Abstract
Yeast Bet1p participates in vesicular transport from the endoplasmic reticulum to the Golgi apparatus and functions as a soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) associated with ER-derived vesicles. A mammalian protein (rbet1) homologous to Bet1p was recently identified, and it was concluded that rbet1 is associated with the Golgi apparatus based on the subcellular localization of transiently expressed epitope-tagged rbet1. In the present study using rabbit antibodies raised against the cytoplasmic domain of rbet1, we found that the majority of rbet1 is not associated with the Golgi apparatus as marked by the Golgi mannosidase II in normal rat kidney cells. Rather, rbet1 is predominantly associated with vesicular spotty structures that concentrate in the peri-Golgi region but are also present throughout the cytoplasm. These structures colocalize with the KDEL receptor and ERGIC-53, which are known to be enriched in the intermediate compartment. When the Golgi apparatus is fragmented by nocodazole treatment, a significant portion of rbet1 is not colocalized with structures marked by Golgi mannosidase II or the KDEL receptor. Association of rbet1 in cytoplasmic spotty structures is apparently not altered by preincubation of cells at 15°C. However, upon warming up from 15 to 37°C, rbet1 concentrates into the peri-Golgi region. Furthermore, rbet1 colocalizes with vesicular stomatitis virus G-protein en route from the ER to the Golgi. Antibodies against rbet1 inhibit in vitro transport of G-protein from the ER to the Golgi apparatus in a dose-dependent manner. This inhibition can be neutralized by preincubation of antibodies with recombinant rbet1. EGTA is known to inhibit ER-Golgi transport at a stage after vesicle docking but before the actual fusion event. Antibodies against rbet1 inhibit ER-Golgi transport only when they are added before the EGTA-sensitive stage. These results suggest that rbet1 may be involved in the docking process of ER- derived vesicles with the cis-Golgi membrane.
Protein transport along the exocytotic and endocytotic pathways is primarily mediated via various types of transport vesicles that bud from one membrane compartment and fuse with a target compartment (Palade, 1975; Pryer et al., 1992; Rothman, 1994; Hong, 1996; Rothman and Wieland, 1996; Schekman and Orci, 1996). Soluble N-ethylmaleimide–sensitive factor (NSF)1 and its yeast counterpart (Sec18p) have been shown to participate in many different transport events (Graham and Emr, 1991; Whiteheart and Kubalek, 1995). Soluble NSF attachment proteins (SNAPs) or the yeast counterpart Sec17p is essential for membrane recruitment and functional activity of NSF (Clary et al., 1990; Griff et al., 1992; Whiteheart and Kubalek, 1995). These soluble factors function in conjunction with SNAP receptors (SNAREs) associated with the vesicles and target membranes. SNAREs are key determinants of the specificity of vesicle docking and fusion events (Rothman, 1994; Whiteheart and Kubalek, 1995).
To account for the specificity of vesicle transport, the SNARE hypothesis predicts that the specific docking and fusion of vesicles with the target compartment is primarily mediated by interaction between v-SNAREs on vesicles with t-SNAREs on the target membrane (Söllner et al., 1993; Ferro-Novick and Jahn, 1994; Rothman, 1994; Rothman and Warren, 1994; Scheller, 1995; Südhof, 1995; Pfeffer, 1996). Yeast Bet1p is an integral membrane protein anchored to the ER membrane by its COOH-terminal hydrophobic membrane anchor. Bet1p is incorporated into ER-derived transport vesicles and functions as a v-SNARE for docking and/or fusion of the vesicle with the early Golgi subcompartment (Newman et al., 1990; Dascher et al., 1991; Ossig et al., 1991; Rexach et al., 1994; Sögaard et al., 1994). By searching the expressed sequence tags (EST) database with the Bet1p sequence, we have identified human and mouse homologues of Bet1p. The rat homologue (rbet1) was recently cloned and reported (Hay et al., 1996). By examining the subcellular localization of an epitope-tagged rbet1 transiently expressed in COS cells, it was concluded that rbet1 is associated with the Golgi apparatus (Hay et al., 1996, 1997). Since Bet1p is primarily associated with the ER and ER-derived vesicles (Newman et al., 1990; Dascher et al., 1991; Ossig et al., 1991; Rexach et al., 1994; Sögaard et al., 1994), the proposed Golgi localization of rbet1 raised the possibility that rbet1 may be involved in a transport event different from that of Bet1p and may therefore not be a functional counterpart. Furthermore, the functional aspect of rbet1 has not been investigated. To clarify these important issues, we have examined the subcellular localization of endogenous rbet1 as well as its involvement in ER-Golgi transport. Our results suggest that rbet1 is preferentially associated with the pre-Golgi intermediate compartment and that rbet1 participates in ER-Golgi transport.
Materials and Methods
cDNA Cloning and Sequencing
EST clones that have open reading frames encoding proteins homologous to Bet1p were revealed using the BLAST program. (These squence data can be found under accession numbers R52442, AA305708, AA112610, AA305267, and W84841 for human, H35645 for rat, and AA245530, W70983, and W18376 for mouse.) The complete coding sequence of EST clones R52442 for human (with oligonucleotide 1 and 2), H35645 for rat (with oligonucleotide 3 and 4), and W70983 for mouse (with oligonucleotide 5 and 6) bet1 were confirmed by sequencing using the following oligonucleotides: 1, 5′CGGGATCCATGAGGCGTGCAGGCCTGGGTGAAGGAGTAC; 2, 5′GGAAGCTTTCACCTTTGGTTTGGCCTCCCCTCTGG; 3, 5′GGGGATCCATGAGCCGTGCAGCCCTGGGTGATGG; 4, 5′GGAAGCTTTCAGGTTTTACCTAGAAATCCAG; 5, 5′ACCCACTATTAGCATAGCCAT; and 6, 5′AATCACAATTTGATTCCACAA.
Expression and Purification of Recombinant Proteins
For the production of recombinant glutathione-S-transferase (GST) fusion proteins, the cytoplasmic regions of hbet (residues 1–86) and rbet (residues 1–81) were retrieved by PCR with oligonucleotides 1 and 2 (R52442 as the template), or 3 and 4 (H35645 as the template). The PCR products were digested with BamHI and EcoRI restriction enzymes and subcloned into BamHI/EcoRI sites of the bacterial expression vector pGEX-KG (Guan and Dixon, 1991). The ligated DNA was transformed into DH5α cells and ampicillin resistant colonies expressing the GST fusion proteins were screened as described (Sambrook et al., 1989). Purification of GST-hbet1 and GST-rbet1 was performed as described previously (Lowe et al., 1996).
Antibodies
For the preparation of polyclonal antibodies against hbet and rbet1, GST-hbet1 or GST-rbet1 proteins (400 μg) emulsified in complete Freund's adjuvant were injected subcutaneously into two local New Zealand rabbits. Booster injections containing similar amounts of the antigens emulsified in incomplete Freund's adjuvant were performed after 2, 4, 6, 9, and 12 wk. Rabbits were bled 10 d after the second and subsequent booster injections. Affinity purification of specific antibodies was performed using the GST-rbet1 protein coupled to cyanogen bromide–activated Sepharose (3 mg/ml Sepharose bead). Briefly, 3 ml of antiserum was diluted with 3 ml of PBS and then incubated with the coupled beads for 2 h at room temperature. The beads were washed extensively with PBS, buffer A (50 mM Tris, pH 7.4, 500 mM NaCl), buffer B (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100), and then PBS. Specific antibodies were eluted with 10 ml of immunopure IgG elution buffer (Pierce Chemical Co., Rockford, IL). Collected fractions (1 ml each) were analyzed by SDS-PAGE to determine fractions containing the antibodies. Fractions containing the antibodies were pooled and either dialyzed against PBS or transport buffer (25 mM Hepes-KOH, pH 7.2, 125 mM KOAc). Rabbit polyclonal antibodies against α2,6-sialyltransferase and monoclonal antibody HFD9 against GS28 have been described previously (Subramaniam et al., 1995, 1996). Polyclonal antibodies against the intermediate compartment (IC) marker p58 (Saraste et al., 1987; Saraste and Svensson, 1991) were kindly provided by J. Saraste (University of Bergen, Norway). Monoclonal antibody against ERGIC-53, the human counterpart of p58 (Schweizer et al., 1988, 1990), was kindly provided by H.-P. Hauri (University of Basel, Switzerland). Monoclonal antibody against mammalian KDEL receptor has been described previously (Tang et al., 1995b ). Monoclonal antibody against Golgi mannosidase II was purchased from Babco (Berkeley, CA). Monoclonal antibody against Rab1 (Plutner et al., 1991) was kindly provided by W.E. Balch. Rabbit polyclonal antibodies against syntaxin 5 were described recently (Subramaniam et al., 1997). Polyclonal antibodies against α-SNAP and syntaxin 6 were raised in rabbit with corresponding purified recombinant proteins. Specific antibodies were affinity-purified as described above for rbet1 antibodies.
Immunofluorescence Microscopy
Immunofluorescence microscopy was performed as described previously (Subramaniam et al., 1995; Lowe et al., 1996; Tang et al., 1997). Briefly, cells grown on coverslips were washed twice with PBSCM (PBS containing 1 mM CaCl and 1 mM MgCl2) and then fixed with 3% paraformaldehyde in PBSCM for 30 min at 4°C. After sequentially washing with PBSCM, 50 mM NH4Cl in PBSCM, and PBSCM, cells were permeabilized with PBSCMS (PBSCM containing 0.2% saponin) for 20 min at room temperature. Incubation with primary antibodies (5–10 μg/ ml) in fluorescence dilution buffer (PBSCM with 5% normal goat serum, 5% FBS, and 2% BSA, pH 7.6) was performed for 1 h at room temperature. After washing three times with PBSCMS, cells were incubated with rhodamine- or FITC- conjugated secondary antibodies for 1 h at room temperature. Cells were then washed five times with PBSCMS, mounted with Vertastain (Vector Laboratories, Burlingame, CA), and then viewed. Conventional fluorescence microscopy was done using a microscope (model Axiophot; Carl Zeiss, Inc., Thornwood, NY) equipped with epifluorescence optics. Confocal microscopy was performed using a scan head (model MRC600; Bio-Rad Labs, Carlsbad, CA) connected to a microscope (model Axiophot; Carl Zeiss, Inc.) with epifluorescence optics.
For temperature treatment of cells, normal rat kidney (NRK) cells were incubated at 15°C for 3 h and then incubated at 37°C for 0, 5, 10, and 30 min before processing for immunofluorescence microscopy. Infection of Vero cells with the ts045 strain of vesicular stomatitis virus (VSV) and the subsequent processing for immunofluorescence microscopy were performed as described previously (Tang et al., 1993, 1995b , 1997). For the treatment of cells with brefeldin A or nocodazole, cells grown on coverslips were incubated in the presence of brefeldin A (10 μg/ml) or nocodazole (10 μg/ml) for 1 h at 37°C, washed twice with PBSCM, and then fixed in 3% paraformaldehyde. Fixed cells were then permeabilized and incubated with antibodies against rbet1 and monoclonal antibodies against mannosidase II, the KDEL receptor, or G-protein of VSV for 1 h at room temperature. After washing three times with PBSCMS, cells were incubated with rhodamine-conjugated goat anti–rabbit IgG (10 μg/ml) and FITC-conjugated sheep anti–mouse (10 μg/ml) for 1 h at room temperature. After washing extensively, coverslips were then mounted as described above.
Immunoprecipitation and Immunoblot Analysis
rbet1-specific antibodies (100 μg) bound to protein A–Sepharose beads (Pharmacia Biotech, Inc., Piscataway, NJ) were incubated overnight with 3 mg of Golgi extracts in incubation buffer (20 mM Hepes, pH 7.2, 100 mM KCl, 1 mM DTT, 10 mM EDTA, 0.2 mM ATP) with 1% Triton X-100 at 4°C. Beads were then washed twice in incubation buffer with 0.5% Triton X-100 and then twice in incubation buffer with 0.2% Triton X-100. Immunoprecipitates were separated on SDS-PAGE and transferred to a Hybond-C extra nitrocellulose filter before sequential incubation with primary antibodies (10 μg/ml) and 125I–protein A (0.1 μCi/ml), followed by autoradiography. Incubation of the filter with primary antibodies and 125I–protein A and washing of the filter were performed in blocking buffer (PBS containing 5% skim milk and 0.05% Tween 20), PBS, and PBST (PBS containing 0.05% Tween 20), respectively.
In Vitro ER-Golgi Transport
The ER to Golgi transport assay using semiintact cells was performed as described previously (Beckers et al., 1987; Balch et al., 1994). Briefly, NRK cells were grown on 10-cm Petri dishes to form a confluent monolayer and infected with a temperature-sensitive strain of the vesicular stomatitis virus, VSVts045, at 32°C for 3–4 h. The cells were pulse-labeled with [35S]methionine (100 μCi/ml) at the restrictive temperature (40°C) for 10 min and perforated on ice by hypotonic swelling and scraping. These semiintact cells were then incubated in a complete assay cocktail of 40 μl containing (in final concentrations) 25 mM Hepes-KOH, pH 7.2, 90 mM KOAc, 2.5 mM MgOAc, 5 mM EGTA, 1.8 mM CaCl2, 1 mM ATP, 5 mM creatine phosphate, 0.2 IU of rabbit muscle creatine phosphokinase, 25 μg of cytosol (Davidson and Balch, 1993), and 5 μl (25–30 μg of protein; 1–2 ×105) of semiintact cells. Additional reagents were added as indicated in Results. For a standard assay, samples were incubated for 90 min at 32°C, and transport was terminated by transfer to ice. The membranes were collected by a brief spin, solubilized in 60 μl of 0.2% SDS, 50 mM Na citrate, pH 5.5. After boiling for 5 min, the samples were digested overnight at 37°C in the presence of 2.5 U of endoglycosidase H (endo H), and the reaction was then terminated by adding 5× concentrated gel sample buffer. The samples were analyzed by 7.5% SDS–polyacrylamide gels. The transport was quantified using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). For antibody inhibition of transport assay, rbet1 antibodies were added into the complete assay cocktail and incubated on ice for 60 min to allow the antibodies to diffuse into semiintact cells. To neutralize antibody inhibition, different amounts of GST-rbet1 fusion proteins were incubated with rbet1 antibody on ice for 30 min before incubation with complete assay cocktail. In the case of the two-stage assay, after Stage I incubation for 60 min at 32°C in a complete assay cocktail supplemented with 10 mM EGTA but without Ca2+, membranes were spun for 20 s at full speed in an Eppendorf table top centrifuge and subsequently resuspended in fresh assay cocktail with Ca2+ by pipetting up and down 10 times with a yellow pipette tip. Additional reagents were added as indicated in the Results. Samples were incubated for 30 min at 32°C, and transport was terminated by transferring to ice.
In Vitro Binding Assays
Golgi membranes (3 mg) were centrifuged at 12,000 g for 15 min at 4°C, and the pellet was extracted with 500 μl of incubation buffer (100 mM KCl, 20 mM Hepes pH 7.3, 2 mM EDTA, 2 mM DTT, 0.2 mM ATP) containing 1% Triton X-100 at 4°C for 1 h with agitation. The extracted membranes were diluted with 500 μl of incubation buffer without Triton X-100 and then centrifuged at 100,000 g at 4°C for 1 h. Beads containing 2 μg GST–α-SNAP or GST alone were washed twice with incubation buffer containing 0.5% TX-100 (1 ml each) and then incubated with different amounts of the Golgi extract in a total volume of 100 μl at 4°C for 3 h with agitation. Beads were then washed three times with incubation buffer with 0.5% Triton X-100, once with incubation buffer with 0.1% Triton X-100, and then processed for immunoblot analysis to detect rbet1.
Results
Mammalian Homologues of Bet1p Are Well Conserved
Searching the EST database with the yeast Bet1p sequence led to the identification of an EST clone (accession number R52442) encoding a putative human homologue. During the course of our study, a rat homologue (rbet1) was published (Hay et al., 1996), and more EST clones for the human (accession numbers AA305708, AA112610, AA305267, and W84841) as well as for mouse (accession numbers AA245530, W70983, and W18376) homologues were subsequently identified in the database. The human and mouse EST clones were sequenced to obtain the coding nucleotide sequence and hence the amino acid sequences of human and mouse bet1. As aligned in Fig. 1, human, rat, and mouse bet1 (hbet1, rbet1, and mbet1, respectively) are highly homologous (hbet1 is ∼93% identical to rbet and mbet1, while rbet1 and mbet1 share over 98% identity). All the mammalian homologues are ∼20% identical to Bet1p and share an overall amino acid sequence similarity of about 38–40% with Bet1p. The recombinant cytoplasmic domain of hbet1 was initially expressed as a fusion protein to GST (GST-hbet1) and was used to immunize rabbits. Polyclonal antibodies against hbet1, however, cross-react poorly with rbet1 in NRK cells, despite the fact that hbet1 and rbet1 are highly homologous. To facilitate our morphological and functional studies in NRK cells, we subsequently expressed the cytoplasmic region (residues 1–81) of rbet1 (GST-rbet1), and affinity-purified rabbit antibodies against GST-rbet1 were used in all subsequent experiments.
Figure 1.
The mammalian bet1 proteins are highly conserved. The amino acid sequences of human, rat, and mouse bet1 are aligned and residues identical among them are shaded.
rbet1 Is a 17-kD Protein Preferentially Associated with Membrane Fractions Enriched in the Golgi and Intermediate Compartment
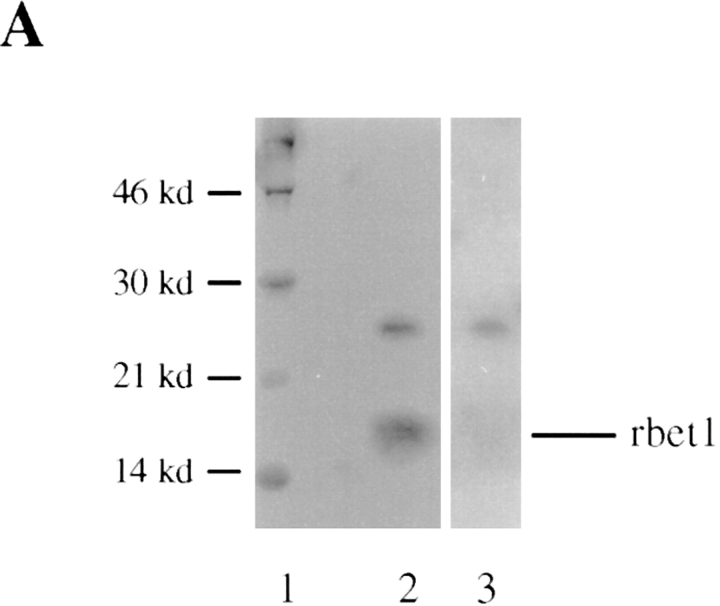
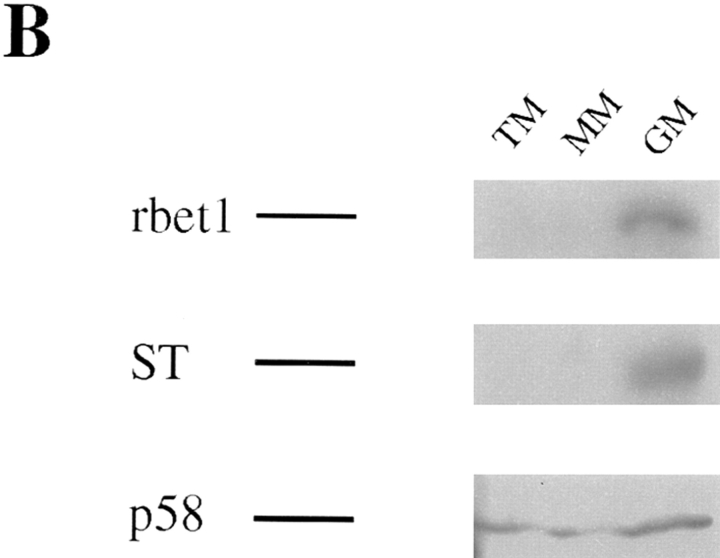
When the total membrane fraction derived from NRK cells was analyzed by immunoblot using rbet1 antibodies, a major polypeptide of about 17-kD was detected (Fig. 2 A, lane 2). Detection of this polypeptide was abolished by preincubation of antibodies with GST-rbet1 (Fig. 2 A, lane 3) but not with GST (data not shown), demonstrating that the 17-kD polypeptide is rbet1. When rat liver total membranes, microsomal membranes, and Golgi membranes (GM) were analyzed by immunoblot, rbet1 was enriched in the GM fraction (Fig. 2 B, upper panel). Markers of the IC are also known to be enriched in the GM fraction when separated by sucrose gradient (Saraste et al., 1987; Schweizer et al., 1988). As shown, similar to α2,6-sialyltransferase (Fig. 2 B, middle panel), the IC marker p58 is also enriched, although to a lesser extent, in the GM fraction (Fig. 2 B, lower panel).
Figure 2.
rbet1 is a 17-kD protein enriched in the membrane fraction of the Golgi and the IC. (A) Total membrane fraction of NRK cells was resolved by SDS-PAGE and transferred to a filter. The filter was incubated with rbet1 antibodies in the absence (lane 2) or presence of GST-rbet1 (lane 3). (B) Total membrane (TM), microsomal membrane (MM), and Golgi membrane (GM) fractions derived from rat liver were analyzed by immunoblot to detect rbet1, α2,6-sialyltransferase (ST), and the IC-enriched protein p58.
rbet1 Is a SNARE and Interacts with GS28 and Syntaxin 5
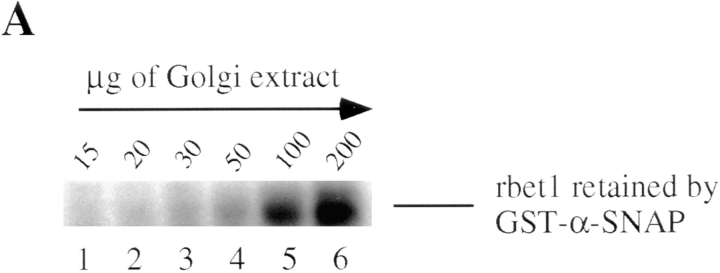
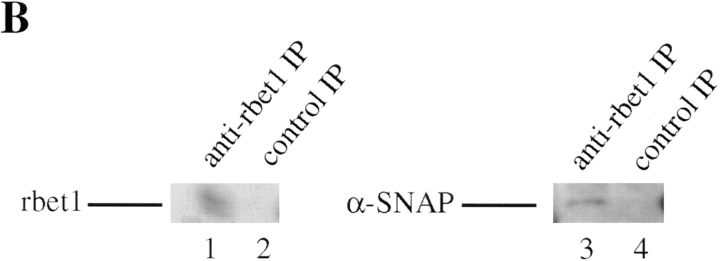
When increasing amounts of Golgi extract were incubated with immobilized recombinant α-SNAP fused to GST (GST–α-SNAP), increased amounts of rbet1 were found to be retained by the beads (Fig. 3 A). Under identical conditions, rbet1 was not retained by immobilized GST or several other GST fusion proteins (data not shown). Furthermore, α-SNAP was detected in the immunoprecipitate of rbet1 antibodies but not in the immunoprecipitate of control antibodies (Fig. 3 B). These results demonstrate that rbet1 functions as a SNARE.
Figure 3.
rbet1 is a SNARE. (A) The indicated amounts of Golgi extract were incubated with 2 μg of immobilized GST–α-SNAP. After extensive washing, the beads were analyzed by immunoblot to detect rbet1. (B) Golgi extract was immunoprecipitated with rbet1 antibodies or control antibodies and the immunoprecipitates were analyzed by immunoblot to detect rbet1 and α-SNAP.
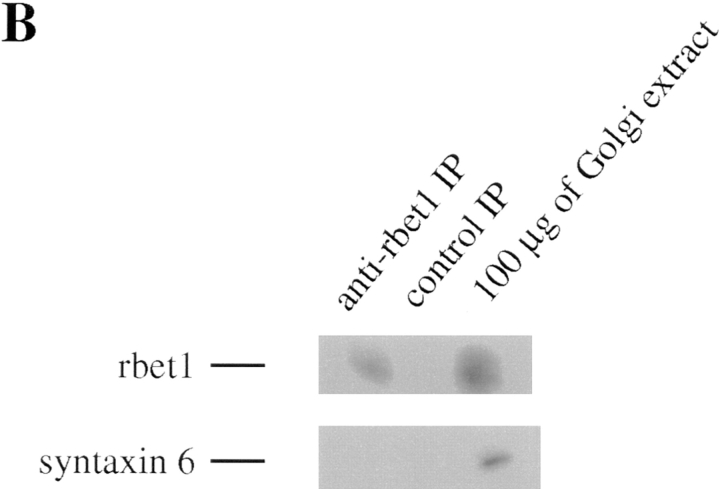
When the immunoprecipitate of rbet1 antibodies was analyzed by immunoblot, GS28 and syntaxin 5 were detected (Fig. 4 A). The antibodies against syntaxin 5 detect specifically two polypeptides of 38 and 32 kD, respectively (Subramaniam et al., 1997). As shown, the 38-kD form was preferentially coimmunoprecipitated with rbet1, while the 32-kD form was barely detectable. GS28 and syntaxin 5 are two cis-Golgi SNAREs involved in ER-Golgi transport (Banfield et al., 1994; Dascher et al., 1994; Nagahama et al., 1996; Subramaniam et al., 1996). The coimmunoprecipitation of GS28 and syntaxin 5 by rbet1 antibodies is specific because neither GS28 nor syntaxin 5 was detected in the immunoprecipitate of control antibodies. Under identical conditions, syntaxin 6, another Golgi SNARE (Bock et al., 1996), was not coimmunoprecipitated by rbet1 antibodies (Fig. 4 B). In addition, we have observed that rbet1 could also be coimmunoprecipitated by GS28 and syntaxin 5 antibodies (data not shown). These results suggest that rbet1 exists in a protein complex that contains GS28 and syntaxin 5.
Figure 4.
rbet1 exists in a protein complex that contains GS28 and syntaxin 5. (A) Golgi extract was immunoprecipitated with rbet1 antibodies or control antibodies. The immunoprecipitates were analyzed by immunoblot to detect rbet1, GS28, and syntaxin 5. (B) The immunoprecipitate of rbet1 and control antibodies, together with 100 μg of Golgi extract, were analyzed by immunoblot to detect rbet1 and syntaxin 6.
Predominant Association of rbet1 with the Intermediate Compartment
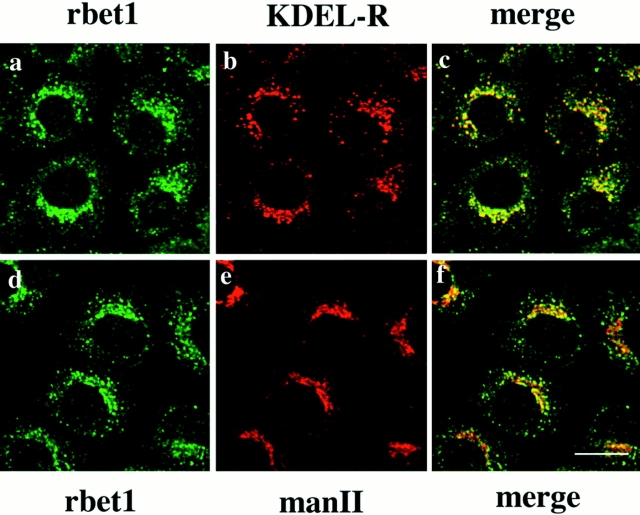
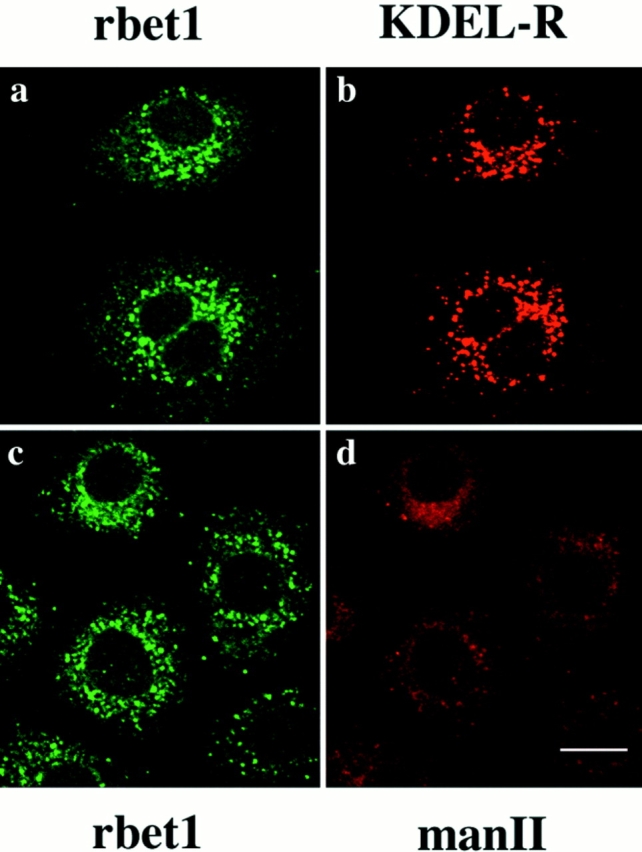
Using affinity-purified antibodies against rbet1, we have examined the subcellular localization of endogenous rbet1 in NRK cells by indirect immunofluorescence microscopy. As shown in Fig. 5, rbet1 is predominantly detected in vesicular structures located throughout the entire cytoplasm with a higher concentration around the perinuclear region (Fig. 5, a and d). This labeling was totally abolished when the antibodies were preincubated with GST-rbet1 (data not shown). A majority of these vesicular structures is also labeled by a monoclonal antibody against the mammalian KDEL receptor (Fig. 5, b and c) (Tang et al., 1995a ), which is known to be enriched in the pre-Golgi IC (Griffiths et al., 1994; Tang et al., 1995a ,b). However, when double-labeled with Golgi mannosidase II (Moreman and Robbins, 1991) (Fig. 5 e), the majority of rbet1-containing structures are devoid of mannosidase II labeling. Even those perinuclear/peri-Golgi rbet1-containing structures have a morphology (more spotty) distinct from that of Golgi mannosidase II (Fig. 5 f), suggesting that a significant portion of these peri-Golgi vesicular structures is juxtaposed to Golgi apparatus but is probably not part of the Golgi apparatus.
Figure 5.
Double-labeling of rbet1 (a and d) with the KDEL receptor (KDEL-R) (b) or Golgi mannosidase II (man II) (e) in NRK cells. The merged pictures (c and f) are also presented. Bar, 10 μm.
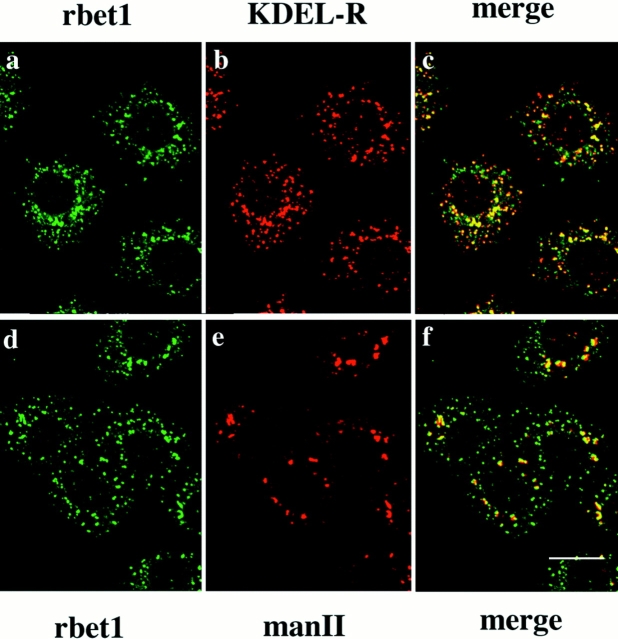
When cells were treated with nocodazole, which is known to fragment the Golgi apparatus (Rogalski and Singer, 1984; Turner and Tartakoff, 1989), rbet1 (Fig. 6, a and d) and the mammalian KDEL receptor (Fig. 6 b) remained predominantly associated with cytoplasmic spotty structures, while the Golgi apparatus marked by mannosidase II became fragmented into several patches (Fig. 6 e). Under this condition, it is interesting to note that a significant portion of the spotty structures marked by rbet1 and the KDEL receptor is no longer colocalized, suggesting that rbet1 and KDEL receptor may reside in distinct structures of the IC. Under this condition, partial colocalization of rbet1 and KDEL receptor in larger dots could be detected, and these structures may either represent the IC or the fragmented Golgi apparatus. Furthermore, it is obvious that the majority of rbet1-containing structures is negative for Golgi mannosidase II labeling (Fig. 6 f). Some rbet1-containing spotty structures are seen in the vicinity of those marked by mannosidase II, although they are not well colocalized, suggesting that this fraction of rbet1 may be associated with a subregion of the fragmented Golgi apparatus that is not enriched in the Golgi mannosidase II.
Figure 6.
NRK cells were treated with 10 μg/ml nocodazole for 1 h, fixed, and double-labeled for rbet1 (a and d) with KDEL-R (b) or man II (e). Also shown are the merged images (c and f). Bar, 10 μm.
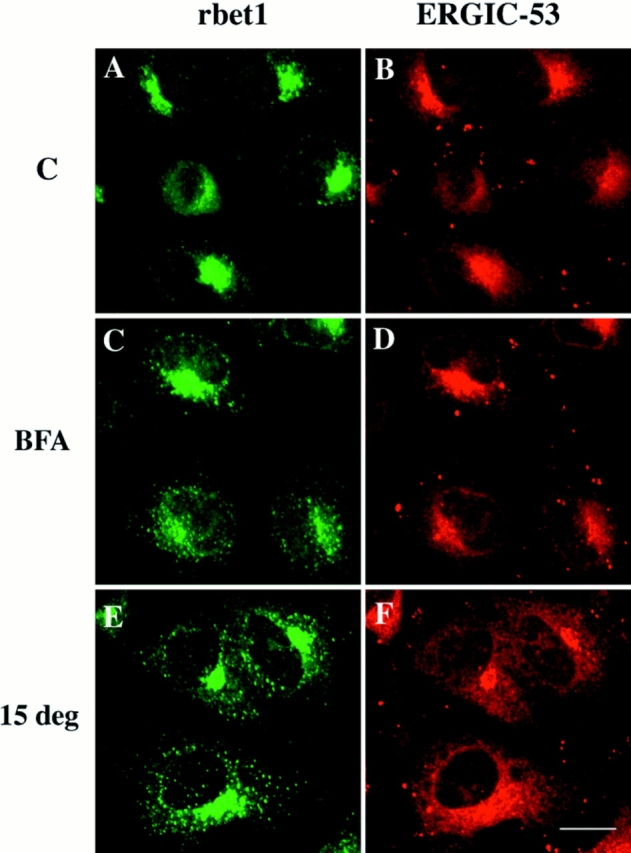
When cells were treated with brefeldin A, which is known to have differential effects on proteins enriched in the IC as compared with resident Golgi proteins (Klausner et al., 1992; Tang et al., 1995a ), rbet1 is redistributed into spotty structures (Fig. 7, a and c) that are also marked by KDEL receptor (Fig. 7 b). Under the same condition, Golgi mannosidase II is redistributed into the ER-like structures (Fig. 7 d). Redistribution by brefeldin A into spotty structures is a characteristic of proteins associated with the IC, including ERGIC-53/p58 and KDEL receptor (Saraste and Svensson, 1991; Tang et al., 1993, 1995a ). These results, taken together, suggest that rbet1 is preferentially associated with the IC, although a fraction is also associated with a subregion (most likely the cis-face) of the Golgi apparatus under steady state and that the majority of rbet1 can be shifted to the IC under some conditions, such as treatment with brefeldin A.
Figure 7.
NRK cells were treated with 10 μg/ml brefeldin A for 1 h, fixed, and double-labeled for rbet1 (a and c) with KDEL-R (b) or man II (d). Bar, 10 μm.
To provide additional evidence for the preferential association of rbet1 with the IC, we have double-labeled cells with antibodies against rbet1 and a monoclonal antibody against ERGIC-53, which is normally enriched in the IC and cycles preferentially between the IC and the ER (Schweizer et al. 1988, 1990). Because only polyclonal antibodies against rbet1 and monoclonal antibody specific for human ERGIC-53/p58 are available, this experiment was only performed in a human cell line (HeLa). As shown in Fig. 8, at steady state rbet1 and ERGIC-53 are colocalized well in spotty structures enriched in the Golgi region (Fig. 8, A and B). In cells pretreated with brefeldin A, they are similarly colocalized in the spotty structures enriched in the perinuclear region (Fig. 8, C and D). When cells were preincubated at 15°C, rbet1 and ERGIC-53 could be seen to concentrate in a compact Golgi-like structure, although rbet1 could be also seen in peripheral spotty structures while association of ERGIC-53 with the ER became more obvious. ERGIC-53 has also been shown previously to shift to a compact Golgi-like structure when cells are preincubated at 15°C (Lippincott-Schwartz et al., 1990). The effect of 15°C preincubation on the IC markers in HeLa cells is different from that observed in NRK cells, in which the IC markers became more enriched in peripheral spotty structures as shown above. These results further support our interpretation that rbet1 is preferentially associated with the IC.
Figure 8.
Double-labeling of rbet1 with ERGIC-53 in control cells (A and B), cells pretreated with 10 μg/ml brefeldin A for 1 h (C and D), and cells preincubated at 15°C for 3 h (E and F). Bar, 10 μm.
Dynamic Distribution of rbet1 between the Peripheral and peri-Golgi IC
Proteins in the IC are dynamically distributed between the peripheral structures and the perinuclear structures around the Golgi apparatus (peri-Golgi), and cargo proteins in the ER are transported to the Golgi apparatus via migration/ maturation of peripheral IC to the peri-Golgi region or directly from the peri-Golgi IC (Saraste and Svensson, 1991; Lotti et al., 1992; Plutner et al., 1992; Lippincott-Schwartz, 1993; Tang et al., 1993, 1995a ; Balch et al., 1994; Tisdale et al., 1997). Incubation of cells at 15°C is known to block transport from the ER to the Golgi at the level of the IC and also causes an increase in the concentration of IC proteins in the peripheral structures (Saraste and Svensson, 1991; Lippincott-Schwartz, 1993; Tang et al., 1993, 1995a ). When cells were preincubated at 15°C, rbet1 and the KDEL receptor were colocalized in the same vesicular structures spreading in the entire cytoplasm (Fig. 9, a and b). When 15°C-arrested cells were warmed up to 37°C for 5–10 min, rbet1 and the KDEL receptor were seen to shift to the peri-Golgi region (Fig. 9, c–f). At later time points, rbet1 and the KDEL receptor reach a steady-state distribution (Fig. 9, g and h). These results suggest that, like other IC proteins, rbet1 is dynamically distributed between the peripheral and peri-Golgi IC. This interpretation is also consistent with our observation that, like other IC proteins, the fractions of rbet1 associated with the peripheral and peri-Golgi IC vary among different cell types (data not shown).
Figure 9.
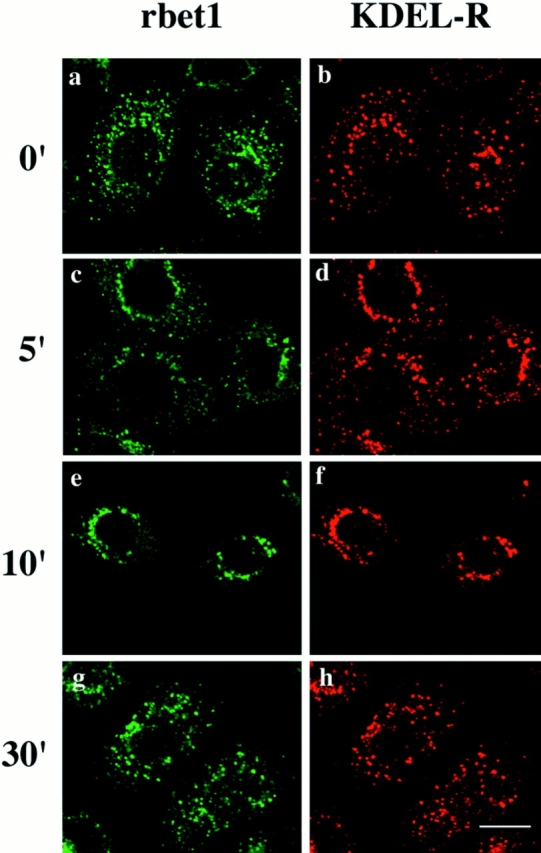
NRK cells were incubated at 15°C for 3 h and then incubated at 37°C for the indicated time. The cells were fixed and double-labeled for rbet1 (a, c, e, and g) with KDEL-R (b, d, f, and h). Bar, 10 μm.
The IC is a dynamic collection of specialized ER regions involved in COPII vesicle budding, budded vesicles, and vesicular-tubular intermediates involved in cargo transport from the ER to the Golgi apparatus (Bonatti et al., 1989; Schweizer et al., 1990; Tang et al., 1993; Krijnse-Locker et al., 1994). To gain more insight into the functional significance of rbet1 association with the IC, we infected Vero cells with the ts045 strain of VSV, the G-protein of which is mutated in such a way that it can not be exported from the ER at the restricted temperature (40°C). The infected cells were maintained at 40°C to restrict G-protein to the ER. Cells were then processed for double-labeling for rbet1 and G-protein. As shown in Fig 10 A, b, G-protein is mainly distributed in the ER with a significant amount colocalized with vesicular structures marked by rbet1 (Fig. 10 A, a and c), suggesting that G-protein could distribute into the structures marked by rbet1. The G-protein associated with these vesicular structures is most likely enriched in the ER region involved in COPII vesicle budding, which was defined as part of the ER export complex (equivalent to the IC) (Bannykh et al., 1996; Bannykh and Balch, 1997). Under this condition, some rbet1 could be detected in the reticular structures of the ER, particularly at the peripheral area of the cell (Fig. 10 A, a). This observation is consistent with the fact that IC proteins can be detected in the ER under certain conditions and/or in some cell types (Saraste and Svensson, 1991; Tang et al., 1995b ). ER labeling for rbet1 is not so obvious in uninfected Vero cells maintained at 37°C (Fig 10 B, Vero), suggesting that viral infection, extensive accumulation of cargo molecule in the ER, and/or incubation of cells at 40°C caused a shift of a fraction of rbet1 from the IC to the ER. Other IC markers, such as the KDEL receptor and ERGIC-53/p58, are also seen to be enhanced in the ER when cells were infected with VSV (Tang et al., 1995a ,b). When VSV-infected cells were shifted from 40°C to a permissive temperature (32°C) for 4 min, G-protein was seen to export from the ER and accumulate in larger peri-Golgi spotty structures (Fig. 10 A, e). Under this condition, a major portion of rbet1 became concentrated in these structures as well (Fig. 10 A, d and f). Upon incubation at 32°C for 12 min, G-protein is seen in typical Golgi structure (Fig. 10 A, h) while rbet1 revents to small spotty structures (Fig. 10 A, g), and a majority of these structures are no longer colocalized with G-protein (Fig. 10 A, i). The dynamic distribution of rbet1 from the peripheral to the peri-Golgi IC correlates well with transport of cargo proteins from the ER to the Golgi via peripheral and peri-Golgi IC.
Figure 10.
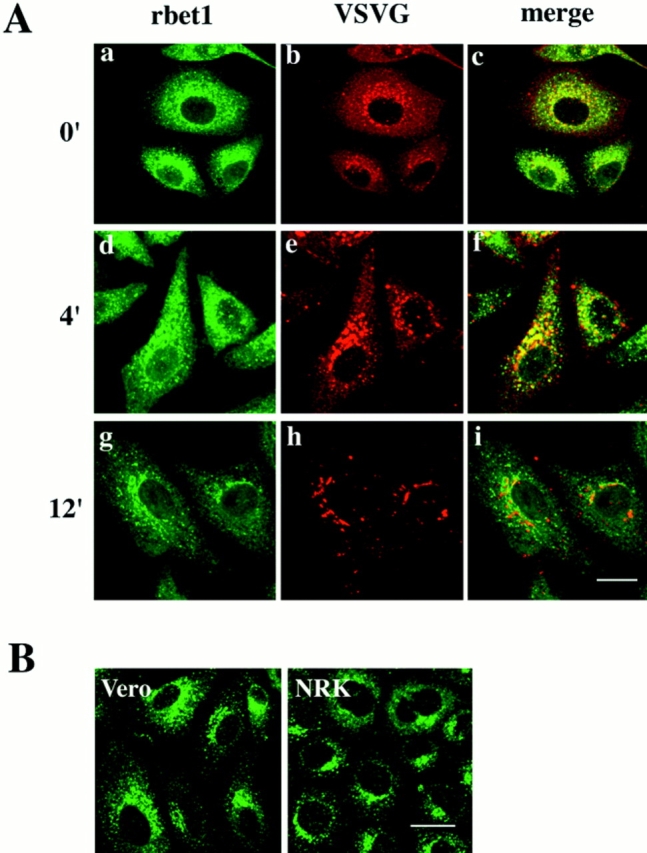
(A) Vero cells were infected with VSV ts045 and incubated at 40°C for 4 h. Cells were then shifted to 32°C for the indicated time and fixed. Double-labeling of rbet1 (a, d, and g) with the envelope protein of VSV (VSVG) (b, e, and h) was performed. The merged images (c, f, and i) are also shown. (B) Control Vero and NRK cells were fixed and labeled with rbet1 antibodies. Bars, 10 μm.
Antibodies against rbet1 Inhibit ER-Golgi Transport
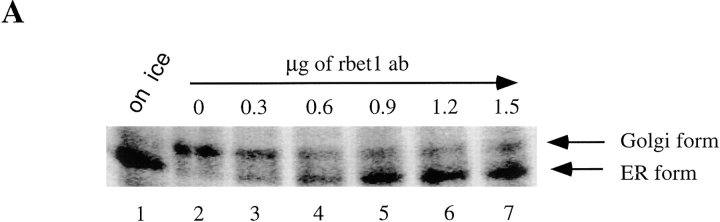
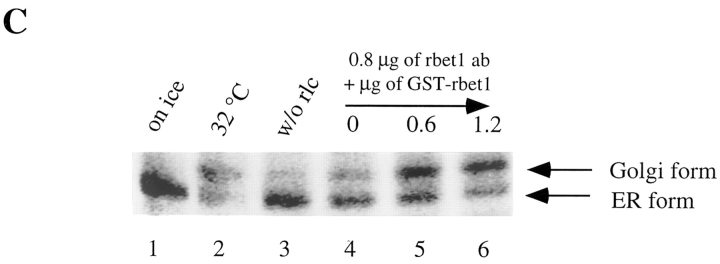
The predominant association of rbet1 with the IC and the observation that rbet1 colocalizes with a cargo protein en route to the Golgi suggest that rbet1 may be involved in protein transport from the ER to the Golgi apparatus in mammalian cells. To establish this point, we have examined whether protein transport from the ER to the Golgi could be inhibited by antibodies against rbet1. We adopted the well-established in vitro ER-Golgi transport system using VSV ts045–infected NRK cells (Beckers et al., 1987; Balch et al., 1994). Infected NRK cells were pulse-labeled with [35S]methionine at 40°C so that the labeled G-protein is restricted to the ER. The plasma membrane was then selectively perforated, and the cells were depleted of endogenous cytosol. G-protein can be transported from the ER to the Golgi when these semiintact cells are incubated at permissive temperature (32°C) supplemented with exogenous cytosol and an ATP-regenerating system. ER-Golgi transport was measured by following the extent of conversion of ER-restricted endo H–sensitive G-protein into endo H–resistant Golgi form. As shown in Fig. 11 A, no transport was detected when semiintact cells were incubated on ice (Fig. 11 A, lane 1). The majority of G-protein is converted into endo H–resistant Golgi form when incubated at 32°C (lane 2). Transport from the ER to the Golgi was however inhibited by antibodies against rbet1 in a dose-dependent manner (lanes 3–7). Inhibition was not so obvious when only 0.3 μg of antibodies was added (lane 3). However, ∼60% of the G-protein was not converted into the Golgi form when 0.6 μg of antibodies was added (lane 4). Transport was almost completely inhibited when 0.9 μg (lane 5) or more (lanes 6–7) of antibodies were added. The inhibition is specific because the same amount of heat-denatured antibodies had no effect on transport (compare Fig. 11 A, lane 4, with Fig 11 B, lane 3) and comparable amounts of antibodies against the KDEL receptor (Tang et al. 1997), and several other control antibodies had no effect on ER-Golgi transport of G-protein (data not shown, also see Subramaniam et al., 1996). Furthermore, inhibition exhibited by antibodies against rbet1 could be neutralized by GST-rbet1 (Fig 11 C). G-protein transport to the Golgi is almost completely inhibited by 0.8 μg of rbet1 antibodies (lane 4). However, preincubation of rbet1 antibodies with 0.6 (lane 5) and 1.2 μg (lane 6) of GST-rbet1 resulted in ∼60 and 80% of G-protein being converted into the Golgi form. These results, taken together, suggest that inhibition of ER-Golgi transport by rbet1 antibodies occurs by specific association with endogenous rbet1. rbet1 is therefore essential for ER-Golgi transport.
Figure 11.
rbet1 antibodies specifically inhibit in vitro ER-Golgi transport. (A) In vitro ER-Golgi transport was performed either on ice (lane 1) or at 32°C (lanes 2–7) supplemented with the indicated amounts of rbet1 antibodies. (B) In vitro ER-Golgi transport was performed either on ice (lane 1) or at 32°C (lanes 2–4) supplemented with 1.2 μg of rbet1 antibodies (lane 3) or the heat-inactivated antibodies (lane 4). (C) In vitro ER-Golgi transport was performed either on ice (lane 1) or at 32°C (lanes 2–6) in the absence (lane 3) or in the presence of rat liver cytosol (rlc) (lanes 1 and 2, and 4–6) supplemented with 0.8 μg of rbet1 antibodies and indicated amounts of GST-rbet1.
rbet1 Antibodies Must Be Present before the EGTA-sensitive Stage to Achieve Inhibition
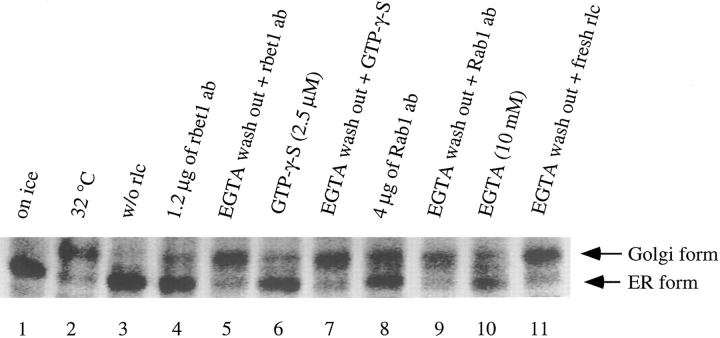
In vitro ER-Golgi transport could be inhibited by EGTA at a stage between vesicle docking and fusion (Rexach and Schekman, 1991; Balch et al., 1994; Pind et al., 1994; Aridor et al., 1995; Lupashin et al., 1996; Subramaniam et al., 1996). To gain additional understanding about the involvement of rbet1 in ER-Golgi transport, we have found that rbet1 antibodies must be present before the EGTA-sensitive stage to exhibit an inhibitory effect (Fig. 12). In this experiment, in vitro ER-Golgi transport was first performed in the presence of EGTA to arrest transport at the EGTA-sensitive stage. Semiintact cells were then washed, resuspended in complete transport cocktail, and resumed with a second stage of incubation to continue the events between the EGTA-sensitive stage to the actual membrane fusion. As shown, G-protein remained in the endo H–sensitive ER form after the first stage of incubation (Fig. 12, lane 10). A second incubation in fresh cytosol and complete transport cocktail allowed almost complete conversion of the EGTA-arrested ER form into the endo H–resistant Golgi form (lane 11). Inclusion of rbet1 antibodies in standard transport assay inhibited the transport to the background level (lane 4). However, when rbet1 antibodies were included only in the second stage of incubation, almost complete transport was achieved, suggesting that rbet1 antibodies could not inhibit the transport when included only in the second stage of transport assay. As shown previously (Nuoffer et al., 1994; Aridor et al., 1995), GTP-γ-S (lanes 6–7) and a Rab1 monoclonal antibody (Plutner et al., 1991) (lanes 8–9) were also no longer inhibitory to ER-Golgi transport when supplemented only at the second stage of incubation. These results suggest that rbet1 antibodies can no longer gain access to rbet1 or that rbet1 is no longer required after the EGTA-sensitive stage.
Figure 12.
rbet1 antibodies must be present before the EGTA-sensitive stage to exhibit the inhibitory effect on in vitro ER-Golgi transport. In vitro ER-Golgi transport was performed either on ice (lane 1) or at 32°C (lanes 2–11) in the absence (lane 3) or presence of rat liver cytosol (rlc) (lanes 1 and 2, and 4–11). The standard transport was performed for lanes 1–4. For lanes 5–11, transport assay was first performed in the presence of 10 mM EGTA to arrest the transport at the EGTA-sensitive stage followed by a washing step and second incubation at 32°C to continue the transport. Reagents were supplemented as indicated.
Discussion
A Role of rbet1 in ER-Golgi Transport
The identification of mammalian proteins that are structurally related to yeast Bet1p raised the issue as to whether these proteins represent true functional counterparts of Bet1p or are members of a similar protein family that participate in different transport events. Since Bet1p participates in vesicular transport from the ER to the Golgi by functioning as a v-SNARE of ER-derived vesicles (Newman et al., 1990; Dascher et al., 1991; Ossig et al., 1991; Rexach et al., 1994; Sögaard et al., 1994), the mammalian proteins in question should play a similar role if they are indeed Bet1p counterparts. However, the functional aspects of rbet1 have not been investigated by previous studies (Hay et al., 1996, 1997). In addition to its association with ER-derived vesicles, the majority of Bet1p is present in the ER. However, an examination of the subcellular localization of a transiently expressed epitope-tagged form of rbet1 has led to the conclusion that rbet1 is primarily associated with the Golgi apparatus (Hay et al., 1996). The apparent Golgi association of rbet1 is inconsistent with the notion that rbet1 is a mammalian counterpart of Bet1p, but it may rather be participating in a transport event mediated by Golgi-derived vesicles. To resolve this discrepancy, we have investigated in detail the subcellular localization of endogenous rbet1. It is clear that rbet1 is primarily associated with the pre-Golgi IC. Since the pre-Golgi IC is a collection of specialized ER regions involved in vesicle budding, budded vesicles, and other vesicular-tubular intermediates mediating ER-Golgi transport (Hong and Tang, 1993; Bannykh et al., 1996), localization of rbet1 in the IC is more consistent with its potential role in ER-Golgi transport. To examine the function of rbet1 in mammalian cells, we showed that antibodies against rbet1 could specifically inhibit in vitro ER-Golgi transport of VSV G-protein. Furthermore, we demonstrated that rbet1 antibodies must be present before the EGTA-sensitive stage to achieve an inhibition. Since EGTA inhibits ER-Golgi transport at a stage between vesicle docking and the actual membrane fusion event (Rexach and Schekman, 1991; Balch et al., 1994; Pind et al., 1994; Aridor et al., 1995; Lupashin et al., 1996; Subramaniam et al., 1996), our results suggest that once ER-derived vesicles have docked onto the cis-Golgi membrane, rbet1 antibodies are no longer inhibitory in the transport assay. This observation can be explained in two alternative ways. One possibility is that rbet1 is only important for the vesicle docking process but not for the fusion event. Alternatively, once vesicles have docked onto the cis-Golgi membrane, rbet1 becomes incorporated into a large SNARE complex in such a way that rbet1 is no longer accessible to the antibodies. Whatever the underlying mechanism is, our results clearly suggest that rbet1 antibodies must be added before ER-derived vesicles have undergone the docking process to inhibit ER-Golgi transport. Our results are thus consistent with the notion that rbet1 is a true mammalian counterpart of Bet1p by functioning as a v-SNARE of ER-derived vesicles and that it participates primarily in the docking process. Our demonstration that rbet1 is a SNARE and exists in a GS28- and syntaxin 5-containing protein complex further supports this conclusion because both GS28 and syntaxin 5 are involved in ER-Golgi transport and syntaxin 5 is a t-SNARE of the cis-Golgi membrane (Hardwick et al., 1992; Banfield et al., 1994; Dascher et al., 1994; Subramaniam et al., 1996). In an independent study, it was found that syntaxin 5 but not GS28 could be coimmunoprecipitated by rbet1 antibodies (Hay et al., 1997). This study is in contrast to our results that GS28 could be coimmunoprecipitated by rbet1 antibodies. What could potentially account for this discrepancy remains to be established. One possibility could be the fact that different rbet1 antibodies were used.
The Role of the IC in ER-Golgi Transport
The IC was originally identified by the accumulation of viral envelope proteins in morphologically distinct structures throughout the entire cytoplasm and concentrated in the peri-Golgi region, when viral-infected cells are incubated at 15°C (Saraste and Kuismanen, 1984; Bonatti et al., 1989; Schweizer et al., 1990; Tang et al., 1993). More and more evidence has accumulated supporting the idea that the IC represents true intermediates in ER-Golgi transport. We proposed several years ago that the IC represents specialized regions of ER devoted to vesicle budding and is equivalent to the transitional elements seen in pancreatic acinar cells (Hong and Tang, 1993). We referred to these specialized ER regions as ER exist sites (ERES). It becomes more clear now that the IC is a dynamic collection of the ERES (equivalent to the transitional elements), vesicles derived from ER, and tubular-vesicular intermediates that are involved in anterograde transport to the Golgi apparatus and retrograde recycling of proteins back to the ER (Aridor et al., 1995; Tang et al., 1995b ; Bannykh et al., 1996). In a recent study, the IC was also referred to as the ER export complex, and a three-tier structure was proposed (Bannykh et al., 1996; Bannykh and Balch, 1997). The first tier consists of closely adjacent buds on a single ER cisternae, and these buds contain COPII coats. A collection of tier I budding sites encompassing a central cavity containing a collection of vesicular tubular clusters (VTC) was defined as tier II. The VTC has COPI coats and can mediate COPI vesicle budding for recycling back to the ER. The entire export complex consisting of a local concentration of ER budding sites and the central VTC was defined as tier III. The ER budding profile defined by tier I and II may be equivalent to the ERES and the ER transitional elements, while the VTC represents the first post-ER and pre-Golgi membrane structure involved in ER-Golgi transport. The VTC exists both in peripheral sites as well as in the Golgi region. The peripheral VTC can be mobilized to the Golgi region. Furthermore, our knowledge of the role of the IC/the ER export complex in ER-Golgi transport has progressed beyond mere morphological description to recent demonstration that proteins involved in ER-Golgi transport are concentrated in the IC. These include components of COPII that mediate vesicle budding from the ER (Orci et al., 1991; Schaywitz et al., 1995; Paccaud et al., 1996; Tang et al., 1997), components of COPI that are involved in coupling ER-derived vesicles to both anterograde transport to the Golgi and recycling of proteins back to the ER (Oprins et al., 1993; Aridor et al., 1995; Griffiths et al., 1995; Lippincott-Schwartz et al., 1995), and small GTPase Rab1 and 2 that regulate vesicle docking and/or fusion events in ER-Golgi transport (Plutner et al., 1991; Nuoffer et al., 1994). The human protein ERGIC-53 and its rat counterpart p58 were one of the first few cellular proteins known to be enriched in the IC and which cycle preferentially between the IC and the ER (Schweizer et al., 1990; Saraste and Svensson, 1991). The recent demonstration that ERGIC-53/p58 is a major component of ER-derived COPII vesicles (Rowe et al., 1996) and is involved in ER-Golgi transport (Tisdale et al., 1997) further supports the notion that proteins participating in ER-Golgi transport are enriched in the IC. In our present study, we have shown that rbet1 is preferentially associated with the IC. Association of rbet1 with the IC suggests that it is present in the ERES (tier I and II structures) and/ or the VTC involved in ER-Golgi transport. This interpretation is further supported by our demonstration that rbet1 is essential for ER-Golgi transport and that its function in ER-Golgi transport cannot be inhibited by antibodies once vesicles have gone beyond the docking stage. Our demonstration that rbet1 is a SNARE and exists, together with GS28 and syntaxin 5, in a protein complex further confirms this conclusion.
Currently, how the VTC is mechanistically involved in ER-Golgi transport remains debatable. Two models have been recently proposed (Bannykh and Balch, 1997). In the first, the VTC is a stable independent compartment located between ER and the cis-Golgi. The COPII coat mediates vesicle formation in the ERES (the transitional elements or the tier I and II structures of the export complex) and the derived vesicles fuse with the VTC. Two types of vesicles are generated from the VTC: one type is generated by the COPI coat and it is involved in retrograde transport back to the ER; the formation of the other type is mediated by an unknown coat, and this type of vesicle is involved in anterograde transport from the VTC to the cis-Golgi. In this model, two consecutive vesicle-mediated transport steps are required for ER-Golgi transport. The second model suggests that the VTC is derived de novo from the homotypic fusion of ER-derived COPII vesicles and undergoes maturation processes to form the cis-most Golgi compartment, during which COPI-mediated vesicles would direct recycling of components back to the ER. In this alternative model, one vesicle budding step and one homotypic fusion of ER-derived vesicles coupled to COPI-mediated recycling is sufficient for ER-Golgi transport. Our studies on rbet1 reported here allow us to propose yet another model. The ER-derived COPII vesicles could undergo homotypic fusion to form the VTC. The ER-derived vesicles could also fuse (in a homotypic manner) with preexisting VTC. The maturation of the VTC is mediated by COPI coat to recycle components back to the ER. The mature VTC then undergoes heterotypic fusion with the cis-Golgi. Our hypothesis is based on the second model but also suggests that a heterotypic fusion of the VTC with the preexisting cis-Golgi is also involved in ER-Golgi transport. According to our model, rbet1 is incorporated into COPII vesicles and exists as a v-SNARE of the VTC. In this way, rbet1 could be involved in heterotypic fusion of VTC with the cis-Golgi by interacting with syntaxin 5 and GS28. rbet1 may also participate in the homotypic fusion during the formation of the VTC. This possibility is consistent with our current knowledge of ER-Golgi transport in the yeast in which one COPII-mediated vesicle budding from the ER coupled to a heterotypic fusion is involved in ER-Golgi transport (Schekman and Orci, 1996). Whether a structure equivalent to the VTC exists and COPI-mediated recycling participates in the maturation of this equivalent structure during ER-Golgi transport in yeast remains to be examined. Alternatively, our results are also consistent with the first model in which two vesicle steps are involved in ER-Golgi transport and the VTC is a distinct stable compartment. In this consideration, rbet1 could function as a v-SNARE for ER-derived COPII vesicles, and it is involved in heterotypic fusion with the VTC.
Acknowledgments
We thank James E. Rothman for the plasmid expressing recombinant HisX6-tagged α-SNAP, Richard Scheller for rat syntaxin 5 and 6 cDNA clones, J. Saraste for p58 antibodies, H.-P. Hauri for ERGIC-53 monoclonal antibodies, W.E. Balch for monoclonal antibody against Rab1, members of Hong's laboratory for critical reading of the manuscript, and Dr. Y.H. Tan for his continuous support.
Note Added in Proof
The sequence data for human and mouse bet1 are available from GenBank/EMBL/DDBJ under accession numbers AF007551 and AF007552, respectively.
Footnotes
1. Abbreviations used in this paper: endo H, endoglycosidase H; ERES, ER exist sites; EST, expressed sequence tags; IC, intermediate compartment; GST, glutathione-S-transferase; NSF, N-ethylmaleimide–sensitive factor; NRK, normal rat kidney; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor; VSV, vesicular stomatitis virus; VTC, vesicular tubular clusters.
Tao Zhang and Siew Heng Wong contributed equally to this work.
Address all correspondence to Dr. Wanjin Hong, Institute of Molecular and Cell Biology, 15 Lower Kent Ridge Road, Singapore 119076, Singapore. Tel.: 65-778-6827. Fax: 65-779-1117. E-mail: mcbhwj@leonis.nus.sg
References
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plunter H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Rabouille C, Warren G, Pelham HRB. Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J Cell Biol. 1994;127:357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic reticulum–Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers CJM, Keller DS, Balch WE. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987;50:523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicle. J Biol Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- Bonatti S, Migliaccio G, Simons K. Palmitylation of viral membrane glycoprotein takes place after exit from the endoplasmic reticulum. J Biol Chem. 1989;264:12590–12595. [PubMed] [Google Scholar]
- Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C, Matteson J, Balch WE. Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J Biol Chem. 1994;269:29363–29366. [PubMed] [Google Scholar]
- Davidson HW, Balch WE. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans-Golgi network. J Biol Chem. 1993;268:4216–4226. [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff IC, Schekman R, Rothman JE, Kaiser CA. The yeast SEC17 gene product is functionally equivalent to mammalian α-SNAP protein. J Biol Chem. 1992;267:12106–12115. [PubMed] [Google Scholar]
- Griffiths G, Ericsson M, Krijnse-Locker J, Nilsson T, Goud B, Soling H-D, Tang BL, Wong SH, Hong W. Localisation of the KDEL receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994;127:1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Pepperkok R, Krijnse-Locker J, Kreis TE. Immunocytochemical localization of β-COP to the ER-Golgi boundary and the TGN. J Cell Sci. 1995;108:2839–2856. doi: 10.1242/jcs.108.8.2839. [DOI] [PubMed] [Google Scholar]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HRB. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC, Harald H, Scheller RH. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 1996;271:5671–5679. doi: 10.1074/jbc.271.10.5671. [DOI] [PubMed] [Google Scholar]
- Hay JC, Chao DS, Kuo CS, Scheller RH. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 1997;89:147–158. doi: 10.1016/s0092-8674(00)80191-9. [DOI] [PubMed] [Google Scholar]
- Hong, W. 1996. Protein Trafficking along the Exocytotic Pathway. R.G. Landes Company, Austin, TX. 197 pp.
- Hong W, Tang BL. Protein trafficking along the exocytotic pathway. Bioassay. 1993;15:231–238. doi: 10.1002/bies.950150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J, Ericsson M, Rottier PJM, Griffiths G. Characterization of the budding compartment of the mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. Bidirectional membrane traffic between the endoplasmic reticulum and Golgi apparatus. Trends Cell Biol. 1993;3:81–88. doi: 10.1016/0962-8924(93)90078-f. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole NB, Marotta A, Conrad PA, Bloom GS. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol. 1995;128:293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti LV, Torrisi MR, Pascale MC, Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SL, Wong SH, Hong W. The mammalian ARF-like protein 1 (Arl1) is associated with the Golgi complex. J Cell Sci. 1996;109:209–220. doi: 10.1242/jcs.109.1.209. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Hamamoto S, Schekman RW. Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes. J Cell Biol. 1996;132:277–289. doi: 10.1083/jcb.132.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreman KW, Robbins PW. Isolation, characterization, and expression of cDNAs encoding murine α-mannosidase II, a Golgi enzyme that controls conversion of high mannose to complex N-glycans. J Cell Biol. 1991;115:1521–1534. doi: 10.1083/jcb.115.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama M, Orci L, Ravazzola M, Amherdt M, Lacomis L, Tempst P, Rothman JE, Söllner TH. A v-SNARE implicated in intra-Golgi transport. J Cell Biol. 1996;133:507–516. doi: 10.1083/jcb.133.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AP, Shim J, Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990;10:3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Oprins A, Duden R, Kreis TE, Geuze HJ, Slot JW. β-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Meda P, Holcomb C, Moore H-P, Hicke L, Schekman R. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci USA. 1991;88:8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte H-H, Schmitt HD, Gallwitz D. The yeast SLY genes products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccaud J-P, Reith W, Carpentier J-L, Ravazzola M, Amherdt M, Schekman R, Orci L. Cloning and functional characterization of mammalian homologues of the COPII component Sec23. Mol Biol Cell. 1996;7:1535–1546. doi: 10.1091/mbc.7.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. Intracellular aspects of the processing of protein synthesis. Science (Wash DC) 1975;189:347–354. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Biol Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, Der CJ, Balch WE. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutner H, Davidson HW, Saraste J, Balch WE. Morphological analysis of protein transport from the ER to the Golgi membrane in digitonin-permeabilized cells: role of the p58 containing compartment. J Cell Biol. 1992;119:1097–1116. doi: 10.1083/jcb.119.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer NK, Wuestehube LJ, Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Rexach MF, Schekman RW. Distinct biochemical requirements for budding, targeting, and fusion of ER-derived transport vesicles. J Cell Biol. 1991;114:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach MF, Latterich M, Schekman RW. Characterization of endoplasmic reticulum-derived transport vesicles. J Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski AA, Singer SJ. Associations of elements of the Golgi apparatus with microtubule. J Cell Biol. 1984;99:1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T, Aridor M, McCaffery JM, Plutner H, Nuoffer C, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratories, Cold Spring Harbor, NY.
- Saraste J, Kuismanen E. Pre-and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984;38:535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987;105:2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1532. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Bachi T, Ginsel L, Hauri H-P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Matter K, Kreis TE, Ginsel L, Hauri H-P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- Shaywitz DA, Orci L, Ravazzola M, Swaroop A, Kaiser CA. Human SEC13Rp functions in yeast and is localized on transport vesicles budding from the endoplasmic reticulum. J Cell Biol. 1995;128:769–777. doi: 10.1083/jcb.128.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sögaard M, Tani K, Ye RB, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Krijnse-Locker J, Tang BL, Ericsson M, Yusoff ARBM, Griffiths G, Hong W. Monoclonal antibody HFD9 identifies a novel 28 kD integral membrane protein on the cis-Golgi. J Cell Sci. 1995;108:2405–2414. doi: 10.1242/jcs.108.6.2405. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Peter F, Philip R, Wong SH, Hong W. GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science. 1996;272:1161–1163. doi: 10.1126/science.272.5265.1161. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Loh E, Hong W. NSF and α-SNAP mediate dissociation of GS28-syntaxin 5 Golgi SNARE complex. J Biol Chem. 1997;272:25441–25444. doi: 10.1074/jbc.272.41.25441. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tang BL, Wong SH, Qi XL, Low SH, Hong W. Molecular cloning, characterization, subcellular localization, and dynamics of p23, the mammalian KDEL receptor. J Cell Biol. 1993;120:325–338. doi: 10.1083/jcb.120.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Low SH, Hong W. Differential response of resident proteins and cycling proteins of the Golgi to brefeldin A. Eur J Cell Biol. 1995a;68:199–205. [PubMed] [Google Scholar]
- Tang BL, Low SH, Hauri H-P, Hong W. Segregation of ERGIC53 and the mammalian KDEL receptor upon exit from the 15°C compartment. Eur J Cell Biol. 1995b;68:398–410. [PubMed] [Google Scholar]
- Tang BL, Peter F, Krijnse-Locker J, Low SH, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Tartakoff AM. The response of the Golgi complex to microtubule alternations: the role of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989;109:2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart SW, Kubalek EW. SNAPs and NSF: general members of the fusion apparatus. Trends Cell Biol. 1995;5:64–69. doi: 10.1016/s0962-8924(00)88948-5. [DOI] [PubMed] [Google Scholar]