Abstract
Given the tolerance of the right heart circulation to mild regurgitation and gradient, we study the potential of using motionless devices to regulate the pulmonary circulation. In addition, we document the flow performance of two mechanical valves. A motionless diode, a nozzle, a mechanical bileaflet valve, and a tilting disk valve were tested in a pulmonary mock circulatory system over the normal human range of pulmonary vascular resistance (PVR). For the mechanical valves, regurgitant fractions (RFs) and transvalvular pressure gradients were found to be weak functions of PVR. On the low end of normal PVR, the bileaflet and tilting disk valves fluttered and would not fully close. Despite this anomaly, the regurgitant fraction of either valve did not change significantly. The values for RF and transvalvular gradient measured varied from 4 to 7% and 4 to 7 mm Hg, respectively, at 5 lpm for all tests. The diode valve was able to regulate flow with mild regurgitant fraction and trivial gradient but with values higher than either mechanical valve tested. Regurgitant fraction ranged from 2 to 17% in tests extending from PVR values of 1 to 4.5 mm Hg/lpm at 5 lpm and with concomitant increases in gradient up to 17 mm Hg. The regurgitant fraction for the nozzle increased from 2 to 23% over the range of PVR with gradients increasing to 18 mm Hg. The significant findings were: (1) the mechanical valves controlled regurgitation at normal physiological cardiac output and PVR even though they failed to close at some normal values of PVR and showed leaflet flutter; and (2) it may be possible to regulate the pulmonary circulation to tolerable levels using a motionless pulmonary valve device.
Keywords: mechanical heart valve, pulmonary vascular resistance, diode, regurgitant fraction
1 Introduction
Congenital pulmonary insufficiency is initially well tolerated [1], However, severe untreated pulmonary valvar insufficiency progresses to right ventricular dilation, loss of biventricular systolic function, and deterioration in symptomatic status of most patients by 35–40 years of age but earlier in some [2,3]. Restoring pulmonary valve sufficiency in young patients improves biventricular function and reduces symptoms [2], although the optimal timing for intervention remains unclear as there is no clear replacement solution [1,4,5]. Bioprosthetic valves and homografts are the choice for valve replacement in older adults, but these will need to be replaced in younger patients. Further, children and adolescents show a high risk of accelerated tissue valve calcification [1]. Mechanical heart valves (MHVs) are permanent, but require lifetime anticoagulation therapy. There are limited independent reports of an elevated risk of thrombosis with associated MHV malfunction for the pulmonary position [3–6], particularly for bileaflet designs [6–8].
While prosthetic heart valves have been studied in extensive detail in left heart applications, there is no in vitro performance database for mechanical valves as pulmonary valves with published studies limited primarily to imaging of valve movement [9,10]. Aberrant leaflet motions, including leaflet flutter and incomplete closure, have been reported and are likely to occur at values of pulmonary vascular resistance (PVR) near and below 2 mm Hg/lpm[9]. The consequences of valvular pulmonic stenosis on the hemodynamics of the pulmonary artery have been studied using bioprostheses [11–13].
Given the tolerance of the right heart circulation to mild regurgitation and pressure gradient, we examine the concept of using a motionless valve to regulate flow in the pulmonary position. We also examine the flow performance of two existing MHVs. In each case, flow performance was documented as a function of PVR. A St. Jude Medical standard bileaflet valve (SJMB) and a MedicalCV Omnicarbon tilting disk valve (OTD) were used as representatives of their class of mechanical valve. For the alternate paradigm, we test a momentum-nozzle fluid diode and a flow nozzle. Tests were done using a pulmonary mock circulatory system [9] over the normal adult range of PVR.
2 Background
2.1 Overview
The operating pressures in the right heart are significantly lower than those of the left heart and with marked differences in the circulation impedances. The pulmonary circulation shows a tolerance for mild regurgitation and pressure gradient [14]. The normal adult pulmonary valve diastolic gradient (vessel to ventricle) is about 5 to 15 mm Hg. It is the diastolic gradient that provides the force to close the leaflet and keep it closed. For the healthy adult, the PVR is between 2 to 3 mm Hg/lpm with a normal range of 1 to 5 mm Hg/lpm. At the lower values of PVR, the diastolic gradient approaches zero. A normal pulmonary compliance is about 4 ml/mm Hg but varies with activity. These values compare with a normal systemic vascular resistance of between 15 and 20 mm Hg/lpm and diastolic pressure gradients generally above 50 mm Hg [15].
Although there are no specific data correlating regurgitant fraction to long term ventricular function over time, clinical experience and practice strongly suggest that mild loading conditions represent little if any risk to long term right ventricular performance. The commonest cause of abnormal right ventricular volume loading conditions is that of left-to-right shunting, such as is seen from secundum atrial septal defects. The pressure load resulting from such defects is very low. As a reference value, a regurgitant fraction of 15% is the equivalent volume load of a left-to-right shunt of < 1.2:1 (ratio of pulmonary blood flow to systemic blood flow). All published guidelines for intervention on left-to-right shunts are in agreement that intervention is mandated for shunt volumes of greater than 2:1 [16]. However, there is complete agreement that left-to-right shunts of < 1.5:1 are clinically inconsequential with respect to the resulting volume load. As such, regurgitant fractions on the level of 20% and transvalvular pressure gradients below 25 mm Hg should be well tolerated in the pulmonary circulation [1].
2.2 Fluid Diodes
Given the low pressures involved in the right heart, the aberrant leaflet motion reported in mechanical leaflet valve studies, and the tolerance of the right heart for mild regurgitation, we speculate on the absolute need for moving leaflets to regulate flow. If not absolutely necessary, then a simpler design paradigm may hold for a replacement pulmonary valve. As an example, a fluid diode is a flow regulating device without moving elements that promotes unidirectional flow by providing low resistance to forward flow but a higher resistance to backflow [17–20]. While a fluid diode can not completely stop reverse flow, a proper design may regulate regurgitation to an acceptable physiological value. Hence, these devices may be useful to limit reverse flow in pulsatile conditions while controlling the inevitable distortion of the valve annular ring that accompanies ventricular dysfunction.
Any motionless flow-regulating device having a preferred direction can be called a fluid diode. A flow nozzle uses a shaped entry and abrupt exit to provide a ratio of the steady forward flow resistance to reverse flow resistance (λ) of λ~2:1 even at Reynolds numbers down to 1000 [18]. The original concept of a momentum diode was posed by Tesla [21], which utilizes confined jet interacting flows to provide a significant resistance to flow in the reverse direction. A momentum diode consists of a central flow with a branching backflow channel [21]. The backflow channel is designed to shunt some reverse flow into a stream that impinges back onto the central flow at such an angle so as to provide a significant resistance to the central flow. Steady flow resistance ratios of λ ~ 4.2:1 are reported [17]. Other designs, such as scroll diodes that use a central obstruction, can show substantially higher values for λ. Models of diode fluid mechanics and supporting experimental data (e.g., [17,18]) predict the reverse-to-forward mass flow ratio as X= 1/(1 +λ) under steady conditions. This suggests the possibility of achieving regurgitant fractions below 20%. While the hydrodynamic merits of fluid diodes suggest their potential as functional heart valves, the hemodynamics of these devices remains untested. These effects require a more detailed flow study than we report here.
3 Methods
3.1 System Setup
Valve tests were conducted in a mock circulatory system (MCS) designed to simulate the pulmonary circulation and near distal pulmonary artery. Extensive details of this system (Fig. 1) have been given in Ref. [9]. The acrylic transparent test section, which houses the test valve, branches just as the pulmonary artery does into the right and left lungs with a geometry derived from the dimensions of healthy pulmonary arteries. It is scaled to a 25 mm tissue annulus. The compliant ventricle pump chamber uses a flexible membrane with pulsation controlled by a timing box using two electromechanical pneumatic actuators. Pressurized compliance chambers, adjustable valves, and head tank are used downstream of the test section to control and to simulate the capacitance and resistance of the pulmonary arterial tree, respectively.
Fig. 1.
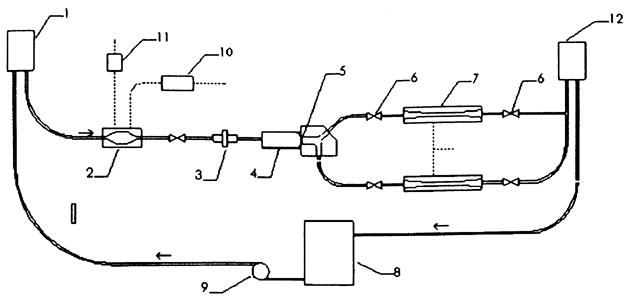
Schematic of the mock circulatory system. Arrows indicate flow direction. 1. Atrial head tank with tricuspid valve. 2. Right ventricular chamber. 3. Flow meter. 4. Test chamber. 5. Mechanical heart valve. 6. Resistance elements. 7. Compliance chambers. 8. Reservoir. 9. Pump. 10. Pressurizing flow valve. 11. Venting flow valve. 12. Resistance head tank.
For these tests, conditions were maintained at a nominal cardiac output of 5 l/min of a blood analog fluid at a pulse rate of 75 bpm and systolic ratio of 45%. The PVR was varied between 1 and 5 mm Hg/lpm by adjusting the downstream resistance to increase the mean pulmonary artery pressure while maintaining flow rate and pulse rate. This required changing stroke volume and pulse pressure. System compliance (stroke volume/pulse pressure) was maintained between 2 ml/mm Hg at the highest values of PVR used up to 6 ml/mm Hg Hg at the lowest values of PVR.
3.2 Measurements
Pulmonary artery pressure (PAP) was measured directly downstream of the test valve using a fluid-filled catheter and Druck® LPM 5480 pressure transducer (−80 to 80 mm Hg). Similarly, right ventricular pressure (RVP) was measured upstream of the valve. Flow rate (Q) was measured with a Carolina Medical Inc. Model 501 flow meter with 25 mm inner diameter flow probe. Instantaneous flow rate and PAP signals were recorded simultaneously at 80 Hz using a data acquisition system. The pulmonary branch volume flow split was held at 50%.
Regurgitant fraction (RF) was estimated from the measured instantaneous flow rate as
| (1) |
The instantaneous peak-peak transvalvular pressure gradient (PPG) was taken as
| (2) |
and the effective orifice area (EOA) was estimated based on a blood analog fluid by [22]
| (3) |
where Qrms is the root-mean-square flow rate (cm3/s), and is the mean systolic-to-diastolic pressure drop. The mean PAP was estimated directly by integration of the pressure signal. Pulmonary vascular resistance values were taken as the magnitude of the zeroth harmonic of the valve-system impedance spectrum as previously reported [9], All values reported are pooled (m = 3) mean values taken over 30 cycles with the ± values referring to the combined 95% uncertainty intervals [23].
3.3 Valve Studies
Two mechanical valves and two diodes were tested: a 25 mm St. Jude Medical bileaftet valve, a 25 mm MedicalCV Omnicarbon tilting disk valve, a momentum-nozzle diode, and a flow nozzle. For either mechanical valve, the fabric sewing ring was removed and the valve was mounted into the test chamber using a holder designed to position the valve so that only the valve and interior ring were exposed to the flow. Each valve was oriented perpendicular to the plane of the pulmonary artery branch and the plane of the leaflets was parallel to gravity. We did not attempt a study of valve orientation on valve behavior.
Each motionless device was designed around an axisymmetric flow nozzle [24] having an elliptical contraction and truncated at the midpoint of its major axis to achieve a profile of L = 12.5 mm. The flow nozzle serves as a base case for regulating backflow with a motionless device. A momentum-nozzle diode was developed from the nozzle by incorporating an enclosed backflow channel, as depicted in Fig. 2. For this diode design, the backflow channels were created by machining an elliptical cusp on the backend of the nozzle, which was then enclosed by adding annular ring c. In principle, during diastole, the flow through the backflow channels is reintroduced to the central flow at an angle that impedes regurgitant flow. The large open central area allows for undisturbed flow during systole. The time-dependent diastolic pulmonary artery pressure provides the mechanism to trigger a high initial resistance to flow but one that diminishes towards late diastole. For the motionless devices, D = 25 mm, d= 12.5 mm, and for the diode valve, b = 2 mm, B = 3 mm, and α = 60 deg.
Fig. 2.
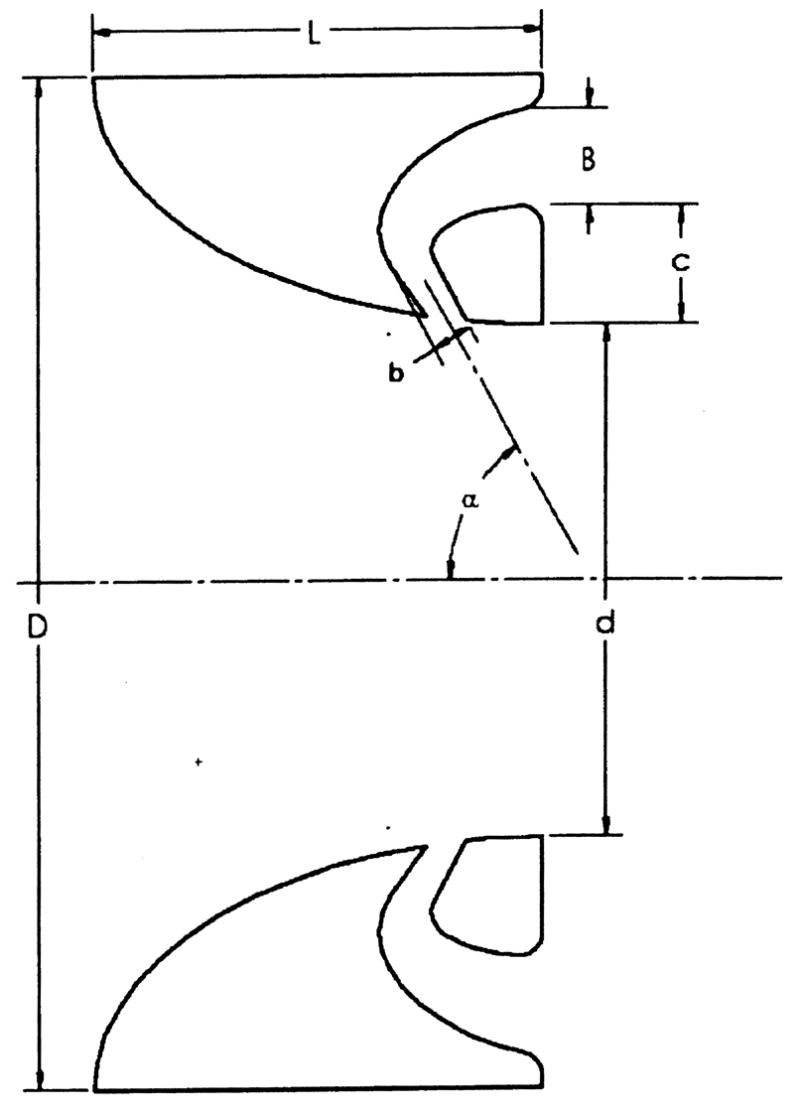
Generic representations for flow nozzle and momentum-nozzle diode designs: Systolic flow is left to right
4 Results and Discussion
4.1 Pressure and Flow Traces
Sample signals for the right ventricular pressure (RVP), pulmonary artery pressure (PAP) and instantaneous flow rate (Q) obtained with the SJMBV and OTD valve are shown in Figs. 3 and 4. Overall, the pressure and flow signals are consistent with physiological values [15]. In either case, the PAP shows a clear dicrotic notch and pressure trends are well correlated with flow rate. Valve regurgitation (negative flow rate) both during closure and by closed leakage is visible, although closed leakage around the OTD valve is essentially zero.
Fig. 3.
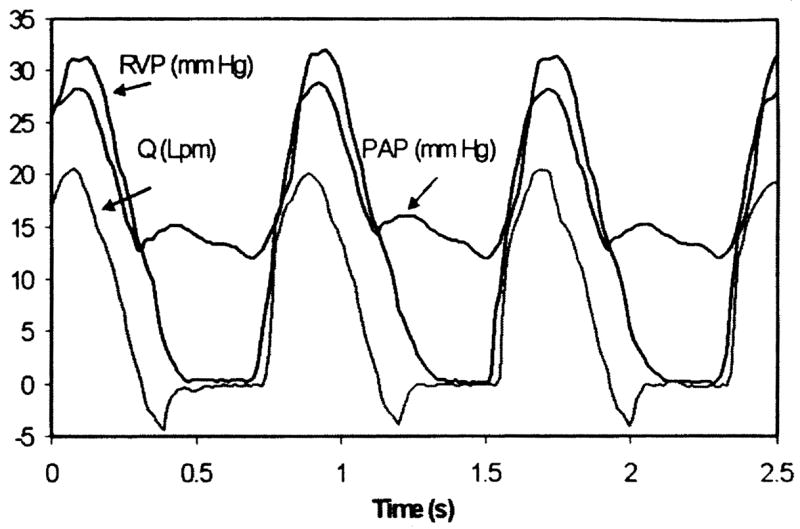
Physiological signals using SJMBV (5 lpm, 75 bpm, 3.9 mm Hg/lpm)
Fig. 4.
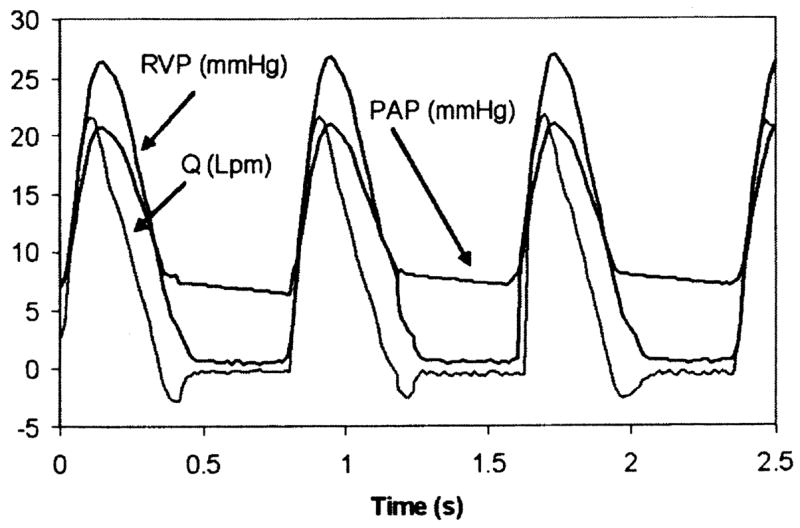
Physiological signals using OTDV (5 lpm, 75 bpm, 2.9 mm Hg/lpm)
A corresponding set of signals obtained with the momentum-nozzle diode valve is shown in Fig. 5. Here again the signals are well correlated. The features of the curves are less abrupt and more rounded than those of Figs. 3 and 4. The onset of diastole is marked by a crossing of the PAP and RVP signals and a change in the direction of the flow rate. Because the central flow area of the diode valve remains constant and open, the diastolic PAP gradually approaches the diastolic RVP, and a diminishing volume of regurgitant flow is sustained throughout diastole.
Fig. 5.
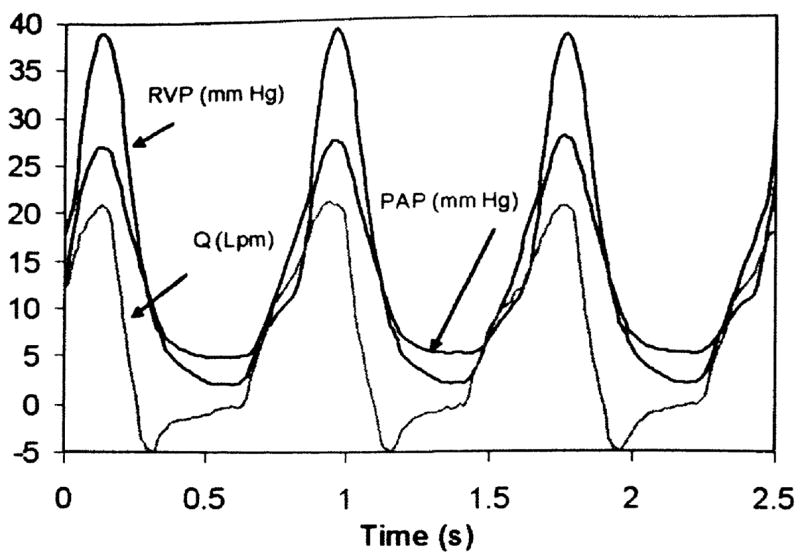
Physiological signals using diode valve (5 lpm, 74 bpm, 3.1 mm Hg/lpm)
4.2 Performance Data
For each valve and device, the test data were analyzed for regurgitation fraction (RF), peak-peak transvalvular pressure gradient (PPG), and effective orifice area (EOA) [4] for a range of PVR settings. Results are summarized in Fig. 6 with EOA values given in Table 1.
Fig. 6.
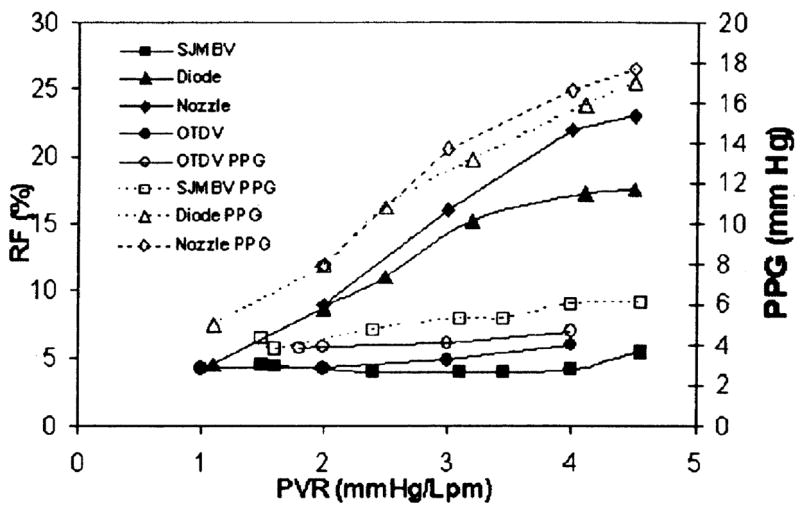
Regurgitant fraction (RF) and peak-peak transvalvular pressure gradient (PPG) results at 5 lpm
Table 1.
Summary of effective orifice area (5 lpm, and 75 bpm).
| Cardiac output [lpm] | PVR [mm Hg/lpm] | EOA [cm2] | |
|---|---|---|---|
| SJM value | 5.1±0.1 | 3.05±0.1 | 3.64 |
| OTD value | 5.2±0.1 | 2.9±0.1 | 2.45 |
| Diode | 5.1±0.1 | 3.0±0.1 | 1.24 |
| Nozzle | 5.1 ±0.2 | 3.0±0.1 | 1.13 |
Both the SJMB valve and the OTD valve flow data reveal only a mild sensitivity to PVR. Values for RF remained between 4 and 6% for the SJMB and between 6 and 7% for the OTD valves. As reported in a previous study [9], the SJMB valve does not fully close below PVR of 2 mm Hg/lpm and the OTD valve does not fully close below PVR of 1.6 mm Hg/lpm. However, the associated changes in RF at low PVR were not significant (p < 0.025). The low diastolic pressure gradient at low PVR prevents significant regurgitant flow despite the open leaflets. Gradients were about 4 to 7 mm Hg for either valve and are essentially insensitive to PVR. The EOA values for both valves are similar, but slightly lower than reported for similar valves in the aortic position [25,26]. Uncertainties in the RF values were estimated at ±0.5% and PPG values at ±0.4 mm Hg at 95% confidence for either valve.
The performances of the diode valve and nozzle show sensitivity to PVR. For the diode, the RF values range from a value of about 2% at PVR=0.9 mm Hg/lpm approaching 17.2% at PVR of 4.5 mm Hg/lpm. This represents a significant (p<0.025) improvement over the flow nozzle for values of PVR >2 mm Hg/lpm. At PVR ≤ 2 mm Hg/lpm, either device compares well against either mechanical valve, where the motionless devices afford a greater resistance to backflow during diastole. For the diode valve, pressure gradient increases with PVR reaching a magnitude of 17 mm Hg. Noted differences in gradients between the diode valve and the nozzle are not significant (p<0.025). The EGA for the diode valve is improved over that of the nozzle. Based only on RF and PPG values, these motionless devices compare favorably with the published performance of some competent mechanical and bioprosthetic valves in the aortic position [25,26]. Uncertainties in the RF values were estimated at ±1.2% and PPG values at ±0.7 mm Hg at 95% confidence for the motionless devices.
Despite the identical central flow areas of both the diode valve and the nozzle, the diode valve showed more forward flow and less regurgitant flow than the nozzle for a given setting of the MCS. The implication is that a backflow channel flow or redirection of diastolic backflow can be used to reduce regurgitant flow. While this performance is encouraging, we note that a goal here was to assess whether a motionless device could regulate a range of pulmonary flows with RF<20% and PPG < 25 mm Hg. These results support this idea and open the possibility for novel bio-compatible designs with improved performance.
5 Conclusion
The flow performances of a St. Jude Medical bileaflet valve and a MedicalCV Omnicarbon tilting disk valve were documented in a pulmonary mock circulatory system over a normal range of pulmonary vascular resistance (PVR). In addition, the concept of flow regulation of the pulmonary circulation by means of a motionless valve was tested. For the mechanical valves, regurgitant fractions (RF) and gradients (PPG) were weak functions of PVR with values in the range of 4 to 7% and 4 to 7 mm Hg. However, at the lower normal values of PVR, the bileaflet and tilting disk valves would not fully close and fluttered. Despite this anomaly, the regurgitant fraction did not change significantly. The finding was that these valves controlled regurgitation at normal physiological cardiac output and PVR even though aberrant leaflet behavior with failure to close was observed.
The results also indicate that it may be possible to regulate the pulmonary circulation with a motionless device. A motionless momentum-nozzle diode valve showed a significant improvement in its ability to regulate regurgitant flow over a flow nozzle. For the diode, the regurgitant fraction ranged from 2 to 17% with gradients up to 17 mm Hg over the normal range of PVR compared with 2 to 23% for the nozzle.
Acknowledgments
We gratefully acknowledge support through NIH grants RRO18823, HL083975, and HL/HD67135 and the SC BRIN Collaborative Research Program.
Footnotes
Contributed by the Bioengineering Division of ASME for publication in the Journal of Biomechanical Engineering.
References
- 1.Bonow RO, Carabello B, de Leon AC, Edmunds LH, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O’Gara PT, O’Rourke RA, Rahimloola SH. ACC/AHA Guidelines on the Management of Patients With Valvular Heart Disease: Executive Summary. Circulation. 1998;98:1949–1984. doi: 10.1161/01.cir.98.18.1949. [DOI] [PubMed] [Google Scholar]
- 2.Hillman ND, Mavroudis C, Backer CL. Adult Congenital Heart Disease. In: Mavroudis C, Backer CL, editors. Pediatric Cardiac Surgery. 3. Mosby; Philadelphia: 2003. chap. 48. [Google Scholar]
- 3.de Ruijter FTH, Weenink I, Hitchcock FJ, Meijboom EJ, Benninck GBWE. Right Ventricular Dysfunction and Pulmonary Valve Replacement After Correction of Tetralogy of Fallot. Ann Thorac Surg. 2000;73:1794–1800. doi: 10.1016/s0003-4975(02)03586-5. [DOI] [PubMed] [Google Scholar]
- 4.Ilbawi MN, Lockhart CG, Idriss FS, DeLeon SY, Muster AJ, Duffy CE, Paul MH. Experience With St. Jude Medical Valve Prosthesis in Children: A Word of Caution Regarding Right-Sided Placement. J Thorac Cardiovasc Surg. 1987;93(1):73–79. [PubMed] [Google Scholar]
- 5.Ilbawi MN, Idriss FS, DeLeon SY, Muster AJ, Duffy CE, Gidding SS, Paul MH. Valve Replacement in Children: Guidelines for Selection of Prosthesis and Timing Surgical Intervention. Ann Thorac Surg. 1987;44(4):398–403. doi: 10.1016/s0003-4975(10)63800-3. [DOI] [PubMed] [Google Scholar]
- 6.Kawachi Y, Masuda M, Tominaga R, Tokunaga K. Comparative Study Between St. Jude Medical and Bioprosthetic Valves in the Right Side of the Heart. Jpn Circ J. 1991;55(6):553–362. doi: 10.1253/jcj.55.553. [DOI] [PubMed] [Google Scholar]
- 7.Rosti L, Murzi B, Colli AM, Festa P, Redaelli S, Havelova L, Menicanti L, Frigiola A. Mechanical Valves in the Pulmonary Position: A. Reappraisal. J Thorac Cardiovasc Surg. 1998;115(5):1074–1079. doi: 10.1016/S0022-5223(98)70407-6. [DOI] [PubMed] [Google Scholar]
- 8.Meuris B, Verbeken E, Fameng W. Mechanical Valve Thrombosis in a Chronic Animal Model: Differences Between Monoleaflet and Bileaflet Valves. J Heart Valve Dis. 2005;14(1):96–104. [PubMed] [Google Scholar]
- 9.Gohean J, Figliola R, Camp T, McQuinn T. Comparative in vitro Study of Bileaflet and Tilting Disk Valve Behavior in the Pulmonary Position. ASME J Biomech Eng. 2006;128:631–635. doi: 10.1115/1.2206204. [DOI] [PubMed] [Google Scholar]
- 10.Kiyota Y, Shiroyama T, Akamatsu T, Yokota Y, Ban T. In Vitro Closing Behavior of the St. Jude Medical Heart Valve in the Pulmonary Position. J Thorac Cardiovasc Surg. 1992;104(3):779–85. [PubMed] [Google Scholar]
- 11.Philpot E, Yoganathan AP, Woo Y, Sung H, French R, Sahn D, Valdes-Cruz L. In vitro Pulsatile Flow Visualization Studies in a Pulmonary Artery Model. ASME J Biomech Eng. 1985;107:368–375. doi: 10.1115/1.3138571. [DOI] [PubMed] [Google Scholar]
- 12.Sung H, Philpot E, Nanda N, Yoganathan A. Axial Flow Patterns in a Pulmonary Artery Model With Varying Degrees of Pulmonic Stenosis—Pulsatile in vitro Studies. J Biomech. 1993;23:563–578. doi: 10.1016/0021-9290(90)90049-9. [DOI] [PubMed] [Google Scholar]
- 13.Sung H, Hsu T, Hsu C, Hsu J. Pulmonary Artery Hemodynamics With Varying Degrees of Valvular Stenosis: In vitro Study. J Biomech. 1998;31:1153–1161. doi: 10.1016/s0021-9290(98)00115-8. [DOI] [PubMed] [Google Scholar]
- 14.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors Associated With Impaired Clinical Status in Long-Term Survivors of Tetralogy of Fallot Repair Evaluated by Magnetic Resonance Imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Rosendorff C, editor. Essential Cardiology: Principles and Practice. W.B. Saunders; New York: 2001. [Google Scholar]
- 16.Backer CL, Mavroudis C. Atrial Septal Defect, Partial Anomalous Pulmonary Venous Connection, and Scimitar Syndrome chap. 16. In: Mavroudis C, Backer CL, editors. Pediatric Cardiac Surgery. Mosby; Philadelphia: 2003. pp. 283–297. [Google Scholar]
- 17.Paul FW. Proceedings IFAC Symposium on Fluidics. Peter Peregrinus Ltd; London: 1969. Fluid Mechanics of the Momentum Flueric Diode; pp. 1–15. Paper A1. [Google Scholar]
- 18.Baker PJ. Comparison of Fluid Diodes. Brit Hydromech Res. 1967;D6:88–115. [Google Scholar]
- 19.Linderoth ET. Aerodynamic Check Valve. 2,727,535 US Patent. 1955
- 20.Conway A. Guide to Fluidics. Macdonald; London: 1971. [Google Scholar]
- 21.Tesla N. Valvular Conduit. 13,295,59 US Patent. 1920
- 22.ANSI/AAMI/ISO 5840. Cardiovascular Implants: Cardiac Valve Prostheses. AAMI; Arlington, VA: 2005. [Google Scholar]
- 23.Figliola RS, Beasley DE. Theory and Design for Mechanical Measurements. 4. Wiley; New York: 2005. chaps. 4 and 5. [Google Scholar]
- 24.Bean HS, editor. Fluid meters: Their Theory and Application. 6. American Society of Mechanical Engineers; New York: 1971. [Google Scholar]
- 25.Yoganathan A, Travis B. Fluid Dynamics of Prosthetic Valves. In: Otto CM, editor. Practice of Clinical Endocardiograhpy. W.B. Saunders; Philadelphia: 2000. [Google Scholar]
- 26.Chandran K. Pulsatile Flow Past SJMBV: In vitro Study. J Thorac Cardiovasc Surg. 1985;89:743–749. [PubMed] [Google Scholar]


