previously (Aniento et al., 1996). Approximately 10% of the tracer was packaged within ECV/MVBs formed in the assay, a value that compares well with our previous in vitro and in vivo observations (Aniento et al., 1996). In contrast to donor early endosomal membranes, ECV/MVBs formed in vitro acquired the capacity to undergo fusion with late endosomes (Aniento et al., 1993, 1996). They contained both β and εCOP (Fig. 9 B) but excluded the early endosomal marker rab5, in agreement with our previous studies (Aniento et al., 1996). These experiments were repeated using cytosol prepared from WT CHO cells incubated at 40°C, corresponding to the ldlF cell restrictive temperature, as a control (Fig. 9). Then, a slight but reproducible increase in ECV/MVB formation was observed, presumably reflecting temperature effects on the activity of some cytosolic factors. In contrast, ECV/MVB formation was inhibited when using cytosol prepared from ldlF cells incubated at 40°C. Inhibition was not complete, perhaps because BHK membranes retained some endogenous εCOP after fractionation (Aniento et al., 1996) or because some forming ECV/MVBs were already committed, beyond the COP-dependent step, at the time of homogenization. Indeed, similar levels of inhibition were observed after depletion of cytosolic coatomer (Aniento et al., 1996).
Figure 9.
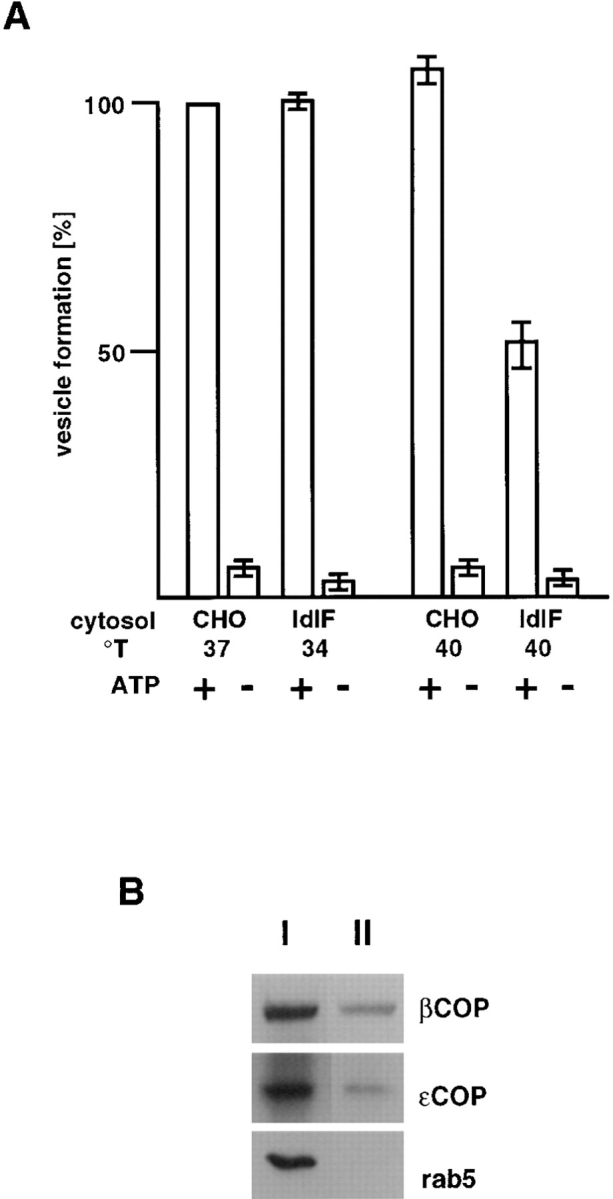
In vitro formation of ECV/MVBs. (A) BHK early endosomes containing HRP internalized for 5 min at 37°C were prepared by flotation on a sucrose gradient and used as donor membranes in the assay (Aniento et al., 1996). Donor endosomes were incubated with (+) or without (−) an ATP-regenerating system and in the presence of cytosol. Cytosols were prepared, as indicated, from ldlF cells incubated at the permissive (34°C) or restrictive (40°C) temperature, or from WT CHO cells incubated at 37 or 40°C. Vesicles formed in vitro were then separated from donor membranes by flotation in a gradient, and the HRP content of both fractions quantified. In the assay, ∼10% of HRP originally internalized into early endosomes was entrapped within vesicles formed in vitro, as shown previously (Aniento et al., 1996). Efficiency of vesicle formation is expressed as a percentage of the control with cytosol from WT CHO cells incubated at 37°C. (B) The assay was carried out in the presence of cytosol from CHO cells incubated at 37°C. Then, donor endosomes (I) and ECV/MVBs formed in vitro (II) were pelleted and analyzed by SDS gel electrophoresis and Western blotting with antibodies against βCOP, εCOP, and rab5, as indicated. Each lane contained 35% of the protein content of the corresponding fraction.
COP Membrane Binding
The striking similarity between the effects of endosomal pH neutralization and εCOP degradation on both endosome ultrastructure and transport suggests that both mechanisms are coupled functionally. We have previously shown that COPs do not bind endosomal membranes in vivo or in vitro after pH neutralization, presumably reflecting the activity of a transmembrane pH sensor (Aniento et al., 1996). We therefore investigated whether εCOP degradation similarly inhibited membrane binding of the remaining COP subunits, or whether these had retained the capacity to bind endosomes. In these experiments, biosynthetic membranes were used as a control.
We used BHK cells as a source of endosomes to ensure that membranes were fully competent to support COP association (Aniento et al., 1996). Cells were pretreated with brefeldin A to reduce possible contamination of endosomes with biosynthetic membranes (see Pelham, 1991). (All subsequent steps were without the drug.) Cells were then homogenized, and fractions were prepared on a step flotation gradient that separates the bulk of biosynthetic membranes from both early and late endosomes (Gorvel et al., 1991; Aniento et al., 1996). As shown in Fig. 10, the distribution of the small GTPase rab5 was unaffected by brefeldin A. However, BHKp23, a transmembrane protein of the 24-kD family that localizes to the intermediate compartment/cis-Golgi network (Rojo et al., 1997), was no longer detected in the fraction after the treatment (Fig. 10).
Figure 10.
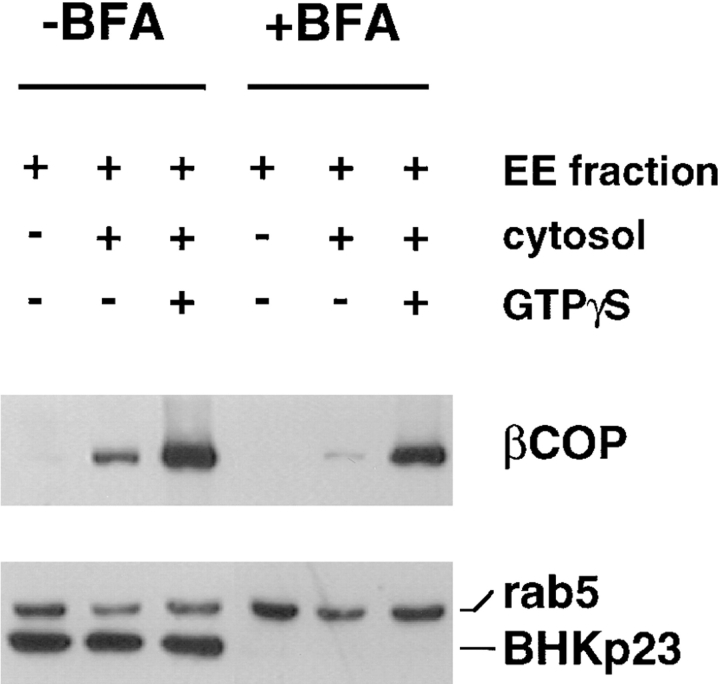
Separation of endosomal and biosynthetic membranes. To achieve optimal conditions for the separation of endosomal and biosynthetic membranes, BHK cells were pretreated with brefeldin A (+BFA). The drug was absent from all subsequent steps. In the control, the drug was omitted (−BFA). After homogenization, the corresponding postnuclear supernatants were fractionated using a sucrose step flotation gradient (Gorvel et al., 1991; Aniento et al., 1996), and fractions enriched in early endosomes were collected. The COP binding capacity of these membranes was measured after incubating 50 μg of each fraction with 500 μg BHK cytosol for 15 min at 37°C in the presence of 10 μM GTPγS, to stimulate COP recruitment. Membranes were then retrieved by flotation on a step gradient and analyzed by SDS gel electrophoresis followed by Western blotting using antibodies against βCOP, rab5, and BHKp23.
We then compared the COP-binding capacity of brefeldin A–treated and untreated early endosomal membranes. The binding assay was initiated by mixing membranes with rat liver cytosol. GTPγS was added to stimulate COP membrane association (Kreis and Pepperkok, 1994; Whitney et al., 1995; Aniento et al., 1996). Membranes were then retrieved by flotation on a step sucrose gradient, and COPs were analyzed by SDS gel electrophoresis and Western blotting. Endogenous βCOP was partially lost during fractionation (Fig. 10) but was revealed after longer exposures of the blot (not shown), as previously observed (Whitney et al., 1995; Aniento et al., 1996). However, after brefeldin A treatment, endosomal membranes retained the capacity to recruit cytosolic COPs, although amounts of bound COPs were reduced when compared with untreated controls, as expected after depletion of biosynthetic membranes (Fig. 10). These membranes were therefore used to test the membrane binding capacity of ldlF COPs.
To our surprise, we observed that COP binding onto endosomal membranes still occurred when εCOP was missing (Fig. 11; EE, early endosome fraction). However, a small COP subset only, consisting of β′, β, and ζCOP, was then recruited onto early endosomes. Previous experiments had shown that γ and δCOP are not present on endosomes (Whitney et al., 1995; Aniento et al., 1996), but in the absence of εCOP, αCOP also failed to interact with endosomes. In contrast, the same cytosol preparation was fully competent to support binding of all subunits, including α, γ, and δCOP, but obviously excluding εCOP, to biosynthetic membranes (Fig. 11; BM, biosynthetic membrane fraction). These experiments demonstrate that some COP subunits, namely β, β′, and ζCOP, are still recruited onto endosomal membranes despite the absence of εCOP. Since this subcomplex did not support membrane transport in vivo (Figs. 3, 4, 7, and 8) or efficient ECV/MVB formation in vitro (Fig. 9), our data suggest that COP function in endosome transport requires the presence of α and/or εCOP. Our data also indicate that recruitment of αCOP onto endosomal membranes, but not biosynthetic membranes, requires the presence of εCOP.
Figure 11.

COP binding to endosomal and biosynthetic membranes. Cytosols were prepared from ldlF cells incubated at the restrictive temperature, as in Fig. 1. The COP binding capacity of biosynthetic (BM) or early endosomal (EE) membranes prepared from BHK cells pretreated with brefeldin A was tested as in Fig 10, except that ldlF cytosol was used with (G and NG) or without (C) 10 μM GTPγS. When indicated (NG), the pH of endosomes was preneutralized with 50 μM nigericin before GTPγS addition. Membranes were retrieved and analyzed using antibodies against each COP subunit. Western blots were developed using the ECL reaction; exposure times were five times longer for EE than for BM membranes to ensure that signals remained in the linear detection range.
Characterization of COP Membrane Binding
Association of COPs to endosomal membranes is inhibited after neutralization of the lumenal pH (Aniento et al., 1996). Neutralization of the pH, however, does not cause the release of pre-bound COPs (not shown), indicating that recruitment only is acidification dependent. That COP association to endosomes involves more than one step is illustrated by the combined effects of pH and GTPγS. COP binding to endosomes was partially inhibited when the endosomal pH was preneutralized with nigericin before addition of GTPγS when compared with GTPγS alone (Fig. 11). The same observations were made using BHK cytosols containing all COP subunits (not shown). The treatment had no effects on biosynthetic membranes, as expected since these compartments are not acidic. The fact that the effects of nigericin and GTPγS were dependent on the order-of-addition suggests that a pH sensor and a GTP-binding protein are sequentially involved in COP binding to endosomal membranes. These experiments also show that COP membrane binding characteristics are fully retained by the β′, β, and ζCOP subset, suggesting that these subunits may mediate association of the coatomer onto endosomes.
Discussion
In this paper, we have studied the role of COP proteins in endosomal membrane transport, using the ldlF cell line with a ts defect in εCOP. At the restrictive temperature, εCOP is degraded, whereas the other subunits are unaffected. Our data show that receptor-mediated endocytosis as well as internalization and recycling of the fluid phase tracer HRP continue at the restrictive temperature but that HRP fails to accumulate intracellularly. Our biochemical and morphological observations indicate that HRP is then internalized into early endosomes, from where it can recycle back to the medium, but that HRP transport from early to late endosomes is inhibited. Concomitant with this transport inhibition, we find that the early endosome ultrastructure is changed into clusters of thin tubules, which lack typical multivesicular domains corresponding to forming ECV/MVBs. That COPs may be directly involved in ECV/MVB biogenesis is supported by our observations that cytosol prepared from ldlF cells incubated at the restrictive temperature, thus lacking εCOP, does not support efficient formation of ECV/MVBs from naive early endosomes of BHK cells. Our data also indicate that COP binding to endosomal and biosynthetic membranes is differentially regulated. In the absence of εCOP, all remaining subunits bind BHK biosynthetic membranes in a pH-independent manner. In contrast, only β, β′, and ζ subunits are recruited onto naive endosomal membranes of BHK cells, and the process depends on the lumenal pH. Since β, β′, and ζCOP retain the characteristics of endosomal COP binding with respect to pH and GTPγS, it is tempting to speculate that these subunits are involved in COP association to endosomes, whereas α and/or εCOP may drive COP function in endosome transport.
Phenotype of ldlF Cells
Originally, Hobbie et al. (1994) observed that the low density lipoprotein (LDL) receptor is relatively unstable in ldlF cells at the restrictive temperature. This processing occurs via an unconventional mechanism since it is insensitive to pH neutralization, in contrast to degradation in lysosomes and to the phenotype of other mutant CHO cell lines, where the LDL receptor is equally unstable. One possible explanation for LDL receptor instability in ldlF cells comes from our observations that the early endosomal content of the lysosomal enzyme β-N-acetyl-glucosaminidase is increased approximately twofold 6 h after temperature shift when compared with unshifted controls (not shown). The presence and activity of hydrolases in mildly acidic early endosomes has been previously reported (Diment and Stahl, 1985; Ludwig et al., 1991). Increased amounts of enzymes may be due to recapture from the medium (Kornfeld, 1992) combined with defective transport to late endosomes. Then, recycling LDL receptor molecules may become progressively processed during their cycle, while passing through early endosomes.
COP-I and Endosome Organization/Dynamics
Within minutes after internalization into early endosomes, molecules destined to be recycled or degraded are rapidly segregated into separate elements (Trowbridge et al., 1993; Gruenberg and Maxfield, 1995; Mellman, 1996). Whereas tubular elements are involved in membrane recycling back to the cell surface, molecules destined to be degraded are selectively incorporated within multivesicular portions (Geuze et al., 1983), which correspond to forming ECV/ MVBs (Aniento et al., 1993, 1996). Very little is known about the mechanisms controlling the morphogenesis of early endosomal membranes into elements with such a strikingly different organization. We find that the absence of a functional COP coat inhibits ECV/MVB biogenesis and therefore also inhibits the accumulation of internal membranes and transport from early to late endosomes. We also find that early endosomal membranes are then changed into typical clusters of thin tubules. Similarly, in the biosynthetic pathway, COPs are required for transport, but also for maintenance of Golgi organization (see Guo et al., 1994; Kreis and Pepperkok, 1994).
The existence of a close relationship between endosome ultrastructure and ECV/MVB biogenesis is further strengthened by our previous observations that neutralization of the endosomal pH causes very similar effects. Then, early endosomes form clusters of thin tubules (50–60-nm diameter) that typically lack multivesicular domains, and both ECV/MVB formation and transport to late endosomes are inhibited (Clague et al., 1994; Aniento et al., 1996). Our data indicate that both pH- and COP-mediated effects are, in fact, coupled functionally. COP association to endosomal membranes is itself pH dependent, both in vivo and in vitro (Aniento et al., 1996), as is the recruitment of the β, β′, and ζCOP subcomplex in the absence of εCOP. Moreover, endosome dynamics, including ultrastructural organization and transport, appear to be affected in the same way when the COP coat is absent after pH neutralization or when a nonfunctional coat is recruited after εCOP degradation. We can conclude that maintenance of early endosome membrane organization and ECV/MVB biogenesis are coupled and that these processes are controlled by the pH-dependent association of a functional COP coat. We previously proposed that pH-dependent COP association may reflect the activity of a transmembrane pH sensor (Aniento et al., 1996). We propose that this pH sensor functions as a molecular checkpoint signaling, via coat recruitment, the onset of the degradation pathway.
Recent studies indicate that, in the biosynthetic pathway, COPs can interact with the cytoplasmic domains of transmembrane proteins (Cosson and Letourneur, 1994; Fiedler et al., 1996; Sohn et al., 1996), suggesting that COPs perform sorting functions, much like the clathrin/ adaptor coats (Pearse and Robinson, 1990; Robinson, 1992). A morphologically visible coat, resembling the biosynthetic COP coat (Orci et al., 1986), has not been detected yet on endosomes, perhaps because of the different composition of endosomal and biosynthetic COPs (Whitney et al., 1995; Aniento et al., 1996). One may, however, speculate that endosomal COPs mediate the selection of proteins destined to be incorporated into forming ECV/MVBs. Indeed, this process is undoubtedly selective, presumably depending on cytoplasmically exposed protein motives (see Gruenberg and Maxfield, 1995). In line with this view, βCOP could be detected at the neck of forming ECV/ MVBs in vitro (Aniento et al., 1996). It is possible that inhibition of this sorting mechanism, via COP inactivation, prevents ECV/MVB biogenesis and therefore also prevents accumulation of internal membranes.
COP-I Subunits Involved in Membrane Binding and Transport
Our data indicate that the β, β′, and ζCOP subunits interact with one or more endosomal membrane proteins, including perhaps the pH sensor itself; our data also indicate that these subunits are selectively recruited from a complete cytosol when εCOP is missing. This recruitment exhibits the characteristic properties of endosomal COP binding, namely pH and GTPγS sensitivity. COP subcomplexes that may mediate membrane association to biosynthetic membranes have been identified. The α, β′, and ε subunits were shown to bind membranes and cytoplasmic KKXX motifs (Lowe and Kreis, 1995). In addition, different COP subcomplexes appear to bind to different peptides derived from the cytoplasmic domains of members of the 20–25-kD protein family (Fiedler et al., 1996). This apparent heterogeneity of COP subcomplexes involved in membrane association, whether in biosynthesis or endocytosis, may reflect the existence of multiple interactions between COPs and different protein motives.
Our data also suggest that COP association to endosomal membranes occurs in more than one functional step. Indeed, neutralization of the pH inhibits COP binding but does not cause the release of prebound COPs. Moreover, pH neutralization inhibits the stimulatory effects of GTPγS on COP recruitment, suggesting that a transmembrane pH sensor (Aniento et al., 1996) and a GTP-binding protein, perhaps of the ARF family, are involved sequentially during COP recruitment. We also find that εCOP is necessary for αCOP recruitment onto endosomal but not biosynthetic, membranes. That these two proteins may be functionally or physically linked is consistent with our observation that cellular amounts of αCOP, but not other COPs, are decreased when εCOP is degraded. Our data also show that COP membrane association and function can be uncoupled. Whereas β, β′, and ζCOP can interact with membrane components, perhaps via a receptor, the α and εCOP subunits are essential for function, possibly reflecting the coat polymerization step. In conclusion, we propose that formation of the COP coat onto endosomal membranes may occur in at least three distinct steps. Membrane recruitment may be mediated by the β, β′, and ζ subunits through the sequential involvement of a pH sensor and a GTP-binding protein. ECV/MVB biogenesis, however, may be dependent on the presence of α and/or εCOP.
Acknowledgments
This work was supported by grant No. 31-37296.93 from the Swiss National Science Foundation (to J. Gruenberg), grant No. 961235 from the National Health and Medical Research Council of Australia (to R.G. Parton), and grant RG 355/94 from the International Human Frontier Science Program (to J. Gruenberg and R.G. Parton).
Footnotes
1. Abbreviations used in this paper: COP, coatomer protein; ECV, endosomal carrier vesicles; HB, homogenization buffer; LDL, low density lipoprotein; Man6P-R, mannose-6-phosphate receptor; MVB, multivesicular body; PNS, postnuclear supernatant; WT, wild-type.
We sincerely wish to thank Marie-Hélène Beuchat for her precious technical help. We wish to thank Monty Krieger for providing us with the ldlF cell line. We also wish to thank Ban-Hock Toh for the gift of anti-EEA1 antibodies, Bernard Hoflack for antibodies against the cation-independent mannose-6-phosphate receptor, Cordula Hater and Felix Wieland for the gift of antibodies against COPs, Jim Rothman for anticoatomer antibodies, Ari Helenius for anticalnexin antibodies, and Thomas Kreis for monoclonal antibodies against βCOP. We are also grateful to Thomas Harder and Volker Gerke for supplying us with the human transferrin receptor cDNA. We wish to thank Monty Krieger, Thomas Kreis, Gisou van der Goot, Katherine Bowers, and all members of the group for critically reading the manuscript and for helpful suggestions.
Address all correspondence to Jean Gruenberg, Biochemistry Department, 30 quai E. Ansermet, 1211-Geneva-4, Switzerland. Tel. and Fax (same number): +41-22-702.6464. E-mail: jean.gruenberg@biochem.unige.ch
F. Aniento's new address is Department Bioquimica y Biologia Molecular, Facultad de Farmacia, Universidad de Valencia. c/ Vicent Andrés Estellés, Burjassot (Valencia), Spain.
References
- Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1388. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton R, Gruenberg J. An endosomal βcop is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman JM, Airhart JA, Woodworth RC, Low RB. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981;91:716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule and motor dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Goodhouse J, Farquhar MG. Mannose-6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol. 1986;103:1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localisation of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Clague M, Urbé S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Diment S, Stahl P. Macrophage endosomes contain proteases which degrade endocytosed protein ligands. J Biol Chem. 1985;260:15311–15317. [PubMed] [Google Scholar]
- Dunn WA, Conolly TP, Hubbard A. Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes: receptor pathway. J Cell Biol. 1986;102:24–36. doi: 10.1083/jcb.102.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF–EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze HJ, Slot JW, Strous GJAM, Lodish HF, Schwartz AL. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab 5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose-6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Gruenberg, J., and J.-P. Gorvel. 1992. In vitro reconstitution of endocytic vesicle fusion. In Protein Targetting, a Practical Approach. A.I. Magee and T. Wileman, editors. Oxford University Press, Oxford, UK. 187–216.
- Gruenberg J, Howell KE. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield F. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg JE, Howell KE. Reconstitution of vesicle fusions occurring in endocytosis with a cell-free system. EMBO (Eur Mol Biol Organ) J. 1986;5:3091–3101. doi: 10.1002/j.1460-2075.1986.tb04615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Vasile E, Krieger M. Disruption in Golgi structure and membrane traffic in a conditional lethal cell mutant are corrected by ε-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Penman M, Trigatti BL, Krieger M. A single point mutation in ε-COP results in temperature-sensitive, lethal defects in membrane transport in a Chinese hamster ovary cell mutant. J Biol Chem. 1996;271:11191–11196. doi: 10.1074/jbc.271.19.11191. [DOI] [PubMed] [Google Scholar]
- Harder T, Gerke V. The subcellular distribution of early endosomes is affected by the annexin II(2) p11(2) complex. J Cell Biol. 1993;123:1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Fisher AS, Lee S, Flint A, Krieger M. Isolation of three classes of conditional lethal Chinese hamster ovary cell mutants with temperature-sensitive defects in low density lipoprotein receptor. J Biol Chem. 1994;269:20958–20970. [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose-6-phosphate/insulin-like growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Koval M, Pagano RE. Lipid recycling between the plasma membrane and intracellular compartments: transport and metabolism of fluorescent sphingomyelin analogues in cultured fibroblasts. J Cell Biol. 1989;108:2169–2181. doi: 10.1083/jcb.108.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE. Micro-injected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO (Eur Mol Biol Organ) J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Pepperkok R. Coat proteins in intracellular membrane transport. Curr Opin Cell Biol. 1994;6:533–537. doi: 10.1016/0955-0674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed]
- Letourneur F, Gaynor E, Hennecke S, Démollière C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the ER. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lowe M, Kreis TE. In vitro assembly and disassembly of coatomer. J Biol Chem. 1995;270:31364–31371. doi: 10.1074/jbc.270.52.31364. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Griffiths G, Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991;115:1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–626. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mu F-T, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo J-P, Tock EPC, Toh B-H. EEA1, an early endosome-associated protein. EEA1 is a conserved α-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1994;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman JE. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Pagano RE, Martin OC, Kang HC, Haugland RP. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J Cell Biol. 1991;113:1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Schrotz P, Bucci C, Gruenberg J. Plasticity of early endosomes. J Cell Sci. 1992;103:335–348. doi: 10.1242/jcs.103.2.335. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dentritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B, Robinson MS. Clathrin, adaptors and sorting. Annu Rev Cell Biol. 1990;6:151–172. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Pelham HRB. Multiple targets for brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis T. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Robinson MS. Adaptins. Trends Cell Biol. 1992;2:293–297. doi: 10.1016/0962-8924(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Robinson, L., F. Aniento, and J. Gruenberg. 1997. Transport from early to late endosomes depends on the N-ethylmaleimide (NEM) sensitive fusion protein (NSF). J. Cell Sci. In press. [DOI] [PubMed]
- Rojo M, Pepperkok R, Emery G, Kellner R, Stang E, Parton RG, Gruenberg J. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J Cell Biol. 1997;139:1119–1135. doi: 10.1083/jcb.139.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe DM, Murphy RF. High-resolution kinetics of transferrin acidification in BALB/c 3T3 cells: exposure to pH 6 followed by temperature-sensitive alkalinization during recycling. Proc Natl Acad Sci USA. 1987;84:7119–7123. doi: 10.1073/pnas.84.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M. Tubular early endosomal network in AtT20 and other cells. J Cell Biol. 1991;115:635–654. doi: 10.1083/jcb.115.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]
- Wada I, Rindness D, Cameron PH, Ou WJ, Doherty LL, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJM. SSRα and associated calnexin are major Ca2+-binding proteins of the endoplasmic reticulum. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.4) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Zerial M, Suomalainen M, Zanetti-Schneider M, Schneider C, Garoff H. Phosphorylation of the human transferrin receptor by protein kinase C is not required for endocytosis and recycling in mouse 3T3 cells. EMBO (Eur Mol Biol Organ) J. 1987;9:2661–2667. doi: 10.1002/j.1460-2075.1987.tb02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]