Abstract
The function of the mlr6739 gene from Mesorhizobium loti MAFF303099 has been identified. This gene encodes 4-Pyridoxic acid dehydrogenase, an enzyme involved in the catabolism of PLP (Vitamin B6). This enzyme was overexpressed in Escherichia coli and characterized. 4-Pyridoxic acid dehydrogenase is a 33 kDa protein that catalyzes the four electron oxidation of 4-pyridoxic acid to 3-hydroxy-2-methylpyridine-4,5-dicarboxylate, using nicotinamide adenine dinucleotide as a cofactor. The kcat for NADH production is 0.01 s−1. The KM values for 4-pyridoxic acid and NAD are 5.8 μM and 6.6 μM respectively.
Introduction
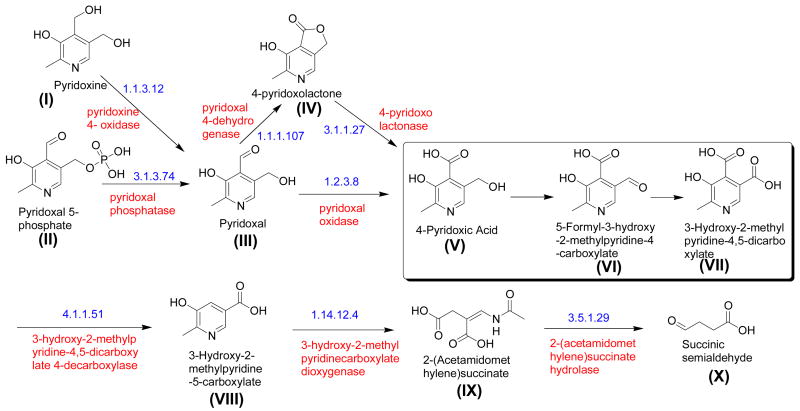
The bacterial pyridoxine (vitamin B6) catabolic pathway is shown in Figure 1 [1], [2]. Of the 10 enzymes used for PLP catabolism, only five of the corresponding genes have been identified. Recently, the genes encoding pyridoxine-4-oxidase, 4-pyridoxolactonase and pyridoxal-4-dehydrogenase were identified in Mesorhizobium loti MAFF303099 [4, 5 & 6]. These genes are not part of an operon, but they are all close to each other on the M. loti chromosome suggesting that other PLP catabolic genes might also be found in this region. In particular, mlr6788 is a likely candidate for the 2-methyl-3-hydroxypyridine-5-carboxylic acid oxygenase gene [7] and mlr6739, annotated as 3-hydroxybutyryl-CoA dehydrogenase (EC 1.1.1.157) [8], is likely to be one of the three missing dehydrogenases. The 309 amino acid gene product is a NAD binding protein with calculated molecular weight of 33 kDa [9]. In this communication, we report the cloning and overexpression of mlr6739 and demonstrate that the purified gene product has a dual dehydrogenase activity catalyzing the 4-electron oxidation of 4-pyridoxic acid (compound V) to 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII).
Figure 1.
The vitamin B6 catabolic pathway in Pseudomonas sp. MA-1 [3]. The reactions shown in the box are catalyzed by 4-pyridoxic acid dehydrogenase.
Materials and Methods
Materials
A dehydrated form of Luria-Bertani (LB) broth was purchased from EMB Chemicals, (Gibbstown, NJ). Ampicillin and isopropyl-β-D-thiogalactopyranoside (IPTG) were obtained from Lab Scientific Inc. (Livingston, N.J.). 4-Pyridoxic acid, NAD, NADH, Lysozyme from chicken egg white, agar, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) molecular weight markers were from Sigma (St. Louis, Mo.). Triethylamine, was from Fisher (Fairlawn, NJ). TFA and methanol (HPLC grade), sodium chloride and imidazole were from Acros Organics (NJ). Sodium dihydrogen phosphate monohydrate was from Mallinckrodt Baker Inc. (Phillipsburg, NJ). Deuterium oxide (D2O) was purchased from Cambridge Isotope Laboratories Inc. (Andover, MA). Microcon YM-10 membranes (10,000 MWCO) and an Amicon Ultra centrifugal filter device (10,000 MWCO) were obtained from Millipore (Billerica, MA). The Supelcosil LC-18-T column for HPLC was from Supelco, (Bellefonte, PA). E. coli strain MachI and the Gateway system were from Invitrogen (Carlsbad, CA). Nucleospin Purification kit, Phusion DNA polymerase, Escherichia coli BL21(DE3) and the Ni-NTA superflow resin were obtained from Macherey-Nagel (Easton, PA), New England Biolabs (Ipswich, MA), Novagen (San Diego, CA) and Qiagen (Valencia, CA) respectively.
Molecular Cloning
Standard methods were used for DNA manipulations [10, 11]. Plasmid DNA was purified with the Qiagen Miniprep kit and DNA fragments were purified from agarose gel with the Nucleospin Purification kit. E. coli strain MachI was used as a recipient for transformations during plasmid construction and for plasmid propagation. Phusion DNA polymerase was used for PCR following the manufacturer’s recommendations. The pENTR-TEV-D-TOPO and the Gateway system were used following the manufacturer’s instructions with slight modifications.
Cloning of M. loti mlr6739
The M. loti mlr6739 gene was amplified from genomic DNA by PCR with the following primer pair: 5′-CAC CAT GAT CCG AAA TAT CGC CAT CAT C-3′ and 5′-CTA CTC CCG GCC TTC CAG GAT GCG TC-3′. The PCR product was purified and used in a topoisomerase mediated reaction with pENTR-TEV-D-TOPO essentially following the manufacturer’s instructions. Clones were screened by PCR and verified by sequencing. A correct clone was used in an LR recombination reaction with the plasmid pDESTF1, which is a Gateway adapted vector based on the pET-system. The plasmid pDESTF1 encodes an N-terminal 6xHis tag and is under the control of the T7lac promoter. Clones were screened by restriction digestion. A correct clone was named pMl5337.XF1.
Overexpression and purification
The plasmid pMl5337.XF1 was used to transform Escherichia coli BL21(DE3). A starter culture was prepared by growing a single colony of transformed cells in 10 ml of LB media containing 100 μg/ml of ampicillin at 37°C with overnight agitation. 1 liter LB medium (20 g/L), containing 100 μg/ml of ampicillin, was inoculated with this starter culture. The cells were grown at 37°C with shaking at 250 rpm until the culture reached an OD590 of 0.6 at which point they were induced by adding IPTG to a final concentration of 0.8 mM, the temperature was lowered to 15°C and the cells were allowed to grow for a further 12 hours. The cells were then harvested by centrifugation at 10000 ×g for 8 min at 4°C.
Cells from 1 liter of culture were re-suspended in 20 ml of binding buffer (50 mM NaH2PO4, 150 mM NaCl, 10 mM imidazole, pH 7.7) and approximately 2 mg of lysozyme was added. The cells were then lysed by sonication (Misonix Sonicator 3000, pulse ‘on’ time 1.0 sec, pulse ‘off’ time 1.0 sec, output level 0.8, 30 cycles) 5 times on ice. The cell debris was removed by centrifugation at 17,000 rpm for 40 minutes at 4°C. The clarified supernatant was loaded on to a 5 ml Ni-NTA-affinity column pre-equilibrated with binding buffer kept at 4°C. The Ni-NTA-affinity column was then washed with 100 ml wash buffer (50 mM NaH2PO4, 150 mM NaCl, 20 mM imidazole, pH 7.7). The protein was eluted from the column with elution buffer (50 mM NaH2PO4, 150 mM NaCl, 200 mM imidazole, pH 7.7) at 4°C. The fractions containing protein were pooled and concentrated using YM-10 Amicon Ultracentrifugal filters at 5000 ×g to a final volume of 500 μl. The concentrated sample was desalted into 100 mM phosphate buffer at pH 8.0 using an Econo-Pac 10DG disposable chromatography column The yield of the purified protein was 6 mg/liter.
Enzymatic assay based on NADH production
The reaction mixture consisted of: enzyme (5 μM), NAD (1 mM) and 4-pyridoxic acid (50 μM) in 100 mM potassium phosphate buffer at pH 8.0. The enzymatic activity was monitored by measuring the increase in absorbance at 340 nm due to the production of NADH. A control reaction without NAD showed negligible background activity.
HPLC analysis
HPLC analysis of the enzymatic reaction mixture was performed on a Hewlett-Packard 1100 instrument using a Supelcosil LC-18-T (15 cm × 4.6 mm, 3.0 μM) column. Solution A contained water with 0.1% TFA, and solution B contained methanol with 0.1% TFA. The following linear gradient mixing solution A with solution B was used: 100% solution A for 0 to 2 min, 100% to 80% solution A from 2–12 min, 80% to 0% solution A in 12–13 min, 0% to 100% solution A in 13–14 min and 100% solution A in 14–17 minutes. Flow rate was 1 ml/min, absorbance was measured at 254 nm (characteristic for NAD) and 320 nm (characteristic of 4-pyridoxic acid, compound V). Under these conditions the following compounds were readily separated (retention time in parenthesis): 4-pyridoxic acid (compound V, 9.8 min), 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII, 7.35 min), NAD (8.75 min).
Reaction time course
A time course was determined with a reaction mixture (1 ml) containing 17 mM NAD, 1.5 mM 4-pyridoxic acid (compound V) and 150 μM of pure enzyme. At various time points, 100 μl of the reaction mixture was quenched by addition to 100 μl of 10% TFA. This mixture was filtered through Microcon YM-10 and 100 μl of the filtrate was analyzed by HPLC.
Product purification
A reaction mixture (5.5 ml) containing 78 mg of NAD (20 mM), 6 mg of 4-pyridoxic acid (6 mM) and freshly purified enzyme (final concentration of 50 μM) was incubated overnight at room temperature. It was then filtered through a YM-10 Microcon centrifugal filter at 14,000 ×g for 30 minutes and the filtrate was analyzed by HPLC. The enzymatic product (retention time 7.35 minutes) was collected over multiple injections. Methanol was removed by rotary evaporation, TFA and water were removed under high vacuum overnight. The resulting white powder was characterized by NMR and ESI-MS analysis.
Steady state kinetic analysis
The steady state kinetic parameters for 4-pyridoxic acid dehydrogenase were determined by monitoring the absorbance at 370 nm over time. To a reaction mixture (500 μl) containing 243 nM enzyme and 400 μM of NAD, varying concentration of 4 –pyridoxic acid (V) was added. The rate of formation of NADH was monitored over 15 minutes at 370 nm for each concentration of the substrate. The extinction coefficient of NAD in 100 mM phosphate buffer at pH 8.0 at 370 nm was determined to be 2600 M−1cm−1. The KM for 4-pyridoxic acid (V) and kcat for the production of NADH by the enzyme was determined by fitting the rate of product formation versus substrate concentration using non-linear regression to the Michaelis-Menten equation using Grafit 5.0.11 (Erithacus Software Limited, Surrey, UK). In order to determine the KM for NAD a similar experiment was done in which the reaction mixture (500 μl) contained 243 nM enzyme and 100 μM of 4-pyridoxic acid (V) and the concentration of NAD was varied. All solutions were made in 100 mM phosphate buffer at pH 8.0.
Results and Discussion
Reaction Time Course
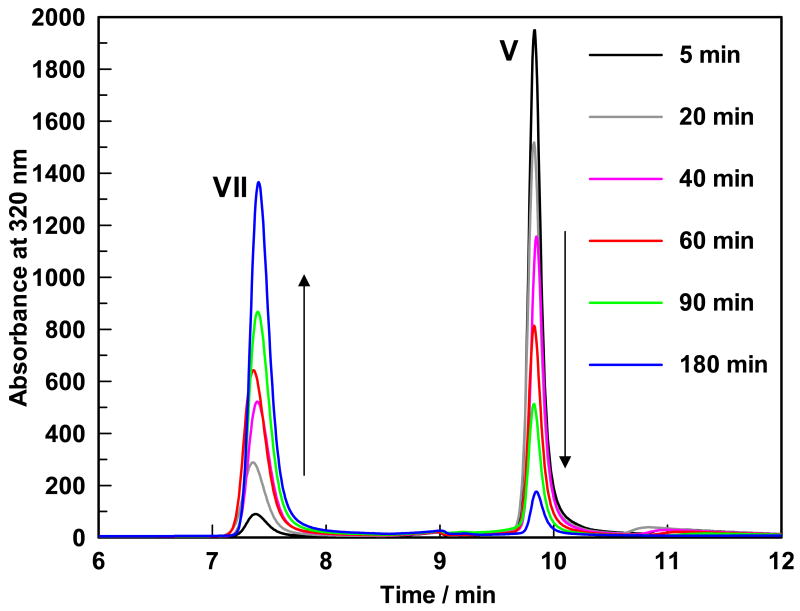
The formation of 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII) and the disappearance of 4-pyridoxic acid (compound V) as a function of time is shown in Figure 2. Neither 320 nm nor 254 nm wavelength showed the presence of any other peaks which indicated that under the reaction condition, the two electron oxidation of 4-pyridoxic acid (compound V) to 5-formyl-3-hydroxy-2-methylpyridine-4-carboxylate (compound VI) was not observed. Under the acidic conditions used to quench the enzymatic reactions NADH is degraded to a non-chromophoric compound at 320 nm and is therefore not detected in the HPLC-chromatogram, monitored at 320 nm.
Figure 2.
HPLC trace showing the disappearance of the reactant (retention time of 9.8 minutes) and the appearance of the product (retention time of 7.35 minutes).
Product characterization
The white colored compound obtained as a result of the enzymatic reaction mixture was identified as 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII) by NMR and MS analysis. 1H NMR (300 MHz, D2O) δ 2.69 (s, 3H, CH3) and 8.42 (s, 1H, C6-H). Negative ion mode ESI-MS (Esquire-LC_00146 instrument, Bruker) showed a species with m/z = 196, which corresponded to the calculated mass of mono anionic 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII). Fragmentation analysis resulted in the formation of a species with m/z =152 (single decarboxylation, -44), which when isolated and fragmented further, showed the formation of the species with m/z = 108 (double decarboxylation, -88).
Steady state kinetic analysis
The steady state kinetic parameters for the enzyme were determined by monitoring the production of NADH in a UV-visible spectrophotometer. The kcat for the NADH production is 0.01 s−1. The KM for 4-pyridoxic acid (compound V) (under saturating concentration of NAD) is 5.8(±0.9) μM. The KM for NAD (under saturating concentration of 4-pyridoxic acid, compound V) is 6.6(±1.3) μM. The absorbance for NADH production was monitored at 370 nm instead of 340 nm (λmax for NADH) since both 4-pyridoxic acid (compound V) and 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII) have some absorbance at 340 nm.
Conclusion
We have demonstrated that the mrl6739 gene product catalyzes the 4-electron oxidation of 4-pyridoxic acid (compound V) to 3-hydroxy-2-methylpyridine-4,5-dicarboxylate (compound VII) using NAD as a cofactor. The intermediate 5-formyl-3-hydroxy-2-methylpyridine-4-carboxylate (compound VI) did not accumulate in the enzymatic reaction mixture. This implies that the enzyme catalyzes the oxidation of VI to VII, faster than the oxidation of V to VI. Since we see no reduction of NAD in absence of the substrate and the reaction product has been unambiguously identified, it is reasonable to assume that two moles of NAD are consumed for each mole of substrate oxidized. Sequence analysis indicates only one nucleotide binding site so we assume that both hydride transfer reactions are occurring at the same active site. The mrl6739 encoded pyridoxic acid dehydrogenase is different from the previously characterized enzyme from Pseudomonas sp. MA-1 which uses FAD as a cofactor and only catalyzes the 2-electron oxidation of 4-pyridoxic acid (compound V) to 5-formyl-3-hydroxy-2-methylpyridine-4-carboxylate (compound VI). FAD is not required by the mrl6739 encoded pyridoxic acid dehydrogenase that has been characterized in this paper.
Acknowledgments
We are grateful to Dr. Yasunobu Ohkawa, Director of Genebank, National Institute of Agrobiological Sciences, Ibaraki, Japan for providing us with the M. loti MAFF303090 strain. This research was supported by a grant from the National Institutes of Health (GM069618).
Abbreviations
- NAD
Nicotinamide adenine dinucleotide
- NADH
reduced form of NAD
- FAD
Flavin adenine dinucleotide
- TEA
Triethylamine, TFA, Trifluoroacetic acid
- IPTG
isopropyl-β-D-thiogalactopyranoside
- LB
Luria-Bertani
- PLP
pyridoxal-5-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snell EE, Haskell BE. In: Comprehensive Biochemistry. Florkin M, Stotz EH, editors. Vol. 21. Elsevier/North Holland; New York: 1971. pp. 47–67. [Google Scholar]
- 2.Burg RW, Rodwell VW, Snell EE. J Biol Chem. 1960;235:1164–1169. [PubMed] [Google Scholar]
- 3.Yagi T, Kishore GM, Snell EE. J Biol Chem. 1983;258:9419–9425. [PubMed] [Google Scholar]
- 4.Yuan B, Yoshikane Y, Yokochi N, Ohnishi K, Yagi T. FEMS Microbiol Lett. 2004;234:225–230. doi: 10.1016/j.femsle.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Funami J, Yoshikane Y, Kobayashi H, Yokochi N, Yuan B, Iwasaki K, Ohnishi K, Yagi T. Biochim Biophys Acta. 2005;1753:234–239. doi: 10.1016/j.bbapap.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Yokochi N, Nishimura S, Yoshikane Y, Ohnishi K, Yagi T. Arch Biochem Biophys. 2006;452:1–8. doi: 10.1016/j.abb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Chaiyen P, Sucharitakul J, Svasti J, Entsch B, Massey V, Ballou DP. Biochemistry. 2004;43:3933–3943. doi: 10.1021/bi035734d. [DOI] [PubMed] [Google Scholar]
- 8.http://theseed.uchicago.edu/FIG/protein.cgi?prot=fig|266835.1.peg.5337&user=&48hr_job=&new_framework=0
- 9.Ausubel FM, Brent R. Current Protocols in Molecular Biology. John Wiley and Sons; New York: 1987. [Google Scholar]
- 10.Sambrook J, Fritsch EF. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Plainview, New York: 1989. [Google Scholar]