Abstract
Cells migrating within tissues may encounter multiple chemoattractant signals in complex spatial and temporal patterns. To understand leukocyte navigation in such settings, we have explored the migratory behavior of neutrophils in model scenarios where they are presented with two chemoattractant sources in various configurations. We show that, over a wide range of conditions, neutrophils can migrate “down” a local chemoattractant gradient in response to a distant gradient of a different chemoattractant. Furthermore, cells can chemotax effectively to a secondary distant agonist after migrating up a primary gradient into a saturating, nonorienting concentration of an initial attractant. Together, these observations suggest the potential for cells' step-by-step navigation from one gradient to another in complex chemoattractant fields. The importance of such sequential navigation is confirmed here in a model system in which neutrophil homing to a defined domain (a) requires serial responses to agonists presented in a defined spatial array, and (b) is a function of both the agonist combination and the sequence in which gradients are encountered. We propose a multistep model of chemoattractant-directed migration, which requires that leukocytes display multiple chemoattractant receptors for successful homing and provides for combinatorial determination of microenvironmental localization.
Cells migrating in a tissue encounter many different signals that can potentially direct their path. Neuronal migration is a well-characterized example; neurons weave a lengthy, tortuous, and precise path through the body as they are guided by attractants, repellents, and adhesion molecules. This complex task is simplified because cells travel in a series of short trajectories, negotiating direction at “choice points” along their journey (21). Like neurons, cells of the immune system require very accurate positioning within tissues to perform their biological functions. However, little is known about how leukocytes navigate through the complex environments they encounter upon entering a lymphoid organ or target tissue. In this study, we investigate how leukocytes behave in complex migratory environments. We have focused on one class of leukocyte guidance cues—the increasingly diverse family of leukocyte chemoattractants.
The number of known leukocyte attractants has expanded in recent years with the discovery of increasing numbers of chemokines (for review see 14, 16). Like classical leukocyte attractants, chemokines are small molecules that bind to G protein–coupled receptors on leukocytes and mediate directional migration when presented in a concentration gradient. Each leukocyte type has the capacity to respond to multiple different chemokines and/or classical attractants. Furthermore, many of the attractants that act on a given cell may be present together in a leukocyte-recruiting tissue. One classical example is a site of bacterial infection, in which a variety of neutrophil chemoattractants are produced by different sources: host endothelial, epithelial, and stromal cells produce arrays of attractants including interleukin-8 (IL-8)1, GRO-α, and others; complement deposition on pathogens releases the attractant C5a; invading bacteria generate N-formyl peptides, which are neutrophil attractants; and activated neutrophils themselves produce attractants (for review see reference 8). Recent reports reveal many analogous examples of differential chemokine expression by different cell types in a range of leukocyte-recruiting tissues (1, 9, 10, for review see references 19, 25). Therefore, once it has entered a tissue, a leukocyte requires mechanisms to integrate the various directional signals it receives from different attractants such that it arrives at its correct destination.
Heterologous desensitization of chemoattractant receptors may be one important mechanism whereby migrating cells integrate information from different chemoattractants (4, 12). Several studies have shown that treating leukocytes with certain chemoattractants diminishes the cells' ability to respond to other attractants. Responses to one attractant that can be suppressed by others include intracellular signaling events (7, 17, 18, 22, 26), cell adhesion (4), orientation (6), actin polymerization (12), and cell migratory behavior (4, 12). Based on observations in neutrophils, we postulated that this cross-inhibition of migratory responses might allow cells to prioritize signals from their end targets over more general recruitment signals from host cells (4).
In this study, we investigate the decision making processes whereby leukocytes navigate through complex fields of overlapping chemoattractant gradients, such as those cells might encounter within tissues. We focus on the spatial guidance of cells through different agonist arrays, in situations that do not involve cross-inhibition as well as in situations that do. We use neutrophils as a model of leukocyte migratory behavior. We have examined neutrophils' responses to four well-characterized attractants produced at sites of inflammation, focusing not only on the regulatory cell–derived lipid leukotriene B4 (LTB4) and the chemokine IL-8, but also including the “classical,” end target–derived attractants, bacterial-derived formyl peptides and complement fragment C5a. Based on our observations, we propose that leukocytes can navigate through complex chemoattractant fields by migrating in a step- by-step manner in response to one agonist source after another, so that their ultimate positions are determined combinatorially by the array of receptors they express and by the sequence of chemoattractant gradients they encounter.
Materials and Methods
Cell Preparation
Blood was collected from healthy adult volunteers. Neutrophils were prepared using conventional 1% dextran sedimentation to remove erythrocytes, followed by Ficoll density gradient centrifugation (Pharmacia Biotech Sevrage, Uppsala, Sweden). Residual erythrocytes were lysed by resuspending in hypotonic salt solution (0.2% NaCl), followed by hypertonic rescue (equivolume 1.6% NaCl). The resulting granulocyte preparation was ⩾95% neutrophils as assessed by histological staining. Neutrophils were resuspended at 107 cells/ml in migration medium (see below) and kept on ice until the start of the assay. All procedures were performed with endotoxin-free solutions.
Under-agarose Assay
Agarose Gel.
The procedures of Nelson et al. (see reference 15) were adapted for our experiments. Falcon 3001 tissue culture dishes (Falcon Plastics, Cockeysville, MD) were filled with 3 ml each of an agarose solution (50:50 HBSS/H2CO3-buffered RPMI-1640 (Sigma Chemical Co., St. Louis, MO), 10% heat-inactivated bovine calf serum, 1.2% agarose (GIBCO BRL, Gaithersburg, MD). After the gel had solidified, the bicarbonate-buffered plates were allowed to equilibrate to physiological temperature and pH in a 37°C CO2 incubator.
Wells were cut in the standard configurations as follows. Guided by a template, 3.5-mm-diam holes were cut 2.2 mm apart in the agarose gel, using a plastic pipette tip attached to a vacuum line to remove the cylindrical plugs.
Migration Medium.
Cells and agonists were suspended in RPMI-1640 + 10% heat-inactivated bovine calf serum (serum inactivated at 57°C, 25 min).
Chemoattractants.
f-Met-Leu-Phe (Sigma Chemical Co.), recombinant human complement fragment C5a (Sigma Chemical Co.), LTB4 (Sigma Chemical Co.), and recombinant human IL-8, a gift from A. Rot (Sandoz Forschungsinstitut, Vienna, Austria).
Preparations for Video Microscopy.
For video microscopy, agarose plates were prepared as described, but with 1.2% agarose in RPMI-1640 without bicarbonate (Sigma Chemical Co.), 10 mM Hepes, pH 7.4, and 10% heat-inactivated bovine calf serum. Migration assays were performed in a humidified chamber, kept at 37°C with a stage warmer.
Assay Procedures
At the start of the standard migration assay, four “cell wells” (experimental wells containing cells) surrounding a central attractant well were each filled with 105 neutrophils (vol = 10 μl). 10 μl of chemoattractant was placed in the central well, and 10 μl of medium alone was placed in four peripheral control wells. The same results were obtained when only two cell wells, on either side of the attractant well, were filled, indicating that our results do not reflect interactions between cells in different cell wells on a plate. Once plates were filled with cells and attractant, they were allowed to incubate in a 37°C CO2 incubator for the times indicated in the Results section. No directional migration was seen towards medium only in peripheral control wells.
To test whether cells could migrate away from a local agonist source in response to a distant one, 10 μl of neutrophils (105) and 10 μl of chemoattractant were placed together into each cell well (total vol = 20 μl), with 10 μl of chemoattractant in the central attractant well. To test the responses of cells to two gradients in sequence, wells on either side of the central attractant well were filled with 105 neutrophils (vol = 10 μl). The central attractant well and a well 2.2 mm to its side (90° from the cell well) were filled with attractant or medium only at t = 0 and refilled at t = 1.5 h. Peripheral control wells were filled with medium only. After a 3-h incubation, plates were flooded with methanol.
Fixation and Staining.
Plates were fixed with methanol for >30 min, followed by 37% formaldehyde for >30 min. Agarose gels were removed and cells, now fixed to the plate, were stained using Field's rapid stain (Gallard-Schlessinger Chemical Manufacturing, Long Island, NY). The distance from the edge of the cell well to the leading edge of the migrating cell front was measured with the aid of a magnifier and reticle. Cell numbers in specified regions were quantitated using a transparent template attached to the bottom of the plate, with plates projected onto a television screen to facilitate counting.
Measurement of Gradients
One 3.5-mm-diam hole was cut in the center of the same type of agarose-filled tissue culture dish used for migration assays. The well was filled with 20 μl of solution containing either 0.25 pmol (0.5 μCi) of 125I-labeled IL-8 (Amersham Corp., Arlington Heights, IL), diluted into migration medium, or 25 pmol of [3H]LTB4 (0.5 μCi; Amersham Corp.). Plates were allowed to incubate at 37°C in the CO2 incubator for 30–120 min. After incubation, liquid remaining in the well was removed, and several 1.7-mm-wide strips of agarose were cut radially from the edge of the well towards the perimeter of the plate, using two parallel razor blades attached to a spacer. Strips were treated as specimens for frozen section, as in reference 24. Serial 60-μm cross-sections were cut from the strip using a microtome cryostat (International Equipment Co., Needham, MA), collected, and then radioactivity in each group of four sections was quantitated using a gamma counter for 125I (Gamma 4000; Becton and Dickinson Co., Mountain View, CA) or Scintillation counter for [3H]LTB4 (LS1801, Becton and Dickinson Co.). For these determinations, the 60-μm thickness of cryostat sections was confirmed by two methods: (a) an agarose gel of defined width and height was permeated by a known amount of evenly distributed radioactive IL-8. This block was sectioned as above, and the length of each section was calculated based on the volume of each slice, as indicated by the cpm. (b) A series of blocks of defined length (1.7 mm, cut with razor blade device) were sectioned as above. The mean number of slices to cut a 1.7-mm distance was 29 (1,700/29 = 58.6 μm).
Sources of experimental imprecision in the measurements used for gradient calculations include: (a) the standard deviation in cpm for “identical” 60-μm slices is ∼15% of the mean. This may include imprecision in section thickness and/or in gamma counter readings. (b) The standard deviation in size of 1.7-mm strips, cut as described, is ∼12% of the mean. (c) The standard deviation in the amount of labeled compound added to each starting well, with a Hamilton syringe, is <3% of the mean.
To determine where the edge of the chemoattractant well was during sectioning, we stained the edges of several wells with India ink, cut strips starting at these wells, and prepared them for frozen sectioning as above. The first 60-μm section that lacked visible India ink was taken to represent the first 60 μm of agarose for the gradient plots in Fig. 2.
Figure 2.
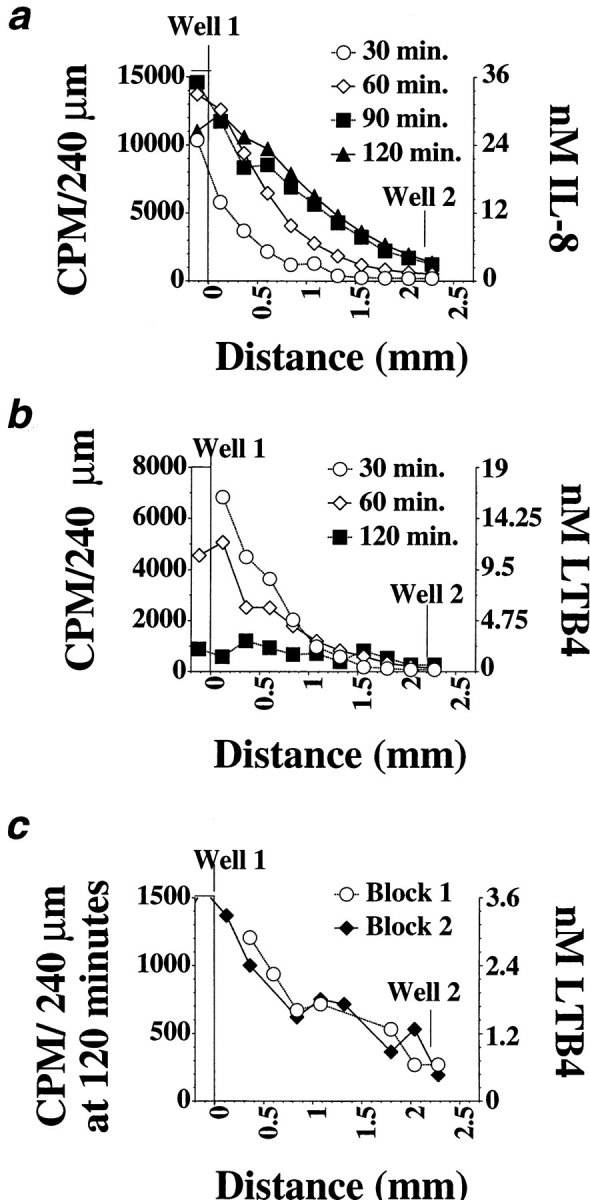
Gradients formed by IL-8 and LTB4 and calculated agonist concentrations for 1 pmol added in well 1. Each curve shows a representative of at least three determinations of the IL-8 (a) or LTB4 (b) concentration gradients in agarose, 30–120 min after addition of radioactive agonist, using the protocol outlined in the Materials and Methods section. Replicate IL-8 gradient measurements were very consistent in shape and magnitude, whereas LTB4 gradient measurements were more variable, especially in magnitude. The concentrations indicated were calculated for a gradient generated by 1 pmol of agonist. (c) Two replicate determinations of the LTB4 gradient at 120 min.
Gradient Calculations
The cpm of the IL-8 or LTB4 added to the agonist well, and the cpm in sections of agarose at known distances from the starting well were determined. The volume of each group of four 60-μm sections (a “240-μm block”) was known to be 0.17 cm × 0.2095 cm × 0.024 cm. Using this information, the amount of radiolabeled compound in each 240-μm block was calculated, allowing calculation of the mean agonist concentration in each block as a function of the amount of agonist added.
We determined the change in the agonist concentration per mm or per cell length as follows: for each gradient, plots of the log agonist concentration vs. distance from the agonist source well displayed a close fit to a straight line, so that the gradient profiles followed the exponential function:
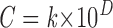
where C = concentration at a given distance, D, from the agonist well, and k = a constant. This equation is consistent with free diffusion from a broad front. The slope of each gradient profile on a semi-log plot (dlog C/dD) was used to determine the fold change in concentration for a given change in distance for ΔD = 1 mm, or for ΔD = 10 μm (approximately one cell length). Fold concentration change over a given distance ΔD was calculated as 10slope × ΔD .
Results
To model the migratory responses of neutrophils navigating within a tissue, we used an “under-agarose” neutrophil migration assay (15). In this system, a chemoattractant is introduced into a well in the agarose, and cells migrate towards the attractant. We chose this system rather than a conventional Boyden chamber–type assay because attractant-containing wells can be introduced in a variety of spatial configurations in the “plane” of the tissue culture dish, and therefore this system can be used to study cellular responses to a complex chemotactic field with two (or more) chemotactic gradients overlapping in different spatial arrays. Furthermore, this system permits ready visual assessment of the distance and direction of neutrophil migration in two dimensions.
Migration Down a Local Concentration Gradient
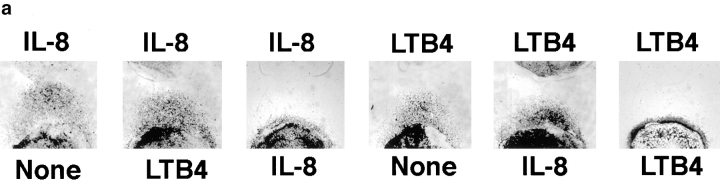
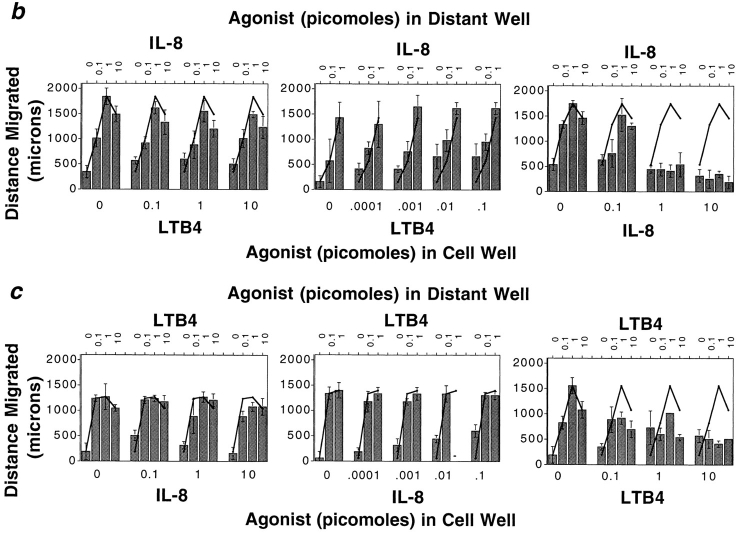
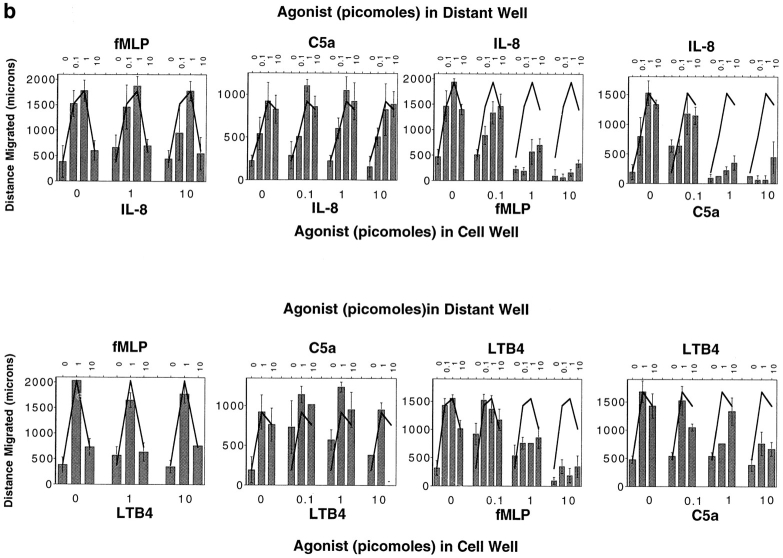
Given the multiple cell types that produce leukocyte chemoattractants, leukocytes responding to a distant attractant from a target site may often be faced with the dilemma of having to migrate away from a local agonist source to reach their destination. To model this situation in the under-agarose assay, we placed neutrophils together with various concentrations of a primary agonist in one well (well 1), and assessed their ability to migrate away from the primary source towards a distant source of the same or of a different chemoattractant (in well 2). Addition of IL-8 to the well containing neutrophils efficiently inhibited chemotaxis towards a distant IL-8 source, as expected (Fig. 1, a and b). In contrast, addition of LTB4 to the cell well had little or no effect on migration to IL-8; only at the highest LTB4 doses was there a minimal effect on the distance of neutrophil migration to IL-8 (Fig. 1, a and b). Similarly, placing LTB4 with cells in well 1 inhibited migration to a distant LTB4 source (well 2), but neutrophils migrated efficiently away from a local IL-8 source towards the distant lipid attractant (Fig. 1, a and c). Thus neutrophils can readily migrate from a well containing a primary agonist towards a distant secondary attractant well.
Figure 1.
Neutrophils migrate away from one chemoattractant source towards another. (a) Photographs of stained neutrophils after 2-h migration towards a distant source of LTB4 or IL-8 (1 pmol), in the presence or absence of an inverse gradient generated by LTB4 or IL-8 (10 pmol). Cells placed with one agonist migrate towards the other agonist almost as well as control cells, but do not migrate well towards a distant source of the same agonist. (b) Dose-response curves illustrate that over a wide range of concentrations of both agonists, cells can migrate away from an IL-8 source in response to a target LTB4 gradient; however, cells do not migrate away from a well containing IL-8 (⩾1 pmol) towards a distant IL-8 source. (c) Conversely, over a wide concentration range of close and distant agonists, neutrophils migrate away from an LTB4 source towards IL-8, but not towards LTB4. Each graph in b and c shows the data from a representative experiment of at least two to five performed with similar results. Error bars in b and c indicate the standard deviation of the distance migrated for four replicates.
That responding neutrophils are in fact migrating down a local gradient during this assay was confirmed by measuring the IL-8 and LTB4 gradients, using radioactively labeled IL-8 and LTB4. IL-8 gradient measurements were very consistent, in shape and magnitude, whereas LTB4 gradient measurements were more variable, especially in magnitude. As illustrated in Fig. 2 a, an inverse IL-8 gradient is present from 30 min (the earliest time point measured), and the inverse gradient is maintained over the entire 2-h time course of the assay. The mean IL-8 concentration near the source well does not decrease over this time, presumably reflecting sustained diffusion of IL-8 into the agarose (some fluid remains in the well for at least 60 min after the start of the assay). An LTB4 gradient is also present throughout the entire assay, consistent with previous studies (24) (Fig. 2, b and c). Unlike the IL-8 gradient, the mean concentration of the LTB4 near well 1 decreases with time, suggesting that LTB4 diffuses faster than IL-8. Although LTB4 levels have decreased by 120 min, a gradient from the source well is still present (Fig. 2 c).
Neutrophils' ability to orient in a gradient of chemoattractant is a function of two parameters: gradient steepness (fold concentration change/cell length) and mean agonist concentration (6, 23, 27, 28). In our experiment, the steepness of the inverse IL-8 gradient ranges from a 20-fold change per mm (1.026-fold/cell length) at 30 min to a fourfold change per mm (1.016-fold/cell length) at 120 min; fold concentration change was calculated as described in Materials and Methods. The inverse LTB4 gradient shape ranges from a decrease of eightfold/mm (1.02-fold/cell length) at 30 min to twofold/mm at 120 min. Furthermore, for all gradients measured, the gradient profile approximates an exponential curve, meaning that the fold concentration change per mm is the same throughout the gradient. Other studies have shown that neutrophils can orient well in response to these concentration differentials; orientation occurs, even at nonoptimal agonist concentrations (10-fold above or below the receptor dissociation constant), in a more than threefold/mm gradient, and can occur at optimal agonist levels (near the receptor dissociation constant) in a gradient that changes only twofold/mm (1.007-fold/ cell length) (27). In addition to these quantitative considerations, the efficient migration towards IL-8 or LTB4 observed in our assays confirms that the steepness of these gradients is in a relevant range for eliciting directional migration.
The ability of neutrophils to migrate down, and apparently largely ignore, the gradients arising from their starting well is also largely independent of the concentration range of the inverse gradients. When cells are placed with 1 pmol of IL-8, the mean concentration at 30 min in the first 0.5 mm of the gel is 10 nM, about 10 times the concentration at which half of the cells' IL-8 receptors are occupied and 10 times the 50% effective concentration (EC50) for migration to IL-8 in a filter assay (Millipore Corp., Waters Chromatography, Bedford, MA) (2, 13). As shown in Fig. 1 b, cells migrate just as effectively away from the IL-8 source towards an optimal dose of LTB4 when the mean IL-8 concentration near the cell well is one, two, or three orders of magnitude less (at 1, 0.1, or 0.01 times the concentration for half-receptor occupancy). This range clearly spans concentrations expected to be optimal for directing cell orientation and migration. For example, neutrophils migrating away from a 0.1 pmol IL-8 source are moving against a 20-fold/mm gradient with a mean concentration of about 1 nM at 30 min (when they are still near the cell well), and a 7-fold/mm gradient with a mean concentration of 2 nM at 90 min (when the leading edge has migrated roughly 1 mm under these conditions). Neutrophils can migrate away from an LTB4 source over a wide range of LTB4 concentrations as well, from concentrations corresponding to 50–100 times half-receptor occupancy and chemotactic EC50 in a Boyden chamber (1–3 nM) (11), to at least three orders of magnitude below the chemotactic EC50 (Fig. 1 c). We conclude that, under a wide range of conditions, leukocytes have the capacity to migrate away from a nearby agonist source in response to a distant chemoattractant.
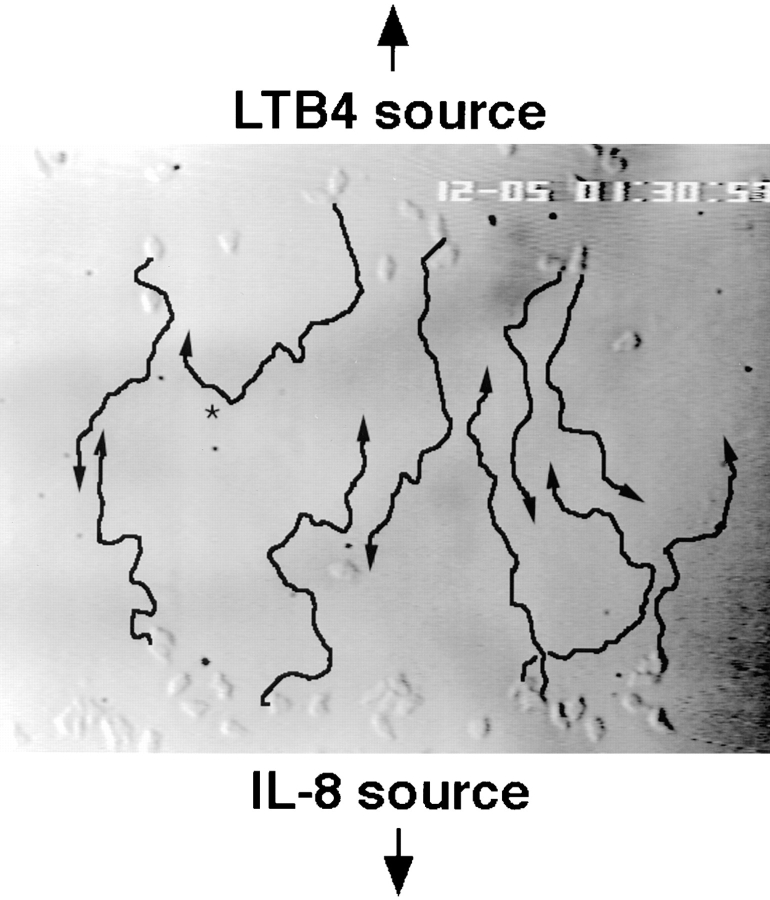
One prediction from these studies would be that, if neutrophils were placed with IL-8 in one well, and other neutrophils with LTB4 in another well 2.2 mm away, the cells should migrate in opposite directions with each cell population responding to the agonist in the distant well. Fig. 3 illustrates traces of neutrophils migrating from an IL-8 to an LTB4 source or (in the opposite direction) from the LTB4 source to the IL-8 source, over a 15-min period (105–120 min) as they approach a midpoint between the two source wells. The traces emphasize the striking, opposing directionality of chemotactic migration of the two populations as they come together.
Figure 3.
Neutrophils can migrate in opposite directions in the same field of overlapping chemoattractant gradients. The migratory behavior of neutrophils placed in IL-8 (originating in a well below the picture) and LTB4 (originating in a well above the picture) was recorded by time-lapse video microscopy. The image shows cells at the leading edge of each population 90 min after the start of the assay, when each population is nearing a common point between the starting wells. Arrows indicate the migration paths of several representative cells over the next 15 min. Most cells that started at the IL-8 source migrate towards the LTB4 source and vice versa; occasionally, a cell is observed to change directions (i.e., cell indicated by *).
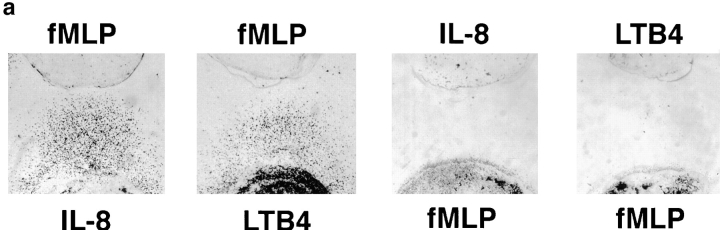
The cells' ability to ignore a local agonist source to respond to a distant one is constrained by the dominance of end target–derived attractants (N-formyl peptide fMet-Leu-Phe [fMLP], C5a) over regulatory cell–derived attractants (IL-8, LTB4), in a manner consistent with previous studies in other model systems (4, 6, 12). Whereas neutrophils could migrate away from a local IL-8 or LTB4 source towards fMLP or C5a (Fig. 4, a and b), cells originating from a high local concentration of fMLP or C5a do not respond efficiently to a distant source of IL-8 or LTB4. Local fMLP also suppressed migration to a distant source of C5a and, although less completely, C5a also suppressed migration to fMLP (data not shown).
Figure 4.
The migratory behavior of cells originating at a source of fMLP or C5a. (a) Photographs of stained cells after 2-h migration to a source of fMLP, IL-8, or LTB4 (1 pmol). Cells originating in a well containing IL-8 or LTB4 (10 pmol) exhibit robust migration towards fMLP (top row); however, cells placed with fMLP (10 pmol) do not migrate towards IL-8 or LTB4. (b) Dose-response curves show the effect of varying concentrations of fMLP and C5a on migration to IL-8 and LTB4, and the effect of varying amounts of IL-8 and LTB4 on migration to fMLP and C5a. Each graph shows the data from a representative experiment of two to five performed with similar results; error bars indicate the standard deviation of the distance migrated for four replicates.
Migration beyond a Threshold High Dose of Chemoattractant
Neutrophils migrating towards an agonist source necessarily encounter increasing concentrations of agonist. In the under-agarose assay, neutrophils migrating towards a greater-than-optimal dose of chemoattractant appear to arrest and collect upon entering a region of excessive agonist concentration (Fig. 5) (5). At increasingly high doses, large numbers of cells continue to leave the cell well, but their migration is arrested at relatively discrete distances, with the distance migrated being inversely related to the concentration of agonist added. These observations are consistent with the well-known high dose inhibition of neutrophil orientation and directed migration (for review see reference 6). Zigmond observes that neutrophils orienting in response to a 10-fold/mm formyl peptide gradient begin to become disoriented in a gradient with a mean peptide concentration >10 times the peptide receptor dissociation constant (27). Extrapolating from our gradient measurements above, it is clear that the concentrations of agonist at the position where the barrier forms exceed those required for receptor saturation. For IL-8, our gradient calculations indicate that the position of the high dose disorientation “barrier” for cells migrating to 50 pmol IL-8 corresponds to a concentration range between 100 nM at 60 min, when the barrier is forming, and 400 nM at 120 min (extrapolated from Fig. 2 a; with concentrations multiplied 50-fold). Cells migrating to 50 pmol LTB4 form a barrier at a position corresponding to ∼100 nM throughout the assay. These measurements are consistent with the prediction that the migration arrest we observe reflects cell disorientation due to receptor saturation.
Figure 5.
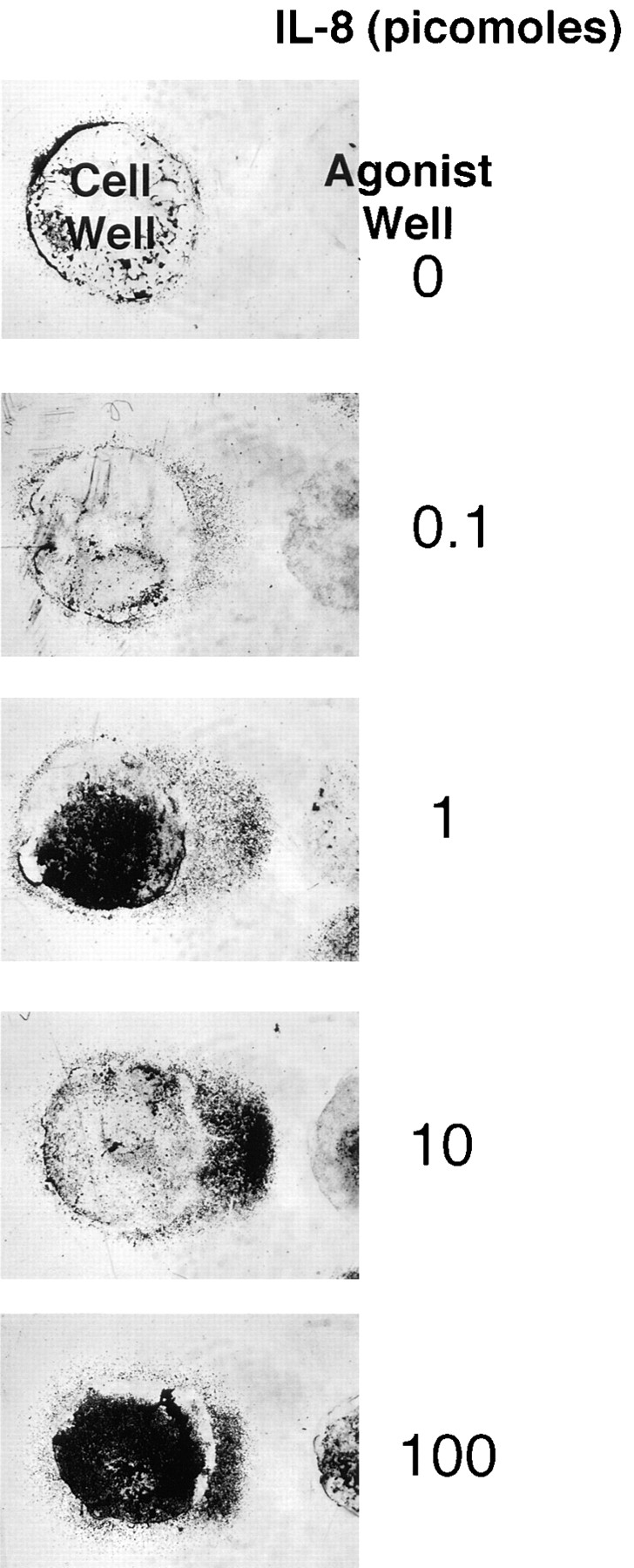
Dose dependence of neutrophil migration under agarose. Neutrophil responses to a range of IL-8 gradients in the standard 2-h under-agarose migration assay are shown. Neutrophils in the cell well migrate in response to a concentration gradient generated by the indicated amounts of IL-8 in the agonist well. On migrating towards gradients generated by >1 pmol IL-8, the migrating front at the leading edge is flat, and cells are densely packed. The distance cells migrate before arresting in this configuration decreases as the IL-8 concentration in the agonist well increases. All chemoattractants used in this study exhibit a qualitatively similar dose- response curve in this migration assay.
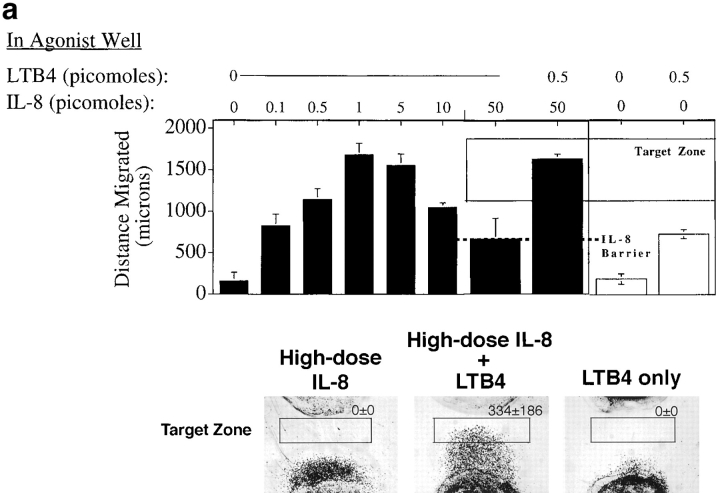
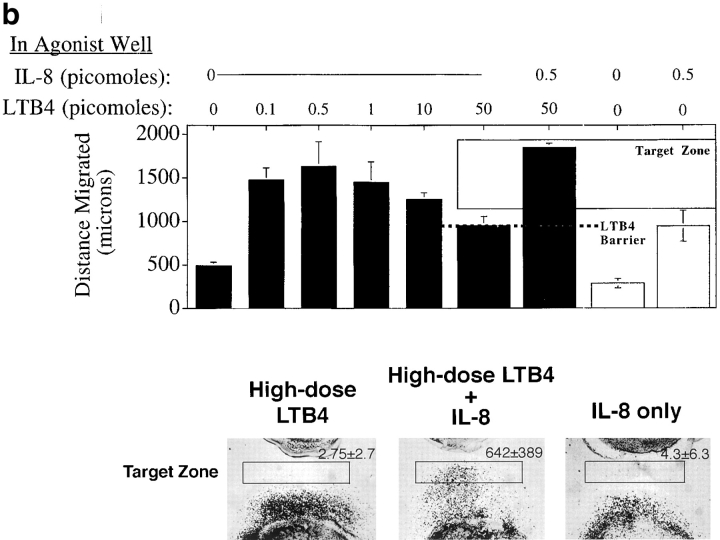
We asked if neutrophils that had reached the high dose barrier to further migration could nonetheless migrate further in response to a different attractant gradient (Fig. 6). The distances of neutrophil migration over 2.5 h to various concentrations of IL-8 are depicted in the bar graph in Fig. 6 a, illustrating the high dose barrier formation at ∼700 μm from the cell well when 50 pmol agonist is added. Halfway through the assay (at 1.25 h), when the barrier has formed, a lower dose of either IL-8 or LTB4 was introduced into the agonist source well. Addition of more IL-8 did not permit further migration of cells, but addition of a distinct attractant, LTB4, to the agonist well resulted in cell reorientation and migration beyond the IL-8 barrier. A representative experiment is illustrated in the lower panel (Fig. 6 a); the number of cells entering a homing “target zone” beyond the IL-8 barrier is given for each condition depicted. In this 2.5-h assay, cells must migrate first to IL-8, and then respond to the LTB4 added halfway through the assay (at 1.25 h) to reach the target zone. Cells that have not first migrated to IL-8 do not enter the target zone, even if they experience the LTB4 gradient for 1.25 h. In parallel experiments, neutrophils arrested in a high dose barrier of LTB4 could be induced to migrate beyond that barrier by the addition of low dose IL-8 (but not LTB4) to the agonist well halfway through the assay. Fig. 6 b summarizes the data from this series of experiments. Disorienting levels of the primary agonists are clearly retained throughout the response to the secondary attractant, as indicated by the fact that a low dose of the primary agonist did not induce further migration when added halfway through the assay. Moreover, even when the high dose primary agonist was re-added halfway through the assay along with the secondary agonist, the secondary agonist could elicit migration into the target zone (data not shown.) We conclude that leukocytes migrating up a primary gradient into disorienting agonist concentrations can reinitiate directed locomotion in response to secondary gradients at lower levels.
Figure 6.
Neutrophils can migrate beyond a high dose barrier of a primary agonist in response to a secondary agonist. (a–c) In a standard migration assay, neutrophils were allowed to migrate towards a gradient generated by an agonist dose high enough (50 pmol IL-8 or LTB4, 5 pmol fMLP) to arrest crawling cells in a flat migrating front at a fixed distance from the agonist well. Halfway through the assay (at 75 min), a lower dose of a second agonist (5 pmol IL-8 or LTB4) was added to the agonist well. The distances cells migrated, in the presence or absence of the second agonist, are graphed in the upper panels as the mean of four replicates, error bars showing the standard error. The dashed line shows the position of the high dose migration barrier. Photographs show cells that have migrated to a first agonist only for 150 min (bottom left), the first agonist for the entire 150 min plus the second agonist for the last 75 min (bottom center), or to the second agonist only for 75 min (bottom right). The number of cells entering a target zone near the agonist well are indicated (mean and range of two replicates from a representative experiment).
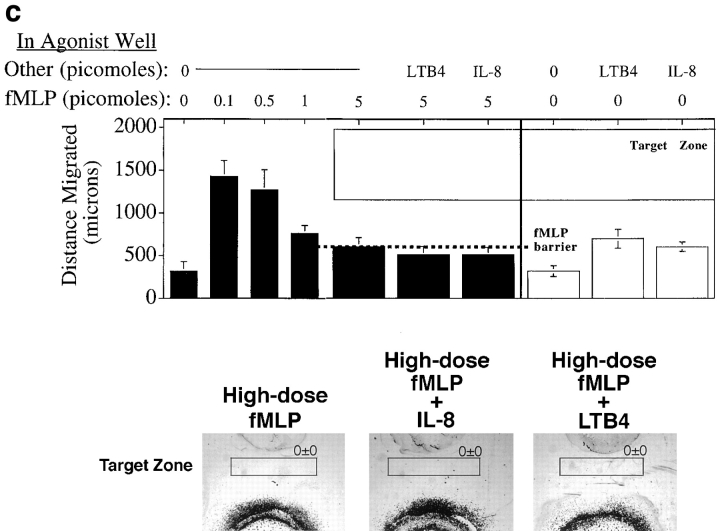
As expected, the capacity for a secondary agonist to induce migration beyond the high dose barrier was constrained by the dominance of end target attractants; neither IL-8 nor LTB4 could induce migration into the target zone when the primary agonist was fMLP (Fig. 6 c).
Step-by-step Navigation through Sequential Chemotactic Fields
The experiments outlined above demonstrate that leukocytes can orient and chemotax efficiently to a distant attractant even when migrating down a local attractant gradient, or after migrating uphill into and beyond a saturating and disorienting level of a primary agonist. This has fundamental implications for leukocyte navigation through tissues, as it suggests that they should be able to migrate sequentially from agonist source to agonist source in a multistep process.
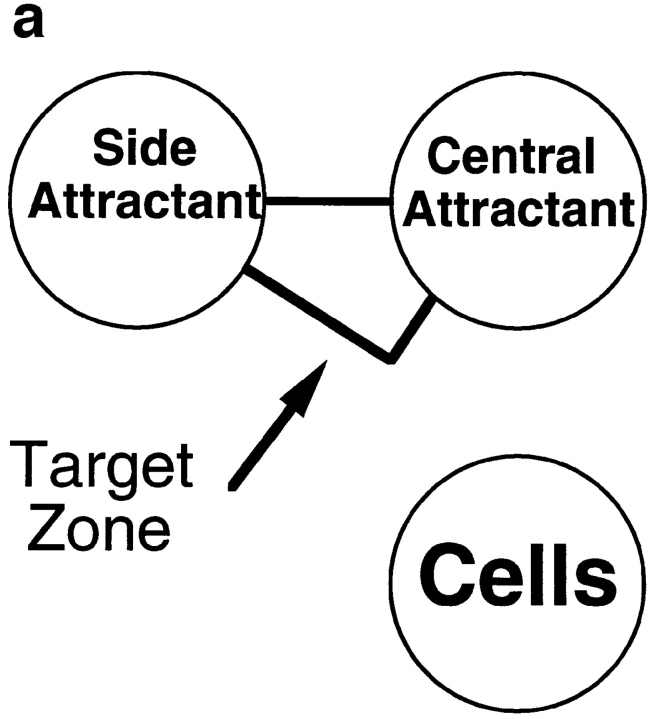
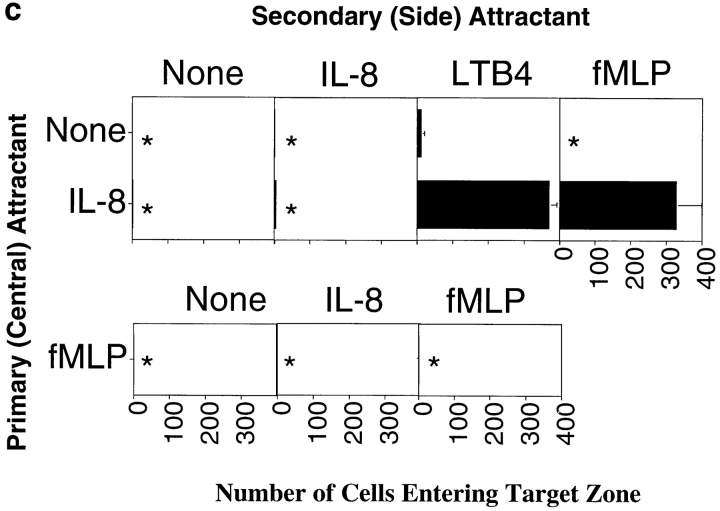
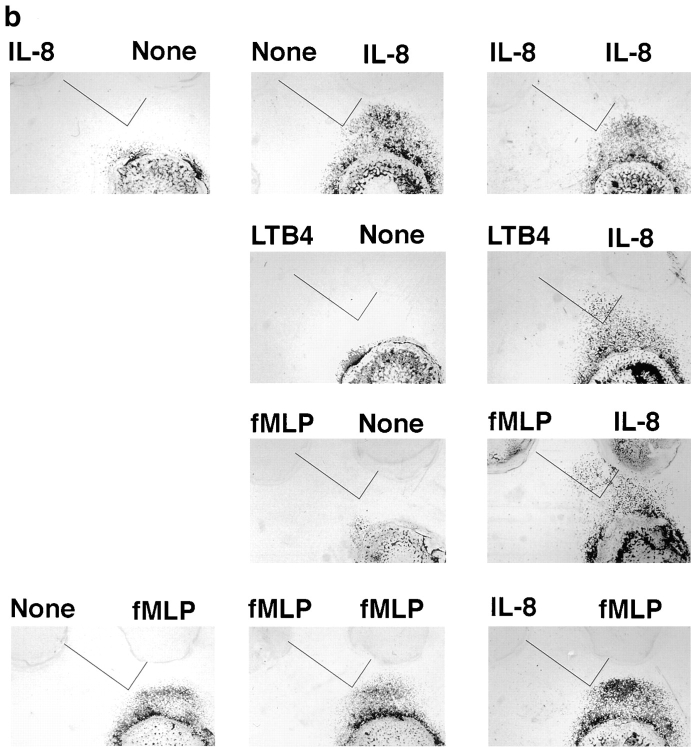
To model this type of scenario, we assessed the behavior of cells responding to attractants presented in two wells, one “central” well located 2.2 mm from the cell well (as in the previous assays), and the second “side” well positioned at a 90° angle, 2.2 mm from the central well and 4.5 mm from the cell well (Fig. 7 a). We defined an arbitrary target zone, as illustrated in the figure, and asked whether neutrophils could be attracted to this target zone by chemoattractants presented in various combinations in the primary (central) and/or the more distant, secondary attractant wells. All agonists were used at optimal chemoattractant concentrations (defined for the proximal well, Fig. 7, legend). As illustrated in Fig. 7 b, and shown quantitatively in Fig. 7 c, cells migrated well in response to IL-8 in the central well, but could not enter the target zone in significant numbers. When IL-8, LTB4, or both were presented in the side well only, cells were able to migrate a short distance directly towards the side well, but did not enter the target zone. When IL-8 was presented in both the central and side wells, cells migrated only towards the closer central well; this was not unexpected, as locally this would be seen as “upgradient” in spite of the additional, more distant source of the same agonist. Finally, when IL-8 was presented in the central well and LTB4 was presented in the side well, neutrophils were able to home to the target zone in large numbers (Fig. 7, b and c).
Figure 7.
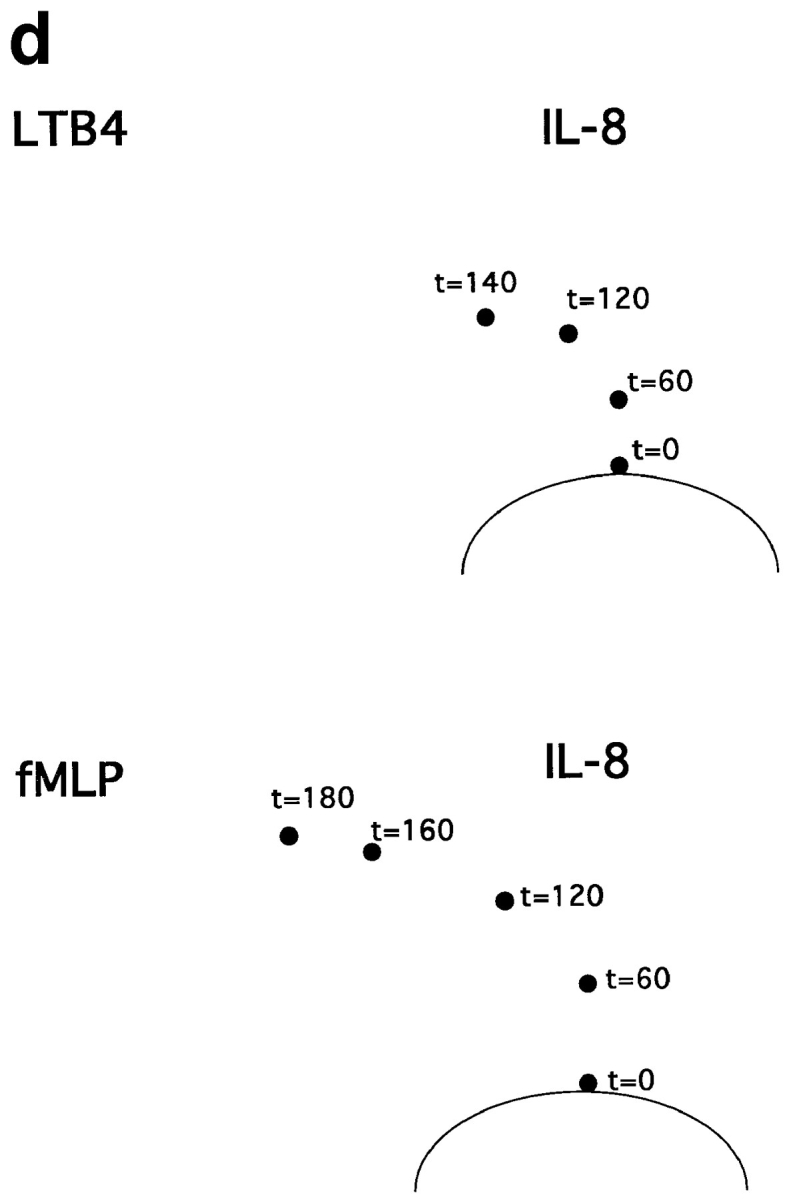
Neutrophil navigation targeted by sequential migration to chemoattractants in a defined spatial array. (a) Diagram illustrating the position of the two agonist wells relative to the cell well, and the outline of the target zone used to quantitate the number of cells migrating sequentially to both agonists (see c). (b) Photographs of fixed, stained cells that have been allowed to migrate for 3 h in the presence or absence of a central agonist (right side of photos) and/or side agonist (left side of photos). The approximate position of the target zone is indicated in each image (for quantitative analyses, target zones were precisely positioned by a template). Successfully homed cells have been accentuated for illustrative purposes by enhancing the contrast in the target region. The amounts of IL-8, LTB4, and fMLP in the agonist wells were 1 pmol, 1 pmol, and 0.5 pmol, respectively. (c) Graph indicating the number of cells migrating into the defined target zone in presence of various agonist combinations. The mean number of cells entering the target zone is indicated. Error bars show the range for two replicate determinations from a representative experiment. Asterisks indicate that the mean number of cells in the target region was <15. (d) Turning behavior of cells migrating to two agonists in sequence. Dots indicate the center of the leading edge of migrating cells at various times for cells responding to a central IL-8 source and side LTB4 source (top) or a central IL-8 source and side fMLP source (bottom).
Similar patterns were elicited with IL-8 and fMLP (Fig. 7, b and c). Only when the attractant in the secondary, side well was distinct from the primary central IL-8 attractant were cells able to localize in significant numbers to the target zone: when fMLP was presented in both the central and the side wells, cells migrated towards the central well only. However, when IL-8 was presented in the central well and fMLP in the more distant side well, cells migrated into the target zone. Tracing the position of the leading edge of migrating neutrophils, shown in Fig. 7 d, demonstrates that the successfully homing neutrophils migrate initially towards the primary IL-8 source, and then turn as they come in range of the secondary attractant allowing their migration into the target region.
In this assay, as in the previous ones, the ability of neutrophils to respond to a secondary agonist is constrained by the dominance of fMLP over IL-8. Although cells migrate into the target zone when IL-8 is presented as the central (proximal) agonist and fMLP is presented in the side well, the converse is not true. Neutrophils migrate well towards the central fMLP source, but do not turn in response to the IL-8 in the side well (Fig. 7, b and c). Thus, in this model of sequential navigation, the targeting of neutrophils is a function of both the chemoattractants and receptors involved, and of the order in which the attractants are encountered by migrating cells.
Discussion
In the last several years, numerous novel leukocyte chemoattractant receptors and their ligands (especially chemokines) have been identified. In general these receptors are expressed in overlapping patterns by several leukocyte subtypes, and each leukocyte subtype itself expresses multiple chemoattractant receptors (14, 16). Moreover, it is clear that the attractants themselves are produced in diverse combinations by host cells in the setting of inflammation, and by lymphoid and tissue stromal cells in sites of constitutive lymphocyte trafficking. In the context of this multiplicity and promiscuity of receptor and agonist expression, it has been difficult to formulate a unifying model of how chemoattractants might participate in the highly specific microenvironmental targeting of leukocyte subpopulations observed in vivo. The mechanisms of cell targeting by chemotaxis, of course, are not relevant only to leukocyte homing but also to the migration of other cell types during, for example, embryogenesis or neurogenesis.
To better understand the role of multiple attractants in directing cell migration within tissues, we have examined how a migrating leukocyte behaves when it encounters a two-dimensional array of attractants for which it bears receptors. We used an in vitro model of neutrophil migration under agarose, a model that allowed us to focus on directional migration responses to chemoattractants in the absence of the numerous other factors, such as extracellular matrix components and cells, which undoubtedly contribute to the regulation of cell navigation in vivo. Using this model, we have examined simplified scenarios where neutrophils are exposed to defined spatial gradients of two different neutrophil chemoattractants. Our findings lead us to propose that leukocytes can progress through complex, varying chemotactic fields in a series of steps with different arrays of attractants combining to dictate specific homing pathways.
Migration Down a Local Concentration Gradient
Situations in which leukocytes need to migrate down a gradient towards their target could arise in diverse settings where primary chemoattractants are produced by stimulated host cells. Mechanisms that allow leukocytes to remain sensitive to new chemotactic signals, even when they are at a local peak in the concentration of one agonist, could permit cells to progress from one gradient source to another rather than remaining at the peak of the first gradient they encounter. For example, leukocytes recruited from the blood by endothelial cell–derived attractants may subsequently need to migrate away from the endothelial agonist source towards their target within a tissue.
In this study, we find a wide range of circumstances in which neutrophils can indeed migrate down a functional gradient of one chemoattractant in response to a second, more distant gradient. As might have been predicted from the hierarchy of chemoattractant receptors (discussed below), neutrophils can migrate down a gradient of IL-8 or LTB4 toward the dominant attractants fMLP and C5a. In vivo, this behavior might permit cells to migrate away from a regulatory cell recruiting neutrophils towards a bacterium or immune complex. In addition, however, neutrophils can migrate down an IL-8 gradient towards LTB4; surprisingly, the converse is also true. This indicates that inhibition of the proximal agonist responses by the distant one is not required for cells to migrate down a local gradient. Moreover, cells can be recruited “downhill” away from an agonist source over an enormous range of concentrations, spanning several orders of magnitude around the EC50 for chemotaxis.
When the proximal gradient has a high mean concentration, it may provoke only a weak orienting signal due to receptor saturation, homologous desensitization, and/or receptor sequestration. When the nearby gradient has a low mean concentration (with few receptors occupied), cells may ignore it in favor of the distant gradient because the slope and concentration range of the distant gradient are more compelling (i.e., because the distant source forms a gradient in a more optimal range for the cell's gradient detection mechanism). Any degree of homologous desensitization to the primary agonist could also contribute to dominance of the response to the distant gradient. More difficult to understand is the ability of cells to migrate even against a proximal gradient that, in the absence of the distant agonist, would be ideal for inducing reorientation and reverse migration. We hypothesize that directional persistence in this context may reflect a more efficient or rapid adaptation of the migrating cell to increasing (distal agonist) vs. decreasing (proximal agonist) attractant concentrations. Whatever the mechanisms involved, the ability of neutrophils to migrate down a gradient of one agonist towards a distant agonist, even in the absence of receptor cross-inhibition, suggests the potential for cells to migrate along a series of gradients in sequence.
Migration beyond a Threshold High Dose of Chemoattractant
To attract cells over long distances, a target site must generate enough attractant for those cells to detect. Cells migrating towards such a potent source, however, may not reach the source, because as they approach they will eventually reach a saturating agonist concentration, beyond which they lose the capacity to orient. This is likely due to receptor saturation and/or receptor desensitization. We have shown that leukocytes migrating up a gradient into disorienting agonist concentrations retain the capacity to resume directional migration in response to a gradient of a different agonist. Thus, two attractants specific for the same cell, rather than simply being redundant, may function together to recruit leukocytes. For example, a target expressing multiple attractants at different levels may be able to attract leukocytes from a greater distance than if it were merely producing a single attractant alone. This may represent an important advantage in regulatory cell recruitment of leukocytes during inflammation; it may also help explain the almost universal observation that activated stromal cells, as well as infiltrating leukocytes, characteristically secrete multiple induced chemoattractant cytokines concurrently.
Step-by-step Navigation through Sequential Chemotactic Fields
A major advantage of a system with multiple receptor– ligand pairs is the potential to mix-and-match the attractants to create different homing pathways, thereby enhancing the specificity targeting and the number of targeting choices. This would be possible if agonists could be “strung together” into different homing sequences, which leukocytes could follow by migrating first towards one agonist, and then to a subsequent agonist, and so on. Our experiments indicate that neutrophils can indeed migrate along a gradient of one agonist, and then turn towards a more distant agonist source presented at a 90° angle to arrive at a specific target site. Interestingly, when two sources of the same agonist are presented in this configuration, cells do not arrive in the target zone; two distinct agonists are required. This phenomenon may reflect the combined gradient shape perceived when a single agonist is used, since neutrophils will always see the closest (central) source as “uphill.” Even though the highest agonist concentration may also be towards the central well when different agonists are used, neutrophils respond completely differently in this situation, eventually turning away from the first agonist source to respond to the second. In this case, the strength of the orienting signal from the first agonist may progressively decrease due to homologous desensitization or progressive receptor saturation as the migrating cells approach the primary (central) well; sensitivity to the secondary agonist would be retained, however, so that its orienting signal would eventually dominate as the migrating cell is drawn within range. The turning behavior of neutrophils in this model emphasizes that, as in the previous case of neutrophil recruitment beyond an inhibitory high dose agonist barrier, two distinct chemoattractants presented in sequence can elicit strikingly different homing patterns than those elicited by any agonist alone (even when presented in the same spatial array). We conclude that multiple agonist–receptor pairs can be engaged in sequence during leukocyte navigation, with resulting potential for enhanced versatility and selectivity in leukocyte targeting.
The Stop Signal
The experiments outlined above demonstrate that neutrophils migrate in sequence from one agonist source to another. How then do cells arrive and remain at appropriate final target sites? In many physiologic situations, it is likely that additional mechanisms such as contact guidance, regulated adhesion, or inhibitory signals may determine the final arrest of navigating cells. However, chemoattractants can also play definitive roles in this context; as mentioned in the Introduction, previous studies have shown that neutrophil response to regulatory attractants such as LTB4 or IL-8 can be suppressed in the presence of uniform fields of C5a and fMLP, agonists that define physiologic end targets for these professional phagocytes (i.e., immune complexes that fix complement and generate C5a, or bacteria or dying cells releasing formyl peptides). This inhibitory effect of fMLP and C5a on regulatory agonist responses is not due to toxicity, as we have previously shown in transwell assays that relatively low concentrations of formyl peptides, within the range capable of supporting migration in response to a gradient of formyl peptide itself, can completely suppress migratory responses to IL-8 gradients (4). In the present studies as well, neutrophils' ability to orient towards a distant agonist is constrained by the dominance of these end-target agonists, thus providing an exception to the rule of sequential responses. In contrast to the ability of cells to migrate away from the regulatory agonist source towards any distant secondary attractant, cells do not migrate away from a strong fMLP or C5a source. In the presence of mechanisms that maintain chemotactic responsiveness to sequential gradients, hierarchical cross- inhibition of responses to certain agonists can be viewed as an important mechanism for ensuring cells' final navigation to their appropriate targets. Once neutrophils clear the source of a dominant agonist (e.g., by phagocytosis of bacteria) they may again be free to respond to regulatory cell–derived or more distant end target attractants.
The ability of N-formyl peptides and C5a to inhibit chemotactic responses to regulatory agonists is thought to reflect the phenomenon of heterologous receptor desensitization (4, 12). Stimulation of N-formyl peptide or C5a receptors induces phosphorylation of the IL-8 receptor, for example, with associated functional uncoupling of the receptor from its signaling apparatus (17, 20). In the context of leukocyte trafficking, it is worth emphasizing that the formyl peptide and C5a receptors are the only receptors known to mediate heterologous desensitization: none of the tested chemokine or lipid attractant receptors examined to date appear to support uncoupling of heterologous responses. Thus it is likely that the diverse family of chemokines and their receptors can participate as intermediates in multistep cellular navigation, as illustrated here for the chemoattractants LTB4 and IL-8.
Implications for the Combinatorial Regulation of Leukocyte Migration
Several years ago, we proposed that the interaction of circulating lymphocytes or other leukocytes with endothelial cells at sites of leukocyte recruitment could be determined combinatorially. In other words, mixing and matching different combinations of the receptor–ligand pairs available to mediate the sequential steps (contact initiation through microvillus receptors, rolling, activation, and activation-dependent arrest) involved in leukocyte–endothelial cell recognition (3). The experiments described here extend the concept of multistep trafficking to the arena of chemotactic responses of leukocytes. They imply the potential for combinatorial control of cellular targeting by association of different receptors and agonists supporting the step-by-step navigation of leukocytes through chemoattractant arrays within tissues.
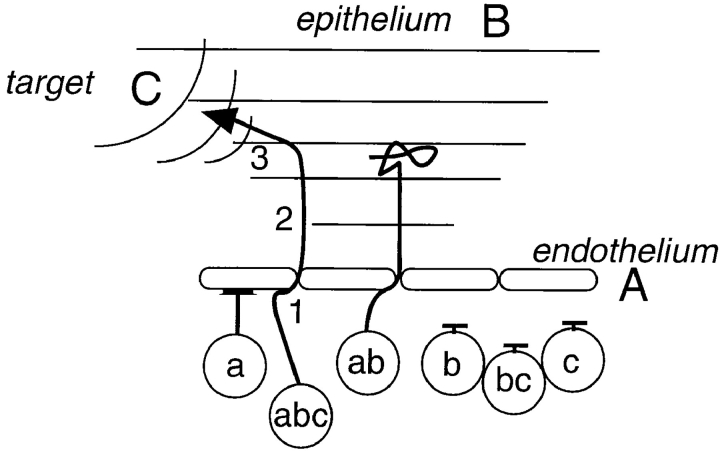
The implications of this model can be considered in the context of a hypothetical scenario, illustrated in Fig. 8, in which leukocyte recruitment from the blood vascular endothelium to a defined tissue target is directed by three chemoattractant sources operating in sequence. In the first step, endothelial-associated agonist(s) (Fig. 8, A), which may have played a role in leukocyte arrest from the blood, support chemokinetic locomotion of the leukocyte on the endothelium and potentially transendothelial migration. In the second step, cells are recruited into the surrounding tissues, away from the vessel wall, by a strong attractant gradient (Fig. 8, B), which might be derived from an overlying stimulated epithelial surface. Cells migrating up this gradient will eventually enter saturating, disorienting concentrations of the epithelial-derived agonist, allowing reorientation and entry into the target zone in response to a local chemoattractant (Fig. 8, C). Only those cells expressing receptors for all three agonists (Fig. 8, a, b, and c) will successfully localize to the target.
Figure 8.
Combinatorial regulation of chemotaxis. Leukocyte homing during step-by-step navigation is determined by the combination and sequence of agonists and receptors engaged. In this example, a cell requires receptor a to acquire motile properties and transmigrate through the endothelium in response to agonist A; receptor b to respond to a gradient from an overlapping epithelial surface that draws the cell into the tissue; and receptor c to detect a signal from its end target, and migrate there. Only cells displaying all three receptors can successfully home to the target region. The same receptors and agonists, displayed in different patterns and/or in combination with other receptors, could participate in a variety of different leukocyte targeting scenarios. This schematic emphasizes that simultaneous expression of multiple receptors is required for homing, and demonstrates how receptors that are displayed relatively indiscriminately (by overlapping subsets of leukocytes) can elicit a specific homing pattern when used in combination.
If, within this scenario, a single agonist is used at each step, and hypothetically four distinct receptor–agonist pairs can be substituted at each step, then it follows that 4 × 4 × 4 = 64 independent leukocyte targeting scenarios can be generated from only 12 chemoattractant receptor–agonist combinations. Moreover, the combinatorial possibilities will increase both with the number of differentially regulated receptors that are available for substitution at each step, and (in more complicated scenarios) with the number of steps involved.
However, as mentioned above, chemoattractant receptors on leukocytes, and their agonists in host stromal cells, tend to be expressed in overlapping rather than mutually exclusive patterns. Additionally, chemoattractant gradients within tissues must overlap spatially for migrating cells to make the transition from one step to the next. These features undoubtedly contribute to the resiliency of cellular targeting in settings of altered agonist expression or function, but at the same time they may limit the operational specificity at any one step. In this context, another important implication of a multistep model is that the specificity of the overall process can greatly exceed that of any of its individual steps. Thus, in our hypothetical scenario, if neutrophils were three times more likely than lymphocytes to respond to the endothelial-associated agonists mediating step 1, twice as likely to migrate into the tissue in response to the chemoattractant profile available at step 2, and five times more efficiently attracted at step 3, then 30 (5 × 2 × 3) times more neutrophils should arrive at the final target site.
These considerations illustrate the potential power of multistep chemotaxis for regulating the segregation and specific homing of leukocytes (and other cells) in the context of complex agonist fields that are likely to be the norm in vivo. Within this model the multiplicity of chemoattractant receptors on most leukocytes, far from implying a lack of selectivity due to redundancy of responses, would instead be essential for efficient and selective navigation through sequential gradients. Moreover, the operation of several steps in sequence, each potentially mediated by only one or a few receptor agonist pairs, also suggests that therapeutic intervention into the overall process of leukocyte homing may be possible by interfering with any of a number of different chemoattractant–receptor pairs.
Conclusion
In conclusion, leukocytes have the capacity to respond sequentially to chemoattractants, making possible a step- by-step navigation through complex chemoattractant fields. We have modeled this multistep migration through a simple in vitro chemoattractant array, demonstrating that sequential migration through two spatially distinct attractant fields can target leukocytes in a unique fashion determined both by the nature and sequence of attractants. The step-by-step model of leukocyte targeting provides a combinatorial mechanism whereby multiple attractants can participate to guide leukocyte subsets to their unique destinations within tissues, and suggests that rather than being redundant, each of the many chemoattractant receptors expressed on leukocyte surfaces may contribute to the versatility and efficiency of leukocyte targeting in vivo.
Acknowledgments
We thank E. Resurreccion for dedicated technical assistance; S. Grossman for assistance in the final editing and typing of this manuscript; L. McEvoy, E. O'Hara, E. Bowman, and the rest of the Butcher lab for helpful discussions. We also thank D. Ostrov for theoretical insights into diffusion; S. Zigmond for critical review of ongoing experiments; M. Szabo for comments on the manuscript; and E. Foxman for his unwavering support and many helpful discussions.
This work was supported in part by a grant from the National Institutes of Health (NIH) and an award from the Department of Veterans Affairs (to E.C. Butcher). E.F. Foxman was supported by Medical Scientist Training Program grant No. GM07365 from the National Institute of General Medical Sciences. J.J. Campbell was supported by the NIH Cancer Etiology, Prevention, Detection, and Diagnosis grant No. 5T32CA090302, NIH Individual National Research Service Award No. IF32AI08930, and is a recipient of an Arthritis Foundation Postdoctoral Fellowship.
Abbreviations used in this paper
- EC50
50% effective concentration
- fMLP
N-formyl peptide fMet-Leu-Phe
- IL-8
interleukin-8
- LTB4
leuko-triene B4
Footnotes
Address all correspondence to E.F. Foxman, c/o Eugene C. Butcher, Department of Pathology, Stanford University School of Medicine, Stanford, CA 94305-5324. Tel.: (650) 852-3369. Fax: (650) 858-3986.
References
- 1.Becker S, Quay J, Koren HS, Haskill JS. Constitutive and stimulated MCP-1, Gro-alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am J Physiol. 1994;266:278–286. doi: 10.1152/ajplung.1994.266.3.L278. [DOI] [PubMed] [Google Scholar]
- 2.Besemer J, Hujber A, Kuhn B. Specific binding, internalization, and degradation of human neutrophil activating factor by human polymorphonuclear leukocytes. J Biol Chem. 1989;264:17409–17415. [PubMed] [Google Scholar]
- 3.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JJ, Foxman EF, Butcher EC. Chemoattractant receptor crosstalk as a regulatory mechanism in leukocyte adhesion and migration. Eur J Immunol. 1997;27:2571–2578. doi: 10.1002/eji.1830271016. [DOI] [PubMed] [Google Scholar]
- 5.Chenoweth DE, Rowe JG, Hugli TE. A modified method for chemotaxis under agarose. J Immunol Methods. 1979;25:337–353. doi: 10.1016/0022-1759(79)90026-7. [DOI] [PubMed] [Google Scholar]
- 6.Devreotes, P.N., and S.H. Zigmond. 1988. Chemotaxis in eukaryotic cells: a focus on leukocytes and dictyostelium. Annu. Rev. Cell. Biol. 4:649–686. [DOI] [PubMed]
- 7.Dobos GJ, Norgauer J, Eberle M, Schollmeyer PJ, Traynor-Kaplan AE. C5a reduces formyl peptide-induced actin polymerization and phosphatidyl(3,4,5)trisphospate formation, but not phosphadidylinositol (4,5) bisphosphate hydrolysis and superoxide production, in human neutrophils. J Immunol. 1992;149:609–615. [PubMed] [Google Scholar]
- 8.Gallin, J.G. 1993. Inflammation. In Fundamental Immunology. W.E. Paul, editor. Raven Press, LTD., New York. 1015–1032.
- 9.Gillitzer R, Ritter U, Spandau U, Goebeler M, Brocker E. Differential expression of Gro-alpha and IL-8 mRNA in psoriasis: a model for neutrophil migration and accumulation in vivo. J Invest Dermatol. 1996;107:778–782. doi: 10.1111/1523-1747.ep12371803. [DOI] [PubMed] [Google Scholar]
- 10.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman DW, Goetzl EJ. Heterogeneity of human polymorphonuclear leukocyte receptors for leukotriene B4. J Exp Med. 1984;159:1027–1041. doi: 10.1084/jem.159.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitayama J, Carr MW, Roth S J, Buccola J, Springer TA. Contrasting responses to multiple chemotactic stimuli in transendothelial migration. J Immunol. 1997;158:2340–2349. [PubMed] [Google Scholar]
- 13.Leonard EJ, Skeel A, Yoshimura T, Noer K, Kutvirt S, Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990;144:1323–1330. [PubMed] [Google Scholar]
- 14.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson RD, Quie PQ, Simmons RL. Chemotaxis under agarose: a new and simple method for measuring migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975;115:1650–1656. [PubMed] [Google Scholar]
- 16.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 17.Richardson RM, Ali H, Tomhave ED, Haribabu B, Snyderman R. Cross-desensitization of chemoattractant receptors occurs at multiple levels. J Biol Chem. 1995;270:27829–27833. doi: 10.1074/jbc.270.46.27829. [DOI] [PubMed] [Google Scholar]
- 18.Sabroe I, Williams TJ, Hebert C, Collins PD. Chemoattractant cross-desensitization of the human neutrophil IL-8 receptor involves receptor internalization and differential receptor subtype expression. J Immunol. 1997;158:1361–1369. [PubMed] [Google Scholar]
- 19.Schroder J. Cytokine networks in the skin. J Invest Dermatol. 1995;105:20s–24s. [PubMed] [Google Scholar]
- 20.Tardif M, Mery L, Brouchon L, Boulay F. Agonist-dependent phosphorylation of N-formylpeptide and activation peptide from the fifth component of complement (C5a) chemoattractant receptors in differentiated HL60 cells. J Immunol. 1993;150:3534–3545. [PubMed] [Google Scholar]
- 21.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 22.Tomhave ED, Richardson RM, Didsbury JR, Menard L, Snyderman R, Ali H. Cross-desensitization of receptors for peptide chemoattractants. J Immunol. 1994;153:3267–3275. [PubMed] [Google Scholar]
- 23.Tranquillo, R.T. 1990. Theories and models of gradient perception. In Biology of the Chemotactic Response. J.P. Armitage and J.M. Lackie, editors. Cambridge University Press, Cambridge. 35–75.
- 24.Uden A, Hafstrom I, Palmblad J. Relation of chemotactic factor gradients to neutrophil migration and orientation under agarose. J Leuk Biol. 1986;39:27–35. doi: 10.1002/jlb.39.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel UO, Abboud HE. Chemokines and renal disease. American Journal of Kidney Diseases. 1995;26:982–994. doi: 10.1016/0272-6386(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 26.Wilde MW, Carlson KE, Manning DR, Zigmond SH. Chemoattractant-stimulated GTPase activity is decreased on membranes from polymorphonuclear leukocytes incubated in chemoattractant. J Biol Chem. 1989;264:190. [PubMed] [Google Scholar]
- 27.Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zigmond SH. Consequences of chemotactic peptide receptor modulation for leukocyte orientation. J Cell Biol. 1981;88:644–647. doi: 10.1083/jcb.88.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]