Abstract
Nitrofuranyl isoxazolines with increased proteolytic stability over nitrofuranyl amides were designed and synthesized leading to discovery of several compounds with potent in vitro anti-tuberculosis activity. However, their in vivo activity was limited by high protein binding and poor distribution. Consequently, a series of non-nitrofuran containing isoxazolines was prepared to determine if the core had residual anti-tuberculosis activity. This led to the discovery of novel isoxazoline 12 as anti-tuberculosis agent with a MIC90 value of 1.56 μg/mL.
Keywords: Isoxazolines, Nitrofurans, Anti-tuberculosis agents
Mycobacterium tuberculosis is a very successful pathogen that infects one third of the world’s population.1 The emergence of multi-drug resistant tuberculosis and extensively drug resistant tuberculosis coupled with an increasing number of tuberculosis patients due to the overlap between tuberculosis and AIDS epidemics has created an urgent need to develop novel therapeutics to treat this deadly disease.1 In order to develop a better chemotherapeutic regime it is believed that drugs are most needed to treat the latent phase of this disease. Unfortunately, latent bacteria are intrinsically more difficult to treat.2 The nitroaromatic class of antibiotics is one of the few classes of antibiotics that have shown activity against latent M. tuberculosis, and nitroimidazoles PA-824 and OPC-67683 are in current clinical trials to treat tuberculosis.3 We chose to investigate a related class, the nitrofurans. Previously, we discovered and developed a series of nitrofuranyl amides with excellent in vitro activity against M. tuberculosis (Figure 1).4 However, this series of compounds did not perform well during in vivo studies due to a short biological half life and rapid elimination. The amide linkage (shown in green, Figure 1) was thought to be the major reason for the observed metabolic instability. Thus in this current study we evaluated the replacement of the amide linker with an isoxazoline linker (shown in pink, Figure 1). The isoxazoline ring system represents a stable bioisosteric replacement for the amide bond that is found among many biologically active molecules and drugs.5
Figure 1.
Discovery of novel isoxazoline compound in the course of developing nitrofuran anti-tuberculosis agents.
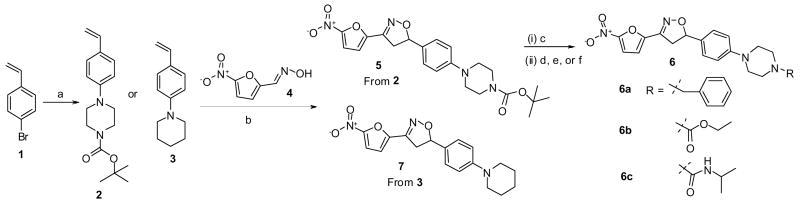
The synthesis of the nitrofuranyl isoxazoline compounds is shown in Scheme 1. First, the olefin 2 was prepared in good yield (88%) by a palladium-catalyzed aromatic amination reaction on p-bromo styrene 1 with N-Boc-piperazine.6 Second, to establish the isoxazoline bridge, the nitrile oxide was generated in situ from oxime 4 following Torsell’s procedure, which upon treatment with olefin 2, underwent a [3+2] regioselective cycloaddition7 to give isoxazoline 5 in 67% yield.8 Boc-deprotection of 5 was achieved by aqueous trifluoroacetic acid treatment to yield the free amine. The free amine was treated with benzyl bromide in the presence of K2CO3 to afford 6a (59%). Compounds 6b and 6c were obtained by reacting the free amine with ethyl chloroformate and isopropyl isocyanate (82% and 86%) respectively.9 7 was synthesized in a similar manner to compound 5 starting by reacting 1 and piperidine to form 3 (86% yield) and then reacting 3 with 4 to give isoxazoline 7 (63% yield).
Scheme 1. Synthesis of nitrofuranyl derivatives with an isoxazoline linker.
aReagents and conditions: a) N-Boc piperazine or piperidine, PdCl2[P(o-tol)3], NaOtBu, Toluene, 100 °C, 3 h; b) N-chlorosuccinimide, pyridine, dry Et3N, CHCl3, 60 °C - rt, 2 h; c) CF3COOH-H2O, THF, rt; d) BnBr, K2CO3, DMF, rt, 6 h; e) EtOCOCl, Et3N, THF, rt, 6 h; f) iPrNCO, Et3N, THF, rt, 6 h.
The anti-tuberculosis activity of compounds 5, 6a–c, and 7 were tested using microbroth dilution (Table 1).10 All compounds in this series demonstrated outstanding MIC activity and compounds 6a–c were advanced for in vivo testing in a short term mouse model of tuberculosis infection.11 Unfortunately, only modest reduction in the bacterial load was observed after a 9 day treatment regime (Table 1) (P < 0.05). This led us to more closely examine the biopharmaceutic and pharmacokinetic properties of the series. Solubility was determined at two different pH values using a miniaturized shake-flask method.12 Metabolic stability of the compounds was assessed in pooled rat liver microsomal preparations by monitoring disappearance of the compound. The percentage of intact parent compound was estimated using an LC-MS/MS assay. Plasma protein binding was determined by equilibrium dialysis using RED® devices (Pierce Biotechnology Inc, Rockford, IL). The results of these studies are also included in Table 1. Compound 6a was selected for further in vivo evaluation of pharmacokinetic properties in rats. 6a was found to have an oral bioavailability of about 35%, an acceptable elimination half-life about 2.6 hours but a relatively small volume of distribution of 2.0 L/Kg.13 The in vivo efficacy of anti-infective agents is usually dictated by their intrinsic antimicrobial activity and their free, unbound concentration in the target tissue, as only free, non-protein bound drug is pharmacologically active. Thus these results seem to suggest that the limiting factor for these highly protein bound compounds is tissue penetration.
Table 1.
Anti-tuberculosis activity and in vitro data of nitrofuran compounds with isoxazoline linkage (NT:Not Tested)
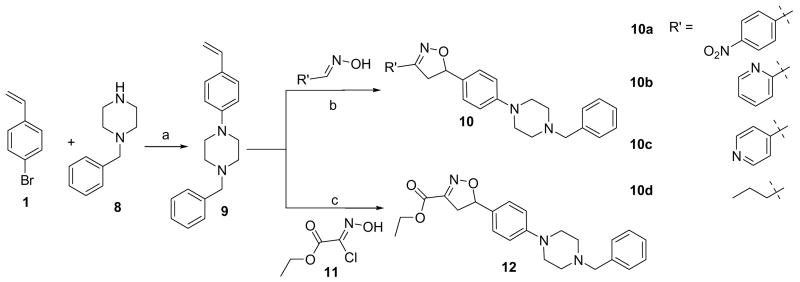
The outstanding anti-tuberculosis potency of 5–7 led us to question if the core isoxazoline scaffold itself had any intrinsic anti-tubercular activity. To test this hypothesis, a subsequent set of isoxazoline compounds was synthesized keeping the main core but altering the nitrofuran portion (Scheme 2) using a similar synthetic strategy. First, the p-bromostyrene was subject to a palladium catalyzed amination reaction with benzylpiperazine under the similar conditions described earlier to give the olefin intermediate 9 in 79% yield. Then 9 was reacted with different oximes in the presence of NaOCl and catalytic triethylamine to give corresponding 3+2 cycloaddition products 10a–d in 45–60% yields. The ester analog was created by reacting 9 with the commercially available building block 11 in the presence of base triethylamine to afford isoxazoline ethyl ester derivative 12 in 71% yield.14
Scheme 2. Synthesis of Isoxazoline compounds by altering the nitrofuran motif.
aReagents and conditions: a) PdCl2[P(o-tol)3], NaOtBu, Toluene, 100 °C, 3 h; b) Oxime, 5% NaOCl, cat. Et3N, CH2Cl2, rt, c) Et3N, CH2Cl2, rt.
The anti-tuberculosis activity of this series was determined and is shown in Table 2. Compound 12 was most active with a MIC90 of 1.56 μg/mL against M. tuberculosis. The remainder of the compounds 10a–d did not show any appreciable activity. Importantly, 12 represents a novel isoxazoline chemotype for which anti-tuberculosis properties have not been previously noted.
Table 2.
Anti-tuberculosis activity of isoxazolines 10a–d and 12
In conclusion, isoxazoline linked nitrofurans were synthesized. These compounds had better anti-tuberculosis activity in vitro and had improved serum half lives over corresponding compounds in the previous nitrofuranyl amide series, demonstrating that the strategy of replacing the amide bond with isoxazoline ring was sucessful.4 However the series still possessed limited in vivo efficacy. A detailed pharmacokinetic analysis of these agents showed them to be limited by low solubility, high serum protein binding and a low volume of distribution. When these results are combined it strongly suggests that in vivo efficacy is limited by poor tissue penetration and low concentrations of free drug at the site of infection. These factors are now being addressed in the design of the next generation of compounds in this series.
As the nitrofuranyl isoxazole series was so potent in vitro we explored if the core isoxazoline had any intrinsic anti-tuberculosis activity. This led to the discovery of a novel isoxazoline compound 12 with MIC90 value of 1.56 μg/mL. This is a new chemotype though less potent than the nitrofurans it does offer some significant potential advantages including increased solubility as the compounds are less crystalline and lower potential side effects as no nitro group is present. Further optimization of this series is ongoing and will be reported subsequently.
Acknowledgments
We thank National Institutes of Health grant AI062415 for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tuberculosis WHO Fact Sheet No 104. Geneva: Health Communications, WHO; 2006. http://www.who.int/mediacentre/factsheets/fs104/en/index.html. [Google Scholar]
- 2.Boshoff HIM, Barry CE., 3rd Nat Rev Microbiol. 2005;3:70. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 3.Barry CE, Boshoff HI, Dowd CS. Curr Pharm Des. 2004;10:3239. doi: 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- 4.Tangallapally RP, Yendapally R, Lee RE, Hevener K, Jones VC, Lenaerts AJM, McNeil MR, Wang Y, Franzblau S, Lee RE. J Med Chem. 2004;47:5276. doi: 10.1021/jm049972y.Tangallapally RP, Yendapally R, Lee RE, Lenaerts AJM, Lee RE. J Med Chem. 2005;48:8261. doi: 10.1021/jm050765n.Tangallapally RP, Lee REB, Lenaerts AJM, Lee RE. Bioorg Med Chem Lett. 2006;16:2584. doi: 10.1016/j.bmcl.2006.02.048. For a review, see: Tangallapally RP, Yendapally R, Daniels AJ, Lee REB, Lee RE. Curr Top Med Chem. 2007;7:509. doi: 10.2174/156802607780059772.
- 5.For recent reviews related to oxazolidinone and oxazolidine antibacterial agents, see: Renslo AR, Luehr GW, Gordeev MF. Bioorg Med Chem. 2006;14:4227. doi: 10.1016/j.bmc.2006.01.068.Sood R, Bhadauriya T, Rao M, Gautam R, Malhotra S, Barman TK, Upadhyay DJ, Rattan A. Infect Disord Drug Targets. 2006;6:343. doi: 10.2174/187152606779025860.Zappia G, Menendez P, Delle Monache G, Misiti D, Nevola L, Botta B. Mini Rev Med Chem. 2007;7:389. doi: 10.2174/138955707780363783.For a paper regarding glycoprotein IIb/IIIa receptor antagonists, see: Sielecki TM, Liu J, Mousa SA, Racanelli AL, Hausner EA, Wexler RR, Olson RE. Bioorg Med Chem Lett. 2001;11:2201. doi: 10.1016/s0960-894x(01)00406-1.For papers regarding factor Xa inhibitors, see: Quan ML, Liauw AY, Ellis CD, Pruitt JR, Carini DJ, Bostrom LL, Huang PP, Harrison K, Knabb RM, Thoolen MJ, Wong PC, Wexler RR. J Med Chem. 1999;42:2752. doi: 10.1021/jm980405i.Lam PYS, Adams JJ, Clark CG, Calhoun WJ, Luettgen JM, Knabb RM, Wexler RR. Bioorg Med Chem Lett. 2003;13:1795. doi: 10.1016/s0960-894x(03)00130-6.For a paper regarding human leukocyte elastase (HLE) inhibitors, see: Groutas WC, Venkataraman R, Chong LS, Yoder JE, Epp JB, Stanga MA, Kim EH. Bioorg Med Chem. 1995;3:125. doi: 10.1016/0968-0896(95)00006-3.For papers regarding antibacterials, see: Pirrung MC, Tumey LN, Raetz CRH, Jackman JE, Snehalatha K, McClerren AL, Fierke CA, Gantt SL, Rusche KM. J Med Chem. 2002;45:4359. doi: 10.1021/jm020183v.Barbachyn MR, Cleek GJ, Dolak LA, Garmon SA, Morris J, Seest EP, Thomas RC, Toops DS, Watt W, Wishka DG, Ford CW, Zurenko GE, Hamel JC, Schaadt RD, Stapert D, Yagi BH, Adams WJ, Friis JM, Slatter JG, Sams JP, Oien NL, Zaya MJ, Wienkers LC, Wynalda MA. J Med Chem. 2003;46:284. doi: 10.1021/jm020248u.
- 6.Guram AA, Rennels RA, Buchwald SL. Angew Chem Int Ed Engl. 1995;34:1348. [Google Scholar]
- 7.Padwa A, editor. 1,3-Dipolar Cycloaddition Chemistry. Wiley; New York: 1984. [Google Scholar]
- 8.Sammelson RE, Miller RB, Kurth MJ. J Org Chem. 2000;65:2225. doi: 10.1021/jo991551e. General procedure for 3+2 cycloaddition to form isoxazoline linkage: Olefin (1.0 eq), aldoxime (1.0–1.5 eq) and Et3N (0.2 eq, catalytic) were dissolved in DCM, and the solution was cooled to 0 °C. Bleach containing 5% NaOCl by weight (3–6 eq) was added dropwise to the vigorously stirring solution. The biphasic mixture was allowed to warm to room temperature and stirred for 8 h overnight. An additional volume of water (equal to the volume of bleach) was added and the layers were separated. The aqueous layer was extracted two to three additional times with DCM, and the combined organic layers were dried with sodium sulfate, rota-evaporated and flash column purified to give the product as enantiomeric mixture. In case of poor solubility of oxime such as nitrofuranyl oxime a 1:1 mixture of DCM and THF was used as reaction solvent. In this case, for workup, after the reaction the reaction mixture was concentrated on rota-evaporator and the crude product obtained was subjected to water work up. Caution: Nifuraxime is light sensitive hence the proper precautions have to be taken during weighing, transferring etc. [DOI] [PubMed] [Google Scholar]
- 9.Representative analytical data of compound 6a. 1H NMR (500 MHz, CDCl3): δ 2.5–2.62 (4H, broad s), 3.1–3.23 (4H, m), 3.32 (1H, dd, J = 9.0, 17.3 Hz), 3.53 (2H, s), 3.66 (1H, dd, J = 11.2, 17.3 Hz), 5.66 (1H, dd, J = 9.0, 11.2 Hz), 6.83 (2H, d, J = 8.7 Hz), 6.95 (1H, d, J = 3.9 Hz), 7.14–7.24 (3H, m), 7.25–7.33 (5H, m); 13C-NMR (300 MHz, CDCl3): ppm 29.14, 40.59, 48.05, 52.27, 62.35, 83.56, 111.82, 112.47, 115.39, 126.59, 127.82, 128.79, 128.97, 146.84, 147.27, 151.08; ESI MS: 455.2 (M+23). HPLC purity: 100%, tR = 5.23 min.
- 10.Lee RE, Protopopova M, Crooks E, Slayden RA, Terrot M, Barry CE., III J Comb Chem. 2003;5:172. doi: 10.1021/cc020071p. [DOI] [PubMed] [Google Scholar]
- 11.Lenaerts AJM, Gruppo V, Brooks JV, Orme IM. Antimicrob Agents Chemother. 2003;47:783. doi: 10.1128/AAC.47.2.783-785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glomme A, März J, Dressman JB. J Pharm Sci. 2005;94:1. doi: 10.1002/jps.20212. [DOI] [PubMed] [Google Scholar]
-
13.Pharmacokinetic studies of compound 6a
Route t1/2 (hr) AUC∞ (μg hr/L) Volume of distribution (L/kg) Clearance (L/hr/kg) Bio-availability IV (10mg/kg) 2.6 19091 2.0 0.53 N/A Oral (100 mg/kg) 4.2 65931 9.3 1.58 34.5% - 14.Synthetic procedure for compound 12. To a stirred mixture of olefin 9 (0.2 g, 0.719 mmol) and Et3N (0.2 mL, 1.438 mmol) in anhydrous CH2Cl2 (10 mL) ethyl chloroximido acetate (0.163 g, 1.079 mmol) was added in portions at 0 °C. The reaction mixture was stirred at RT for 8 h and washed with water (2 × 10 mL), dried (anhyd. Na2SO4) and concentrated under reduced pressure. The crude product was purified by flash chromatography to give 12 (0.2 g, 71%) as a yellow solid. 1H NMR (500 MHz, CDCl3): δ 1.37 (3H, t, J = 7.0 Hz), 2.6 (4H, t, J = 4.8 Hz), 3.17–3.25 (5H, m), 3.5–3.51 (3H, m), 4.36 (2H, q, J = 7.3 Hz), 5.7 (1H, dd, J = 9.2, 11.4 Hz), 6.89 (2H, d, J = 8.7 Hz), 7.21 (2H, d, J = 8.7 Hz), 7.25–7.29 (2H, m), 7.31–7.37 (3H, m); 13C NMR (500 MHz, CDCl3): δ 14.24, 40.84, 48.73, 52.98, 62.07, 63.06, 85.33, 115.79, 127.22, 127.31, 128.35, 129.23, 129.56, 138.01, 151.28, 151.69, 160.78; MS: 394.4 (M+1); HPLC purity: 100%, tR = 5.13 min.