Abstract
Purpose
Preschool vision screenings often include refractive error or visual acuity (VA) testing to detect amblyopia, as well as alignment testing to detect strabismus. The purpose of this study was to determine the effect of combining screening for eye alignment with screening for refractive error or reduced VA on sensitivity for detection of strabismus, with specificity set at 90% and 94%.
Methods
Over 3 years, 4040 preschool children were screened in the Vision in Preschoolers (VIP) Study, with different screening tests administered each year. Examinations were performed to identify children with strabismus. The best screening tests for detecting children with any targeted condition were noncycloplegic retinoscopy (NCR), Retinomax autorefractor (Right Manufacturing, Virginia Beach, VA), SureSight Vision Screener (Welch-Allyn, Inc., Skaneateles, NY), and Lea Symbols (Precision Vision, LaSalle, IL and Good-Lite Co., Elgin, IL) and HOTV optotypes VA tests. Analyses were conducted with these tests of refractive error or VA paired with the best tests for detecting strabismus (unilateral cover testing, Random Dot “E” [RDE] and Stereo Smile Test II [Stereo Optical, Inc., Chicago, IL]; and MTI PhotoScreener [PhotoScreener, Inc., Palm Beach, FL]). The change in sensitivity that resulted from combining a test of eye alignment with a test of refractive error or VA was determined with specificity set at 90% and 94%.
Results
Among the 4040 children, 157 were identified as having strabismus. For screening tests conducted by eye care professionals, the addition of a unilateral cover test to a test of refraction generally resulted in a statistically significant increase (range, 15%–25%) in detection of strabismus. For screening tests administered by trained lay screeners, the addition of Stereo Smile II to SureSight resulted in a statistically significant increase (21%) in sensitivity for detection of strabismus.
Conclusions
The most efficient and low-cost ways to achieve a statistically significant increase in sensitivity for detection of strabismus were by combining the unilateral cover test with the autorefractor (Retinomax) administered by eye care professionals and by combining Stereo Smile II with SureSight administered by trained lay screeners. The decision of whether to include a test of alignment should be based on the screening program’s goals (e.g., targeted visual conditions) and resources.
Strabismus has been reported to affect approximately 4% of children. 1–3 It can result in psychosocial, developmental, and psychological, as well as visual sequelae (e.g., amblyopia and/or loss of depth perception).2,4–6 Referral can be delayed due to the misconception that children will outgrow strabismus.4,6 To promote early detection and treatment of vision problems such as amblyopia and strabismus, the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine7 recommended a series of eye evaluations, including infant fixation and cover test, leading to eye evaluations in children 3 years of age or older to include “age-appropriate visual acuity measurement” and “ocular motility assessment.” The U.S. Preventive Services Task Force8 recommends screening “to detect amblyopia, strabismus, and defects in visual acuity in children younger than 5 years.”
Phase I of the Vision in Preschoolers (VIP) Study evaluated 11 preschool vision screening tests and showed that tests of refractive error and visual acuity (VA) performed best overall in identifying preschool children with one or more targeted conditions (amblyopia, strabismus, significant refractive error, and/or unexplained reduced VA) as well as the most severe conditions.9,10 Phase II of the VIP Study showed that combining a test of stereopsis with a test of VA or refractive error did not improve sensitivity for detecting one or more targeted conditions.11 However, vision screenings currently used in preschool children often include eye alignment testing to detect strabismus and binocularity problems7,9,12–17 and the effect of including a test of ocular alignment on sensitivity for specifically detecting strabismus has not yet been determined. Therefore, the purpose of this study was to determine the effect of combining tests of eye alignment with tests of refractive error or VA (which performed best overall in identifying preschool children with one or more targeted conditions) on sensitivity for detecting strabismus in preschool children. Effects on overall sensitivity for detection of one or more targeted conditions and the most severe conditions are also considered.
Methods
The methods of phases I and II of the VIP Study have been described previously.9,11 Only the methodologic features with direct relevance for evaluating the results presented in this report are provided herein.
Subjects
Participants were 4040 preschool children who were enrolled in Head Start near a VIP clinical center (Berkeley, CA; Boston, MA; Columbus, OH; Philadelphia, PA; or Tahlequah, OK) and who were 3 to <5 years of age on September 1 of a data-collection year (2001, 2002, or 2003). All children who failed and a sample of children who passed the routine Head Start visual screening were invited to participate.9,11 The research adhered to the tenets of the Declaration of Helsinki and was approved by the local institutional review board(s). Each child’s parent or guardian provided written informed consent. Each child was eligible to participate only once.
Procedures
Vision Examination
Each child who participated received a standardized comprehensive eye examination by a licensed eye care professional (optometrist or ophthalmologist) who was experienced in working with young children, was masked to the child’s screening results, and had completed training in performing the examination according to VIP Study standardized protocols. The vision examination included assessment of monocular threshold VA, stereoacuity, cover testing (unilateral and alternate), and cycloplegic retinoscopy.9,18 Children were classified as having one or more targeted conditions (amblyopia, strabismus, significant refractive error, or unexplained reduced VA) or normal vision. Definitions for the targeted visual conditions have been published previously9,18 and are summarized and clarified in Table 1. Severity of conditions was also ranked hierarchically (Table 1).
Table 1.
Definition of VIP Targeted Disorders by Hierarchy
| Group 1: Very important to detect and treat early |
| Amblyopia |
| Presumed unilateral: ≥3 line interocular difference, a unilateral amblyogenic factor, and worse eye VA ≤ 20/64 |
| Suspected bilateral: a bilateral amblyogenic factor, worse eye VA < 20/50 for 3-year-olds or < 20/40 for 4-year-olds, contralateral eye VA |
| worse than 20/40 for 3-year-olds or 20/30 for 4-year-olds |
| Strabismus: constant in primary gaze |
| Refractive error |
| Severe anisometropia (interocular difference > 2 D hyperopia, > 3 D astigmatism, or > 6 D myopia) |
| Hyperopia ≥ 5.0 D |
| Astigmatism ≥ 2.5 D |
| Myopia ≥ 6.0 D |
| Group 2: Important to detect early |
| Amblyopia |
| Suspected unilateral: 2-line interocular difference and a unilateral amblyogenic factor |
| Presumed unilateral: ≥3 line interocular difference, a unilateral amblyogenic factor, and worse eye VA > 20/64 |
| Strabismus: intermittent in primary gaze |
| Refractive error |
| Anisometropia (interocular difference > 1D hyperopia, > 1.5D astigmatism, or > 3D myopia)* |
| Hyperopia > 3.25 D and < 5.0 D AND interocular difference in SE ≥ 0.5 D |
| Astigmatism > 1.5 and < 2.5 D |
| Myopia ≥ 4.0 and < 6.0 D |
| Group 3: Detection clinically useful |
| Unexplained reduced VA |
| Bilateral: no bilateral amblyogenic factor, worse eye VA < 20/50 for 3-year-olds or < 20/40 for 4-year-olds, contralateral eye VA worse than 20/40 for 3-year-olds or 20/30 for 4-year-olds |
| Unilateral: no unilateral amblyogenic factor, worse eye VA < 20/50 for 3-year-olds or < 20/40 for 4-year-olds or ≥ 2 line difference between eyes (except 20/16 and 20/25) |
| Refractive error |
| Hyperopia > 3.25 and < 5.0 D AND interocular difference in SE < 0.5 D |
| Myopia > 2.0 and < 4.0 D |
Modified from Ophthalmology, 111, The Vision in Preschoolers Study Group, Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision in Preschoolers Study, 637–650, Copyright 2004, with permission from American Academy of Ophthalmology.
Vision Screening
Children were administered screening tests of refractive error, VA, and ocular alignment. In each year, the screeners were masked to whether the child had passed the Head Start screening and whether the child wore glasses. Licensed eye care professionals, who had completed training in performing screening tests according to VIP Study standardized protocols, administered 11 screening tests during years 1 and 2 (phase I). In phase I of the VIP Study, the sensitivities of the screening tests were compared (at specificities set at 90% and 94%) to determine which tests performed best in the detection of one or more targeted conditions, the most severe (group 1) conditions, and each targeted condition. For the purposes of this report, the tests included in the analysis are the tests with the highest sensitivity for detection of one or more targeted conditions (each of which involves assessment of refractive error or VA) and the tests of ocular alignment with the highest sensitivity for the detection of strabismus.
In year 1, the best tests for detection of one or more targeted conditions were noncycloplegic retinoscopy (NCR), Retinomax autorefraction (Right Manufacturing, Virginia Beach, VA), and Lea Symbols (Precision Vision, LaSalle, IL and Good-Lite Co., Elgin, IL) and HOTV VA tests (both in a crowded, isolated line format), and the best tests for the detection of strabismus included these four tests as well as cover-uncover test (CT) and Random Dot “E” (RDE; Stereo Optical, Inc., Chicago, IL) stereoacuity. In year 2, the best tests for detection of one or more targeted conditions were the Retinomax autorefractor and SureSight Vision Screener (Welch-Allyn, Inc., Skaneateles, NY), and the best tests for strabismus included these two tests as well as the MTI PhotoScreener (PhotoScreener, Inc., Palm Beach, FL) and the Stereo Smile Test II (Stereo Optical, Inc.).9
Screening tests in year 3 (phase II) were administered by nurse and lay screeners who had completed training in performing screening tests according to VIP Study standardized protocols. The screening tests selected for phase II (Retinomax, SureSight Vision Screener, and Lea Symbols VA testing) were those tests that had performed best in years 1 and 2 when administered by a licensed eye care professional but did not require extensive training. Stereo Smile Test II stereoacuity testing was also included because of its sensitivity in detecting strabismus in phase IA.11
Statistical Analysis
The change in sensitivity that resulted from combining the results of each of the best tests of refractive error or VA with the results of each of the best tests of ocular alignment was determined for the detection of strabismus, one or more targeted conditions, and the most severe (group 1) conditions, compared with the sensitivity from a test of refractive error or VA alone. Analyses were performed with specificity set at 90% and at 94%. When the results of two screening tests were combined, failure criteria were adjusted so that failure on either of the tests provided the desired level of specificity. Analysis could be performed only of the results of tests administered within the same data-collection year (i.e., on the same children).
Because multiple tests were performed for the test of change in sensitivity due to combining tests, we used the Hochberg procedure (a less conservative and more powerful procedure than the Bonferroni method) to adjust the probabilities from multiple comparisons and to control the overall type I error (0.05, two-sided).19 This procedure was executed with commercial software (PROC MULTTEST in SAS/STAT 9.1; SAS Institute, Inc., Cary, NC).
Results
In VIP years 1 to 3, 157 children with strabismus were identified by standardized comprehensive eye examination (Table 2). For each year of the study, the characteristics of the strabismus (e.g., frequency, direction, and magnitude) and the prevalence of coexisting significant refractive error or amblyopia are shown in Table 2. These characteristics may affect the likelihood that children with strabismus would be identified by a test of refractive error or VA alone. Table 3 shows the prevalence of amblyopia among children with various forms of strabismus. Of 17 intermittent strabismic children with amblyopia, 10 (58.8%) had a coexisting amblyogenic refractive error. Table 4 shows the distribution of strabismus and other VIP targeted conditions in children who passed and failed the initial Head Start vision screening. The majority of children (range, 72%–93%) with VIP targeted conditions had failed Head Start vision screening. Some children were not testable on each screening test of ocular alignment and the distribution of constant strabismus and strabismic amblyopia among testable and untestable children is shown in Table 5.
Table 2.
Characteristics of Strabismus in Years 1–3
| Year
|
|||
|---|---|---|---|
| Characteristic | 1 (n= 48) | 2 (n = 62) | 3 (n = 47) |
| Frequency | |||
| Constant | 26 (54%) | 41 (66%) | 31 (66%) |
| Intermittent | 22 (46%) | 21 (34%) | 16 (34%) |
| Direction | |||
| Esotropia | 29 (60%) | 40 (65%) | 33 (70%) |
| Exotropia | 18 (38%) | 21 (34%) | 14 (30%) |
| Unknown | 1 (2%) | 1 (1%) | 0 |
| Magnitude* | |||
| <10Δ | 5 (10%) | 13 (21%) | 6 (13%) |
| ≥10Δ | 42 (88%) | 48 (77%) | 40 (85%) |
| Unknown | 1 (2%) | 1 (1%) | 1 (2%) |
| Amblyopia† | |||
| Present | 16 (33%) | 22 (35%) | 22 (47%) |
| Absent | 32 (67%) | 40 (65%) | 25 (53%) |
| Significant refractive error‡ | |||
| Present | 24 (50%) | 34 (55%) | 32 (68%) |
| Absent | 24 (50%) | 28 (45%) | 15 (32%) |
Table 3.
The Prevalence of Amblyopia in Children with Various Forms of Strabismus
| n | Amblyopia n (%) | |
|---|---|---|
| Strabismus | 157 | 60 (38.2) |
| Constant | 98 | 43 (43.9) |
| Intermittent | 59 | 17 (28.8) |
| Esotropia | 102 | 45 (44.1) |
| Exotropia | 53 | 14 (26.4) |
| <10Δ | 24 | 9 (37.5) |
| ≥10Δ | 130 | 50 (38.5) |
Table 4.
The Distribution of VIP Targeted Conditions in Head Start Vision Screening Failures and Passers
| Head Start Fail (n = 2227) | Head Start Pass (n = 1813) | ||
|---|---|---|---|
| n | n (%) | n (%) | |
| Group 1 | 521 | 461 (88.5) | 60 (11.5) |
| Amblyopia | 155 | 144 (92.9) | 11 (7.1) |
| Strabismus-constant | 98 | 87 (88.8) | 11 (11.2) |
| Refractive error | 467 | 416 (89.1) | 51 (10.9) |
| Strabismus | 157 | 133 (84.7) | 24 (15.3) |
| Constant | 98 | 87 (88.8) | 11 (11.2) |
| Intermittent | 59 | 46 (78.0) | 13 (22.0) |
| Esotropia | 102 | 93 (91.2) | 9 (8.8) |
| Exotropia | 53 | 38 (71.7) | 15 (28.3) |
| Amblyopic strabismics | 60 | 56 (93.3) | 4 (6.67) |
| Amblyopia | 264 | 233 (88.3) | 31 (11.7) |
| Anisometropia | 309 | 257 (83.2) | 52 (16.8) |
| Hyperopia | 472 | 397 (84.1) | 75 (15.9) |
| Myopia | 92 | 83 (90.2) | 9 (9.80) |
| Astigmatism | 505 | 425 (84.2) | 80 (15.8) |
| Significant refractive error | 919 | 759 (82.6) | 160 (17.4) |
Table 5.
The Distribution of Constant Strabismus and Strabismic Amblyopia among Testable and Untestable Children for Screening Tests of Alignment
| Testable | Constant Strabismus | Strabismic Amblyopia | Untestable | Constant Strabismus | Strabismic Amblyopia | |
|---|---|---|---|---|---|---|
| Screening Test | n | n (%) | n (%) | n | n (%) | n (%) |
| Cover-uncover | 1117 | 24 (2.15) | 16 (1.43) | 24 | 2 (8.33) | 0 (0.00) |
| Random Dot E | 1026 | 23 (2.24) | 14 (1.36) | 111 | 3 (2.70) | 2 (1.80) |
| MTI | 1360 | 38 (2.79) | 21 (1.54) | 84 | 3 (3.57) | 1 (1.19) |
| Stereo Smile II | ||||||
| Phase IA | 1422 | 39 (2.74) | 20 (1.41) | 21 | 1 (4.76) | 1 (4.76) |
| Phase II lay | 1427 | 31 (2.17) | 22 (1.54) | 23 | 0 (0.00) | 0 (0.00) |
| Phase II nurse | 1427 | 31 (2.17) | 22 (1.54) | 22 | 0 (0.00) | 0 (0.00) |
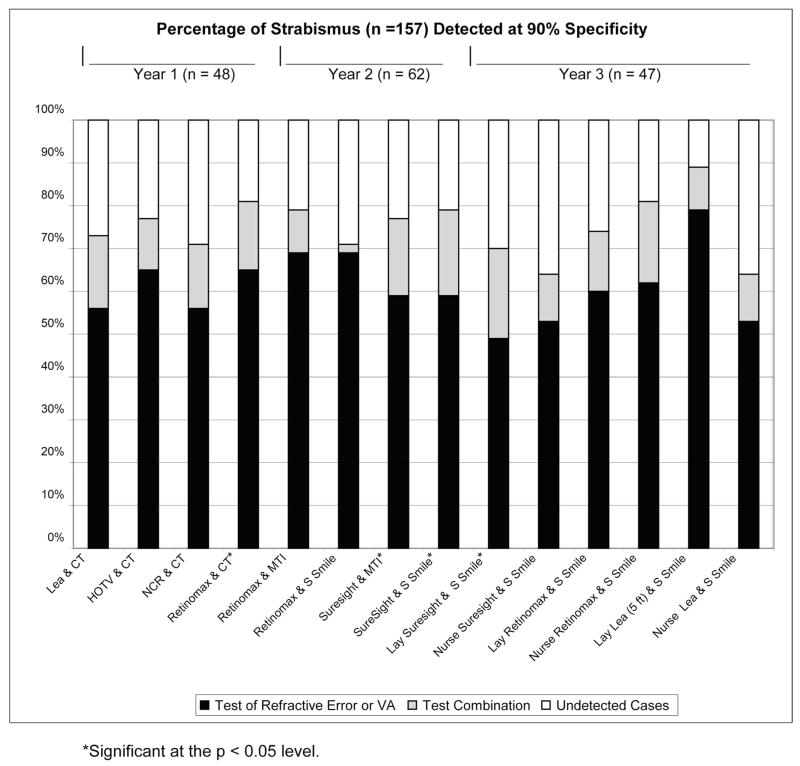
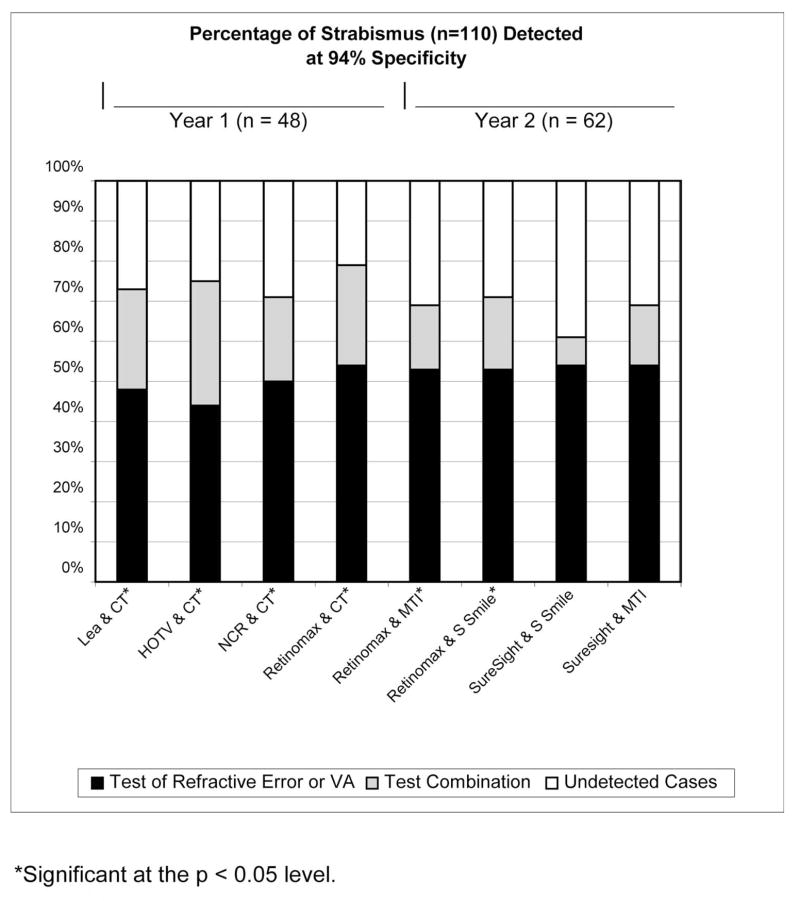
Figures 1 and 2 show the change in sensitivity for detection of strabismus resulting from combining each of the best-performing tests of ocular alignment with each of the best-performing tests of refractive error or VA for each year at 90% and 94% specificity, respectively. Analysis was performed for RDE, but the results are not presented because combinations with the RDE stereoacuity test generally resulted in no change or a decrease in sensitivity. In addition, the set specificity level of 90% or 94% often could not be attained in combinations with the RDE.
Figure 1.
Percentage of strabismus detected at 90% specificity.
Figure 2.
Percentage of strabismus detected at 94% specificity.
At 90% specificity, statistically significant increases in sensitivity for detection of strabismus were found for the combinations of Retinomax and CT (16% increase, P = 0.038), SureSight Vision Screener and MTI PhotoScreener (18% increase, P = 0.014), SureSight Vision Screener and Stereo Smile Test II (20% increase, P = 0.01), and Lay screener administration of the SureSight Vision Screener and Stereo Smile Test II (21% increase, P = 0.023).
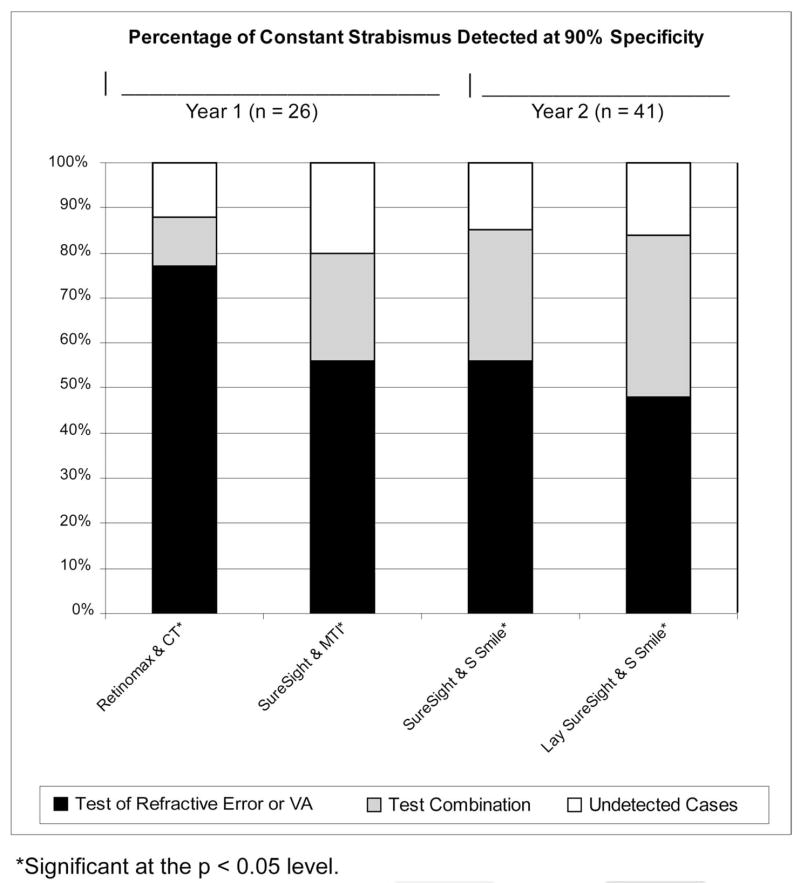
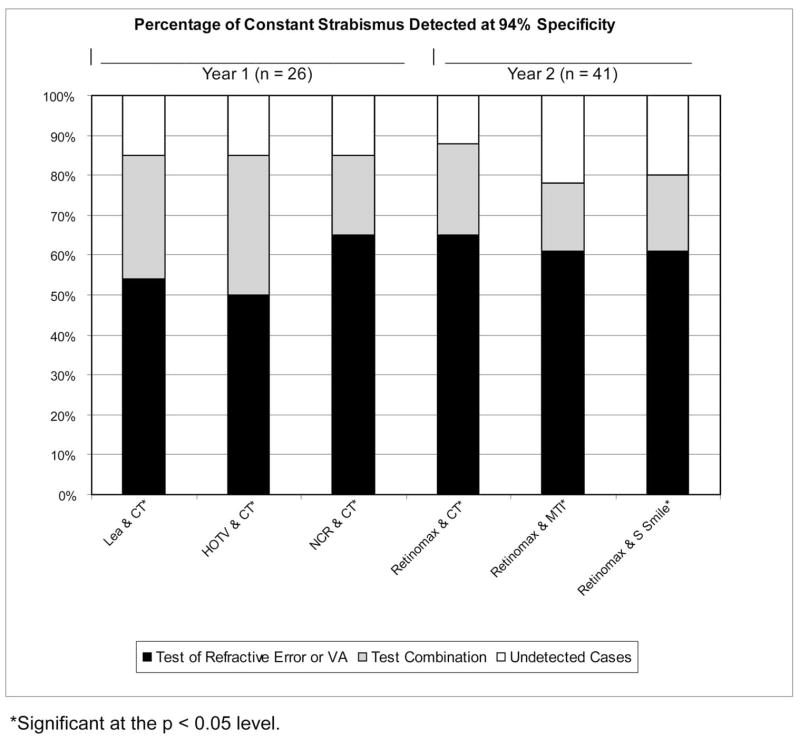
With specificity set at 94% (or the closest possible specificity that was attainable), statistically significant increases in sensitivity for detection of strabismus were found for the combinations of NCR and CT (21% increase, P = 0.008), Retinomax and CT (25% increase, P = 0.008), Lea Symbols VA and CT (25% increase, P = 0.008), HOTV VA and CT (31% increase, P = 0.0008), Retinomax and MTI Photo-Screener (16% increase, P = 0.04), and Retinomax and Stereo Smile Test II (18% increase, P = 0.009). Specificity of 94% could not be precisely attained for the combinations of Retinomax and CT (93%), Lea Symbols VA and CT (93%), HOTV VA and CT (91%), and Retinomax and MTI Photo-Screener (93%). In year 3 (lay screener administration of tests), 94% specificity was not attainable for any test combinations. For test combinations that resulted in a statistically significant increase in sensitivity for detection of strabismus, further analysis was performed to determine the change in sensitivity for detection of constant strabismus resulting from combining each of the best-performing tests of ocular alignment with each of the best-performing tests of refractive error or VA for each year at 90% and 94% specificity (see Figs. 3 and 4).
Figure 3.
Percentage of constant strabismus detected at 90% specificity.
Figure 4.
Percentage of constant strabismus detected at 94% specificity.
As shown in Table 6, analysis of sensitivity for detection of one or more targeted conditions and the most severe conditions at 90% specificity revealed statistically significant increases in detection of the most severe conditions for lay screener administration of the Retinomax plus Stereo Smile Test II (6% increase, P = 0.014) and lay screener administration of the SureSight Vision Screener plus Stereo Smile Test II (7% increase, P = 0.006). As shown in Table 7, analysis of sensitivity for detection of one or more targeted conditions and the most severe conditions at 94% specificity revealed statistically significant increases for HOTV VA and CT (13% increase, P = 0.0006; 17% increase, P = 0.0006, respectively). However, it should be reiterated that 94% specificity was not attainable for HOTV VA and CT (91% was the maximum attainable specificity).
Table 6.
Increase or Decrease in Sensitivity at 90% Specificity with Adjusted Probability
| Combination of Tests | Overall | Group 1 | Strabismus | Specificity |
|---|---|---|---|---|
| Year 1 | (n = 346) | (n = 139) | (n = 448) | (n = 796) |
| NCR and CT | 0.02 (0.48) | 0.03 (0.32) | 0.15 (0.10) | 0.90 |
| Retinomax and CT | 0.03 (0.20) | 0.04 (0.10) | 0.16 (0.038) | 0.90 |
| Lea VA and CT | −0.06 (0.21) | −0.01 (1.00) | 0.17 (0.10) | 0.90 |
| HOTV VA and CT | 0.00 (0.84) | 0.00 (1.00) | 0.12 (0.07) | 0.90 |
| Year 2 | (n = 409) | (n = 172) | (n = 62) | (n = 1037) |
| Retinomax and MTI | 0.02 (0.33) | 0.02 (0.26) | 0.10 (0.12) | 0.90 |
| SureSight and MTI | −0.01 (0.90) | 0.06 (0.10) | 0.18 (0.014) | 0.90 |
| Retinomax and Stereo Smile II | 0.01 (0.90) | 0.04 (0.26) | 0.06 (0.17) | 0.90 |
| SureSight and Stereo Smile II | −0.05 (0.21) | 0.07 (0.10) | 0.20 (0.01) | 0.90 |
| Year 3 | (n = 462) | (n = 210) | (n = 47) | (n = 990) |
| Lay Retinomax and Stereo Smile II | 0.03 (0.32) | 0.06 (0.014) | 0.14 (0.10) | 0.90 |
| Lay SureSight and Stereo Smile II | 0.02 (0.59) | 0.07 (0.006) | 0.21 (0.023) | 0.90 |
| Lay Lea VA (5 ft) and Stereo Smile II | 0.00 (0.59) | 0.00 (0.83) | 0.10 (0.10) | 0.90 |
| Nurse Retinomax and Stereo Smile II | −0.05 (0.08) | 0.01 (0.83) | 0.19 (0.10) | 0.90 |
| Nurse SureSight and Stereo Smile II | −0.06 (0.054) | −0.02 (0.83) | 0.11 (0.23) | 0.90 |
| NURSE Lea VA and Stereo Smile II | −0.02 (0.59) | 0.00 (0.83) | 0.11 (0.23) | 0.90 |
Probabilities were adjusted by the Hochberg procedure for multiple comparison. Changes significant at the P < 0.05 level are indicated in bold type.
Table 7.
Increase or Decrease in Sensitivity at 94% Specificity with Adjusted Probability
| Combination of Tests | Overall | Group 1 | Strabismus | Specificity |
|---|---|---|---|---|
| Year 1 | (n = 346) | (n = 139) | (n = 448) | (n = 796) |
| NCR and CT | 0.01 (0.87) | 0.01 (0.87) | 0.21 (0.008) | 0.94 |
| Retinomax and CT | 0.03 (0.40) | 0.01 (0.87) | 0.25 (0.008) | 0.93† |
| Lea VA and CT | 0.00 (0.87) | 0.06 (0.80) | 0.25 (0.008) | 0.93† |
| HOTV VA and CT | 0.13 (0.0006) | 0.17 (0.0006) | 0.31 (0.0008) | 0.91† |
| Year 2 | (n = 409) | (n = 172) | (n = 62) | (n = 1037) |
| Retinomax and MTI | 0.04 (0.14) | 0.04 (0.39) | 0.16 (0.04) | 0.93† |
| SureSight and MTI | 0.04 (0.23) | 0.06 (0.19) | 0.15 (0.10) | 0.93† |
| Retinomax and Stereo Smile II | −0.02 (0.23) | 0.01 (0.55) | 0.18 (0.009) | 0.94 |
| SureSight and Stereo Smile II | −0.03 (0.23) | 0.03 (0.55) | 0.07 (0.37) | 0.94 |
Probabilities were adjusted using the Hochberg procedure for multiple comparisons. Changes significant at the P < 0.05 level are indicated in bold type.
94% specificity is not attainable.
As shown in Tables 8 and 9, the adjustments to failure criteria required to maintain the same level of specificity (which is necessary for a fair comparison of sensitivities) were generally small.9
Table 8.
Failure Criteria for the Year 1 Test of VA from the Single or Combined Screening Test
| Age
|
|||||
|---|---|---|---|---|---|
| Specificity | Setting | 3 y | 4 y | 5 y | |
| Lea VA | 90% | Single test | 10/32 | 10/20 | 10/20 |
| 90% | Combined with CT | 10/16 | 10/32 | 10/20 | |
| 94% | Single test | 10/32 | 10/25 | 10/20 | |
| 93% | Combined with CT | 10/32 | 10/32 | 10/20 | |
| HOT VA | 90% | Single test | 10/25 | 10/25 | 10/20 |
| 90% | Combined with CT | 10/32 | 10/25 | 10/20 | |
| 93% | Single test | 10/32 | 10/32 | 10/25 | |
| 91% | Combined with CT | 10/32 | 10/32 | 10/20 | |
Any child whose acuity score was equal to or worse than the value shown was scored as failing the screening test.
Table 9.
Failure Criteria for Test of Refraction from the Single and Combined Screening Tests
| Specificity | Setting | Hyperopia | Myopia | Astigmatism | Anisometropia | |
|---|---|---|---|---|---|---|
| Year 1 | ||||||
| NCR | 90% | Single test | ≥2.75 D | ≥2.75 D | ≥1.25 D | ≥1.50 D |
| 90% | Combined with CT | ≥2.75 D | ≥2.75 D | ≥1.50 D | ≥1.50 D | |
| 94% | Single test | ≥2.50 D | ≥2.75 D | ≥2.00 D | ≥1.50 D | |
| 94% | Combined with CT | ≥2.75 D | ≥2.75 D | ≥2.00 D | ≥2.25 D | |
| Retinomax | 90% | Single test | ≥1.50 D | ≥2.75 D | ≥1.50 D | ≥2.00 D |
| 90% | Combined with CT | ≥1.75 D | ≥2.75 D | ≥1.50 D | ≥2.25 D | |
| 94% | Single test | ≥1.75 D | ≥2.75 D | ≥2.00 D | ≥2.75 D | |
| 94% | Combined with CT | ≥1.75 D | ≥2.75 D | ≥2.25 D | ≥2.75 D | |
| Year 2 | ||||||
| Retinomax | 90% | Single test | ≥1.50 D | ≥2.75 D | ≥1.50 D | ≥1.75 D |
| 90% | Combined with MTI | ≥1.50 D | ≥4.25 D | ≥1.50 D | ≥1.75 D | |
| 90% | Combined with Stereo Smile II | ≥2.75 D | ≥4.25 D | ≥1.50 D | ≥3.00 D | |
| 94% | Single test | ≥2.50 D | ≥2.75 D | ≥1.75 D | ≥2.50 D | |
| 93% | Combined with MTI | ≥2.00 D | ≥4.25 D | ≥2.00 D | ≥2.75 D | |
| 94% | Combined with Stereo Smile II | ≥2.25 D | ≥4.25 D | ≥2.25 D | ≥3.00 D | |
| SureSight | 90% | Single test | ≥4.00 D | ≥1.00 D | ≥1.50 D | ≥3.00 D |
| 90% | Combined with MTI | ≥4.75 D | ≥1.00 D | ≥1.75 D | ≥3.00 D | |
| 90% | Combined with Stereo Smile II | ≥4.25 D | ≥1.00 D | ≥2.25 D | ≥3.00 D | |
| 94% | Single test | ≥4.25 D | ≥1.00 D | ≥1.75 D | ≥3.50 D | |
| 93% | Combined with MTI | ≥5.50 D | ≥1.00 D | ≥2.25 D | ≥4.25 D | |
| 94% | Combined with Stereo Smile II | ≥5.75 D | ≥1.00 D | ≥2.50 D | ≥3.25 D | |
Any child with refractive error of the listed value or greater was scored as failing the screening test.
Information was not collected regarding whether children had been receiving eye care, but was collected regarding spectacle wear. Approximately 32% of the children with strabismus were wearing glasses (and therefore presumably had seen an eye care professional). The majority (range, 60%–100%; average 73%) of children newly identified by the addition of a test of alignment were not currently wearing spectacles, and it is not known whether these children were currently under care for strabismus.
Discussion
Vision screenings currently used in preschool children often include eye alignment testing to detect strabismus and binocularity problems7,9,12–17 and it is important to consider the effect of adding a test of ocular alignment on sensitivity for specifically detecting strabismus. While the goal of combining tests is often to increase sensitivity, the possible disadvantage of increasing false positives and unreadable or uninterpretable rates and costs must also be considered.
Because amblyopia and strabismus have been found to be associated frequently with significant refractive error (Table 2),9,10 many children with strabismus were identified by the best tests of refraction or VA alone. However, combining the unilateral CT, MTI PhotoScreener, or Stereo Smile Test II with a refractive error or a VA screening test generally resulted in increased sensitivity for the detection of strabismus (increases ranged from 6% to 31%). The greatest and most consistent increases in sensitivity for detection of strabismus were found with the addition of the unilateral CT. Therefore, these results agree with Brooks’ report regarding screening tests for strabismus that the unilateral cover test was “the most sensitive and specific.”4 Combinations with the RDE stereoacuity test generally resulted in decreased sensitivity; however, this may be at least in part due to the reduced testability when the RDE was used with some of the youngest children in this population.9
Approximately 38% of the children with strabismus were amblyopic. It is not known how many of those in this study had had the condition identified by parents20 or other professionals and were under care versus those in whom it was newly identified by the vision screening. Approximately one third of the children with strabismus in this population were wearing spectacles and were presumably under the care of an eye care professional. This refractive treatment alone could provide substantial reductions in amblyopia.21 Furthermore, earlier detection of certain refractive errors may prevent the development of amblyopia.22 Therefore, such children may be detected as having the amblyogenic factor, but no longer have the amblyopia detectable with an acuity test. The majority of children detected by the addition of a test of alignment were not wearing spectacles (range, 60%–100%; average, 73%) and it is not known if any of these children were currently under care. One factor that must be considered in the decision of whether to add a test of alignment to increase sensitivity for the detection of strabismus is the expected prevalence of undetected strabismus in the population of children to be screened.
One should also consider the added time and expense of adding a screening test of alignment. The administration time for unilateral cover testing (without prism neutralization) is approximately 1 minute, and the administration times for the Stereo Smile Test II and MTI PhotoScreener have been reported to be 3 minutes11 and 2.5 minutes,23 respectively. When administering the MTI PhotoScreener, the screener must also allow time (3 minutes) for the Polaroid film to develop properly and ensure that the image is acceptable. Although this additional time would not be expected to result in fatigue in the children being screened, it could have a significant impact on personnel time and costs if a large number of children must be screened. The cost of the personnel needed to administer the test must also be considered. A CT can be reliably performed only by a highly trained examiner; therefore tests, such as the Stereo Smile Test II which can be administered by trained lay-staff screeners may have a lower total personnel cost. Expenses include the cost of the equipment as well as the cost of additional personnel time. The cost of the tests of alignment included in this study varies widely from approximately $30 for an occluder and pediatric fixation targets, to approximately $500 for the Stereo Smile Test II, to approximately $5000 for the MTI PhotoScreener. Additional costs for the MTI include the Polaroid film (for each child screened), photograph interpretation (per child screened if the screener is not trained/skilled in interpretation) and possibly a carrying case, extra battery, and maintenance contract. Depending on the screening environment, additional costs to control lighting may be incurred. For example, a stand lamp may be needed to ensure adequate lighting for stereoacuity testing or curtains may be needed for MTI Photo-Screener administration to ensure that the area is adequately dark. Finally, antibacterial wipes may be needed to clean screening equipment between children (such as the occluder in the CT).
For test combinations that resulted in a significant increase in sensitivity for detection of strabismus, the effect of a test combination on sensitivity for detection of one or more targeted conditions must also be considered. Although a statistically significant increase in detection of both strabismus and one or more targeted conditions would be ideal, any increase in sensitivity for detection of strabismus should be accompanied by at least a corresponding small increase in sensitivity for detection of one or more targeted conditions, unless the failure criteria associated with the new test combination resulted in the detection of strabismus at the expense of failing to detect one or more other conditions. For example, if there were 500 children with one or more targeted conditions, 50 children with strabismus and a test combination resulted in a 20% increase in sensitivity of strabismus (10 children), a 2% (10 child) increase in detection of one or more targeted conditions should be observed. If an increase of approximately that magnitude is not observed, then some of the children with newly detected strabismus were identified at the expense of failing to detect one or more other conditions. Similarly, the effect of a test combination on sensitivity for detection of the most severe conditions must also be considered. Although a statistically significant increase in detection of both strabismus and the most severe conditions would be ideal, an increase in sensitivity for detection of strabismus may not be associated with a statistically significant increase in detection of the most severe conditions, because not all strabismus was classified as one of the most severe conditions (Table 1). Nevertheless, one should not see a decrease in sensitivity for detection of the most severe conditions. For licensed eye care professionals, addition of CT to screening with Retinomax met these criteria at 90% and 94% specificity, although the increases in sensitivity for detection of one or more targeted conditions and the most severe conditions were not statistically significant. For trained lay screeners, the Sure-Sight Vision Screener combined with the Stereo Smile Test II best met the criteria. This combination resulted in a significant increase in detection of strabismus (approximately nine children), a small increase in detection of one or more targeted conditions (approximately six children) and a significant increase in the detection of the most severe conditions (approximately nine children). Although the increase in detection of one or more targeted conditions was slightly lower than expected, it is noteworthy that any increases in detection of strabismus were at the expense of failing to detect children with group 2 or 3, rather than group 1, conditions (Table 1).
Conclusion
For vision screening tests of refraction or VA administered by eye care professionals, the most efficient and low-cost way to achieve a statistically significant increase (16%–25%) in the sensitivity for detection of strabismus was achieved by the addition of the unilateral cover test to the Retinomax. For trained lay screeners, the addition of Stereo Smile Test II to the Sure Sight Vision Screener resulted in a statistically significant increase (21%) in the sensitivity of detection of strabismus at 90% specificity and a statistically significant increase (7%) in the sensitivity for detection of the most severe conditions. The decision of whether to include a test of alignment should be based on the screening program’s goals (e.g., targeted visual conditions) and resources.
Acknowledgments
Supported by National Eye Institute Grants U10EY12534, U10EY12545, U10EY12547, U10EY12550, U10EY12644, U10EY12647, and U10EY12648. Paulette Schmidt was principal investigator for a grant (<$20,000) issued to The Ohio State University Research Foundation from Welch Allyn, Inc. for testing a prototype of the SureSight Vision Screener. Pennsylvania College of Optometry receives royalties <$10,000 annually on sales of the Stereo Smile Test.
APPENDIX
The Vision in Preschoolers Study Group
Executive Committee
Paulette Schmidt (Chair), Agnieshka Baumritter, Elise Ciner, Lynn Cyert, Velma Dobson, Beth Haas, Marjean Taylor Kulp, Maureen Maguire, Bruce Moore, Deborah Orel-Bixler, Ellen Peskin, Graham Quinn, Maryann Redford, Janet Schultz, and Gui-shuang Ying.
Writing Committee
Marjean Taylor Kulp (Chair), Gui-shuang Ying, Maureen Maguire, Velma Dobson, Graham Quinn, Elise Ciner, Paulette Schmidt, Lynn Cyert, Agnieshka Baumritter, and Deborah Orel-Bixler.
Participating Centers
AA, Administrative Assistant; BPC, Back-up Project Coordinator; GSE, Gold Standard Examiner; NS, Nurse Screener; LS, Lay Screener; LPS, LEP Screener; PI, Principal Investigator; PC, Project Coordinator; PL, Parent Liaison; PR, Programer; VD, Van Driver; NHE, Nurse/Health Coordinator.
University of California Berkeley School of Optometry (Berkeley, CA)
Deborah Orel-Bixler (PI/GSE/LPS), Pamela Qualley (PC), Dru Howard (BPC/PL), Sarah Fisher (GSE/LPS), Darlene Fong (GSE), Cindy Hsiao (GSE), Selim Koseoglu (GSE), A. Mika Moy (GSE), Sharyn Shapiro (GSE), Lisa Verdon (GSE), Tonya Watson (GSE), Sara Frane (LPS), Nina Friedman (LPS), Jennifer Seino (LPS), Sean McDonnell (LS/VD), Erika Paez (LS), Coriemae Perea (LS), Darlene Sloan (LS), Evelyn Smith (LS), Leticia Soto (LS), Angela Stelly-Leonard (LS/NHE), Lempi Miller Suzuki (BPC), Beatrice Moe (NS), Robert Prinz (LS/VD).
New England College of Optometry (Boston, MA)
Bruce Moore (PI GSE/LPS), Joanne Bolden (PC), Sandra Umaña (PC), Justin Smith (BPC), Nicole Quinn (GSE/LPS), Nancy Carlson (GSE/LPS), Melissa Suckow (GSE/LPS), Amy Croteau (GSE), Barry Kran (GSE), Jean Ramsey (GSE), Erik Weissberg (GSE), Daniel Kurtz (LPS), Daniel Laby (LPS), Stacy Lyons (LPS), Marthedala Chery (LS/PL), Leticia Gonzalez (LS/PL), Edward Braverman (LS/VD), Susan Crowley (NHE), Paul Dennehy (VD), Benny Jaramillo (VD), Amy Silbert (BPC), Nicole Quinn (GSE), Heather Bordeau (GSE), Micki Flynn (GSE), Maria Diaz (LS), Rosalyn Johnson (LS/PL), Charlene Henderson (LS/PL), Maria Bonila (PL), Cathy Doherty (NS), Cynthia Peace-Pierre (NS), Ann Saxbe (NS), Vadra Tabb (NS).
The Ohio State University College of Optometry (Columbus, OH)
Paulette Schmidt (PI), Marjean Taylor Kulp (Co-investigator, GSE/LPS), Molly Biddle (PC), Jo Haynes (PC), Jason Hudson (BPC), Kristyne Edwards (GSE/LPS), Heather Gebhart (GSE/LPS), Ann Hickson (GSE/LPS), LeVelle Jenkins (GSE/LPS), Sandra Anderson (GSE), Nancy Evans (GSE), Jay Henry (GSE), Richard Hertle (GSE), Jeffrey Hutchinson (GSE), Andrew Toole (GSE), Michael Earley (LPS), Sherry Crawford (LPS), Kathy Reuter (LPS), Keith Johnson (LS/VD), Beth Haas (LS), Tonya James (LS), Denise Martin (LS), Sandra Dorton (NHE), Youlanda Grace (NHE), Trina Hisle (NHE), Cheryl Jones (NHE), Betty Smith (NHE), Robert Bower (VD), Melanie Ackerman (GSE), Richard Shoemaker (VD), Rita Atkinson (LS), Fran Hochstedler (LS), Tasha Jones (LS), June Kellum (LS), Christina Dunagan (NS), Joy Cline (NS), Sue Rund (NS).
Pennsylvania College of Optometry (Philadelphia, PA)
Elise Ciner (PI, GSE/LPS), Angela Duson (PC/LS), Lydia Parke (BPC), Mark Boas (GSE/LPS), Shannon Burgess (GSE/LPS), Penelope Copenhaven (GSE/LPS), Ellie Francis (GSE/LPS), Michael Gallaway (GSE/LPS), Graham Quinn (GSE/LPS), Janet Schwartz (GSE/LPS), Brandy Scombordi-Raghu (GSE/LPS), Janet Swiatocha (GSE/LPS), Edward Zikoski (GSE/LPS), Jennifer Lin (GSE), Sheryl Menacker (GSE), Rose Little (LS/PL), Geneva Moss (LS/PL), Jose Figueroa (LS/VD), Barbara Hall (LS), Eric Nesmith (LS), Gwen Gold (BPC, NHE, PL), Deborah Ciner (PL), Elizabeth Jordan (PL), David Harvey (VD), Leslie Kennedy (LS/PL), Rosemary Little (LS/PL), Latricia Rorie (LS), Shirley Stokes (LS/PL), Ashanti Carter (PL), Sandra Hall (NS), Lisa Hildebrand (NS), Margaret Lapsley (NS), Cecilia Quenzer (NS), Lynn Rosenbach (NHC/NS).
Northeastern State University Oklahoma College of Optometry (Tahlequah, OK)
Lynn Cyert (PI, GSE/LPS), Linda Cheatham (PC/VD), Anna Chambless (BPC, PL), Colby Beats (GSE), Debbie Coy (GSE), Jeffrey Long (GSE), Jerry Carter (GSE), Shelly Rice (GSE), James Dunn (LPS), Elisabeth Harrington (LPS), Leslie Trimble (LPS), Shelly Dreadfulwater (LS/PL), Cindy McCully (LS/PL), Rod Wyers (LS, VD), Edith Bingham (LS), Vicky Taylor (LS), Glenda Byfield (PL), Pat Gower (NHE), Kathryn Roastingear (NHE), Elizabeth Ross (NHE), Ramona Blake (LS/PL), Jamey Boswell (LS/PL), Anna Brown (LS/PL), Jeff Fisher (NS), Jody Larrison (NS).
Study Center: The Ohio State University College of Optometry (Columbus, OH)
Paulette Schmidt (PI) and Beth Haas (Study Coordinator).
Coordinating Center: University of Pennsylvania, Department of Ophthalmology (Philadelphia, PA)
Maureen Maguire (PI), Agnieshka Baumritter (Project Director), Mary Brightwell-Arnold (Systems Analyst), Christine Holmes (AA), Andrew James (PR), Aleksandr Khvatov (PR), Chengcheng Liu (Biostatistician), Lori O’Brien (AA), Ellen Peskin (Project Director), Claressa Whearry (AA), Gui-shuang Ying (Biostatistician).
National Eye Institute (Bethesda, MD)
Maryann Redford.
Footnotes
Disclosure: P. Schmidt, Welch Allyn, Inc. (F); E. Ciner, Stereo Optical, Inc. (F); all others in The Vision in Preschoolers Study Group, None
References
- 1.Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiol. 2005;12:243–250. doi: 10.1080/09286580590967772. [DOI] [PubMed] [Google Scholar]
- 2.Lavrich JB, Nelson LB. Diagnosis and treatment of strabismus disorders. Pediatr Clin North Am. 1993;40:737–752. doi: 10.1016/s0031-3955(16)38584-4. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein AM, Scully J. Epidemiological aspects of squint. BMJ. 1967;3:334–338. doi: 10.1136/bmj.3.5561.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks SE. Strabismus and amblyopia in children: the role of primary care. Compr Ther. 1997;23:60–66. [PubMed] [Google Scholar]
- 5.Fletcher MC, Silverman SJ. Strabismus, I: a summary of 1,110 consecutive cases. Am J Ophthalmol. 1966;61:86–94. [PubMed] [Google Scholar]
- 6.Castiglia PT. Strabismus. J Pediatr Health Care. 1994;8:236–238. doi: 10.1016/0891-5245(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics. Eye examination in infants, children, and young adults by pediatricians. Pediatrics. 2003;111:902–907. [PubMed] [Google Scholar]
- 8.US Preventive Services Task Force. Screening for visual impairment in children younger than age 5 years: recommendation statement. Ann Fam Med. 2004;2:263–266. doi: 10.1370/afm.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vision in Preschoolers Study Group. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision in Preschoolers Study. Ophthalmology. 2004;111:637–650. doi: 10.1016/j.ophtha.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Vision in Preschoolers Study Group. Sensitivity of screening tests for detecting vision in preschoolers-targeted vision disorders when specificity is 94% Optom Vis Sci. 2005;82:432–438. doi: 10.1097/01.OPX.0000162660.14378.30. [DOI] [PubMed] [Google Scholar]
- 11.Vision in Preschoolers Study Group. Preschool vision screening tests administered by nurse screeners compared with lay screeners in the vision in preschoolers study. Invest Ophthalmol Vis Sci. 2005;46:2639–2648. doi: 10.1167/iovs.05-0141. [DOI] [PubMed] [Google Scholar]
- 12.Robinson B, Bobier WR, Martin E, Bryant L. Measurement of the validity of a preschool vision screening program. Am J Public Health. 1999;89:193–198. doi: 10.2105/ajph.89.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman DK, East MM. Preschool vision screening: negative predictive value for amblyopia. Br J Ophthalmol. 1999;83:676–679. doi: 10.1136/bjo.83.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry JC, Konig HH. Test characteristics of orthoptic screening examination in 3 year old kindergarten children. Br J Ophthalmol. 2003;87:909–916. doi: 10.1136/bjo.87.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shallo-Hoffmann J, Coulter R, Oliver P, et al. A study of pre-school vision screening tests’ testability, validity and duration: do group differences matter? Strabismus. 2004;12:65–73. doi: 10.1080/09273970490515874. [DOI] [PubMed] [Google Scholar]
- 16.Chui L, Fraser T, Hoar K, LaRoche GR. Negative predictive value of a vision screening program aimed at children aged 3 to 4 years old. J AAPOS. 2004;8:566–570. doi: 10.1016/j.jaapos.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Broderick P. Pediatric vision screening for the family physician. Am Fam Physician. 1998;58:691–700. [PubMed] [Google Scholar]
- 18.Vision in Preschoolers Study Group. The electronic visual acuity tester: testability in preschool children. Optom Vis Sci. 2004;81:238–244. doi: 10.1097/00006324-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika. 1988;75:800–803. [Google Scholar]
- 20.Rosner J, Rosner J. Parents as screeners for strabismus in their children. J Vis Impair Blind. 1988;82:193–194. [Google Scholar]
- 21.PEDIG. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113:895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue S. Relationship between anisometropia, patient age, and the development of amblyopia. Am J Ophthalmol. 2006;142:132–140. doi: 10.1016/j.ajo.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Salcido AA, Bradley J, Donahue SP. Predictive value of photoscreening and traditional screening of preschool children. J AAPOS. 2005;9:114–120. doi: 10.1016/j.jaapos.2003.10.011. [DOI] [PubMed] [Google Scholar]