Abstract
Proliferation, differentiation, and other processes must be coordinated during the development of multi-cellular animals. A discrete and regulated cell division, the Second Mitotic Wave (SMW), occurs concomitantly with early cell fate decisions in the Drosophila developing retina. Signals from the Epidermal Growth Factor Receptor (EGFR) are required to promote cell cycle arrest of specified cells and antagonize S-phase entry in the SMW. Cells that do not receive any EGFR activity enter S-phase in the SMW in response to the Notch pathway. To identify genes with potential roles in the SMW, we used microarrays and genetic manipulation of the EGFR pathway to seek transcripts regulated during the SMW. RNA in situ hybridization of 126 differentially transcribed genes revealed genes that have novel expression patterns in cells closely associated with the SMW. In addition, other genes’ transcripts were regulated in the differentiating photoreceptor cells, retinal basal glia, the peripodial epithelium, and blood cells (plasmatocytes) associated with the developing retina. These novel targets suggest that during eye development, EGFR activity coordinates transcriptional programs in other tissues with retinal differentiation.
Introduction
The development of multicellular animals requires the co-ordination of multiple signals that control proliferation and differentiation. Often, the extracellular signaling pathways required for differentiation also regulate the cell cycle. How these signals are choreographed together to produce properly sized and patterned tissue is just beginning to be understood (Baker, 2007).
In the Drosophila eye, the cell-cell signals that control differentiation have been well characterized (Zipursky and Rubin, 1994; Freeman, 1997; Greenwood and Struhl, 1999; Curtiss and Mlodzik, 2000; Simon, 2000) and several of these signals are also required to regulate proliferation in the developing retina (Penton et al., 1997; Horsfield et al., 1998; Baonza and Freeman, 2005; Firth and Baker, 2005). The retina and other limbs each develop from epithelial sacs or imaginal discs (Auerbach, 1936; Cohen, 1993). Each imaginal disc is made of 2 opposing epithelial layers. The layer that forms the adult eye or limb is composed of columnar cells, the disc proper (DP) and overlaying the DP is a layer of squamous epithelial cells, the peripodial epithelium (PE) (Cohen, 1993) (Figure 6). Although the PE does not form part of the adult eye, signals from the PE to the DP are important for retinal development (Cho et al., 2000; Gibson and Schubiger, 2000).
Figure 6.
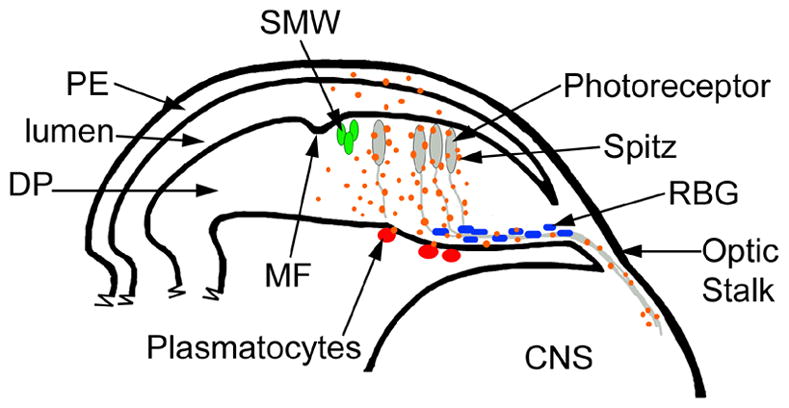
EGFR signaling in the DP regulates the expression of genes in cells within and outside of the retinal epithelium. A cartoon depicting a cross section through the eye imaginal disc, optic stalk and the CNS; posterior is to the right. The PE overlays the apical surface of the DP. At the posterior edge of the MF the SMW occurs (green cells). Posterior to the MF, photoreceptors are differentiating (grey) and their axons extend to the lamina in the CNS via the optic stalk. Retinal basal glia (RBG; blue) are associated with the axons. Plasmatocytes (red) are found on the basal surface outside of the eye imaginal disc. Vertical EGFR signals from the DP to the PE alter gene expression in the PE; this maybe due to an increase of EGFR signaling in the DP or due to the non-autonomous signal Spi (orange). EGFR signaling from the DP regulates gene expression in plasmatocytes. Within the retinal epithelium, Spi signals from axons alter gene expression in the RBG and affect their localization and migration. EGFR also regulates the composition of the basal lamina (not shown).
Differentiation starts at the posterior edge of the presumptive retinal epithelium and progresses anteriorly (Wolff and Ready, 1993). The front of this wave of differentiation is marked by the apical constriction of cells called the morphogenetic furrow (MF). The MF separates the undifferentiated and differentiating portions of the eye. Anterior to the MF, cells are randomly proliferating. Just ahead of the furrow all cells undergo a prolonged G1 arrest during which time signals to specify early cell fate decisions are received and neurogenesis begins (Thomas et al., 1994). Some cells then re-enter the cell cycle and undergo a single round of proliferation, the Second Mitotic Wave (SMW) (Wolff and Ready, 1991). Not all cells enter the SMW however. Groups of cells remain arrested in G1, permanently withdraw from the cell cycle and differentiate (Wolff and Ready, 1993).
During eye development signals from the Epidermal Growth Factor Receptor (EGFR) are required at numerous stages. First, EGFR is activated by the ligand Spitz (Spi) in four cells surrounding the founding photoreceptor cell R8, resulting in the G1 arrest and recruitment of these four cells (R2, R5, R3 and R4) into the photoreceptor precluster (Freeman, 1994; Dominguez et al., 1998; Kumar et al., 1998; Baker and Yu, 2001). The surrounding cells, without any EGFR activity, reenter the cell cycle and perform DNA synthesis. After S-phase, progression from G2 phase into mitosis requires EGFR activity. EGFR is activated in G2 cells that are in contact with precluster cells by Spi (Baker and Yu, 2001). Later expression of Spi recruits these post-mitotic cells into the remaining retinal cell fates (Freeman, 1996). Interestingly, Spi protein travels down the photoreceptor axons into the brain lamina, where it triggers the differentiation of the synaptic cartridge units (Huang et al., 1998). Retinal basal glia cells are closely associated with the developing photoreceptor axons and are important for their guidance down the optic stalk to the brain lamina (Choi and Benzer, 1994; Rangarajan et al., 1999). Also, larval hemocytes/plasmatocytes are found on the outer surface of the eye disc. Both of these cell types are of a different linage to the imaginal disc.
Only some gene targets of EGFR signaling have been identified. The canonical Ras/MAPK pathway downstream of EGFR transduces the EGFR signal to the ETS transcription factor, Pointed (Pnt) (Brunner et al., 1994; O Neill et al., 1994; Yang and Baker, 2003). Phyllopod also appears to be a target during photoreceptor differentiation (Chang et al., 1995; Dickson et al., 1995; Wassarman et al., 1995). EGFR signaling targets the Cdc25 homolog, String (Stg), for progression from G2 to mitosis in the SMW (Baonza et al., 2002; Baker and Yu, 2001). The signal to enter S-phase in the SMW is dependant on the Notch (N) pathway; cells defective for N signaling are unable to progress from G1 phase into S-phase (Baonza and Freeman, 2005; Firth and Baker, 2005). In the absence of both EGFR and N, cells remain in G1 despite lacking EGFR activity. Both N and EGFR signaling are thought to regulate S-phase entry through transcriptional targets, but these targets remain unknown (Firth and Baker, 2005).
To understand the events surrounding the SMW we have taken a genome wide approach to isolate genes expressed within and around the SMW. Other groups have analyzed the gene expression profile during wild type eye development using microarrays or SAGE analysis of FACs sorted cells (Jasper et al., 2002; Klebes et al., 2002; Michaut et al., 2003) and showed that many hundreds of genes are transcriptionaly upregulated during eye development. It is likely that many are targets of EGFR or N regulation.
We designed a microarray gene expression screen to identify genes regulated specifically during the SMW, rather than all the targets of EGFR or N signaling during eye development. The SMW was eliminated genetically through the manipulation of the EGFR pathway and the gene expression profile was compared to similar retinas with a SMW. This approach gave a number of candidate genes. We then determined the mRNA expression patterns of differentially transcribed genes by RNA in situ hybridization. We have identified genes whose expression is regulated by EGFR signaling that are transcribed in cells participating in the SMW or the cell cycle arrested differentiating cells as expected, but also many genes transcribed in the PE, glia and the plasmatocytes. We think that our specific strategy also selected for genes that EGFR regulates in other tissues in response to the eye disc.
Materials and Methods
RNA isolation, probe preparation for array and data analysis
Total RNA was extracted from 100 eye/antennal discs using Trizol and analyzed for quality on the Agilent Bioanalyzer (www.chem.agilent.com). For both GMR>RasV12 and GMR>sSpi, 3 independent samples of total RNA were prepared for the GeneChip® arrays according to the manufacturer’s specifications and hybridized to DrosGenome1 expression arrays (www.affymetrix.com) (Platform accession no. GPL72). The raw data reported in this paper has been submitted to the NCBI Gene Expression Omnibus, www.ncbi.nlm.nih.gov/geo (accession series no. GSE6300). Data analysis was performed using Microarry Suite 5.0 (MAS 5.0), the Data Mining Tool software (Affymetrix) and Microsoft Excel. Single array analyses for each array were performed in MAS 5.0. All nine comparison replicates were performed in the Data Mining Tool with GMR>sSpi as the baseline and GMR>RasV12 as the experiment. A Signal Log Ratio (SLR) greater than 1 is the same as a Fold Change of 2 (Wodicka et al., 1997). Gene expression changes that satisfied both the T-Test and the non-parametric Mann-Whitney Test (p <0.05) were used to evaluate gene expression changes between the GMR>RasV12 and GMR>sSpi (Affymetrix, 2003). A cut off was applied and genes that consistently had a Signal Log Ratio of 1 or greater were deemed significantly upregulated in GMR>RasV12 (or downregulated in GMR>sSpi) and those with a Signal log ratio less than −1 were deemed significantly downregulated in GMR>RasV12 (or upregulated in GMR>sSpi).
RNA in situ probe design, preparation and hybridization
RNA probes were designed against the contiguous cDNA sequence of differentially expressed genes. A PCR strategy for rapid generation of template DNA for synthesis of labeled RNA probes was used. Probes were designed to be between 400–800 base pairs long and correspond to unique sequences of the cDNA as determined by Blast (http://flybase.net/blast). Primers were designed using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3). The following linkers were added to the 5′ end of each primer: Forward Primer: 5′ GGCCGCGG 3′; Reverse Primer: 5′ CCCGGGGC 3′. Primers for each sequence amplified can be found in the supplementary table 1. PCR was checked by gel electrophoresis. If a single product was obtained, 5μl of the PCR reaction was treated with ExoSAP-IT (USB Corp. #78202). 1–2μl of this was used for a second PCR reaction with universal primers containing sequences that hybridize to the linker sequence and promoter sequences for the T7 and SP6 polymerases. Universal forward primer sequence (adds T7 promoter and EcoR1 restriction site for subcloning): 5′GAGAATTCTAATACGACTCACTATAGGGCCGCGG 3′. Universal reverse primer sequence (adds SP6 promoter and a BamH1 restriction site for subcloning): 5′AGGGATCCATTTAGGTGACACTATAGAACCCGGGGC 3′. A standard PCR program with an annealing temperature of 45°C was used. The second PCR products, containing the SP6 and T7 promoter sequences, were cleaned up by gel extraction. Sense (T7) and anti-sense (SP6) RNA DIG probes were made directly from this PCR product (Roche DIG RNA labeling Kit #1 277 073). In cases where the first PCR reaction gave multiple bands, the correct band was gel extracted before the second PCR reaction. DIG probe was precipitated with LiCl and re-suspended in 100μl of 50% Formamide in HSW solution (see below). RNA in situ hybridization was performed as described (Cornell et al., 1999).
Drosophila strains used
GMR Gal4 (Hay et al., 1994; Freeman, 1996); UAS sSpi (Schweitzer et al., 1995); UAS RasV12 (Karim and Rubin, 1998).
Immunofluorescence
Labeling of eye discs was performed as described (Firth et al., 2006). Preparations were examined on the BioRad Radiance 2000 Confocal microscope. Images were processed using Adobe Photoshop 4.0 and NIH Image J software. Rabbit anti-phosphoHistone3 was from Cell Signaling Technology (#9701). Rabbit anti-Caspase 3 (CM1) (Srinivasan et al., 1998). Anti-MDP-1 (Hortsch et al., 1998). Anti-arm (N2 7A1), anti-Dlg (4F3), anti-Repo and anti-ElaV were from the Developmental Studies Hybridoma Bank, maintained by the University of Iowa, Department of Biological Sciences, Iowa City IA52242, USA under contract N01-HD-7-3263 from the NICHD.
Results
Screen design and strategy for identifying genes expressed in the SMW during Drosophila eye development
Using the Drosophila developing eye a microarray gene expression screen was designed to identify genes involved in the SMW. Since the cells in the SMW could not reliably be dissected out from the surrounding tissue, an approach where the SMW was ablated genetically was taken. The SMW was blocked in the developing eye by expressing the EGFR activating ligand, Spitz (Spi). Spi was over expressed in all cells in the differentiating part of the retina posterior to the MF using the UAS-Gal4 system; GMR>sSpi (GMRGal4/+; UAS sSpi/+). GMR Gal4 is active in all cells posterior to the MF (Hay et al., 1994; Freeman, 1996). Expression of sSpi promotes G1 arrest and as a result there is no SMW (Yang and Baker, 2003). Due to increased activity of the EGFR/Ras pathway ectopic differentiation also occurs (Fig. 1A) (Freeman, 1996). The program of eye development and photoreceptor differentiation leads to the induction of many genes (Jasper et al., 2002; Klebes et al., 2002; Michaut et al., 2003). To circumvent the expected enrichment of all EGFR targets throughout the retinal eye field in GMR>sSpi, the gene expression profile of GMR>sSpi eye/antennal discs was compared to that of GMR>RasV12 (GMRGal4/UAS RasV12), rather than to wild type discs. Hyper-activation of the EGFR pathway with oncogenic RasV12 results in a similar ectopic differentiation phenotype; the effects of GMR>RasV12 are autonomous, however and the EGFR/Ras/MAPK pathway is activated after the SMW begins, so that GMR>RasV12 does not prevent the SMW (Fig. 1B) (Baker and Yu, 2001). Due to the absence of cells participating in the SMW, we hypothesized that transcripts of genes expressed in the SMW would be downregulated in GMR>sSpi when compared to GMR>RasV12. There also might be other differences between GMR>sSpi and GMR>RasV12, such as differences in genes specific to the early columns of retinal specification that occur during the SMW. For example, GMR>sSpi contains very few R8 photoreceptors, whereas normal numbers of R8 cells are specified in GMR>RasV12 (Lesokhin et al., 1999; Baker and Yu, 2001).
Figure 1.
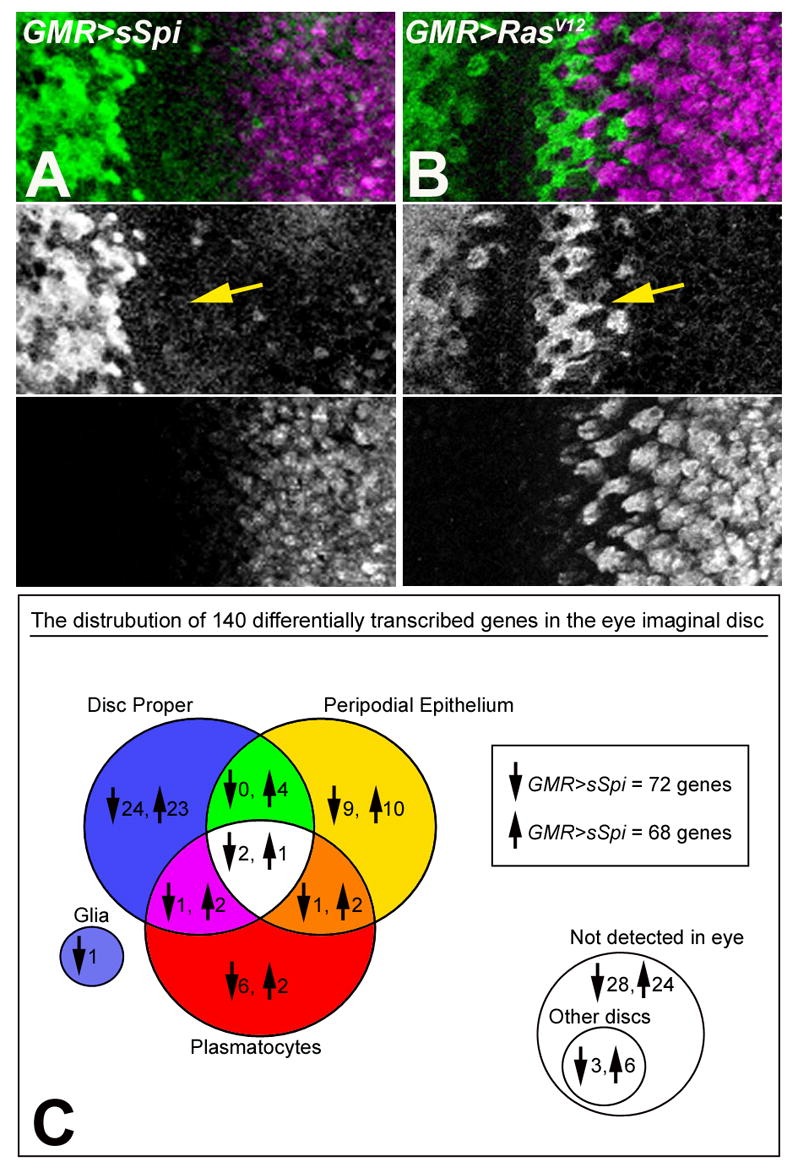
Eye imaginal discs from GMR>sSpi (A) and GMR>RasV12 (B) labeled with Cyclin B (green) to monitor the cell cycle and Elav (magenta) to label differentiating cells. The SMW occurs in GMR>RasV12 expressing eye discs (arrow in B) but is absent in GMR>sSpi (arrow in A). (C) Venn diagram showing the distribution of genes that were up and downregulated in GMR>sSpi compared to GMR>RasV12 and their location of expression in the DP, PE, plasmatocytes and glia of the eye imaginal disc. 54 genes were not expressed in the eye imaginal disc; 9 of these were expressed in other imaginal discs, including 2 expressed in the antennal disc. 7 genes remain untested due to technical difficulties.
The mRNA expression profiles of GMR>sSpi and GMR>RasV12 whole eye/antennal imaginal discs were determined using oligonucleotide arrays representing approximately 13,500 known and predicted genes in the Drosophila genome (GeneChip® expression array DrosGenome1, Affymetrix). For both genotypes, 3 independent RNA samples were prepared and hybridized to the arrays. Using Affymetrix Data Mining Tool Software whole genome gene expression changes between GMR>sSpi and GMR>RasV12 were evaluated (Affymetrix, 2003). 140 genes were differentially transcribed between GMR>sSpi and GMR>RasV12. 72 of these genes were downregulated and 68 were upregulated in GMR>sSpi compared to GMR>RasV12 (Figure 1C).
Characterized genes associated with eye development
Known transcripts downregulated in GMR>sSpi compared to GMR>RasV12
72 transcripts were downregulated in GMR>sSpi compared to GMR>RasV12. 14 of these genes have been previously characterized during eye development. Because the SMW is absent in GMR>sSpi, we expected genes expressed in the SMW cells to be included in this group. As expected 4 of the 14 genes characterized previously are known to be expressed more highly in the cycling cells of the SMW and the in the unspecified cells i.e. in cells for 2/3 columns at the posterior edge of the MF: lozenge (lz), BarH1 (B-H1), Traf1 and Spn43A (Higashijima et al., 1992; Daga et al., 1996; Flores et al., 1998; Lai et al., 2000; Preiss et al., 2001). Three of the downregulated genes are required for R8 photoreceptor development: atonal (ato), bearded (brd) and big brain (bib) (Jarman et al., 1994; Singson et al., 1994; Li and Baker, 2001). R8 specific transcripts were expected to be downregulated because there are fewer R8s in GMR>sSpi (Lesokhin et al., 1999). Another 4 genes downregulated in GMR>sSpi are reported to be present in other differentiating photoreceptors: SoxNeuro (SoxN/SoxB1), Fasciclin 2 (Fas2), klingon (klg) and tartan (trn) (Chang et al., 1993; Butler et al., 1997; Pignoni et al., 1997; Cremazy et al., 2001). Finally, transcripts of 4 downregulated genes are expressed in a pattern associated with the MF: E(Spl)m2, brd, arc (a) and spineless (ss) (Singson et al., 1994; Duncan et al., 1998; Lai et al., 2000; Liu and Lengyel, 2000).
As anticipated, the array analysis identified genes already known to be expressed in cells within the SMW or required for the development R8 photoreceptor cells. The remaining 58 genes, whose transcription was uncharacterized or genes uncharacterized with respect to eye development are described later in the results. They include both, SMW and R8-associated transcripts, as well as genes expressed in other tissues.
Known transcripts upregulated in GMR>sSpi compared to GMR>RasV12
68 transcripts were upregulated in GMR>sSpi compared to GMR>RasV12. Since GMR>sSpi promotes G1 arrest and interferes with the differentiation of R8 photoreceptors, we expect this group to contain genes required for photoreceptor neurons other than R8, possibly required for G1 arrest, or negative regulators of the SMW. Such genes should be expressed in groups of cells corresponding to the precluster cells just posterior to the MF. Six genes known to be necessary for eye development were upregulated in GMR>sSpi; 5 of these are expressed in differentiating cells in the eye and required for neuronal development: kekkon (kek), seven-up (svp), βTub60D and neuroglian (nrg) and pointed (pnt) (Mlodzik et al., 1990; Bellen et al., 1992; Desai et al., 1994; O Neill et al., 1994; Musacchio and Perrimon, 1996; Hoyle et al., 2000). Transcripts for one negative cell cycle regulator, scribble (scrib) (Bilder and Perrimon, 2000), were up regulated in GMR>sSpi.
As expected, the microarray analysis identified transcripts known to be expressed in differentiating photoreceptor cells. The remaining 62 transcripts enriched in GMR>sSpi were uncharacterized with respect to eye development and are described later in the results. They include not only genes transcribed in photoreceptors, but also genes expressed in other tissues (Figure 1C).
Identification of genes’ expression patterns during Drosophila eye development
Using RNA in situ hybridization we examined the wild type expression patterns of 126 genes whose transcripts were determined to be significantly different between GMR>sSpi and GMR>RasV12 (Fig. 1C). We found 27 genes downregulated and 30 genes upregulated in GMR>sSpi that were expressed in the cells of the DP (Figs. 2A–2E, 2K–2T). A single gene, CG31676, downregulated in GMR>sSpi, was expressed in the retinal basal glia (Fig. 2F). Surprisingly, 12 of the genes downregulated and 17 genes upregulated in GMR>sSpi were expressed in the PE of the eye imaginal disc (Figs. 2G and 2H). In addition, transcripts of 10 downregulated and 6 upregulated genes in GMR>sSpi were expressed in the larval plasmatocytes present on the basal outer surface of the eye imaginal disc (Figs. 2I and 2J) (Wolff and Ready, 1993). Some of the genes are expressed in overlapping locations. For example, 3 genes, Pde8, CG8502 and Neu3 were expressed in the DP, PE and plasmatocytes. The Venn diagram in fig. 1C summarizes the expression pattern data and tables 1 and 2 list the novel and known expression patterns of the differentially transcribed genes that are downregulated and upregulated in GMR>sSpi compared to GMR>RasV12 respectively.
Figure 2.
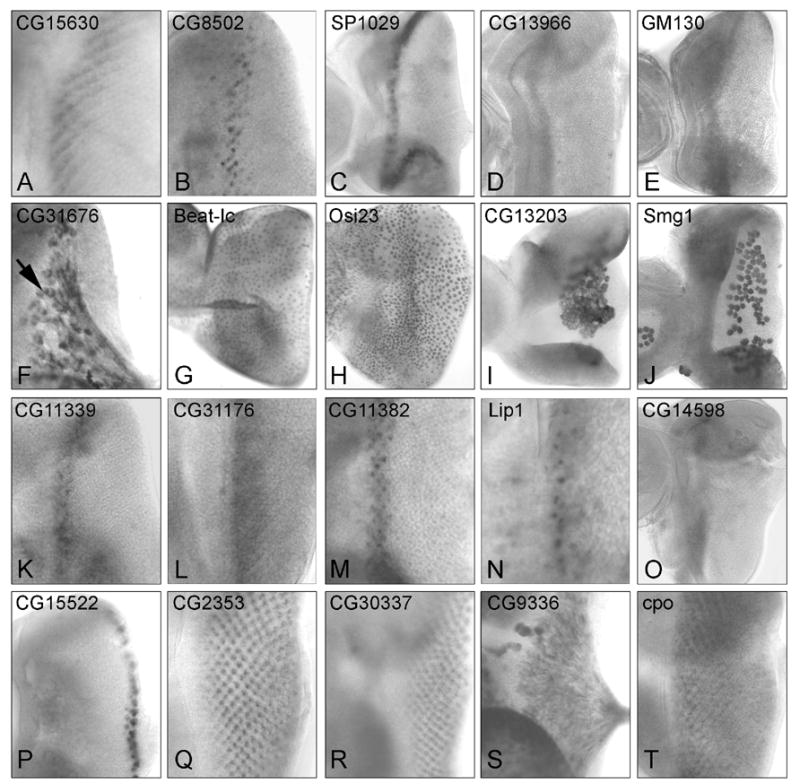
RNA in situ hybridization of differentially expressed genes in 3rd instar eye imaginal discs: (A–J) Transcripts enriched in GMR>RasV12. (K–T) Transcripts enriched in GMR>sSpi. (A–E) Transcripts expressed in a band associated with the MF or SMW: (A) CG15630 and (B) CG8502 are expressed near the SMW; (C) SP1029,(D) CG13966 and (E) GM130 are expressed anterior to the MF; (F) CG31676 is expressed in glia; (G) Beat-Ic and (H) Osi23 are expressed in the peripodial epithelium; (I) CG13203 and (J) Smg are expressed in larval plasmatocytes on the basal surface of the eye imaginal disc. Transcripts for (K) CG11339, (L) CG31176, (M) CG11382 and (N) Lip are expressed in coincident with early fate specifications; (O) CG14598 is expressed ahead of the MF; (P) CG15522 transcripts are present in peripheral ommatidia; (Q) CG30337, (R) SP2353, (S) CG9336 and (T) Cpo are expressed in differentiating cells. The mRNA for CG9336 is present in the axons of photoreceptors.
Table 1.
RNA in situ hybridization patterns of genes downregulated in GMR>sSpi vs. GMR>RasV12
| Gene | SLR | Expression pattern | Function/homology | Reference |
|---|---|---|---|---|
| Disc Proper (27) | ||||
| SoxN | −5.65 | Differentiating photoreceptors posterior to MF | Nervous system development | (Cremazy et al., 2001) |
| lz | −4.06 | Subset of cells posterior to the morphogenetic MF | Eye cell fate specification and differentiation | (Flores et al., 1998) |
| Spn43A | −3.67 | Cells anterior to the MF and in posterior inter-ommatidial cells | Serine-type endopeptidase inhibitor activity | (Green et al., 2000) |
| CG15630 | −3.49 | Subgroups of cells in 3/4 columns posterior to MF (Figure 2A) | Cell adhesion | |
| SP1029 | −3.45 | Band anterior to the MF; inner tarsal ring of leg imaginal disc (Figure 2B) | Proteolysis | |
| CG31676 | −3.22 | Retinal basal glial cells (Figure 2F) | - | |
| Fas2 | −2.91 | Differentiating photoreceptors | Cell adhesion; axonal fasciculation; synaptic plasticity | (Pignoni et al., 1997) |
| B-H1 | −2.87 | Subset of photoreceptors and accessory cells | Photoreceptors R1 and R6, pigment and lens cell differentiation | (Higashijima et al., 1992) |
| thisbe | −2.81 | Individual differentiating cells posterior to MF | FGFR ligand. FGFR signaling in mesoderm, hindgut and heart | |
| CG31291 | −2.71 | Ubiquitous | - | |
| Pde8 | −2.37 | Band just anterior to MF; PE; plasmatocytes; lumen and nuclei of salivary glands. Nuclei of fat body | Signal transduction; cyclic nucleotide metabolism; mesoderm dev. | |
| Klg | −2.14 | Three rows of cells ahead of MF; ommatidial cluster; refines to R7 | R7 photoreceptor cell fate commitment and differentiation | (Butler et al., 1997) |
| CG13966 | −2.03 | Band anterior to MF (Figure 2C) | - | |
| ato | −1.95 | Stripe anterior to MF, refines to R8 cells only | R8 cell specification. | (Jarman et al., 1994) |
| GM130 | −1.92 | Anterior to MF (Figure 2D) | Golgi organization and biogenesis; mitosis; protein targeting | |
| trn | −1.91 | Band of cells just anterior to MF refining to clusters of cells and a subset of differentiating cells. | Target of Egfr/PntP1 signaling in embryonic ventral ectoderm | (Chang et al., 1993) |
| Obp44a | −1.84 | Photoreceptor axons as exiting optic stalk and optic lobe | - | |
| CG8502 | −1.71 | Individual cells near posterior edge of MF for 2/3 columns; eye, leg and wing PE; plasmatocytes (Figure 2E) | Structural constituent of larval cuticle | |
| CG5653 | −1.71 | Ubiquitous | - | |
| CG15911 | −1.69 | Anterior D/V margins of eye disc;plasmatocytes | - | |
| E(spl) m2 | −1.68 | Clusters of cells in the MF region | Notch signaling pathway | (Lai et al., 2000) |
| CAP | −1.64 | Expressed within MF | MAPKKK cascade; cytoskeleton organization and biogenesis | |
| CG5929 | −1.61 | Broad band anterior to MF | Nucleic acid metabolism; RNA-dependent transcription | |
| Ppn | −1.58 | Diffusely expressed in eye imaginal disc | Component of the ECM; metallopeptidase activity | (Kramerova et al., 2000) |
| Traf1 | −1.56 | SMW cells and clusters within MF; ZNP cells in the wing | In vivo regulator of JNK pathway | (Priess and Hirsch, 1986) |
| Brd | −1.5 | Expressed within MF | Sensory organ precursor cell fate determination; Notch pathway | (Singson et al., 1994) |
| Bib | −1.5 | Required during R8 specification | Lateral inhibition of R8 specification; embryonic neurogenesis | (Rao et al., 1990) |
| arc | −1.4 | Clusters along MF | Adheren junction associated protein required for eye development | (Liu and Lengyel, 2000) |
| ss | −1.34 | MF; expressed in all other imaginal discs | Antennal morphogenesis; transcription factor | (Duncan et al., 1998) |
| Peripodial Epithelium (12) | ||||
| Cyp4e2 | −3.19 | PE of the eye/antennal and wing imaginal discs | Electron transport; steroid metabolism | |
| Osiris 23 | −3.16 | PE of the eye and leg imaginal discs (Figure 2H) | - | |
| CG11073 | −2.59 | PE of the eye, leg and wing imaginal discs | - | |
| CG3893 | −2.56 | PE of the eye imaginal disc | - | |
| Pde8 | −2.37 | Band just anterior to MF; PE; plasmatocytes; lumen and nuclei of salivary glands; nuclei of fat body | Signal transduction; cyclic nucleotide metabolism; mesoderm development. | |
| beat-Ic | −2.33 | Peripodial cell’s nucleoli (Figure 2G) | Axon choice point recognition; cell adhesion; defasciculation | |
| wbl | −1.71 | PE of the eye imaginal disc | Exocytosis; protein-Golgi targeting | |
| CG8502 | −1.71 | Individual cells posterior to MF for 2/3 columns; eye, leg and wing PE; plasmatocytes | Structural constituent of larval cuticle | |
| CG13203 | −1.7 | PE of the eye imaginal disc; plasmatocytes | - | |
| CG4408 | −1.7 | PE of the eye imaginal disc | Proteolysis: metallocarboxypeptidase activity | |
| CG2657 | −1.5 | PE of the eye and wing discs; cells in wing disc | Transport: Glutamate-gated ion channel activity | |
| CG15370 | −1.34 | PE of the eye imaginal disc | - | |
| Glia (1) | ||||
| CG31676 | −3.22 | Retinal basal glial cells (Figure 2F) | - | |
| Plasmatocytes (10) | ||||
| Pde8 | −2.37 | Band just anterior to MF; peripodial cells; plasmatocytes; lumen and nuclei of salivary glands; nuclei of fat body | Signal transduction; cyclic nucleotide metabolism; mesoderm dev. | |
| twe | −1.85 | Plasmatocytes | Protein tyrosine phosphatase activity; mitosis | |
| CG12508 | −1.73 | Plasmatocytes | - | |
| CG8502 | −1.71 | Individual cells posterior to MF for 2/3 columns; eye, leg and wing peripodial cells; plasmatocytes | Structural constituent of larval cuticle | |
| CG13203 | −1.7 | PE of the eye imaginal disc; plasmatocytes (Figure 2I) | - | |
| CG15911 | −1.69 | Anterior D/V margins of eye disc;plasmatocytes | - | |
| PGRP-SC2 | −1.55 | Wing pleurites and plasmatocytes | Peptidoglycan recognition molecule | |
| Smg1 | −1.44 | Plasmatocytes (Figure 2J) | Nonsense-mediated decay; protein kinase activity | |
| robl62A | −1.44 | Plasmatocytes | Microtubule-based movement; Dynein complex; ATPase activity | |
| CG18547 | −1.39 | Plasmatocytes | Oxidoreductase activity | |
| Other imaginal discs or tissues (5) | ||||
| Ugt86Di | −3.58 | Expressed in the wing disc | - | (Butler et al., 2003) |
| Obp44a | −1.84 | Photoreceptor axons as exiting optic stalk and optic lobe | - | |
| CG8483 | −1.63 | Expressed in the wing disc | - | (Butler et al., 2003) |
| PGRP-SC2 | −1.55 | Wing plurites and plasmatocytes | - | |
| ss | −1.34 | MF; Expressed in all other imaginal discs | Antennal morphogenesis; transcription factor | (Duncan et al., 1998) |
Table 2.
RNA in situ hybridization patterns of genes upregulated in GMR>sSpi vs. GMR>RasV12
| Gene | SLR | Expression pattern | Function/homology | Reference |
|---|---|---|---|---|
| Disc Proper (30) | ||||
| CG14275 | 4.77 | Axon tracts of differentiating neurons. | - | |
| CG15522 | 4 | Peripheral ommatidia (Figure 2K) | - | |
| CG11339 | 2.87 | Narrow band of cells posterior to MF; inner ring of antennal disc (Figure 2P) | Cytoskeleton organization and biogenesis | |
| CG31176 | 2.36 | Band of cells at posterior edge of MF (Figure 2Q) | - | |
| T48 | 2.35 | Ubiquitous | - | |
| CG4096 | 2.2 | Expressed at the dorsal anterior margin in the eye disc and in wing and leg discs also | - | |
| CG14598 | 2.2 | Anterior to MF; plasmatocytes (Figure 2T) | - | |
| CG30337 | 2.17 | Later differentiating cells (Figure 2N) | Cell cycle; DNA metabolism; intracellular protein transport | |
| kek1 | 2.06 | All cells posterior to MF; 2nd and 3rd antennal segments; presumptive femur in leg imaginal disc. | Negative regulation of EGF receptor activity | |
| CG30188 | 2.04 | Differentiating cells posterior to MF | Cell adhesion; signal transduction | |
| CG9487 | 1.97 | Differentiating cells; PE | - | |
| CG30022 | 1.95 | Dorsal and ventral anterior eye margin; plasmatocytes | - | |
| Neu3 | 1.94 | Differentiating cells; plasmatocytes; PE | Cell adhesion; proteolysis and peptidolysis; signal transduction | |
| svp | 1.93 | R1, R3, R4 and R6 photoreceptors | R3/R4, R1/R6 cell fate commitment; requires Ras signaling | (Mlodzik et al., 1990) |
| CG11382 | 1.92 | Eye: 3/4 columns of ommatidia posterior to MF. Wing: pleurites, myoblasts, cells in wing pouch but restricted from presumptive margin (Figure 2R) | - | |
| CG9336 | 1.87 | Axons projections; PE (Figure 2O) | ||
| Tsp42El | 1.85 | Anterior to MF | Transmission of nerve impulse; neurogenesis; ectoderm dev. | |
| CG31163 | 1.84 | Ubiquitous expression in eye; Wing pouch, restricted from presumptive margin | - | |
| ena | 1.71 | n.d. Ena receptor, Abl, is transcribed in all imaginal epithelial cells with increased levels over the differentiating photoreceptors. | Axonogenesis; regulation of actin polymerization; cytoskeleton organization | (Bennett and Hoffmann, 1992; Gertler et al., 1995) |
| scab | 1.71 | Random cells in eye and wing DP | - | |
| sqz | 1.69 | Late differentiating cells; nuclei of fat body; gut cells; salivary gland | Cell proliferation; regulation of transcription; oogenesis | |
| SP2353 | 1.67 | Differentiating cells of the eye; maybe predominantly single cell type of ommatidium; optic lobe (Figure 2L) | Signal transduction; cell-matrix and cell-cell adhesion | |
| CG32030 | 1.63 | Differentiating cells including clusters at posterior edge of MF | Cell organization and biogenesis | |
| βTub60D | 1.57 | Differentiating cells posterior to MF | Axonogenesis and guidance; GTPase activity; microtubule-based process | (Hoyle et al., 2000) |
| Nrg | 1.51 | Late differentiating cells | Neuron adhesion; Epidermal growth factor receptor signaling | (Hortsch et al., 1990) |
| scrib | 1.5 | MF | Negative regulation of cell proliferation; zonula adherens assembly | (Bilder and Perrimon, 2000) |
| eiger | 1.5 | Random cells in eye and leg PE and DP | Induction of apoptosis; JNK cascade; TNF receptor binding | (Igaki et al., 2002) |
| Lip1 | 1.41 | In eye DP column of single cells at the posterior edge of MF express Lip1. Random cells of eye and wing DP, PE (Figure 2S) | Lipid metabolism | |
| cpo | 1.34 | Apical punctate expression on differentiating neurons and outer antennal ring; leg and wing discs; CNS (Figure 2M) | Peripheral nervous system development | (Bellen et al., 1992) |
| pnt | 1.33 | n.d. | Transcription factor target of Egf receptor/MAPK signaling | (Brunner et al., 1994) |
| Peripodial epithelium (17) | ||||
| CG13532 | 2.37 | PE eye; plasmatocytes; expressed within wing pouch | - | |
| CG13041 | 2.19 | PE; plasmatocytes | - | |
| CG32354 | 1.99 | PE eye/antennal imaginal disc. | Endopeptidase inhibitor activity | |
| CG9487 | 1.97 | Differentiating cells; PE | - | |
| Neu3 | 1.94 | Differentiating cells; plasmatocytes; PE | Cell adhesion; proteolysis and peptidolysis; signal transduction | |
| CG9336 | 1.87 | Axons projections; PE | - | |
| CG18854 | 1.83 | PE eye/antennal imaginal disc | Inositol-trisphosphate 3-kinase activity | |
| sda | 1.68 | PE eye/antennal imaginal disc | Proteolysis and peptidolysis; mechanosensory behavior | |
| Rac2 | 1.67 | PE eye/antennal imaginal disc | Rhabdomere development; small GTPase signal transduction | |
| Sulf1 | 1.67 | PE eye/antennal and wing disc | Pattern specification; metabolism | |
| CG9699 | 1.55 | PE eye/antennal imaginal disc | Cytokinesis; mitosis; structural constituent of cytoskeleton | |
| eiger | 1.5 | Random cells in eye and leg PE and DP | Induction of apoptosis; JNK cascade; TNF receptor binding | (Igaki et al., 2002) |
| Aplip1 | 1.48 | PE eye/antennal imaginal disc | Regulation of JNK cascade; rhodopsin-like receptor activity | |
| Lip1 | 1.41 | In eye DP column of single cells at the posterior edge of MF express Lip1. Random cells of eye and wing DP, PE. | Lipid metabolism | |
| tun | 1.4 | Nuclei of peripodial cells | Olfactory learning | |
| Dscam | 1.36 | PE eye/antennal, wing and leg imaginal disc | PNS development; axon guidance; cell adhesion | |
| CG13890 | 1.22 | PE | Fatty acid beta-oxidation | |
| Plasmatocytes (7) | ||||
| CG32406 | 3.2 | Plasmatocytes; Expressed in posterior half of wing pouch | Intracellular signaling cascade; contains an SH2 motif | |
| CG13532 | 2.37 | PE eye; plasmatocytes; expressed within wing pouch | - | |
| CG14598 | 2.2 | Anterior to MF; plasmatocytes | - | |
| CG13041 | 2.19 | PE; plasmatocytes | - | |
| CG30022 | 1.95 | Dorsal and ventral anterior eye margin; plasmatocytes | Hydrolyase activity; lysase activity; defense response | |
| Neu3 | 1.94 | Differentiating cells; plasmatocytes; PE | Cell adhesion; proteolysis and peptidolysis; signal transduction | |
| CG33275 | 1.8 | Plasmatocytes | Guanyl exchange factor activity; MAPK cascade | |
| Other imaginal discs or tissues (15) | ||||
| CG3244 | 5.06 | Specific pattern on CNS. | Sugar binding | |
| CG7900 | 3.43 | Expressed in wing pouch | - | |
| CG32406 | 3.2 | Plasmatocytes; Expressed in posterior half of wing pouch | Intracellular signaling cascade; contains an SH2 motif | |
| CG13606 | 2.98 | Expressed in the wing DP | - | |
| CG13532 | 2.37 | PE eye; plasmatocytes; expressed within wing pouch | - | |
| Al | 2.37 | Pattern in leg disc; 2nd antennal segment | - | (Schneitz et al., 1993) |
| CG4096 | 2.2 | Expressed at the dorsal anterior margin in the eye disc; wing and leg discs. | - | |
| Lim1 | 2.95 | 3rd antennal segment; outer tarsal segments of leg disc | - | |
| CG11339 | 2.87 | Narrow band of cells posterior to MF; inner ring of antennal disc; specific expression in wing disc | Cytoskeleton organization and biogenesis | |
| kek1 | 2.06 | All cells posterior to MF; 2nd and 3rd antennal segments; presumptive femur in leg imaginal disc. | Negative regulation of EGF receptor activity | |
| CG11382 | 1.92 | Eye: 3/4 columns of ommatidia just posterior to MF. Wing: pleurites, myoblasts, cells in wing pouch but restricted from presumptive margin | - | |
| scab | 1.71 | Random cells in eye and wing DP | - | |
| sqz | 1.69 | Late differentiating cells; nuclei of fat body; gut cells; salivary gland | Cell proliferation; regulation of transcription; oogenesis | |
| SP2353 | 1.67 | Differentiating cells of the eye, maybe predominantly a single cell type per ommatidium; brain CNS | Signal transduction; cell-matrix and cell-cell adhesion | |
| CG31163 | 1.84 | Ubiquitous expression in eye; Wing pouch, restricted from presumptive margin | - | |
N.B.
Transcripts of genes in bold face were detected in multiple tissue and cell types.
DP = disc proper; MF = morphogenetic furrow; PE = peripodial epithelium; SLR = Signal Log Ratio
Transcripts were not detected for the following 24 GMR>sSpi downregulated genes: mthl8; CG18278; Sox100B;Miple; CG9416; CG4914; CG3781; rn; CG33515; CG3525; CG9134; CG17278; pyr; CG1102; CG17919l dbe; disco-r; CG8965; CG14567; Mob1; CG4686; CG2264; prosalpha1; CG18600.
Transcripts were not detected for the following 14 genes upregulated in GMR>sSpi: CG4306; CG15756; CG8303; Obp56d; CG4341; CG4098; CG15117; comm.; CG14170; CG10433; lbm; Nrv1; sano; tna.
We have established the expression pattern of 35 uncharacterized genes expressed in the DP, 29 genes expressed in the PE, 15 genes expressed in the plasmatocytes and 1 uncharacterized gene expressed in glia, suggesting that EGFR signaling may regulate gene expression in these different cell types. The RNA in situ hybridization patterns of all genes examined can be found in Supplementary Table 2. This includes expression patterns in some other imaginal discs and larval tissues. 45 of the differentially regulated transcripts were not detected during normal eye development; 9 of these genes were expressed in other imaginal discs or tissues however including 2 (Lim1 and al) in the antennal disc. The genes for which no transcript was detected in the eye disc are either expressed at levels too low to detect by in situ hybridization, not detected by the probe generated or else ectopically induced by either GMR>RasV12 and GMR>sSpi. Seven genes remain untested due to technical difficulties.
Transcripts of uncharacterized genes associated with the SMW and MF
Six uncharacterized genes are expressed in a patterned band of cells associated with the SMW. Two of these genes, CG15630 and CG8502 appear to be expressed in the dividing cells, and are down regulated in the absence of the SMW in GMR>sSpi. CG15630 is expressed in groups of 2 or 3 cells and CG8502 in single cells, both at the posterior edge of the MF for 2–3 columns (Figs. 2A and 2B). The other four genes were upregulated when the SMW was blocked by GMR>sSpi, and are expressed in a band cells associated with the SMW: CG11339, CG31176, CG11382 and Lip1 (Figs. 2K–2N). CG11339, CG11382 and Lip1 are expressed in the non-dividing cells whereas CG31176 appears to be expressed in all cells near the SMW. These four genes maybe associated with maintaining the G1 arrest of differentiating cells or early photoreceptor differentiation. The gene CAP, which was downregulated in GMR>sSpi, was expressed in the MF prior to the onset of the SMW. E(Spl)m2, brd, arc and ss are also reported to be expressed within the MF (Singson et al., 1994; Duncan et al., 1998; Lai et al., 2000; Liu and Lengyel, 2000).
We also report the expression of 8 uncharacterized genes in the anterior part of the eye disc ahead of the MF. Four of these, SP1029, Pde8, CG13966 and CG14598 were expressed a band of cells anterior to the MF (Figs. 2C, 2D and 2O). The other four genes, GM130, CG5929, Neu3 and Tsp42E1 were expressed throughout the anterior part of the eye disc (Fig. 2E).
Transcripts of uncharacterized genes expressed in differentiating cells
8 genes downregulated and 17 genes upregulated in GMR>sSpi are expressed in the differentiating photoreceptors (Tables 1 and 2). The expression of 7 out of the 8 photoreceptor expressed genes downregulated in GMR>sSpi is known (Table 1). These genes may be regulated specifically in cells that arrest and differentiate in the first 4–5 columns, before the SMW is complete. Transcripts of the remaining gene, Obp44a, were detected in the axons of late differentiating photoreceptors and as they exit the eye disc via the optic stalk. The expression of 11 out of the 17 genes upregulated in GMR>sSpi is novel and 8 of these genes are uncharacterized: CG14275, CG15522, CG30337, CG30188, CG9487, SP2353, CG9336 and CG32030 (Figs. 2P–2S); 2 genes, squeeze (sqz) and couch potato (cpo), have previously implicated in the development of the nervous system but not shown to be expressed in the photoreceptors (Fig. 2T) (Bellen et al., 1992; McGovern et al., 2003) and 1 gene, Neu3 is known to be expressed in plasmatocytes but it’s expression in the photoreceptors has not been reported before (Asha et al., 2003) (Table 2). Transcripts of CG9336 and CG14275 were both detected in the axons of differentiating cells (Fig. 2S). A noteworthy expression pattern is that of CG15522 which was upregulated in GMR>sSpi and is expressed in the groups of cells along the posterior edge of the presumptive eye field (Fig. 2P). These groups of cells may correspond to a distinct subset of peripheral ommatidia; an expression pattern of this kind has not been previously reported.
Consequences of increased Spitz and Ras activity for cells outside of the retina
Many of the differentially regulated transcripts were predominantly expressed outside of the retinal epithelium: in the PE, retinal basal glia or larval plasmatocytes. We have therefore investigated the role of Spi and Ras signaling with respect to these different eye imaginal disc cell types.
Genes expressed in the peripodial epithelium
Transcripts of 29 differentially regulated genes were expressed in PE cells; 12 genes downregulated and 17 genes upregulated in GMR>sSpi (Tables 1 and 2). A change in the relative sizes of the retina and the PE would lead to a consistent increase or decrease in the relative abundance of PE-expressed genes, so these results instead suggest more specific regulation of particular genes. Consistent with this idea, there was no expansion or reduction of the PE in response to RasV12 expression in the retina (data not shown). The morphology of the PE in GMR>RasV12 and GMR>sSpi eye discs was also investigated. To examine the size and shape of the PE cells eye discs were labeled with Armadillo (Arm), which localizes to the adheren junctions (Riggleman et al., 1990). Although the PE of both GMR>RasV12 and GMR>sSpi contained more cells than wild type no discernable difference in cell size or shape between the genotypes was observed (Figs. 3A–3C). The difference in genes expressed in the PE between GMR>RasV12 and GMR>sSpi is not due to gross differences in cellular morphology, cell size or PE size.
Figure 3.
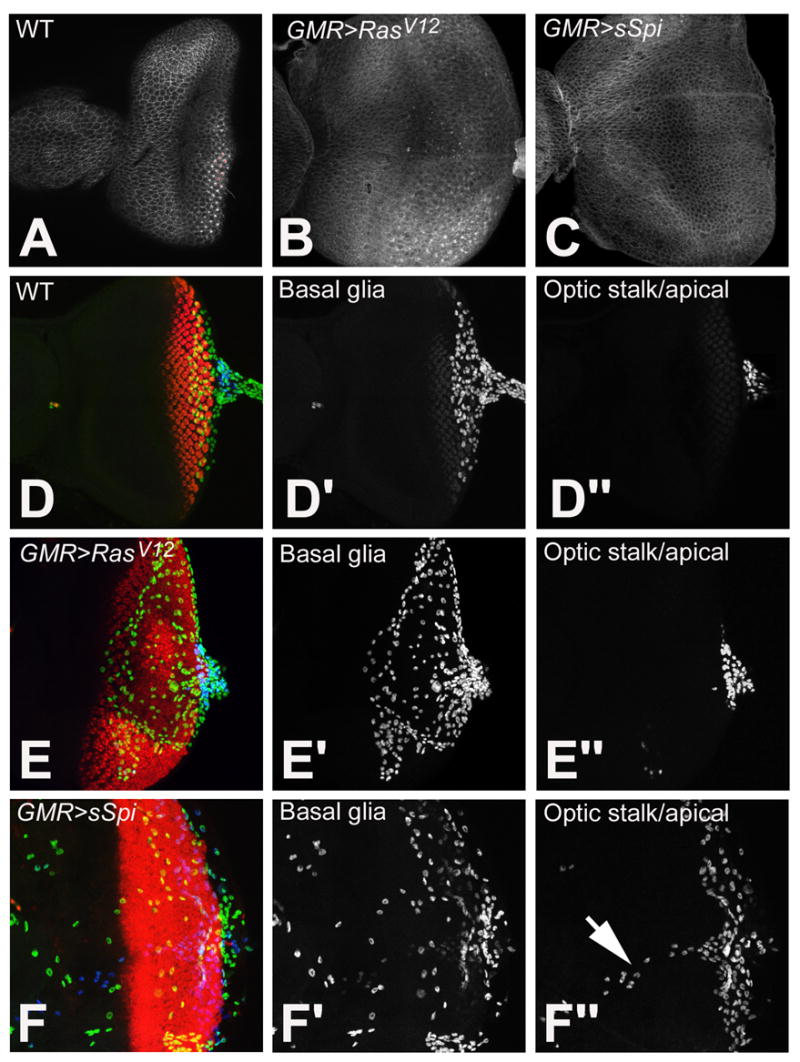
Examination of the PE in wild type (A), GMR>RasV12, (B) and GMR>sSpi (C) eye imaginal discs with Arm. Retinal basal glia in wild type (D-D″), GMR>RasV12 (E-E″) and GMR>sSpi (F-F″) eye imaginal discs. Differentiating photoreceptors are labeled with ElaV (red). Glia are identified with Repo. Glia in the basal part of the eye disc are in the green channel (D′, E′ and F′). Glia located apical and in the optic stalk are in the blue channel (D″, E″ and F″). Normally glia are located basal to photoreceptors and within the optic stalk (D′ and D″). This is unaffected in GMR>RasV12 (E′ and E″). In GMR>sSpi, basal glia migrate more anteriorly (F′), some glia are also located apically and along the Bolwig nerve (arrow) (F″).
The majority of the PE-expressed genes are uncharacterized or little characterized previously (Tables 1 and 2). Notably, all the transcripts were also expressed in the PE of other imaginal discs examined, the leg and or wing discs (Supplementary Fig. 1). At third instar the eye/antennal PE is composed of squamous epithelial cells. The leg and wing PE are made of up both squamous cells and at the margins of the PE, cuboidal cells (margin cells) (Auerbach, 1936; Cohen, 1993). The difference in squamous and margin cells is reflected in the expression of some of the PE specific transcripts. For instance, transcripts of CG8502 are only present in the squamous cells (Supplementary Fig. 1, A–B). In contrast, CG3893, CG2657, Osi23 and dScam transcripts were more readily detected in the margin cells (Supplementary Fig. 1, C–G). The other genes were transcribed throughout the PE (Supplementary Fig. 1, H–I).
The PE-expressed genes can be divided into 2 categories: (I). Genes expressed in PE cells only. This includes most of the PE-expressed genes. Nine genes downregulated in GMR>sSpi: Cyp4e2, Osi23, CG11073, CG3893, beat-Ic, wbl, CG4408, CG2657 and CG15370, and 10 genes upregulated in GMR>sSpi: CG32354, CG18854, sda, Rac2, Sulf1, CG9699, Aplip1, tun, Dscam and CG13890. (II). Genes expressed in the PE and elsewhere in the eye disc and/or plasmatocytes. Three genes downregulated in GMR>sSpi: Pde8, CG8502 and CG13203, and 7 genes upregulated in GMR>sSpi: CG13532, CG13041, CG9487, Neu3, CG9336, eiger, and Lip1 were expressed in this manner (Tables 1 and 2). For example, CG9487, CG9336 are expressed in the PE and DP whereas CG13203 and CG13041 are expressed in the PE and plasmatocytes. As these genes are also expressed outside of the PE, the microarray may have detected the altered expression levels due to either changes in the PE or elsewhere.
Because GMR Gal4 drives UAS transgene transcription in DP cells and not PE cells (data not shown), changes in Group I genes that are only expressed in the PE, indicate EGFR-dependent signaling from the DP to the PE. The simplest hypothesis is that direct targets of EGFR are activated in PE cells by Spi from the DP. However, the results would also be consistent with indirect effects on the PE of EGFR signaling in the DP, if the PE is affected by the SMW, early- differentiating photoreceptors, or cell survival differences that distinguish GMR>RasV12 from GMR>sSpi.
CG31676 is expressed in the retinal basal glia
CG31676 is downregulated in GMR>sSpi and is expressed in the retinal basal glial (Fig. 2F). CG31676 message was also detected along the Bolwig nerve (Supplementary Table 2). In the differentiating eye disc, glia are present along the basal surface of the eye epithelium associated with photoreceptor axons where they are required for guiding axons into the optic stalk (Fig. 3D) (Choi and Benzer, 1994; Rangarajan et al., 1999). In situ hybridization of the CG31676 probe to the original microarray genotypes revealed that the levels of CG31676 mRNA were comparable to wild type in GMR>RasV12 but greatly reduced in GMR>sSpi (Figs. 5G and 5H). Due to non-autonomous Spi secretion it is likely that CG31676 was down regulated in the glia in response to EGFR/Ras signaling. We hypothesize that activation of EGFR in the glia by Spi blocked the expression of CG31676.
Figure 5.
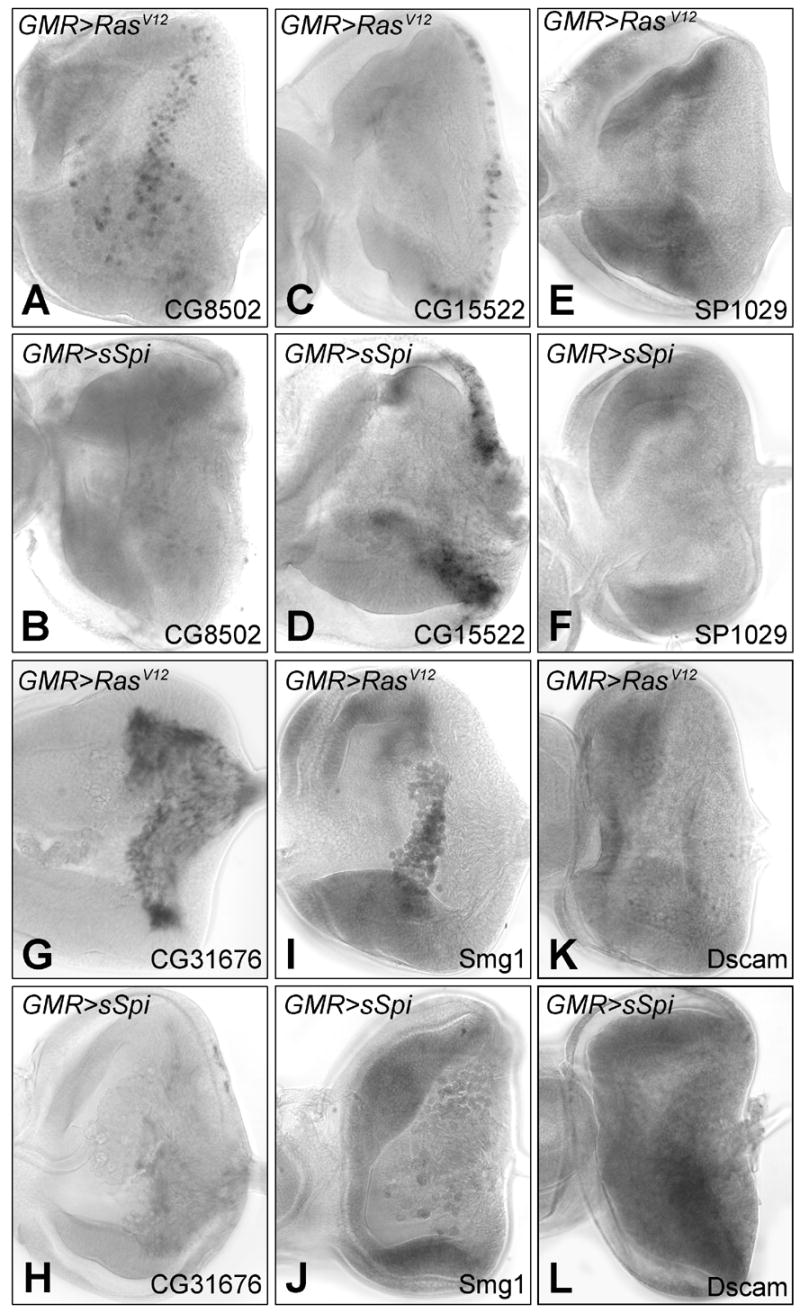
mRNA in situ hybridization of differentially transcribed genes in GMR>RasV12 and GMR>sSpi eye imaginal discs: CG8502 in GMR>RasV12 (A) and GMR>sSpi (B); CG15522 in GMR>RasV12 (C) and GMR>sSpi (D); SP1029 in GMR>RasV12 (E) and GMR>sSpi (F). CG8502, CG15522 and SP1029 are expressed in the DP. CG31676 in GMR>RasV12 (E) and GMR>sSpi (F) in the glia. Smg1 in GMR>RasV12 (I) and GMR>sSpi (J) in the plasmatocytes. The mRNA levels of Dscam in the PE were lower in GMR>RasV12 (K) than GMR>sSpi (L).
To investigate the effects of ectopic Spi on glia, we examined the retinal basal glia in GMR>RasV12 and GMR>sSpi with the glial cell marker, Repo. The glia in GMR>RasV12 retinas were comparable to wild type (Fig. 3E). The glia in GMR>sSpi displayed defects in both migration and localization (Fig. 3F). Normally the anterior border of glial cell migration is posterior to the MF around column 6, the point at which axons begin to turn posteriorly, known as the axonal boundary. Basal glia in GMR>sSpi retinas migrated anterior to the MF past differentiating photoreceptors (Fig. 3F′). In addition, glia were also found apical to the photoreceptors and along the Bolwig nerve (Fig. 3F″). We propose that activation of EGFR in glia, by Spi from the photoreceptor axons, promotes the motility of and alters the localization of retinal basal glia, possibly through CG31676.
Genes transcribed in the larval plasmatocytes
Ten genes downregulated in GMR>sSpi are expressed in the plasmatocytes present on the basal surface of the wild type retinal eye field: Pde8, twe, CG12508, CG8502, CG13203, CG15911, PGRP-SC2, Smg1, robl62A and CG18547 (Figs. 2I and 2J). Seven genes enriched in GMR>sSpi were also expressed in the plasmatocytes: CG13521, CG32406, CG14598, CG13041, CG30022, CG33275 and Neu3. Other genes expressed in larval plasmatocytes such as, serpent (srp), croqumort (crq) or peroxidasin (pxn) were not differentially regulated in our microarray analysis (Nelson et al., 1994; Franc et al., 1996; Rehorn et al., 1996). Although some genes, such as CG8502 and Pde8, are expressed in other eye disc cells, 7 genes are expressed only in the plasmatocytes, implying a difference in the plasmatocytes between GMR>RasV12 and GMR>sSpi.
To identify the plasmatocytes on the developing retina we labeled GMR>RasV12 and GMR>sSpi discs with Arm. More plasmatocytes were present in both GMR>RasV12 and GMR>sSpi than in wild type; there was little difference in number between GMR>RasV12 and GMR>sSpi, however (Figs. 4A–C). To investigate further we labeled wild type, GMR>RasV12 and GMR>sSpi developing retinas with the mature plasmatocyte marker MDP-1 (Hortsch et al., 1998). MDP-1 is present in plasmatocytes on the surface of wild type eye imaginal discs (Fig. 4D′) and plasmatocytes in GMR>RasV12 and GMR>sSpi both expressed MDP-1 suggesting that they mature in both genotypes (Figs. 4E′ and 4F′).
Figure 4.
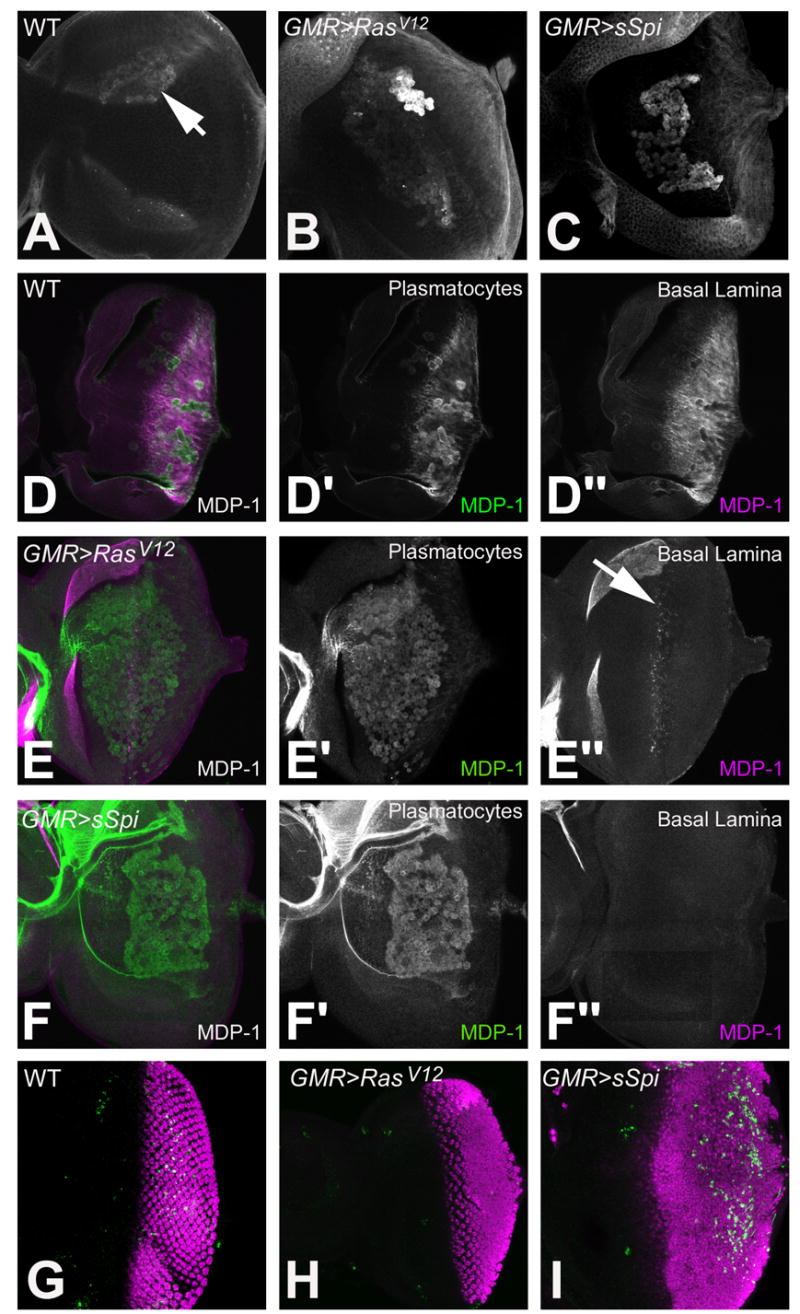
Identification of plasmatocytes in 3rd instar eye imaginal discs. Plasmatocytes identified on the basal surface of the eye imaginal disc with anti-Arm in wild type (A), GMR>RasV12 (B) and GMR>sSpi (C). MDP-1 labeling of wild type (D), GMR>RasV12 (E) and GMR>sSpi (F) eye imaginal discs. MDP-1 in plasmatocytes is green (D′, E′ and F′). MDP-1 in the basal lamina is magenta (D″, E″ and F″). CM1 (green) and Elav (magenta) labels the dying and differentiating photoreceptors in wild type (G), GMR>RasV12 (H) and GMR>sSpi (I) eye imaginal discs .
During development plasmatocytes are required for the phagocytosis of apoptotic cells (Rizki, 1978). EGFR signaling serves as a survival signal, and activation of the EGFR/Ras/MAPK pathway promotes cell survival in the developing retina (Bergmann et al., 1998; Kurada and White, 1998). To examine cell death in GMR>RasV12 and GMR>sSpi we labeled developing retinas with the CM1 antibody that recognizes activated Drice (Srinivasan et al., 1998; Yu et al., 2002). As previously reported, no cell death occurred in GMR>RasV12 (Fig. 4H) (Yang and Baker, 2003). Some cells in GMR>sSpi eye discs labeled positively for CM1, however; the apoptosis was observed in the posterior regions of the retinal eye field (Fig. 4I). Apoptosis in GMR>sSpi is one potential explanation for changes in gene expression in plasmatocytes.
Retinal EGFR signaling regulates basal lamina composition
Transcripts of the proteoglycan papilin (ppn) were downregulated in GMR>sSpi (Table 1). Ppn is transcribed by imaginal disc cells and accumulates in the extracellular matrix where it forms part of the basal lamina (Campbell et al., 1987; Kramerova et al., 2000) (Supplementary Table 2). Ppn is recognized by the monoclonal antibody MDP-1 that we used to label plasmatocytes (Hortsch et al., 1998) (J. Fessler, Pers. Comm.). In wild type retinas, Ppn was detected in the basal lamina under epithelial cells posterior to the MF (Figs. 4D′ and 4D″). Ppn was not detected in the basal lamina of GMR>sSpi eye imaginal discs (Fig. 4F″). In GMR>RasV12 eye discs, Ppn was only present in the in the basal lamina next to cells at the posterior edge of the MF (Fig. 4E″). If the ppn mRNA expression level is a reflection of Ppn in the basal lamina in the microarray analysis, a difference in ppn expression in the DP between GMR>RasV12 and GMR>sSpi may have been detected. Since Ppn in the basal lamina is anterior to ectopic RasV12 activity in the DP, the increased activation of the EGFR pathway in the retinal epithelium possibly negatively regulates ppn transcription in retinal epithelial cells, thereby affecting the composition of the basal lamina.
Microarray predicted changes translate to alterations of transcript levels in vivo
To verify our array results, the RNA in situ hybridization pattern of six genes were re-examined in the original microarray genotypes, GMR>RasV12 and GMR>sSpi: CG8502, CG15522, SP1029, CG31676, Smg1 and Dscam. CG8502, CG15522 and SP1029 are all expressed in the DP. CG8502 was downregulated in GMR>sSpi with a signal log ratio (SLR) of −1.7. Transcripts of CG8502 are expressed in 3 columns of cells at the posterior edge of the MF in both wild type and GMR>RasV12 eye discs; in agreement with the array data, this expression was not detected in GMR>sSpi (Figs. 5A and 5B). The microarray analysis determined a SLR of +4 for CG15522. By in situ hybridization, we also observed an up regulation in the expression level and an expansion of cells expressing CG15522 in GMR>sSpi compared to GMR>RasV12 (Figs. 5C and 5D). The normal expression of SP1029 in a band of cells anterior to the MF was absent in GMR>sSpi (Figs. 5E and 5F) also consistent with the predicted downregulation of the message in GMR>sSpi (SLR = −3.45). In wild type eye discs, CG31676 is expressed in the retinal basal glia. We could not detect any message by in situ for CG31676 in GMR>sSpi eye discs. The in situ for CG31676 in GMR>RasV12 was comparable to wild type. This is consistent with a SLR of −3.2 for CG31676 in the array analysis (Figs. 5G and 5H). The microarray analysis predicted changes of Smg1, a plasmatocyte expressed gene, and Dscam a PE expressed gene (SLR of −1.44 and +1.36 respectively) were both consistent with changes observed between GMR>sSpi and GMR>RasV12 by in situ hybridization (Figs. 5I–5L). Because the gene expression changes observed by microarray analysis, were confirmed in each of these cases, it is likely that the microarray results provide a generally accurate picture of transcriptional differences between GMR>RasV12 and GMR>sSpi in the DP, PE, glia and plasmatocytes.
Discussion
We have used microarray to identify genes transcribed in the SMW during Drosophila eye development. By manipulating the EGFR pathway, we blocked the SMW, and compared the RNA profile of these developing retinas to retinas with a SMW. Both genotypes activated EGFR signaling posterior to the SMW, controlling for many of the other roles of EGFR. We performed RNA in situ on most of the 140 differentially transcribed genes. Together this analysis has identified of uncharacterized genes that are expressed in SMW and differentiating cells.
Although our strategy avoided identifying many thousands of EGFR-dependant genes, many of the genes found were not expressed during the SMW. Instead, these genes are expressed in cells of the PE, larval plasmatocytes and glia. Having investigated the significance of ectopic EGFR signaling on these different cell types, we suggest that : (1) There are targets of EGFR signaling in the PE; (2) Ectopic EGFR signaling leads to the differential regulation of several genes expressed in plasmatocytes; (3) Ectopic EGFR signaling in the glia effects the migration and localization of glia in the developing retina and (4) EGFR signaling also regulates the composition of the the basal lamina. These genes are probably identified because they are targets of Spi secreted from the DP, although some could be regulated indirectly, in response to the SMW or other differences between GMR>sSpi and GMR>RasV12 discs. Thus an unexpected bonus of the approach was to uncover genes that are candidates to mediate responses in other tissues to Spi secretion from the DP. In future it will be interesting to explore these predictions by direct investigation of the non-autonomous role of retinal Spi.
Identification of genes transcribed in cells associated with the SMW
Our primary goal was to identify genes transcribed in the SMW. By comparing eye discs with and without a SMW we have identified 10 genes that are expressed in cells associated with the SMW; 6 of these genes have novel expression patterns in the SMW. The SMW is a specific patterned cell cycle regulated by the developmental signaling pathways EGFR and N. The transcriptional targets of these pathways in the SMW remain unknown. Cell cycle regulators such as Stg, Cyclin B, or Dacapo (Dap) were not differentially regulated between the sSpi and RasV12 expressing eye discs; this was probably due to the many cycling cells in the anterior part of the eye disc that are unaffected by GMR>sSpi and GMR>RasV12.
We expected genes expressed in the SMW cells to be transcribed in a patterned band of cells at the posterior edge of the MF for 2–3 columns of retinal development. The 6 uncharacterized genes are expressed in this manner: CG8502 and CG15630 were down regulated in GMR>sSpi and CG11339, CG11382, CG31167 and Lip were upregulated in GMR>sSpi. The 4 characterized genes that were all downregulated in GMR>sSpi are lz, B-H1, Traf1 and Spn43A their potential cell cycle roles have not been previously examined. Since early cell fate decisions occur simultaneously with the SMW, further work will be required to distinguish whether expression of these genes is associated with cell cycle progression or arrest, or the differentiation of R8 or other early-specified cells.
Ten genes were expressed ahead of the MF in the region of the eye disc where the cells are unspecified and asynchronously cycling: Spn43A, SP1029, Pde8, Klg, CG13966, GM130, trn and CG5929 were downregulated in GMR>sSpi, and CG14598 and Tsp42E1 were upregulated in GMR>sSpi. One of these genes, SP1029, a metallopeptidase located 3′ of stg that exhibits a similar mRNA expression pattern to stg (Alphey et al., 1992). We speculate that SP1029 may come under the regulation of the SMW enhancer of stg; this enhancer not yet been mapped and could be 3′ to stg (Lehman et al., 1999). Five of the genes downregulated in response to sSpi are expressed within the MF: E(Spl)m2, CAP, Traf1, Brd and ss. The EGFR pathway might have transcriptional targets anterior to and within the MF, although this has not been reported previously. Alternatively these targets may be indirect and depend on secreted signals produced during the SMW.
EGFR targets in differentiating cells
Even though GMR>sSpi and GMR>RasV12 lead to ectopic photoreceptor specification to similar degrees, 25 genes expressed in the differentiating cells were uncovered. SoxN, lz, Fas2, B-H1, thisbe (ths), klg, trn and Obp44a were downregulated in GMR>sSpi, and CG14275, CG15522, CG30337, kek1, CG30188, CG9487, CG30022, Neu3, svp, CG9336, sqz, SP2353, CG32030, βTub60D, Nrg, cpo and pnt were upregulated in GMR>sSpi. These genes are potential targets of the EGFR during photoreceptor differentiation. More of the genes expressed in differentiating cells are upregulated in GMR>sSpi than downregulated. Although photoreceptor differentiation occurs only slightly earlier in GMR>sSpi, this maybe sufficient to increase the proportion of transcripts from such cells; alternatively there may also be changes in the type of photoreceptor specified. For example, Spi interferes with R8 photoreceptor development (Lesokhin et al., 1999; Frankfort and Mardon, 2004).
The mRNAs of three genes affected by EGFR signaling, Obp44a, CG9336 and CG14275, were detected in the axons of photoreceptors neurons. The transport of mRNAs to the axon of young neurons has an important role regulating nerve cell maturation (Mohr and Richter, 2000). It will be interesting to determine whether EGFR regulates the transport and/or localization of mRNAs.
EGFR activity in the DP also regulates the composition of extracellular structures preventing the addition of glycoprotein, Ppn, to the adjacent basal lamina. Eight genes expressed in the differentiating cells have putative or known roles either cell adhesion or cytoskeletal organization. EGFR signaling alters the adhesive properties of cells in the eye disc so that normal cell shape re-arrangements occur (Brown et al., 2006). These genes maybe downstream of EGFR in maintaining proper cell adhesion during the G1 arrest of differentiating cells.
Possible targets of the EGFR/Ras pathway in the peripodial epithelium
In this study we have identified 29 genes that were not previously known to be expressed in the PE. 17 are uncharacterized genes that are expressed exclusively in some or all cells of the PE and not elsewhere in the imaginal disc. Until now Ultrabithorax (Ubx) was the only gene expressed in all cells of the wing PE but not the DP, although Ubx is expressed in the DP other imaginal discs (Brower, 1987). Coronin-Gal4 is also detected in the wing PE only but in a subset of cells (Pallavi and Shashidhara, 2003). Thus, we uncovered an unexpected pool of PE-specific genes, suggesting that gene expression in the PE is regulated by Spi secreted from the DP, either directly or in response to the SMW or R8 differentiation.
It is well established that signals from the PE affect the development of the DP (Cho et al., 2000; Gibson and Schubiger, 2000; Pallavi and Shashidhara, 2003; Pallavi and Shashidhara, 2005). Our data suggests that the reverse may also be true; verticals signal from the DP to the PE have an important role in regulating the development and interactions between two disc epithelia. As PE cells do not change in size and morphology between the two genotypes it will be interesting to discover the nature of the response.
Spitz affects glial cell migration and localization
CG31676 was the only gene found to be altered in the retinal basal glia. We speculate that activation of EGFR in the glia, by Spi secreted from the axons, negatively regulates the expression of CG31676. Since only one glia gene was affected, this is unlikely to reflect a change in the relative number of glial cells after Spi expression. We found that migration and localization of glia was affected in GMR>sSpi. Glia normally migrate up to the axonal boundary, the point at which the axons of differentiating photoreceptors turn posteriorly to the optic stalk (Choi and Benzer, 1994). Glia in GMR>sSpi migrated beyond the axonal boundary and photoreceptor differentiation. In addition, glia were present apical to photoreceptor and along the Bolwig nerve. The actual molecular mechanism of glia migration is unknown (Rangarajan et al., 1999). We speculate that CG31676 maybe directly involved in glia migration and/or localization in response to sSpi from photoreceptor cells, as it was the only gene whose transcription was affected.
Spitz affects larval plasmatocytes
Ectopic Spi in the retinal epithelium affected gene expression in plasmatocytes that are associated with the eye imaginal disc. 7 of 17 differentially transcribed genes were not expressed elsewhere in the eye imaginal discs. Most of the plasmatocyte expressed genes uncharacterized. However, Neu3 and PGRP-SC2, have previously been demonstrated by microarray to be expressed in plasmatocytes (De Gregorio et al., 2001; Asha et al., 2003). We propose that Spi from the DP must affect the plasmatocytes directly or indirectly. Interestingly, we detected no difference in number or differentiation (as assessed by the MDP-1 antigen).
EGFR activity coordinates development of the eye disc with other associated tissues
In summary, combining microarray and RNA in situ hybridization has led to the identification of genes that are transcribed in cells associated with the SMW, but we also uncovered as many targets of EGFR in the other cell types associated with the eye imaginal disc. Because our microarray was designed to identify a narrow subset of targets, and appears to have excluded the majority of genes directly or indirectly regulated by EGFR in the eye disc as a whole, we think that most of these genes are different because they are targets of Spi in cells where GMRGal4 does not express. Thus, we inadvertently selected for genes in other tissues that respond to Spi made in the DP. These findings suggest that, in addition to regulating multiple aspects of retinal differentiation, and regulating brain differentiation in response to retinal innervation (Huang et al., 1998), changes in EGFR activity during eye disc differentiation could also serve to coordinate the developmental programs of the glia, PE, and plasmatocytes with the eye disc proper together comprising an organ system of cells from multiple origins (Fig. 6).
Supplementary Material
Acknowledgments
We thank C. Hindnavis for technical assistance; M. Wilkin and D. Dimova for helpful technical advice; M. Hortsch for the MDP-1 antibody and U. Banerjee for helpful advice. The manuscript was improved by suggestions from W. Li and E. Bach. Confocal microscopy was performed at the Analytical Imaging Facility and array processing was performed at the Affymetrix facility at the Albert Einstein College of Medicine. This work was supported by grant GM47892 from the National Institutes of Health. NEB is a Scholar of the Irma T. Hirschl Foundation for Biomedical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affymetrix. (2003). GeneChip Expression Analysis. Data Analysis Fundamentals.
- Alphey L, Jiminez J, White-Cooper H, Dawson I, Nurse P, Glover DM. twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell. 1992;69:977–988. doi: 10.1016/0092-8674(92)90616-k. [DOI] [PubMed] [Google Scholar]
- Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–15. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach C. The development of the legs, wing and halteres in wild type and some mutant strains of Drosophila melanogaster. Proc R Soc Edinb B. 1936;58:787–815. [Google Scholar]
- Baker NE. Patterning and proliferation in Drosophila imaginal discs. Current Opinion in Genetics and Development. 2007;17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Control of cell proliferation in the Drosophila eye by Notch signalling. Developmental Cell. 2005;8:529–539. doi: 10.1016/j.devcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Baonza A, Murawsky CM, Travers AA, Freeman M. Pointed and tramtrack69 establish an EGFR-dependent transcriptional switch to regulate mitosis. Nature Cell Biology. 2002;4:976–980. doi: 10.1038/ncb887. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Kooyer SD, Evelyn D, Pearlman J. The Drosophila couch potato protein is expressed in nuclei of peripheral neuronal precursors and shows homology to RNA-binding proteins. Genes Dev. 1992;6:2125–36. doi: 10.1101/gad.6.11.2125. [DOI] [PubMed] [Google Scholar]
- Bennett RL, Hoffmann FM. Increased levels of the Drosophila Abelson tyrosine kinase in nerves and muscles: subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development. 1992;116:953–66. doi: 10.1242/dev.116.4.953. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Brower DL. Ultrabithorax gene expression in Drosophila imaginal discs and larval nervous system. Development. 1987;101:83–92. doi: 10.1242/dev.101.1.83. [DOI] [PubMed] [Google Scholar]
- Brown KE, Baonza A, Freeman M. Epithelial cell adhesion in the developing Drosophila retina is regulated by Atonal and the EGF receptor pathway. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, Hafen E, Scholz H, Klambt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–9. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Butler MJ, Jacobsen TL, Cain DM, Jarman MG, Hubank M, Whittle JR, Phillips R, Simcox A. Discovery of genes with highly restricted expression patterns in the Drosophila wing disc using DNA oligonucleotide microarrays. Development. 2003;130:659–70. doi: 10.1242/dev.00293. [DOI] [PubMed] [Google Scholar]
- Butler SJ, Ray S, Hiromi Y. klingon, a novel member of the Drosophila immunoglobulin superfamily, is required for the development of the R7 photoreceptor neuron. Development. 1997;124:781–92. doi: 10.1242/dev.124.4.781. [DOI] [PubMed] [Google Scholar]
- Campbell AG, Fessler LI, Salo T, Fessler JH. Papilin: a Drosophila proteoglycan-like sulfated glycoprotein from basement membranes. J Biol Chem. 1987;262:17605–12. [PubMed] [Google Scholar]
- Chang HC, Solomon NM, Wassarman DA, Karim FD, Therrien M, Rubin GM. Phyllopod functions in the fate determination of a subset of photoreceptors in Drosophila. Cell. 1995;80:2121–2129. doi: 10.1016/0092-8674(95)90497-2. [DOI] [PubMed] [Google Scholar]
- Chang Z, Price BD, Bockheim S, Boedigheimer MJ, Smith R, Laughon A. Molecular and genetic characterization of the Drosophila tartan gene. Developmental Biology. 1993;160:315–332. doi: 10.1006/dbio.1993.1310. [DOI] [PubMed] [Google Scholar]
- Cho KO, Chem j, Izaddoost S, Choi KW. Novel signaling from the peripodial membrane is essential for eye disc patterning in Drosophila. Cell. 2000;103:331–342. doi: 10.1016/s0092-8674(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Choi KW, Benzer S. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron. 1994;12:423–31. doi: 10.1016/0896-6273(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Imaginal disc development. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. II. CSHL Press; 1993. pp. 747–841. [Google Scholar]
- Cornell M, Evans DA, Mann R, Fostier M, Flasza M, Monthatong M, Artavanis-Tsakonas S, Baron M. The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics. 1999;152:567–76. doi: 10.1093/genetics/152.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremazy F, Berta P, Girard F. Genome-wide analysis of Sox genes in Drosophila melanogaster. Mech Dev. 2001;109:371–5. doi: 10.1016/s0925-4773(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Curtiss J, Mlodzik M. Morphogenetic furrow initiation and progression during eye development in Drosophila: the roles of decapentaplegic, hedgehog and eyes absent. Development. 2000;127:1325–1336. doi: 10.1242/dev.127.6.1325. [DOI] [PubMed] [Google Scholar]
- Daga A, Karlovich CA, Dumstrei K, Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev. 1996;10:1194–205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A. 2001;98:12590–5. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai CJ, Popova E, Zinn K. A Drosophila receptor tyrosine phosphatase expressed in the embryonic CNS and larval optic lobes is a member of the set of proteins bearing the HRP carbohydrate epitope. J Neurosci. 1994;14:7272–83. doi: 10.1523/JNEUROSCI.14-12-07272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ, Dominguez M, van der Straten A, Hafen E. Control of Drosophila photoreceptor cell fates by phyllopod, a novel nuclear protein acting downstream of the Raf kinase. Cell. 1995;80:453–62. doi: 10.1016/0092-8674(95)90496-4. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Wassarman JD, Freeman M. Multiple functions of the EGF receptor in Drosophila eye development. Current Biology. 1998;8:1039–1048. doi: 10.1016/s0960-9822(98)70441-5. [DOI] [PubMed] [Google Scholar]
- Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12:1290–303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–51. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Firth LC, Li W, Zhang H, Baker NE. Analyses of RAS Regulation of Eye Development in Drosophila melanogaster. Methods Enzymol. 2006;407:711–21. doi: 10.1016/S0076-6879(05)07056-4. [DOI] [PubMed] [Google Scholar]
- Flores GV, Daga A, Kalhor HR, Banerjee U. Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development. 1998;125:3681–7. doi: 10.1242/dev.125.18.3681. [DOI] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–43. doi: 10.1016/s1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. Senseless represses nuclear transduction of Egfr pathway activation. Development. 2004;131:563–70. doi: 10.1242/dev.00941. [DOI] [PubMed] [Google Scholar]
- Freeman M. The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mechanisms of Development. 1994;48:25–33. doi: 10.1016/0925-4773(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- Gertler FB, Comer AR, Jaung JL, Ahern SM, Clark MJ, Liebl EC, Hoffmann FM. enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 1995;9:521–33. doi: 10.1101/gad.9.5.521. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Schubiger G. Peripodial cells regulate proliferation and patterning of Drosophila imaginal discs. Cell. 2000;103:343–50. doi: 10.1016/s0092-8674(00)00125-2. [DOI] [PubMed] [Google Scholar]
- Green C, Levashina E, McKimmie C, Dafforn T, Reichhart JM, Gubb D. The necrotic gene in Drosophila corresponds to one of a cluster of three serpin transcripts mapping at 43A1.2. Genetics. 2000;156:1117–27. doi: 10.1093/genetics/156.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–5808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Kojima T, Michiue T, Ishimaru S, Emori Y, Saigo K. Dual Bar homeobox genes of drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes and Development. 1992;6:50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- Horsfield J, Penton A, Secombe J, Hoffman FM, Richardson H. decapentaplegic is required for arrest in G1 phase during Drosophila eye development. Development. 1998;125:5069–5078. doi: 10.1242/dev.125.24.5069. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Bieber AJ, Patel NH, Goodman CS. Differential splicing generates a nervous system-specific form of Drosophila neuroglian. Neuron. 1990;4:697–709. doi: 10.1016/0896-6273(90)90196-m. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Olson A, Fishman S, Soneral SN, Marikar Y, Dong R, Jacobs JR. The expression of MDP-1, a component of Drosophila embryonic basement membranes, is modulated by apoptotic cell death. Int J Dev Biol. 1998;42:33–42. [PubMed] [Google Scholar]
- Hoyle HD, Turner FR, Raff EC. A transient specialization of the microtubule cytoskeleton is required for differentiation of the Drosophila visual system. Dev Biol. 2000;221:375–89. doi: 10.1006/dbio.2000.9674. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shilo BZ, Kunes S. A retinal axon fascicle uses spitz, an EGF receptor ligand, to construct a synaptic cartridge in the brain of Drosophila. Cell. 1998;95:693–703. doi: 10.1016/s0092-8674(00)81639-6. [DOI] [PubMed] [Google Scholar]
- Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. Embo J. 2002;21:3009–18. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jasper H, Benes V, Atzberger A, Sauer S, Ansorge W, Bohmann D. A genomic switch at the transition from cell proliferation to terminal differentiation in the Drosophila eye. Dev Cell. 2002;3:511–21. doi: 10.1016/s1534-5807(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal discs. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- Klebes A, Biehs B, Cifuentes F, Kornberg TB. Expression profiling of Drosophila imaginal discs. Genome Biol. 2002;3:RESEARCH0038. doi: 10.1186/gb-2002-3-8-research0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerova IA, Kawaguchi N, Fessler LI, Nelson RE, Chen Y, Kramerov AA, Kusche-Gullberg M, Kramer JM, Ackley BD, Sieron AL, Prockop DJ, Fessler JH. Papilin in development; a pericellular protein with a homology to the ADAMTS metalloproteinases. Development. 2000;127:5475–85. doi: 10.1242/dev.127.24.5475. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Tio M, Hsiung F, Akopyan S, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- Lai EC, Bodner R, Posakony JW. The enhancer of split complex of Drosophila includes four Notch-regulated members of the bearded gene family. Development. 2000;127:3441–55. doi: 10.1242/dev.127.16.3441. [DOI] [PubMed] [Google Scholar]
- Lehman DA, Patterson B, Johnston LA, Balzer T, Britton JS, Saint R, Edgar BA. Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development. 1999;126:1793–1803. doi: 10.1242/dev.126.9.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesokhin AM, Yu SY, Katz J, Baker NE. Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila. Dev Biol. 1999;205:129–44. doi: 10.1006/dbio.1998.9121. [DOI] [PubMed] [Google Scholar]
- Li Y, Baker NE. Proneural enhancement by Notch overcomes Suppressor-of-Hairless repressor function in the developing Drosophila eye. Current Biology. 2001;11:330–338. doi: 10.1016/s0960-9822(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Lengyel JA. Drosophila arc encodes a novel adherens junction-associated PDZ domain protein required for wing and eye development. Dev Biol. 2000;221:419–34. doi: 10.1006/dbio.2000.9689. [DOI] [PubMed] [Google Scholar]
- McGovern VL, Pacak CA, Sewell ST, Turski ML, Seeger MA. A targeted gain of function screen in the embryonic CNS of Drosophila. Mech Dev. 2003;120:1193–207. doi: 10.1016/s0925-4773(03)00159-x. [DOI] [PubMed] [Google Scholar]
- Michaut L, Flister S, Neeb M, White KP, Certa U, Gehring WJ. Analysis of the eye developmental pathway in Drosophila using DNA microarrays. Proc Natl Acad Sci U S A. 2003;100:4024–9. doi: 10.1073/pnas.0630561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik m, Hiromi Y, Weber U, Goodman CS, Rubin GM. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990;60:211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- Mohr E, Richter D. Axonal mRNAs: functional significance in vertebrates and invertebrates. J Neurocytol. 2000;29:783–91. doi: 10.1023/a:1010987206526. [DOI] [PubMed] [Google Scholar]
- Musacchio M, Perrimon N. The Drosophila kekkon genes: novel members of both the leucine-rich repeat and immunoglobulin superfamilies expressed in the CNS. Dev Biol. 1996;178:63–76. doi: 10.1006/dbio.1996.0198. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. Embo J. 1994;13:3438–47. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’ Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–47. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Pallavi SK, Shashidhara LS. Egfr/Ras pathway mediates interactions between peripodial and disc proper cells in Drosophila wing discs. Development. 2003;130:4931–41. doi: 10.1242/dev.00719. [DOI] [PubMed] [Google Scholar]
- Pallavi SK, Shashidhara LS. Signaling interactions between squamous and columnar epithelia of the Drosophila wing disc. J Cell Sci. 2005;118:3363–70. doi: 10.1242/jcs.02464. [DOI] [PubMed] [Google Scholar]
- Penton A, Selleck SB, Hoffmann FM. Regulation of cell cycle synchronization by decapentaplegic during Drosophila eye development. Science. 1997;275:203–206. doi: 10.1126/science.275.5297.203. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zipursky SL. Identification of genes required for Drosophila eye development using a phenotypic enhancer-trap. Proc Natl Acad Sci U S A. 1997;94:9220–5. doi: 10.1073/pnas.94.17.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss A, Johannes B, Nagel AC, Maier D, Peters N, Wajant H. Dynamic expression of Drosophila TRAF1 during embryogenesis and larval development. Mech Dev. 2001;100:109–13. doi: 10.1016/s0925-4773(00)00506-2. [DOI] [PubMed] [Google Scholar]
- Rangarajan R, Gong Q, Gaul U. Migration and function of glia in the developing Drosophila eye. Development. 1999;126:3285–92. doi: 10.1242/dev.126.15.3285. [DOI] [PubMed] [Google Scholar]
- Rao Y, Jan LY, Jan YN. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990;345:163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- Rehorn KP, Thelen H, Michelson AM, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–31. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990;63:549–60. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- Rizki TM. The circulatory system and associated cells and tissues. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. 2b. Academic Press; New York: 1978. pp. 397–452. [Google Scholar]
- Schneitz K, Spielmann P, Noll M. Molecular genetics of Aristaless, a prd-type homeobox gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes and Development. 1993;7:114–129. doi: 10.1101/gad.7.1.114. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Shaharabany M, Seger R, Shilo BZ. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ectoderm determination. Genes and Development. 1995;9:1518–1529. doi: 10.1101/gad.9.12.1518. [DOI] [PubMed] [Google Scholar]
- Simon MA. Receptor tyrosine kinases: specific outcomes from general signals. Cell. 2000;103:13–15. doi: 10.1016/s0092-8674(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Singson A, Leviten MW, Bang AG, Hua XH, Posakony JW. Direct downstream targets of proneural activators in the imaginal disc include genes involved in lateral inhibitory signaling. Genes Dev. 1994;8:2058–71. doi: 10.1101/gad.8.17.2058. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli K. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death and Differentiation. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- Thomas BJ, Gunning DA, Cho J, Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Wassarman DA, Therrien M, Rubin GM. The Ras signaling pathway in Drosophila. Current Opinion in Genetics and Development. 1995;5:44–50. doi: 10.1016/s0959-437x(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Wodicka L, Dong H, Mittmann M, Ho MH, Lockhart DJ. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:1359–67. doi: 10.1038/nbt1297-1359. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compond eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Vol. 2. Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- Yang L, Baker NE. Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Developmental Cell. 2003;4:359–369. doi: 10.1016/s1534-5807(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, Baker NE. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annual Review of Neuroscience. 1994;17:373–397. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


