Abstract
3-O-3′(or 2′)-methylsuccinyl-betulinic acid (MSB) derivatives were separated by using recycle HPLC. The structures of four isomers were assigned by NMR and asymmetric synthesis. 3-O-3′S-Methylsuccinyl-betulinic acid (3′S-MSB, 4) exhibited potent anti-HIV activity with an EC50 value of 0.0087 μM and a TI value of 6.3×103, which is comparable to the data for bevirimat (DSB, PA-457), a current clinical trials drug that was also derived from betulinic acid. The anti-HIV potency of 4 was slightly better than that of AZT.
Highly active antiretroviral therapy (HAART) combines several anti-HIV drugs with different targets, and its use has significantly improved AIDS treatment.1-3 However, it has also led to some serious problems, such as increased toxicities and emergence of multi-drug resistant HIV viral strains.4-7 Therefore, new classes of compounds with novel modes of action against HIV are still urgently needed.
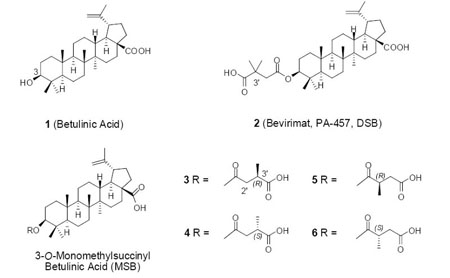
We initially identified betulinic acid (BA, 1) as an anti-HIV lead compound with an EC50 1.4 μM and therapeutic index (TI) 9.3 from the leaves of Syzygium claviflorum.8 Our subsequent modification study resulted in the discovery of 3-O-(3′,3′-dimethylsuccinyl)-betulinic acid (bevirimat, DSB, PA-457, 2). Bevirimat exhibits extremely potent anti-HIV activity with an EC50 value of <0.00035 μM and a TI value of >20,000.9,10 It was found to inhibit HIV-1 replication by interfering with a previously unidentified viral target, HIV-1 P24/P25 processing, resulting in the production of a noninfectious HIV-1 particle.11,12 Therefore, bevirimat represents a unique first in a class of anti-HIV compounds termed maturation inhibitors (MIs). Bevirimat has recently succeeded in Phase IIa clinical trials and is currently in Phase IIb clinical trials.13,14
Our prior structure-activity relationship (SAR) study of 2 and other 3-O-acyl-betulinic acid derivatives suggested that the proper length, a terminal carboxylic acid, and C-3′ dimethyl substitution of the C-3 side chain contribute to enhanced anti-HIV activity.9,15 However, it is still unclear which, if either, C-3′ methyl group within the C-3 side chain of 2 contributes more toward activity. Therefore, 3-O-monomethylsuccinyl betulinic acid (MSB) derivatives were designed to investigate the impact of these methyl groups. This paper reports their synthesis, isolation, structural assignment, and SAR study.
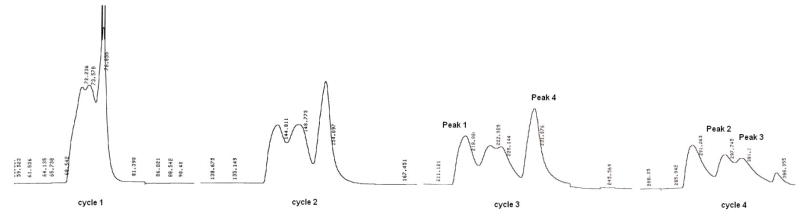
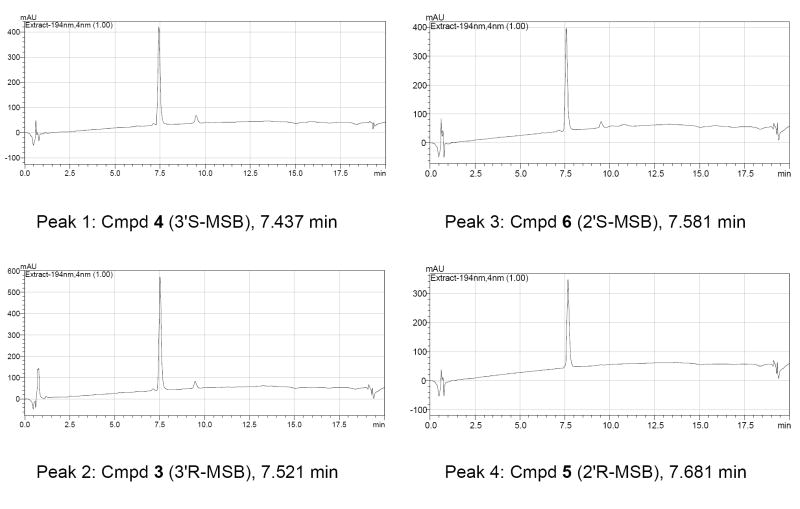
Betulinic acid was treated with 2-methylsuccinic anhydride and DMAP in pyridine at reflux to furnish the 3′-MSB racemic isomers, as well as the undesired 2′-MSB isomers. The separation of the four compounds (3-6)16 was challenging, since they differ only by the presence of a 2′ or 3′ (R or S) methyl group, but was successfully achieved by using recycle preparative HPLC (Figure 1).17 The purities of 3-6 were ascertained by analytical HPLC (Figure 2). By comparing the 1H-NMR spectra of the purified isomers with those of 2 and 3-O-2′,2′-dimethylsuccinyl BA, peaks 1 and 2 were assigned as the 3′-MSBs, and peaks 3 and 4 as the 2’-MSBs. However, we still needed to determine the stereochemistry of the C-3′ and C-2′ chiral centers. Therefore, the 3′R and 3′S isomers were also prepared through asymmetric synthesis as described below.
Figure 1.
JAI LC-918 Recycling Preparative HPLC. Mobile phase: 85% ACN 0.1% TFA; flowrate: 5.0 mL/min; Detector: Refractive Index (RI); Column: Alltima C18 5μ 250 mm × 22 mm. Peak 1: 3-O-(3′S-methylsuccinyl)betulinic acid (4); Peak 2: 3-O-(3′R-methylsuccinyl)-betulinic acid (3); Peak 3: 3-O-(2′S-methylsuccinyl)-betulinic acid (6); Peak 4: 3-O-(2′R-methylsuccinyl)-betulinic acid (5)
Figure 2.
Purity confirmation of the four MSB (3-6) isomers by Shimadzu LC-20AT. Column: Alltima C18 5μ 150 mm × 2.1 mm.
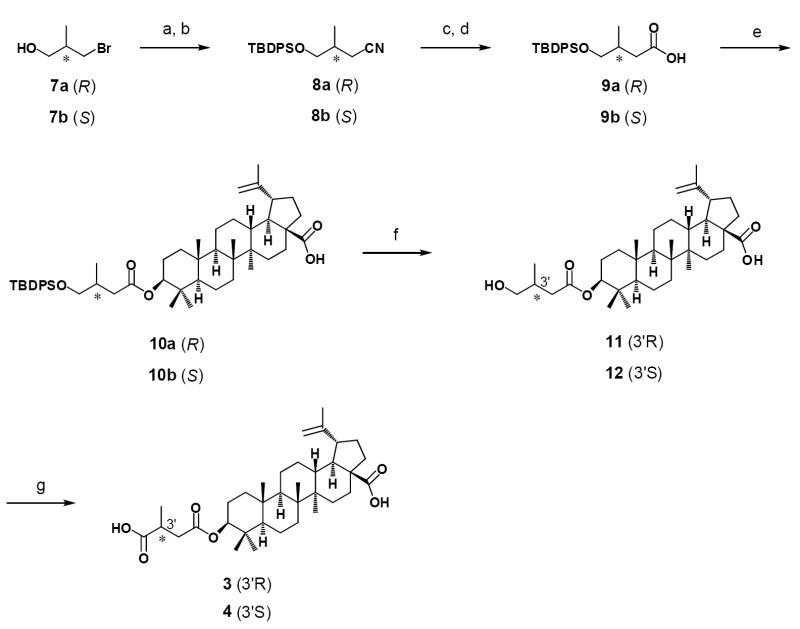
As shown in Scheme 1, the hydroxy group of 3R-bromo-2-methylpropanol was first protected by reaction with TBDPSCl in DMF. The bromide group was then replaced with an aldehyde group by reaction first with sodium cyanide in DMSO, followed by reduction using DIBALH.18 The aldehyde was oxidized to a carboxylic acid in the presence of NaClO2 and NaH2PO4 in DMSO to yield 9a. Reaction of 1 with 9a in the presence of EDCI and DMAP led to esterification of the 3β-hydroxy group of 1 with the carboxylic acid of 9a to provide 10a. After cleavage of the TBDPS group with TBAF in THF, the primary hydroxy group of 11 was oxidized to a carboxylic acid in the presence of TEMPO, PhI(OAc)2 in a solution of H2O-CH2Cl2 to give 3-O-(3′R-methylsuccinyl)-betulinic acid (3′R-MSB, 3). The 3′S-MSB isomer (4) was synthesized using the same method starting with 3S-bromo-2-methylpropanol. By comparing the HPLC retention times and 1H-NMR spectra of these compounds, we determined that peak 1 is the 3′S diastereoisomer and peak 2 is the 3′R diastereoisomer.
Scheme 1.
Reagents and conditions: a) TBDPSCl, imidazole, quant; b) NaCN, DMSO, 93%; c) DIBALH, 95%; d) NaClO2, NaH2PO4, DMSO, quant; e) EDCI, DMAP, BA, 25-48%; f) TBAF, THF, quant; g) TEMPO, PhI(OAc)2, H2O, CH2Cl2, 75-88%
Furthermore, the reaction of 2R-methylsuccinic anhydride with betulinic acid yielded the 3′R-MSB (3) and 2′R-MSB (5) isomers. The HPLC retention time indicated that peak 4 is the 2′R isomer (5). Similarly, peak 3 was confirmed as the 2′S isomer (6). Thus, the stereochemistry of the 3′ or 2′ chiral center in 3–6 was fully determined.
Anti-HIV activities of all four synthetic compounds (3–6) and 2 were evaluated according to the procedure described in Refs 19 and 20. The synthetic intermediate 12 and a 1:1 mixture (13) of the 3′R- and 3′S-MSB isomers (3 and 4) were also tested.
The anti-HIV activities of the synthesized compounds are summarized in Table 1. Among these derivatives, the 3′S-MSB isomer (4) showed very potent anti-HIV activity with a TI of 6.3×103 and EC50 of 0.0087 μM, which is comparable to that of 2 (EC50: 0.0013 μM) and slightly better than that of AZT (EC50: 0.034 μM) in this assay system. The 3′R-MSB isomer (3) exhibited only moderate anti-HIV activity with a TI of 9.6×102 and EC50 of 0.12 μM. The antiviral activity (EC50: 0.016 μM) of the mixture (13) of the two stereoisomers falls in between those of 3 and 4. This result indicates that the two C-3′methyl groups in the DSB side chain contribute differently to the extremely potent anti-HIV activity of this compound class. We postulate that an interaction of the 3′S-methyl group with the viral target might be essential to the anti-HIV activity of 2. The interaction of the 3′R-methyl group within the target is less significant, but still necessary, since 2, with dimethyl substitution at C-3′, is slightly more potent than the 3′S-MSB isomer (4). In comparison with 3 and 4, both 2′-MSBs (5, 6) exhibited no or little activity in the same assay. These results agree with our previous SAR study, indicating that methyl substitution at the C-3′ position improves the antiviral activity much more significantly than at the C-2′ position.
Table 1.
Anti-HIV activities for 3-O-monomethylsuccinyl-betulinic acid derivatives in HIV-1 infected MT-2 cell line.18
| Cmpd | EC50 (μM)a | IC50 (μM)b | TI (IC50/EC50) |
|---|---|---|---|
| AZT | 0.034 | 1,870 | 55,330 |
| 2 | 0.0013 | > 42.78 | 30,555 |
| 3 | 0.12 | > 43.83 | 961 |
| 4 | 0.0087 | 32.78 | 6,274 |
| 5 | No Suppression | > 43.83 | – |
| 6 | 0.44 | > 43.83 | 100 |
| 12 | No Suppression | > 44.93 | – |
| 13 | 0.016 | > 44.93 | 5,323 |
: concentration that inhibits HIV-1 replication by 50%
: concentration that is toxic to 50% of mock-infected MT-2 cells
In addition, compound 12, which differs structurally from 3 only in the replacement of a primary hydroxyl group for a terminal carboxylic acid in the 3-O-acyl side chain, had no activity. This result is also consistent with our findings in prior studies that the terminal carboxylic acid is essential for enhanced antiviral activity.15
In conclusion, 3′ MSBs and 2′MSBs were prepared in our study to investigate the SAR of the C-3′ chiral center of the anti-HIV clinical trials agent bevirimat (2). Our results suggest that the 3′S-methyl group of 2 is the major contributor to the compound’s extremely high anti-HIV potency, since 2 and 3′S-MSB (4) exhibit comparable anti-HIV activity, while the 3′R isomer shows much lower activity.
Acknowledgments
This investigation was supported by Grant AI-33066 from the National Institute of Allergy and Infectious Diseases (NIAID) awarded to K.H. Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. N Eng J Med. 1998;338:853. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Mohri H, Bonhoeffer S, Monard S, Perelson AS, Ho DD. Science. 1998;279:1223. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 3.Klein MB, Willemot P, Murphy T, Lalonde RG. AIDS. 2004;18:1895. doi: 10.1097/00002030-200409240-00005. [DOI] [PubMed] [Google Scholar]
- 4.Piscitelli SC, Flexner C, Minor JR, Polis MA, Masur H. Clin Infect Dis. 1996;23:685. doi: 10.1093/clinids/23.4.685. [DOI] [PubMed] [Google Scholar]
- 5.Louie M, Markowitz M. Antiviral Res. 2002;55:15. doi: 10.1016/s0166-3542(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 6.Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Jones E, Tsay J, Ip J, Farthing C, Limoli K, Parkin N, Markowitz M. J Am Med Assoc. 1999;282:1135. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 7.Condra JH. Haemophilia. 1998;4:610. doi: 10.1046/j.1365-2516.1998.440610.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen IS, Lee KH. J Nat Prod. 1994;57:243. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 9.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. J Med Chem. 1996;39:1016. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto F, Kashiwada Y, Cosentino LM, Chen CH, Garrett PE, Lee KH. Bioorg Med Chem. 1997;5:2133. doi: 10.1016/s0968-0896(97)00158-2. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. Proc Natl Acad Sci USA. 2003;100:13555. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Chen CH, Aiken C. Retrovirology. 2004;1:15. doi: 10.1186/1742-4690-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beatty GLJ, Eron J, Pollard R, Saag M, Doto J, Salzwedel K, Wild C, Allaway G, Jacobson J, Martin D. Presented at the 45th Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. December 2005. [Google Scholar]
- 14.Panacos. [03/12/2007]; http://www.panacos.com/ne_press_releases.htm.
- 15.Yu D, Wild CT, Martin DE, Morris-Natschke SL, Chen CH, Allaway GP, Lee KH. Expert Opin Investig Drugs. 2005;6:681. doi: 10.1517/13543784.14.6.681. [DOI] [PubMed] [Google Scholar]
- 16.Spectroscopic data for 3-O-(3′R-methylsuccinyl)-betulinic acid (3): white amorphous powder. Mp 268–271 °C. MS (ESI-) m/z: 569.38 (M- - H) for C35H54O6. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.54 (1H, dd, J = 9.9, 5.7 Hz, H-3), 3.01 (1H, m, H-19), 2.87-2.95 (1H, m, H-3′), 2.84, 2.42 (1H each, dd, J = 15.9, 8.3, 5.6, H-2′), 2.10-2.20 (1H, m, H-13), 1.69 (3H, s, H-30), 1.28, 1.26 (3H, d, J = 6 Hz, CH3-3′), 0.97 (6H, s, 2 × CH3), 0.86, 0.83, 0.80 (3H each, s, 3 × CH3). 3-O-(3′S-methylsuccinyl)betulinic acid (4): white amorphous powder. Mp 279–281 °C. MS (ESI-) m/z: 569.38 (M- - H) for C35H54O6. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.51 (1H, dd, J = 11.1, 4.8 Hz, H-3), 2.96-3.03 (1H, m, H-19), 2.86-2.93 (1H, m, H-3′), 2.70, 2.61 (1H each, dd, J = 16.2, 6.3, 4.8 Hz, H-2′), 2.06-2.16 (1H, m, H-13), 1.69 (3H, s, H-30), 1.27, 1.25 (3H, d, J = 6 Hz, CH3-3′), 0.97, 0.94, 0.86, 0.85, 0.81 (3H each, s, CH3-23, 24, 25, 26, 27). 3-O-(2′R-methylsuccinyl)-betulinic acid (5): white amorphous powder. Mp 250–252 °C. MS (ESI-) m/z: 569.38 (M- - H) for C35H54O6. 1H NMR (300 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.52 (1H, t, J = 7.8 Hz, H-3), 2.90-3.10 (2H, m, H-19, H-2′), 2.67, 2.42 (1H each, dd, J = 16.8, 9.6, 4.2, H-3′), 2.13-2.20 (1H, m, H-13), 1.68 (3H, s, H-30), 1.26, 1.23 (3H, d, J = 7.2 Hz, CH3-2′), 0.97 (6H, s, 2 × CH3), 0.86, 0.83, 0.81 (3H each, s, 3 × CH3). 3-O-(2′S-methylsuccinyl)-betulinic acid (6): white amorphous powder. Mp 256–258 °C. MS (ESI-) m/z: 569.38 (M- - H) for C35H54O6. 1H NMR (300 MHz, CDCl3): δ 4.74, 4.61 (1H each, s, H-29), 4.48 (1H, dd, J = 10.8, 4.8 Hz, H-3), 2.80-3.01 (2H, m, H19, H-2′), 2.76, 2.46 (1H each, dd, J = 16.9, 9.5, 4.7, H-3′), 2.10-2.18 (1H, m, H-13), 1.69 (3H, s, H-30), 1.25, 1.23 (3H, d, J = 6 Hz, CH3-2′), 0.97, 0.92 (3H each, s, 2 × CH3), 0.84-0.86 (9H, m, 3 × CH3).
- 17.Peak 4 (compound 5) was collected during cycle 3, and peak 1 (compound 4) during cycle 4. Peak 2 (compound 3) and peak 3 (compound 6) were collected together during cycle 4, and subjected again to recycle preparative HPLC, which yielded pure compounds at cycle 7.
- 18.Keyling-Bilger F, Schmitt G, Beck A, Luu B. Tetrahedron. 1996;47:14891. [Google Scholar]
- 19.Yu D, Sakurai Y, Chen CH, Chang FR, Huang L, Kashiwada Y, Lee KH. J Med Chem. 2006;49:5462. doi: 10.1021/jm0601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. J Immunol Methods. 1991;142:257. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]