Abstract
Purpose
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) is an inhibitory receptor on T cells. Knocking out CTLA4 in mice causes lethal lymphoproliferation, and polymorphisms in human CTLA4 are associated with autoimmune disease. Trials of the anti-CTLA4 antibody ipilimumab (MDX-010) have resulted in durable cancer regression and immune-mediated toxicities. A report on the diagnosis, pathology, treatment, clinical outcome, and significance of the immune-mediated enterocolitis seen with ipilimumab is presented.
Patients and Methods
We treated 198 patients with metastatic melanoma (MM) or renal cell carcinoma (RCC) with ipilimumab.
Results
The overall objective tumor response rate was 14%. We observed several immune mediated toxicities including dermatitis, enterocolitis, hypophysitis, uveitis, hepatitis, and nephritis. Enterocolitis, defined by grade 3/4 clinical presentation and/or biopsy documentation, was the most common major toxicity (21% of patients). It presented with diarrhea, and biopsies showed both neutrophilic and lymphocytic inflammation. Most patients who developed enterocolitis responded to high-dose systemic corticosteroids. There was no evidence that steroid administration affected tumor responses. Five patients developed perforation or required colectomy. Four other patients with steroid-refractory enterocolitis appeared to respond promptly to tumor necrosis factor alpha blockade with infliximab. Objective tumor response rates in patients with enterocolitis were 36% for MM and 35% for RCC, compared with 11% and 2% in patients without enterocolitis, respectively (P = .0065 for MM and P = .0016 for RCC).
Conclusion
CTLA4 seems to be a significant component of tolerance to tumor and in protection against immune mediated enterocolitis and these phenomena are significantly associated in cancer patients.
INTRODUCTION
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) is a cell surface receptor initially cloned from a cDNA library from a murine cytotoxic T-lymphocyte.1 Its ligands are CD80 and CD86 which also participate in lower affinity interactions with the costimulatory T-cell receptor CD28. Rather than costimulate, CTLA4 functions as an inducible receptor with T-cell inhibitory activity.2–5 Thus its primary role is to down-regulate T-cell activation. CTLA4 was also found constitutively expressed on inhibitory CD25+ CD4+ regulatory T cells (Treg) and CTLA4 signaling was necessary in Treg control of intestinal inflamation.6 Targeted destruction of the CTLA4 gene in mice causes lymphoproliferation and autoimmune disease and antimurine CTLA4 antibodies induced antitumor activity, particularly when combined with antitumor vaccination.5,7,8 This led to clinical trials of a fully human immunoglobulin G1 antibody against CTLA4, ipilimumab (formerly MDX-010; Medarex Inc, Princeton, NJ). In patients with melanoma or ovarian cancer who also had antitumor vaccination, tumor necrosis and cellular infiltration was reported after ipilimumab administration,9 and other studies have also documented durable tumor regression by standard criteria. Phan et al reported 14 patients with melanoma who received anti-CTLA4 antibody (3 mg/kg every 3 weeks) in combination with antimelanoma peptide vaccines. Three patients experienced objective cancer regression, and two patients experienced mixed responses.10 Grade 3/4 autoimmune toxicities were seen in six (43%) of 14 patients. Further trials established that tumor regression could also be seen without added vaccination. A number of grade 3/4 immune-mediated toxicities, unanticipated by preclinical testing in nonhuman primates, were encountered in patients given ipilimumab.11–13 These included dermatitis, enterocolitis, hypophysitis, uveitis, and hepatitis. Mice with their CTLA4 genes knocked out show lethal lymphoproliferation as well as myocarditis and pancreatitis.14 Administration of anti-CTLA4 antibody in mice also enhanced experimental autoimmune myasthenia gravis,15 precipitated and exacerbated autoimmune diabetes16 and experimental autoimmune encephalomyelitis,17 and induced autoimmune gastritis.18 Population-based studies found that specific polymorphisms in the human CTLA4 gene were associated with increased risks of autoimmune diabetes and thyroid disease.19 Therefore, these ipilimumab-associated toxicities were thought to be possible autoimmune manifestations of CTLA4 blockade. To further investigate this hypothesis, we studied the most frequent ipilimumab-associated toxicity, enterocolitis, to determine its clinicopathologic characteristics, contributing factors, response to therapy, and association with tumor regression. A total of 234 patients with metastatic melanoma (MM) or renal cell carcinoma (RCC) have received ipilimumab in the Surgery Branch of the National Cancer Institute. One hundred thirty-seven of these patients had melanoma and received antibody with or without melanoma peptide vaccines. Sixty-one patients with metastatic clear-cell RCC were given ipilimumab without vaccination. Thirty-six additional patients receiving ipilimumab in combination with high-dose interleukin-2 (IL-2) are not included in this report. Enterocolitis was the most frequent significant adverse occurrence, but we also observed dermatitis, hypophysitis, uveitis, hepatitis, nephritis, and one case of autoimmune meningitis. This report presents the clinicopathologic results and outcome analysis on the 41 patients who developed enterocolitis in association with ipilimumab treatment.
PATIENTS AND METHODS
Patients
One hundred ninety-eight patients were treated with intravenous human immunoglobulin anti-CTLA4 monoclonal antibody ipilimumab, from March 19, 2002, to July 15, 2005. All patients had a histologic diagnosis of stage IV cutaneous melanoma or stage IV clear-cell RCC, and had measurable disease. All patients had a life expectancy ≥ 3 months and an Eastern Cooperative Oncology Group performance status ≤ 2. A normal CBC, creatinine, hepatic panel, hepatitis, HIV, and autoimmunity screen was required. Patients receiving a peptide vaccine were constrained to be human leukocyte antigen (HLA) -A0201–positive. Patients with RCC were either IL-2 refractory or IL-2 ineligible. Patients with any other major malignancy, a history of autoimmune disease, a requirement for immunosuppressive agents, who were pregnant or nursing, or who had received prior ipilimumab treatment were excluded.
Treatment With Ipilimumab
Patients were treated on three separate protocols approved by the investigational review board of the National Cancer Institute and in accordance with an assurance filed with and approved by the US Food and Drug Administration: ipilimumab + vaccines for melanoma (56 patients), ipilimumab dose escalation ± vaccines for melanoma (81 patients), and ipilimumab for RCC (61 patients). Ipilimumab was given intravenously every 3 weeks in doses ranging from 1 mg/kg to 9 mg/kg, depending on protocol. Vaccines used were the modified gp100209-217 (210M) and gp100280-288 (288V) peptide vaccines (provided by the Cancer Therapy Evaluation Program, National Cancer I) emulsified with Montanide ISA-51 (Seppic Inc, Fairfield, NJ) and injected subcutaneously in the extremities every 3 weeks at the time of ipilimumab dosing.
Melanoma patients treated with ipilimumab + vaccines in the first protocol received ipilimumab at a dose level of 3 mg/kg for all doses in cohort I or an initial loading dose of ipilimumab at 3 mg/kg, followed by all subsequent doses at 1 mg/kg in cohort II.
Melanoma patients treated with ipilimumab dose escalation ± vaccines were treated with intrapatient dose escalation until objective clinical response, ≥ grade 3 autoimmunity or other dose limiting toxicity was reached. Doses started at 3 mg/kg in cohort I or 5 mg/kg in cohort II. Doses were administered every 3 weeks, escalated to 5 mg/kg or 9 mg/kg every other dose and continued until response, adverse event, or disease progression on 9 mg/kg of antibody was seen. HLA-A0201–positive patients on this protocol were randomized to receive or not receive concurrent peptide vaccination as described above. HLA-A0201–negative patients received only ipilimumab without vaccine.
The treatment regimen for RCC consisted of two cohorts: cohort I received MDX at 3 mg/kg with subsequent doses of 1 mg/kg every 3 weeks and cohort II were treated with 3 mg/kg every 3 weeks for all doses.
Diagnosis of Enterocolitis
Assessment of enterocolitis varied in the first 10 presenting patients. One additional patient presented with colonic perforation and no antecedent symptoms. The remaining 30 patients were admitted for work-up on onset of diarrhea, stopped oral intake, and were given intravenous hydration. Stool was sent for standard microbiological and parasite examinations in order to rule out an infectious etiology. Colonoscopy or flexible sigmoidoscopy with biopsies was performed in 40 of the 41 patients. Many patients also underwent esophagogastroduodenoscopy with biopsies. Patients were considered to have enterocolitis if they had either biopsy findings showing enterocolitis or the clinical scenario of sudden onset diarrhea, no alternate etiology identified, and a response to steroid therapy.
Treatment Regimen for Enterocolitis
The first 10 patients who developed enterocolitis were treated with a variety of treatment regimens. A standardized high-dose steroid regimen was developed for subsequent patients. Patients found to have enterocolitis on gross endoscopic examination (erythema or ulceration) were immediately treated with high-dose steroids. In patients with grossly normal mucosa on endoscopy, treatment with steroids was withheld until enterocolitis was histologically demonstrated by pathologic review of biopsies. High-dose steroid therapy consisted of 4 mg of intravenous dexamethasone every 6 hours. Diet was slowly advanced after resolution of symptoms following initiation of steroids. High-dose steroids were continued for approximately 7 days, followed by a taper over 17 days. Four patients with steroid refractory colitis were treated with a single dose of infliximab at 5 mg/kg (Centocor, Horsham, PA), and one patient received it as sole therapy.
Evaluation of Clinical Response to Ipilimumab
Patients given ipilimumab + vaccines for melanoma and ipilimumab for RCC were evaluated for clinical response by computed tomography, magnetic resonance imaging, and physical examination 3 weeks after every fourth dose. Patients in the ipilimumab dose escalation ± vaccines cohort were evaluated by computed tomography, magnetic resonance imaging, and physical examination for clinical response 3 weeks after every other dose to monitor the results of dose escalation.
Response was evaluated using Response Evaluation Criteria in Solid Tumors. A partial response (PR) was defined as a ≥ 30% decrease in the sum of the longest diameters of target lesions (in the absence of new disease). Complete response (CR) was defined as disappearance of all target and nontarget lesions. Progressive disease (PD) was defined as a ≥ 20% increase in the sum of longest diameters compared with baseline, or the appearance of one or more new lesions. Only patients with a PR or CR were considered objective responders.
Statistical Analysis
Statistical analysis was carried out using Fisher’s exact test with a two-tailed P value ≤ .05 indicating significance.
RESULTS
Patient Characteristics
A total of 137 patients with melanoma received ipilimumab; 56 patients were treated with ipilimumab + vaccines and 81 patients with escalating doses of ipilimumab with HLA-A0201 patients randomized to receive or not receive vaccines. Sixty-one patients with RCC were treated with ipilimumab alone (Table 1).
Table 1.
Patient Demographics
| Patients With Previous IL-2
|
Patients With Enterocolitis
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis and Protocol | No. of Patients | Mean Age (years) | Sex Ratio (female:male) | No. | % | Patients Given Vaccine | No. | % |
| Melanoma | 137 | 49 | 45:92 | 67 | 49 | 24 | 18 | |
| Ipilimumab+vaccines | 56 | 51 | 19:37 | 21 | 38 | 56 | 8 | 14 |
| Cohort I 3 mg/kg→1 mg/kg | 27 | 53 | 10:17 | 6 | 29 | 27 | 4 | 15 |
| Cohort II 3 mg/kg→3 mg/kg | 29 | 49 | 9:20 | 15 | 52 | 29 | 4 | 14 |
| Ipilimumab dose escalation | 81 | 48 | 26:55 | 46 | 57 | 16 | 16 | 20 |
| Cohort I 3 mg/kg→9 mg/kg | 38 | 48 | 10:28 | 26 | 68 | 6 | 5 | 13 |
| Cohort II 5 mg/kg→9 mg/kg | 43 | 48 | 16:27 | 20 | 47 | 10 | 11 | 26 |
|
| ||||||||
| Renal cell carcinoma | ||||||||
| Ipilimumab renal cell carcinoma | 61 | 54 | 16:45 | 41 | 67 | 0 | 17 | 28 |
| Cohort I 3 mg/kg→1 mg/kg | 21 | 56 | 4:17 | 21 | 100 | 0 | 3 | 14 |
| Cohort II 3 mg/kg→3 mg/kg | 40 | 53 | 12:28 | 20 | 50 | 0 | 14 | 35 |
NOTE. Ipilimumab is formerly MDX-010.
Abbreviation: IL-2, interleukin-2.
Enterocolitis
Forty-one patients were diagnosed with enterocolitis for an overall incidence of 21%. Incidence by protocol was as follows: 14% for ipilimumab + vaccines for melanoma, 20% for ipilimumab dose escalation ± vaccines for melanoma, and 28% for RCC. There was no significant difference in incidence of enterocolitis between protocols (all pairwise comparisons between protocols NS, P > .1) nor between those patients who received ≤ 3 mg/kg per dose of ipilimumab and those who received doses of ≥ 5 mg/kg (Table 1). The difference between all patients with melanoma versus those with RCC was also not statistically significant (P = .128).
Presenting Symptoms
Patients presented with complaints ranging from three soft stools per day up to 20 watery stools daily. In addition, some patients presented with complaints of abdominal pain, nausea and vomiting, fever, and anal pain (Table 2). The hallmark symptom, however, was diarrhea.
Table 2.
Symptoms on Presentation in 41 Patients With Grade 3/4 Gastrointestinal Toxicity
| Presenting Symptom | No. of Patients |
|---|---|
| Diarrhea | 40 |
| Abdominal pain | 8 |
| Nausea/vomiting | 6 |
| Fever | 5 |
| Anal pain | 4 |
| Rectal bleeding | 1 |
| Constipation | 1 |
The median number of days of symptoms on admission was found to be 5 (range, 1 to 64 days, data available on 37 patients) with 86% of patients presenting within 7 days of onset. For the 39 patients with available data, the median number of days from the last dose of ipilimumab to the onset of symptoms was 11 (range, 0 to 59 days). All but four patients developed symptoms within 21 days of their last dose (Fig 1). Patients received between one and 10 doses of ipilimumab before the onset of enterocolitis, with no predictable pattern (Fig 2).
Fig 1.
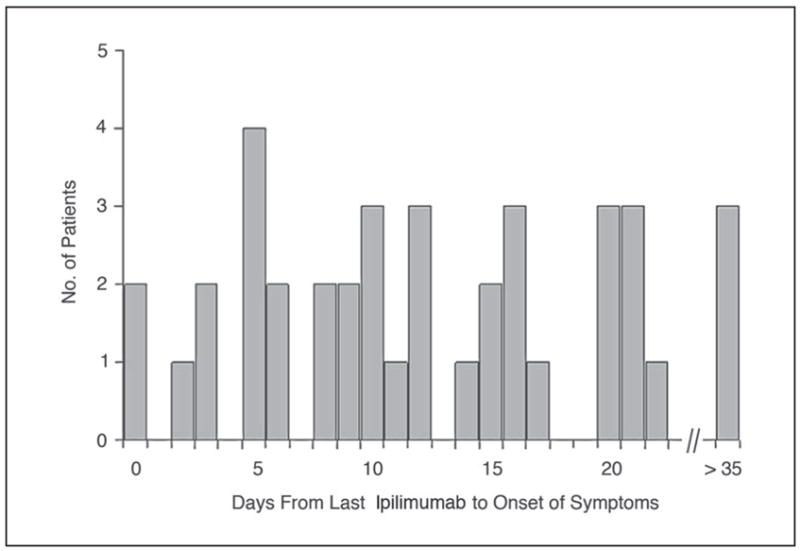
The interval from last dose of ipilimumab (MDX-010) to the onset of symptoms of enterocolitis. An interval of 0 days indicates a patient who received ipilimumab while having some symptoms, and those with intervals of more than 21 days had stopped ipilimumab dosing but went on to develop enterocolitis later.
Fig 2.
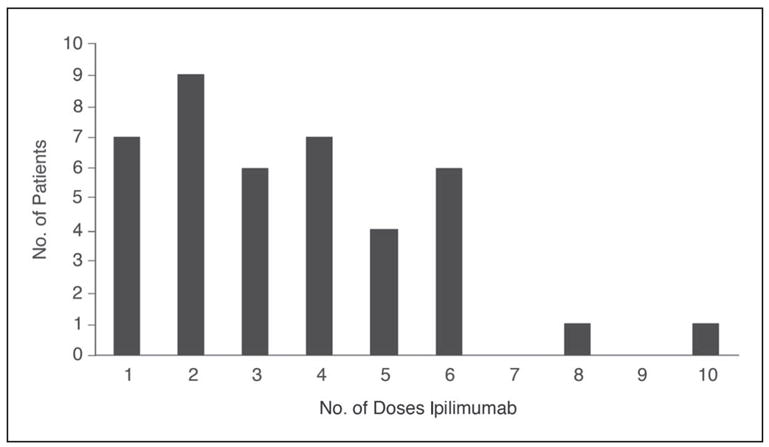
The number of doses of ipilimumab given before the onset of symptoms of enterocolitis.
Endoscopic Findings
Of the 40 patients who underwent flexible sigmoidoscopy or colonoscopy, reported gross findings were available for 36. Twenty-three had erythema or ulceration. Thirty-six of 40 patients had histologically proven colitis, including all 23 patients with gross abnormalities. One additional patient (the only one without diarrhea) was diagnosed with colitis retrospectively from a colectomy specimen after perforating. Eighteen patients also underwent esophagogastroscopy, and reports of gross findings were available for 16. Ten patients had grossly positive endoscopies. Fourteen patients had histologically proven gastritis or duodenitis on pathological review, including two with histologically negative colonic biopsies. In total, 39/41 patients had histologically proven enterocolitis. Two patients, including one patient with perforation, did not have histologic findings of enterocolitis, but were diagnosed by their clinical course.
Histolopathologic Features of Enterocolitis
In the 39 patients with histologic evidence of enterocolitis, three patterns were seen: neutrophilic inflammation only (46%), lymphocytic inflammation only (15%), or combined neutrophilic and lymphocytic inflammation (38%; Fig 3). Neutrophilic inflammation was predominantly characterized by cryptitis, and crypt abscesses were present in 33% of cases. In occasional cases, particularly where lymphocytic inflammation was also present, crypts showed prominent eosinophils. Granulomas were seen in three cases. Lymphocytic inflammation was characterized by increased CD8+ T-cells within the crypt epithelium, and increased CD4+ cells in the lamina propria, though the latter finding was nonspecific. The patterns described were present in biopsies from the stomach, duodenum, and all areas of colon and rectum. Eighty-three percent of patients had more than one positive biopsy site; 32% of patients had three or more positive biopsy sites. Two patients had findings confined to the stomach or duodenum despite multiple lower GI biopsies.
Fig 3.
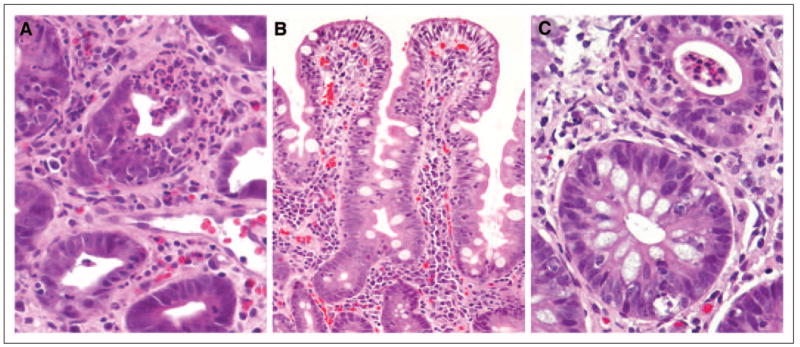
Representative photomicrographs of histopathologic features of enterocolitis. (A) Neutrophilic infiltration with colonic crypt destruction (hematoxylin and eosin, ×400). (B) Small bowel mucosa showing markedly increased surface intraepithelial lymphocytes and expansion of lamina propria with mononuclear cells (hematoxylin and eosin, ×200). (C) Two adjacent colonic glands showing cryptitis in the upper gland and intraepithelial lymphocytosis and crypt cell apoptosis in the lower gland (hematoxylin and eosin, ×400).
Treatment
Thirty-four of the 41 patients were treated with corticosteroids. The median time between onset of symptoms and initiation of steroid therapy was available for 31 patients and was found to be 8 days (range, one to 66 days). One patient who developed symptoms 66 days before steroid therapy first had spontaneous improvement, was re-treated with ipilimumab, and then relapsed.
Twelve patients treated with steroids had refractory enterocolitis as defined by a failure to respond to steroid therapy within 7 days (five patients) or an initial response to steroids followed by a relapse requiring reinstitution of high-dose steroids (seven patients).
Seven patients were not treated with steroids because enterocolitis developed before establishing a consistent steroid based treatment regimen (one patient) the initial presentation was perforation (one patient), mild symptoms rapidly resolved spontaneously (four patients), or infliximab alone was effective (one patient). Four additional patients with enterocolitis refractory to high-dose steroids (more than 10 to 69 days) received a single dose of infliximab at 5 mg/kg as second-line therapy, and all showed rapid and durable resolution of symptoms (Table 3).
Table 3.
Treatment of Patients With Enterocolitis With Infliximab
| Interval (days)
|
|||||
|---|---|---|---|---|---|
| Diagnosis | Age (years) | Ipilimumab Doses per Pateint | Symptoms Prior to Steroids | Steroids Prior to Infliximab | Infliximab to Resolution |
| Renal cell carcinoma | 51 | 3 | — | — | 1 |
| Melanoma | 49 | 5, 5 | 4 | 69 | 1 |
| Melanoma | 63 | 5, 5, 9, 9, 9, 9 | 8 | 40 | 2 |
| Melanoma | 57 | 5, 5, 9 | 8 | 13 | 3 |
| Melanoma | 24 | 5, 9, 9 | 10 | 10 | 2 |
Complications
Four patients experienced colonic perforation secondary to enterocolitis, three with RCC, and one with melanoma. Three of these perforations were in patients who were refractory to their initial treatment with high-dose steroids. Perforation occurred after one, four, six, and six doses of ipilimumab, respectively. Two patients died after perforation—one with overwhelming sepsis and one electing for comfort care due to cancer progression. One additional patient with RCC required colectomy for persistent gastrointestinal bleeding secondary to steroid refractory enterocolitis. Thus, the incidence of perforation or colectomy in patients being treated for RCC was 6.6% (four of 61 patients) and 0.7% (one of 137 patients) in patients with melanoma (P = .032). The mortality rate among patients who developed enterocolitis was 5% (two of 41 patients). The mortality rate among all treated patients was 1% (two of 198 patients).
Other Immune Mediated Toxicities
Although enterocolitis was the most common grade 3/4 immune-mediated toxicity seen with ipilimumab, patients also developed grade 3/4 hypophysitis (13 patients, 7%), dermatitis (eight patients, 4%), arthritis (four patients, 2%), uveitis (two patients, 1%), hepatitis, nephritis, and aseptic meningitis (one patient each). In those patients who developed enterocolitis, other grade 3/4 toxicities were hypophysitis (three patients), dermatitis (two patients), and arthritis (one patient). The frequency of other immune-mediated toxicities was not significantly different between patients with and without enterocolitis (P = .632).
Clinical Response to Ipilimumab
Of 198 patients treated, 189 were assessable for response, and eight were pending (six with MM and two with RCC). One patient ultimately found to have sarcoma instead of melanoma was not assessable for response. The overall response rate (RR) for these 189 patients was 14%. Of the patients who developed enterocolitis, 39 patients are assessable for response, and two patients remain in follow-up. The RR for the 39 assessable patients with enterocolitis was 36% (14 of 39 patients). This association of enterocolitis with objective tumor regression was significant for both patients with melanoma and those with RCC. The objective RR was 36% for melanoma patients with enterocolitis and 35% for RCC patients with enterocolitis, compared with 11% and 2%, respectively, in patients who did not develop enterocolitis, (P = .0065 for MM and P = .0016 for RCC). There was no consistent temporal relationship between the onset of enterocolitis and the onset of tumor regression (data not shown).
DISCUSSION
Several clinical studies now confirm that ipilimumab, an antibody to human CTLA4, can cause the durable regression of both MM and RCC, even in patients who had previously not responded to other immunotherapies such as interleukin-2. This antibody also induces immune-mediated toxicities in several normal organs and tissues, including the upper and lower gastrointestinal tract, anteriorpituitary, skin, uveal tract, and liver. Of these sites, major toxicity was seen most frequently in the gastrointestinal tract. Overall, 21% of all patients receiving ipilimumab developed stage III/IV clinical enterocolitis or had biopsy confirmation of enterocolitis. It was seen in patients with melanoma as well as RCC, though the latter patients had a higher frequency of severe complications. An acute histological picture with neutrophilic infiltrates as well as a chronic picture with infiltrating lymphocytes, and even granulomata, can be seen and does not help to define the pathophysiology. The clinical hallmark is diarrhea, which responds rapidly to withholding oral feedings, and in most cases, high-dose steroids.
The enterocolitis associated with ipilimumab has features similar to both graft-versus-host disease as well as inflammatory bowel disease. Enterocolitis from graft-versus-host disease was significantly associated with the regression of RCC in patients treated with a mini-allotransplant regimen.20 A randomized study has suggested that a contributing factor to enterocolitis in this setting may be intestinal microflora and bacterial antigens,21,22 and this may be an area of future investigation for prophylaxis of enterocolitis after ipilimumab.
The enterocolitis after ipilimumab is also similar to inflammatory bowel disease in its clinical picture of acute and chronic inflammatory changes, skip areas, and its response to infliximab. Recent data have implicated polymorphisms in the CARD15/NOD2 gene, as well as antibody responses to bacterial antigens as risk factors in developing inflammatory bowel disease.23–25 These factors and known polymorphisms in the CTLA4 gene are currently being investigated in our patients to determine if they affect the risk of developing enterocolitis after receiving ipilimumab.
Another potential mechanism for generating enterocolitis after anti-CTLA4 antibody involves CD25+ CD4+ regulatory T cells (Treg). These immunosuppressive regulatory cells constitutively express high levels of CTLA4, unlike other T cells, where CTLA4 is only induced after activation.6 Mice lacking Treg cells (through a germline disruption of the FoxP3 gene) and patients with the IPEX syndrome (immune-dysfunction, polyendocrinopathy, enteropathy, and X-linked inheritance) who have a mutation in FoxP3, both show autoimmune disease.26–28 Furthermore, transfer of CD25+ CD4+ T cells into mice with an experimental immune-mediated colitis leads to resolution of colitis.29 Therefore, it has been postulated that antibody to CTLA4 might deplete Treg cells and thus induce autoimmunity. However preliminary data from our patients receiving ipilimumab have not shown a decrease in Treg number or function in peripheral blood after ipilimumab.30 Nevertheless, examination of Treg cells in tumor or other relevant tissues may be needed to fully investigate this issue.
A short course of high-dose steroids was a definitive treatment for enterocolitis in the majority of our patients. Reinstituting steroids successfully treated most of those suffering a relapse, but infliximab also seemed to be an effective therapeutic option in four patients with steroid-refractory enterocolitis. Further investigation into the impact of tumor necrosis factor alpha neutralization on the antitumor response is warranted before routinely recommending infliximab in this setting.
The mortality in patients who developed autoimmune colitis was 5%. Several patients with protracted colitis and major complications either did not receive prompt treatment or were poorly compliant in taking steroids. Instruction in symptom recognition, timely diagnostic studies and prompt treatment with high-dose steroids with compliance monitoring may reduce the risks of colonic perforation, bleeding, or death. The 2.5% overall risk of perforation or colectomy and the 1% overall risk of death should be weighed against the 14% response rate, often of significant duration, in patients with widespread melanoma or RCC.
One of the most intriguing findings was the association between enterocolitis and tumor regression. Enterocolitis could be a surrogate marker of drug efficacy, or there may be a causal relationship. There could be common antigens expressed by tumor and bowel, though they would have to be found on both melanoma and RCC. It is also possible that immune-mediated enterocolitis nonspecifically supports an independent antitumor immune response, either through cytokine production or dendritic cell activation by CD4 cells of autoimmune origin. Alternatively, there could be a genetic predisposition in responding patients for autoimmunity that extends to enterocolitis and tumor rejection mediated by “self-antigens.” These hypotheses require further laboratory investigation. Clearly, amelioration of this significant toxicity would facilitate the utilization of ipilimumab, an active immunotherapeutic reagent that has shown promise in treating melanoma and RCC. A better understanding of the significant role of CTLA4 in tumor tolerance should also lead to new approaches and immunological strategies for inducing durable tumor rejection.
Acknowledgments
Supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
| Authors | Employment | Leadership | Consultant | Stock | Honoraria | Research Funds | Testimony | Other |
| Israel Lowy | Medarex Inc (N/R) | Medarex Inc (C) | ||||||
| Michael Yellin | Medarex Inc (N/R) | Medarex Inc (C) | ||||||
| Dollar Amount Codes (A) < $10,000 (B) $10,000–99,999 (C) ≥ $100,000 (N/R) Not Required | ||||||||
Author Contributions
Conception and design: Kimberly E. Beck, Joseph A. Blansfield, Khoi Q. Tran, Andrew L. Feldman, Marybeth S. Hughes, Richard E. Royal, Udai S. Kammula, Suzanne L. Topalian, Richard M. Sherry, David Kleiner, Martha Quezado, Israel Lowy, Michael Yellin, Steven A. Rosenberg, James C. Yang
Administrative support: Israel Lowy, Michael Yellin, Steven A. Rosenberg
Provision of study materials or patients: Kimberly E. Beck, Joseph A. Blansfield, Khoi Q. Tran, Andrew L. Feldman, Marybeth S. Hughes, Richard E. Royal, Udai S. Kammula, Suzanne L. Topalian, Richard M. Sherry, David Kleiner, Martha Quezado, Steven A. Rosenberg, James C. Yang
Collection and assembly of data: Kimberly E. Beck, Joseph A. Blansfield, Khoi Q. Tran, Andrew L. Feldman, Marybeth S. Hughes, Richard E. Royal, Udai S. Kammula, Suzanne L. Topalian, Richard M. Sherry, David Kleiner, Martha Quezado, Israel Lowy, Michael Yellin, Steven A. Rosenberg, James C. Yang
Data analysis and interpretation: Kimberly E. Beck, Joseph A. Blansfield, Khoi Q. Tran, Andrew L. Feldman, Marybeth S. Hughes, Richard E. Royal, Udai S. Kammula, Suzanne L. Topalian, Richard M. Sherry, David Kleiner, Martha Quezado, Israel Lowy, Michael Yellin, Steven A. Rosenberg, James C. Yang
Manuscript writing: Kimberly E. Beck, Steven A. Rosenberg, James C. Yang
Final approval of manuscript: Kimberly E. Beck, Joseph A. Blansfield, Khoi Q. Tran, Andrew L. Feldman, Marybeth S. Hughes, Richard E. Royal, Udai S. Kammula, Suzanne L. Topalian, Richard M. Sherry, David Kleiner, Martha Quezado, Israel Lowy, Michael Yellin, Steven A. Rosenberg, James C. Yang
References
- 1.Brunet JF, Dosseto M, Denizot F, et al. The inducible cytotoxic T-lymphocyte-associated gene transcript CTLA-1 sequence and gene localization to mouse chromosome 14. Nature. 1986;322:268–271. doi: 10.1038/322268a0. [DOI] [PubMed] [Google Scholar]
- 2.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 3.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers CA, Krummel MF, Boitel B, et al. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 6.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 8.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by CTLA-4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keler T, Halk E, Vitale L, et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J Immunol. 2003;171:6251–6259. doi: 10.4049/jimmunol.171.11.6251. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson K, Scotland R, Lee P, et al. Auto-immunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 13.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang HB, Shi FD, Li H, et al. Anti-CTLA-4 antibody treatment triggers determinant spreading and enhances murine myasthenia gravis. J Immunol. 2001;166:6430–6436. doi: 10.4049/jimmunol.166.10.6430. [DOI] [PubMed] [Google Scholar]
- 16.Luhder F, Hoglund P, Allison JP, et al. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrin PJ, Maldonado JH, Davis TA, et al. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 18.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 20.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 21.Beelen DW, Elmaagacli A, Muller KD, et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: Final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- 22.Guthery SL, Heubi JE, Filipovich A. Enteral metronidazole for the prevention of graft versus host disease in pediatric marrow transplant recipients: Results of a pilot study. Bone Marrow Transplant. 2004;33:1235–1239. doi: 10.1038/sj.bmt.1704474. [DOI] [PubMed] [Google Scholar]
- 23.Hampe J, Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 24.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Arnott ID, Landers CJ, Nimmo EJ, et al. Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–2384. doi: 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]
- 26.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 27.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–538. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 29.Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+ CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 30.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockage. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]


