Abstract
Mechanisms controlling the survival of neural precursor cells (NPCs) are critical during brain development, in adults for neuron replenishment, and after transplantation for neuron replacement. This investigation found that glycogen synthase kinase 3 (GSK3) promotes apoptotic signaling in cultured NPCs derived from embryonic mouse brain subjected to two common apoptotic conditions, trophic factor withdrawal and genotoxic stress. Trophic factor withdrawal activated GSK3 and the key apoptosis mediators Bax and caspase-3. Pharmacological inhibition of GSK3 activity produced dramatic reductions in the activation of Bax and caspase-3 and NPC death after trophic factor withdrawal. Trophic factor withdrawal-induced apoptosis was delayed in Bax knock-out NPCs, but GSK3 inhibitors provided additional protection. Genotoxic stress induced by camptothecin treatment of NPCs stabilized p53, which formed a complex with GSK3βand activated Bax and caspase-3. Camptothecin-induced activation of caspase-3 was reduced by GSK3 inhibitors in both bax+/+and bax−/− NPCs. Thus, NPCs are sensitive to loss of trophic factors and genotoxic stress, and inhibitors of GSK3 are capable of enhancing NPC survival.
Neural precursor cells (NPCs),2 which through the process of neurogenesis give rise to daughter cells that develop into mature neurons, are vital for brain development and also for the maintenance of normal brain function during the lifespan of mammals (1, 2). Consequently, an increasing number of studies find that impaired neurogenesis is associated with several neurological (3–5) and psychiatric diseases (6, 7), and therapeutic interventions for these diseases can increase neurogenesis in rodents (8–11). These findings indicate that bolstering neurogenesis has significant therapeutic potential. Increased neurogenesis can theoretically be accomplished by increasing NPC proliferation, differentiation, or survival. The latter is a particularly attractive site for intervention because before maturing to functional neurons a significant portion of NPCs is lost due to death by apoptosis, although the precise percentage that dies remains uncertain (12–14), and after transplantation most NPCs die by apoptosis (15). Additionally, many apoptosis signaling pathways are now well characterized (16, 17), providing information about potential targets for reducing loss of NPCs by apoptosis.
An important regulator of apoptosis is glycogen synthase kinase 3 (GSK3) (18, 19), which promotes mitochondria-mediated apoptotic signaling induced by a broad range of insults (20), including conditions modeling a variety of neurodegenerative diseases (21). In addition to facilitating apoptosis induced by many insults, GSK3 is further an attractive potential target for reducing apoptosis because it is inhibited by lithium (22, 23), a drug with a long history of therapeutic use in humans for bipolar disorder (24). Lithium directly inhibits GSK3 (22) and also promotes inhibitory phosphorylation on serine-9 and serine-21 of the two isoforms of GSK3, GSK3β and GSK3α, respectively (25), a modulation that is often catalyzed by Akt (26). In addition, several structurally diverse inhibitors of GSK3 have recently been developed (27), providing an armory of tools available to inhibit GSK3.
Because many NPCs may be lost to apoptosis, GSK3 promotes apoptosis, and GSK3 inhibitors are available to block GSK3-facilitated apoptosis, GSK3 may be a favorable target for bolstering neurogenesis. To investigate this possibility with apoptotic conditions that NPCs may encounter either during development or with cytotoxic stresses, we exposed NPCs to trophic factor withdrawal or to the DNA damaging agent camptothecin. The results demonstrate that NPCs die by apoptosis involving Bax and caspase-3 activation after trophic factor withdrawal or DNA damage, and GSK3 inhibitors attenuate apoptotic signaling in NPCs exposed to each of these conditions.
MATERIALS AND METHODS
Cell Culture and Treatments
NPCs were prepared at gestational day 12–13 from the telencephalons of C57BL/6 mice (National Cancer Institute, Frederick, MD) or knock-out bax−/− mice and matched controls (28). Cells were dissociated for 18 min at 37 °C in Hanks’ balanced salt solution (Invitrogen) containing 0.05% trypsin, 0.02% EDTA, 0.001% DNase I, and 0.2% BSA (Sigma). After treatment with trypsin, an equal volume of Hanks’ balanced salt solution with 10% fetal bovine serum was added, and cells were further dissociated by trituration four times. Cells were washed and resuspended in Dulbec-co’s modified Eagle’s medium containing 20 ng/ml fibroblast growth factor 2 (FGF2; Research Diagnostics, Flanders, NJ), 8 μg/ml heparin, 5 μg/ml insulin, 100 μg/ml apotransferrin, 30 nM sodium selenite, 20 nM progesterone, 100 μM putrescine, 6 g/liter glucose, 2 mM glutamine, 94.4 μM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma), pH 7.4. NPCs were expanded as neurospheres, and experimental procedures were carried out with monolayers of NPCs. To prepare monolayers, the neurospheres were resuspended and incubated for 2 min at 37 °C in Hanks’ balanced salt solution with 0.05% trypsin, 0.02% EDTA, 0.2% BSA, diluted with 2 volumes of Dulbecco’s modified Eagle’s medium with 10% fetal calf serum, and plated on poly-L-lysine/laminin-coated culture plates and grown as monolayers for ~24 h. Trophic factor withdrawal was established by placing NPCs in Dulbecco’s modified Eagle’s medium/F-12 media without any additions. DNA damage was induced by treating cells with 10 μM camptothecin (Sigma) in NPC growth factor-containing media with FGF2. Where indicated, cells were treated with the selective GSK3 inhibitors 20 mM lithium, 5 μM kenpaullone (Sigma), 5 μM 2-thio(3-iodobenzyl)-5-(1-pyridyl)-[1,3,4]-oxadiazole (marketed as GSK3 Inhibitor II; Calbiochem), 20 μM indirubin-3′-monoxime (Alexis Biochemicals, San Diego, CA), or 5 μM SB216763 (Tocris Cookson Inc., Ballwin, MO).
Immunoblot Analysis and Immunoprecipitation
For immunoblotting, cells were washed twice with PBS and lysed with 100 μl of lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 100 μM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin A, 1 nM okadaic acid, and 1% Triton-X-100). The lysates were centrifuged at 14,000 rpm for 20 min at 4 °C. Protein concentrations were determined using the Bradford method (29). Immunoprecipitations were performed using sheep anti-mouse IgG Dynabeads (Dynal Biotech, Oslo, Norway). The Dynabeads, 10 μl of slurry per sample, were washed twice in PBS, 0.1% BSA using a magnetic particle separator. The beads were incubated with 1 μg of anti-GSK3β antibody overnight at 4 °C with gentle agitation followed by incubation with protein samples for 1 h at 4 °C, and the immune complexes were washed 3 times. Samples were mixed with Laemmli sample buffer (2% SDS) and placed in a boiling water bath for 5 min. Proteins were resolved in SDS-polyacrylamide gels, transferred to nitrocellulose, and incubated with primary antibodies to poly(ADP-ribose) polymerase (PARP), proteolyzed PARP 85-kDa fragment (1:1000; dilution; Pharmingen/Transduction Laboratories), total GSK3α/β (1:2000), phospho-Ser-9-GSK3β (1:1000), phospho-Ser21-GSK3α (1:1000), GSK3β (1:2000), total Akt (1:1000), phospho-Thr-308-Akt (1:1000), phospho-Ser-473-Akt (1:1000), Bak (1:1000), Bcl-XL (1:1000), BAX (1:1000), or cleaved, active casapse-3 (1:500; Cell Signaling, Beverly, MA). Immunoblots were developed using horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG followed by detection with enhanced chemiluminescence, and the protein bands were quantitated with a densitometer. Quantitative measurements from at least three independent experiments were tested for statistical significance using analysis of variance.
Immunocytochemistry
NPCs cultured on poly-L-lysine/laminin-coated glass coverslips were treated as described under “Results.” Cells were fixed in 4% paraformaldehyde and then permeabilized and blocked in PBS, pH 7.4, containing 0.2% Triton X-100 and 3% BSA. Cells were incubated overnight at 4 °C with primary antibodies diluted in PBS containing 3% BSA, including mouse anti-nestin (1:40,000; Developmental Studies Hybridoma Bank, Iowa City, Iowa), mouse anti-glial fibrillary acidic protein (1:10,000; Chemicon), mouse anti-β-tubulin iso-type III (1:10,000; Sigma), rabbit anti-activate caspase-3 (1:50; Cell Signaling), or mouse anti-active Bax (1:1000; Pharmingen/Transduction Laboratories), a conformational-specific Bax antibody that recognizes an epitope on Bax that is internalized in non-activated Bax but is externalized after apoptosis signaling-induced activation of Bax (30). The primary antibody was removed, the cells were washed three times with PBS and then incubated with either tyramide signal amplification (PerkinElmer Life Sciences) with cyanine 3-tyramide-conjugated or with fluorescein isothiocyanate-conjugated secondary antibodies for 1 h at room temperature. Nuclei were labeled with 1 μg/ml bisbenzimide (Sigma) for 10 min at room temperature. NPCs were then washed three times with PBS, and coverslips were adhered to glass slides in mounting medium (0.1% p-phenylenediamine in 75% glycerol in PBS). Cells were viewed and photographed with an inverted Nikon fluorescence microscope. Positively stained cells were counted in at least 12 fields from a minimum of 3 different NPC preparations, and the total number of cells counted was always greater than 5000. Quantitative measurements from at least three independent experiments were tested for statistical significance using analysis of variance.
Cell Viability
Chromatin condensation was determined by fluorescence microscopy after nuclear staining with Hoechst dye. Dead cells were quantified by propidium iodide staining and fluorescence-activated cell sorter analysis. For this, after treatments NPCs were trypsinized, fixed in ethanol at −20 °C for 10 min, and rehydrated by adding PBS at room temperature for 15 min. Cells were stained with 3 μM propidium iodide in staining buffer (100 mM Tris, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.1% Nonidet P-40) for 15 min at room temperature and analyzed on a single-laser flow cytometer (BD Biosciences). Quantitative measurements from at least three independent experiments were tested for statistical significance using analysis of variance except for the data in supplemental Fig. 2b where Student’s t test was used to determine statistical significance.
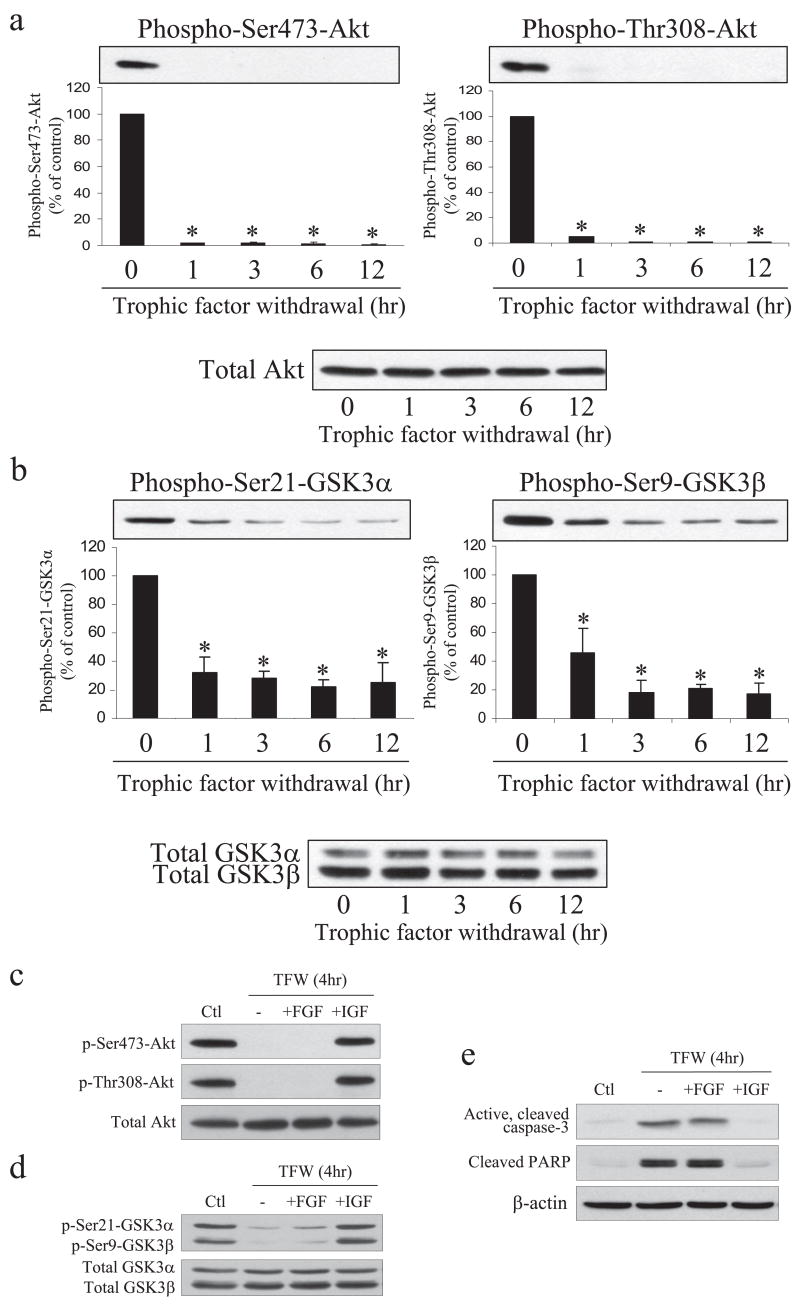
FIGURE 2. Trophic factor withdrawal causes dephosphorylation of Akt and GSK3 in NPCs.
Trophic factors were withdrawn and NPCs were harvested after 0, 1, 3, 6, or 12 h. Extracts were immunoblotted for phospho-Ser-473-Akt, phospho-Thr-308-Akt, and total Akt (a) or phospho-Ser-21-GSK3α, phospho-Ser-9-GSK3β, and total GSK3α/β (b). Quantitative values were obtained by densitometric measurements of immunoblots and are the means ± S.E.; n = 3; *, p < 0.05. c, NPCs were cultured in trophic factor-containing media (Ctl) or trophic factors were withdrawn (TFW) from the culture media, and NPCs were incubated for 4 h with no addition or with 50 ng/ml FGF2 or 50 ng/ml IGF-1. Cell extracts were immunoblotted for phospho (p)-Ser-473-Akt, phospho-Thr-308-Akt, and total Akt (c), phospho-Ser21-GSK3α, phospho-Ser9-GSK3β, and total GSK3α/β (d), or active cleaved caspase-3, cleaved PARP, and β-actin (e).
RESULTS
Characterization of NPCs and Trophic Factor Withdrawal-induced Apoptosis
Isolated NPCs were expanded as neurospheres and then plated as monolayers (supplemental Fig. 1) for experimental manipulations, as previously described (31). Monolayers of NPCs were predominantly nestin-positive (>99%; a marker of NPCs) and less than 1% of cells were glial fibrillary acidic protein-positive (a marker of astrocytes), and no cells stained with the neuronal marker β-tubulin III (supplemental Fig. 1).
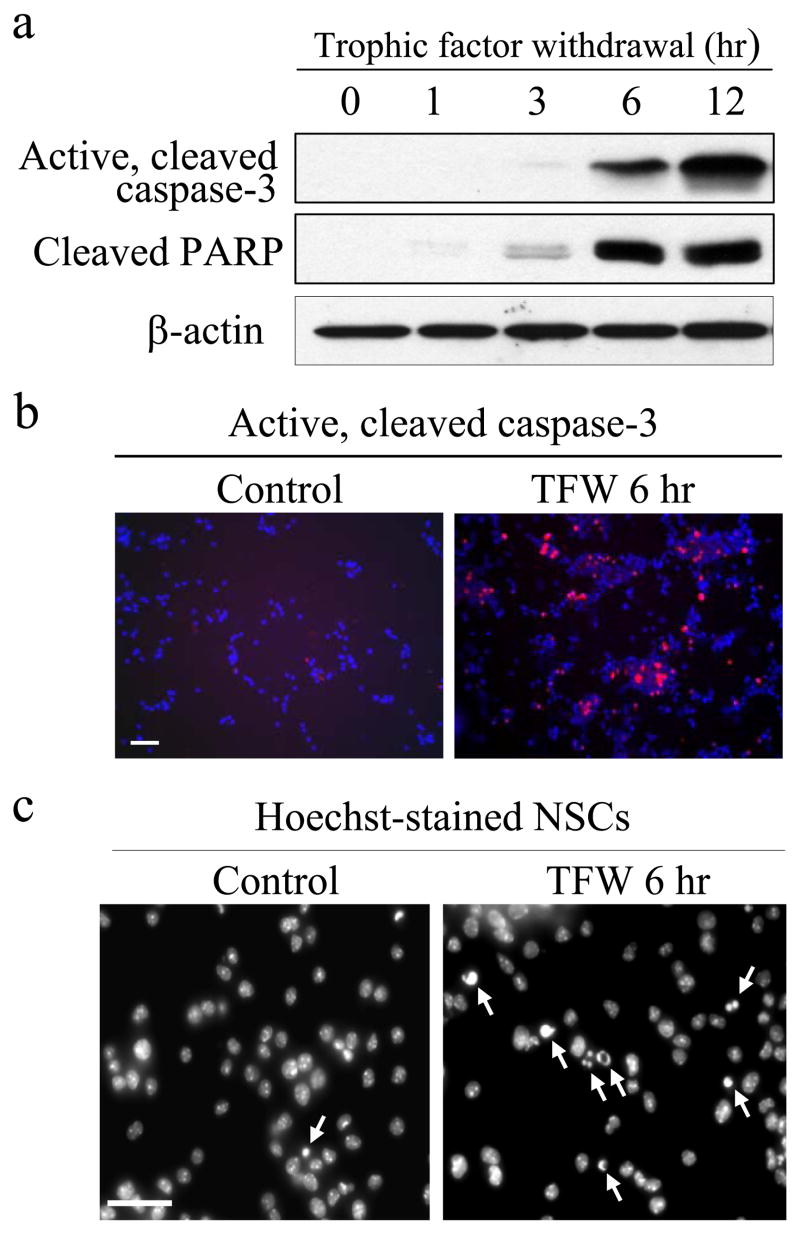
FIGURE 1. Trophic factor withdrawal causes apoptosis of NPCs.
Trophic factors were withdrawn from the culture media, and NPCs were harvested after 0, 1, 3, 6, or 12 h. a, extracts were immunoblotted for active, cleaved caspase-3, cleaved PARP, or β-actin. b, active caspase-3 was detected by immunocytochemistry in control NPCs or 6 h after trophic factor withdrawal (TFW). Scale bar, 50 μm. c, morphological features of apoptosis, including condensed nuclei (arrows), were prominent in NPCs 6 h after trophic factor withdrawal. Scale bar, 20 μm.
Trophic factor withdrawal caused NPCs to undergo time-dependent activation of caspase-3 and cleavage of the caspase-3 substrate PARP beginning ~3 h after trophic factor withdrawal (Fig. 1a). Immunocytochemistry verified the widespread activation of caspase-3 in NPCs 6 h after trophic factor withdrawal (Fig. 1b). Furthermore, morphological changes characteristic of apoptotic cells also were observed, such as chromatin condensation evident in Hoechst-stained NPCs 6 h after trophic factor withdrawal (Fig. 1c).
Trophic Factor Withdrawal Induces Dephosphorylation of Akt and GSK3
Growth factors often maintain cell viability in part by activating the phosphatidylinositol 3-kinase/Akt signaling pathway (26). When activated, Akt is phosphorylated on Ser-473 and Thr-308, and Akt catalyzes phosphorylation of GSK3 on a regulatory serine which inhibits its activity (26, 32). Therefore, we examined if this pathway was inactivated after trophic factor withdrawal by using phospho-dependent antibodies to detect phosphorylation of the regulatory sites on Akt and GSK3 in immunoblot analyses. Trophic factor withdrawal caused a rapid dephosphorylation of Akt on both Ser-473 and Thr-308, whereas the total level of Akt remained unchanged (Fig. 2a). Concomitant with the dephosphorylation of Akt, there was loss of serine phosphorylation of the two isoforms of GSK3, phospho-Ser-21-GSK3α and phospho-Ser-9-GSK3β, whereas the total protein levels of GSK3α/β were unchanged after trophic factor withdrawal (Fig. 2b). Quantitative analyses of these changes suggests that Akt is likely an important kinase for phosphorylating these serines in GSK3 in NPCs since there was concomitant dephosphorylation of Akt and GSK3 but also that other kinases contribute to serine phosphorylation of GSK3 since the dephosphorylation of serine-phosphorylated GSK3 was not as complete as was the dephosphorylation of Akt. These results demonstrate that trophic factor withdrawal led to a rapid inactivation of Akt and decrease of inhibitory serine phosphorylation of GSK3 before activation of caspase-3.
We tested if inclusion of a single growth factor was sufficient to block the trophic factor withdrawal-induced dephosphorylations of Akt and GSK3 and if this attenuated apoptotic signaling. For this, NPCs were incubated for 4 h in complete trophic factor withdrawal media or with the addition of 50 ng/ml FGF2 or 50 ng/ml insulin-like growth factor-1 (IGF-1). Stimulation with IGF-1, but not FGF2, was sufficient to maintain phosphorylation of Akt on both Ser-473 and Thr-308 (Fig. 2c) and inhibition-associated phosphorylation of GSK3α on Ser-21 and GSK3β on Ser-9 (Fig. 2d). Stimulation with IGF-1 also abrogated the activation of caspase-3 and cleavage of PARP, whereas FGF2 was without effect (Fig. 2e).
Inhibition of GSK3 Protects NPCs from Trophic Factor Withdrawal-induced Caspase-3 Activation
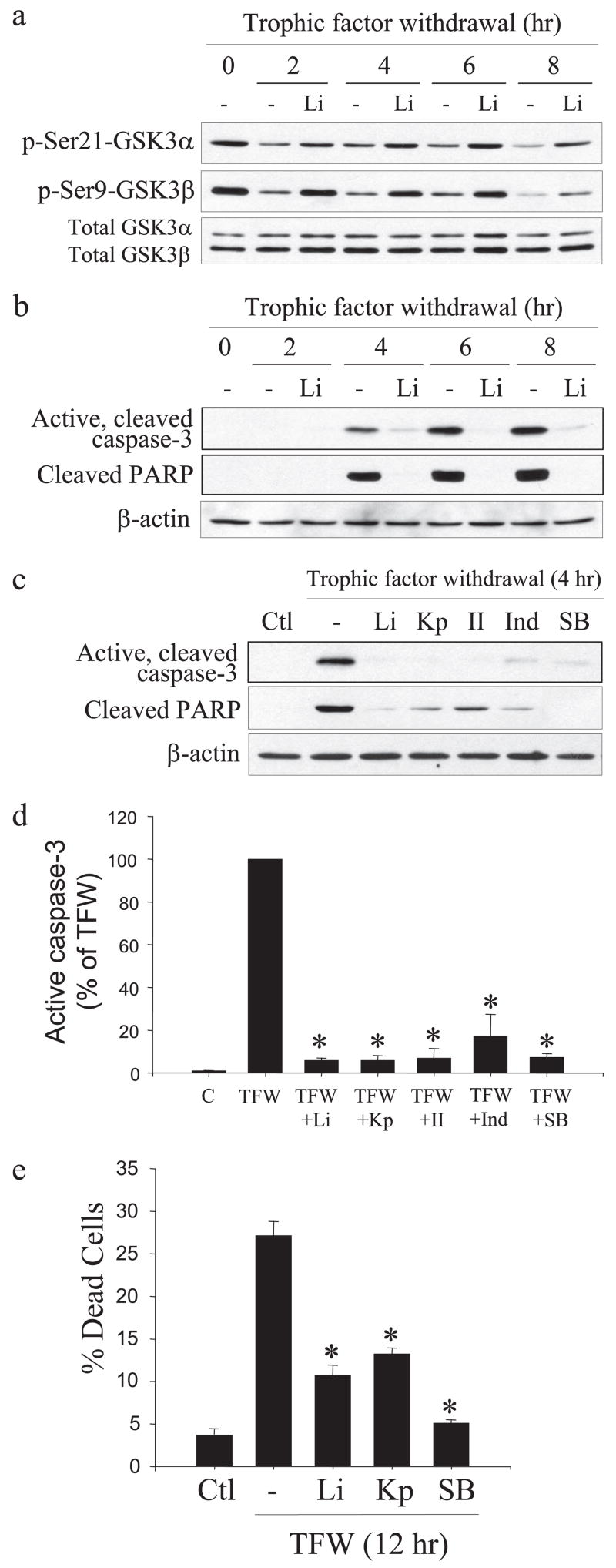
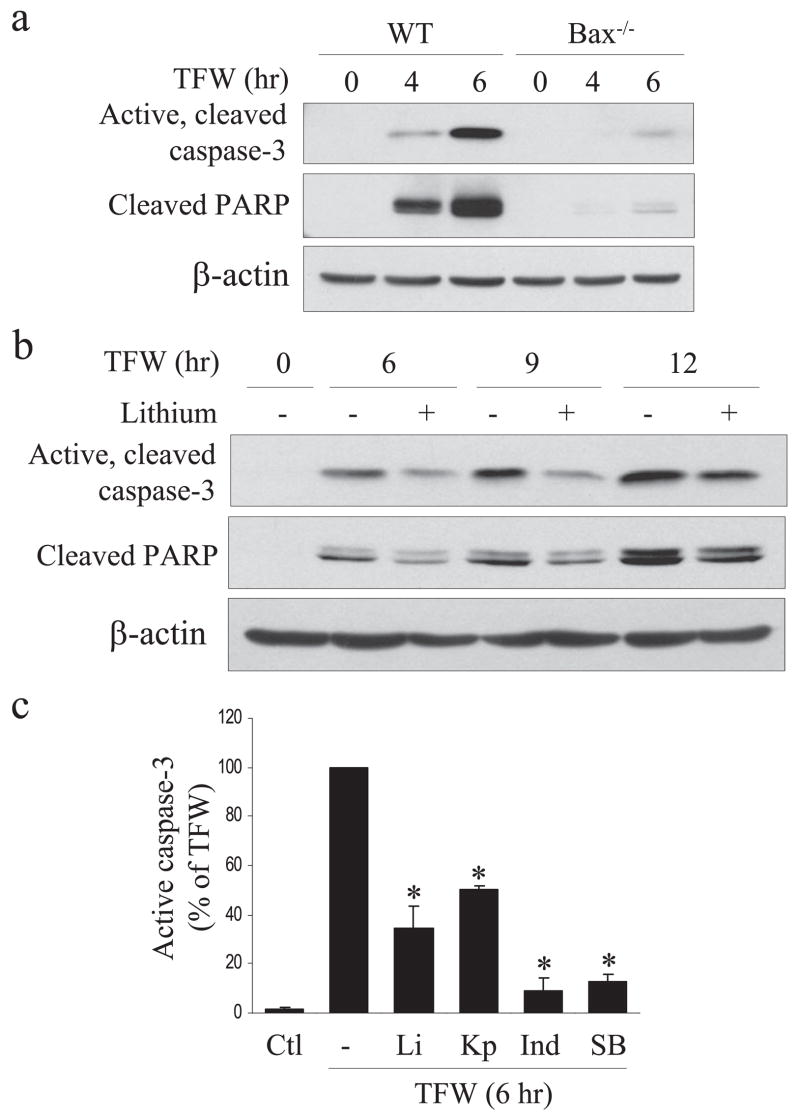
Because GSK3 is known to be an important contributor of apoptotic signaling in many types of cells (20), we tested if inhibition of GSK3 protected NPCs from trophic factor withdrawal-induced caspase-3 activation. Treatment with the selective GSK3 inhibitor lithium (22) reduced trophic factor withdrawal-induced decreases of the inhibitory serine phosphorylation of both isoforms of GSK3 (Fig. 3a) and strongly inhibited trophic factor withdrawal-induced caspase-3 activation and PARP proteolysis between 4 and 8 h after withdrawal (Fig. 3b). To verify that this protective effect of lithium was due to inhibition of GSK3, four other selective inhibitors of GSK3 were tested along with lithium. These experiments demonstrated that NPCs were protected from trophic factor withdrawal-induced caspase-3 activation by 5 μM kenpaullone, 5 μM GSK3 inhibitor II, 20 μM indirubin-3′-monoxime, and 5 μM SB216763 (33–35), similar to the protection provided by 20 mM lithium (Fig. 3c). Quantitation showed these five selective GSK3 inhibitors provided 80–95% protection from caspase-3 activation after 4 h of trophic factor withdrawal (Fig. 3d), verifying that inhibition of GSK3 provides NPCs protection from trophic factor withdrawal.
FIGURE 3. GSK3 inhibitors reduce caspase-3 activation in NPCs after trophic factor withdrawal.
a, treatment of NPCs with 20 mM lithium (Li) attenuated trophic factor withdrawal-induced dephosphorylation of phospho (p)-Ser-21-GSK3α and phospho-Ser-9-GSK3β but did not change the total levels of GSK3α/β b, active caspase-3, cleaved PARP, and β-actin were measured by immunoblot analysis in NPCs after 0, 2, 4, 6, or 8 h of trophic factor withdrawal with and without treatment with 20 mM lithium. c, active caspase-3, cleaved PARP, and β-actin were measured in NPCs after 4 h of trophic factor withdrawal (TFW) with no treatment (Ctl) or with 20 mM lithium, 5 μM kenpaullone (Kp), 5 μM GSK3 inhibitor II (II), 20 μM indirubin-3′-monoxime (Ind), or 5 μM SB216763 (SB). d, quantitative values were obtained by densitometric measurements of immunoblots of active caspase-3 and are the means ± S.E.; n = 3; *, p < 0.001. C, control. e, fluorescence-activated cell sorter analysis of cell death indicated treatment with the GSK3 inhibitors 20 mM lithium, 5 μM kenpaullone, or 5 μM SB216763 reduced cell death 12 h after trophic factor withdrawal. Means ± S.E.; n = 3; *, p < 0.001.
NPC viability was measured after 4, 8, and 12 h of trophic factor withdrawal by fluorescence-activated cell sorter analysis with propidium iodide staining to test if GSK3 inhibition maintained NPC viability (supplemental Fig. 2a). Lithium treatment reduced cell death by 50% after trophic factor withdrawal (supplemental Fig. 2b), and comparison of three GSK3 inhibitors showed that all provided at least 50% protection from cell death 12 h after trophic factor withdrawal (Fig. 3e). Taken together, these results demonstrate that in NPCs trophic factor withdrawal causes rapid dephosphorylation of Akt and GSK3 and caspase-3 activation, and GSK3 inhibitors substantially reduce caspase-3 activation and enhance the viability of NPCs, supporting the conclusion that GSK3 facilitates apoptotic signaling in NPCs.
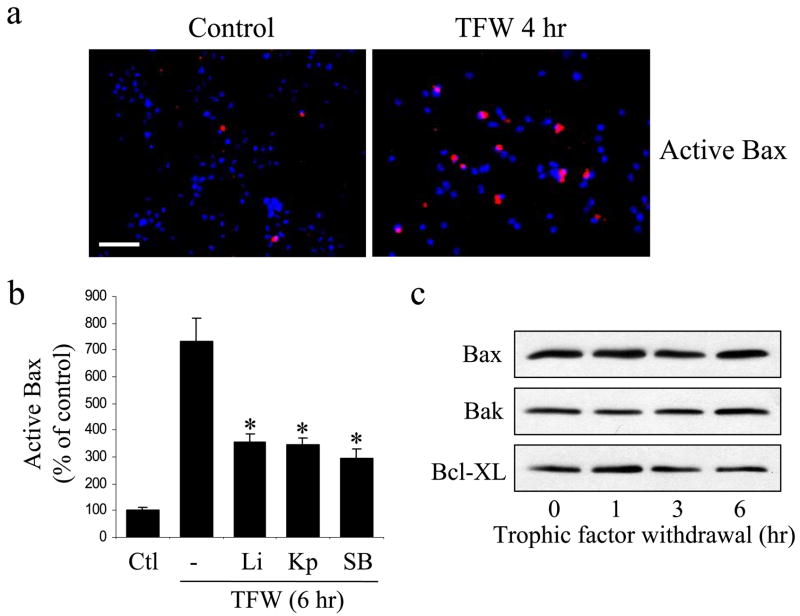
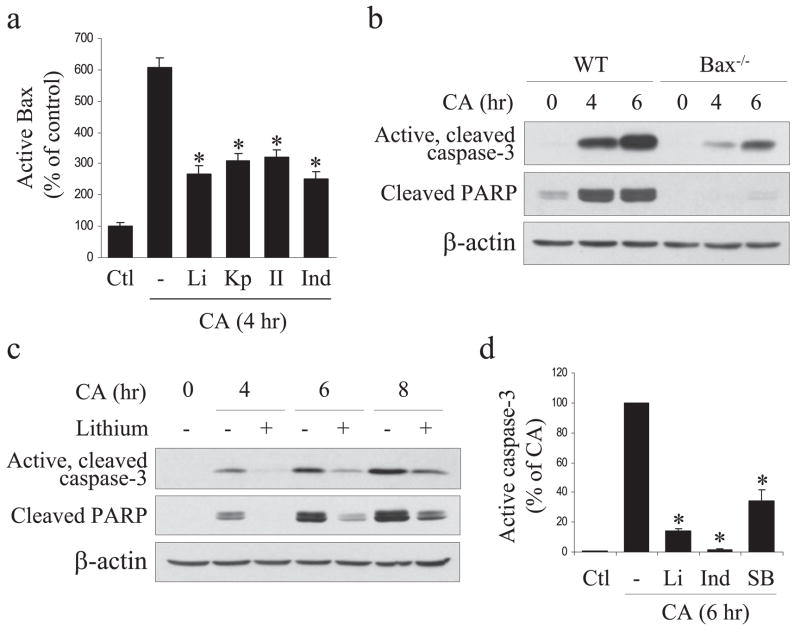
The activation of Bax is a key step in the apoptotic signaling cascade (17, 36). When Bax is activated in the cytosol it translocates to the mitochondria where it promotes the disruption of mitochondria and the ensuing activation of caspases. Apoptosis-associated activated Bax can be detected with a conformational-specific Bax antibody (30). Immunocytochemistry demonstrated a large increase of activated Bax in NPCs after trophic factor withdrawal (Fig. 4a). Trophic factor withdrawal-induced Bax activation was significantly attenuated by treatment with three different GSK3 inhibitors, 20 mM lithium, 5 μM kenpaul-lone, or 5 μM SB216763 (Fig. 4b). Trophic factor withdrawal did not alter the cellular level of Bax or two other bcl-2 family members, Bak and Bcl-XL (Fig. 4c). These results demonstrate that GSK3 promotes the apoptotic signaling cascade at least in part by promoting apoptotic signaling upstream of Bax activation in NPCs subjected to trophic factor withdrawal.
FIGURE 4. GSK3 inhibitors reduce trophic factor withdrawal-induced Bax activation.
a, immunocytochemical comparison of Bax activation (red) in control NPCs and after 4 h of trophic factor withdrawal. Nuclei were labeled with bisbenzimide (blue). Scale bar, 40 μm. b, treatment with 20 mM lithium (Li), 5 μM kenpaullone (Kp), or 5 μM SB216763 (SB) attenuated trophic factor withdrawal-induced Bax activation (means ± S.E.; n = 3; *, p < 0.001). c, cellular protein levels of Bax, Bak, and Bcl-XL were not changed during trophic factor withdrawal.
Because trophic factor withdrawal-induced apoptosis activated Bax, GSK3 has been reported to contribute to Bax activation (37) and GSK3 inhibitors attenuated Bax activation, we tested if Bax was required for trophic factor withdrawal-induced caspase-3 activation or protection by GSK3 inhibitors. These questions were examined by using NPCs isolated from bax−/− mouse embryos. Caspase-3 activation and the associated proteolysis of PARP caused by trophic factor withdrawal were substantially delayed in Bax-null NPCs compared with matched wild-type control NPCs (Fig. 5a). However, caspase-3 activation was still attenuated by inhibition of GSK3 in Bax-null NPCs (Fig. 5, b and c). This indicates that although GSK3 promotes Bax activation, GSK3 also promotes apoptotic signaling by additional mechanisms independent of Bax that are upstream of caspase-3 activation in the trophic factor withdrawal-induced apoptotic signaling pathway.
FIGURE 5. GSK3 inhibitors protect from apoptosis even in the absence of Bax.
a, comparison of the time courses of caspase-3 activation and PARP cleavage in NPCs from wild-type and Bax knock-out embryos during trophic factor withdrawal (TFW). b, lithium treatment reduced caspase-3 activation and PARP proteolysis in NPCs from Bax knock-out embryos. β-Actin was used as loading control. c, caspase-3 activation caused by trophic factor withdrawal (6 h) in NPCs from Bax knock-out embryos was reduced by treatment with the GSK3 inhibitors 20 mM lithium (Li), 5 μM kenpaullone (Kp), 20 μM indirubin-3′-monoxime (Ind), or 5 μM SB216763 (SB) (means ± S.E.; n = 3; *, p < 0.001). Ctl, control.
DNA Damage-induced Apoptosis in NPCs
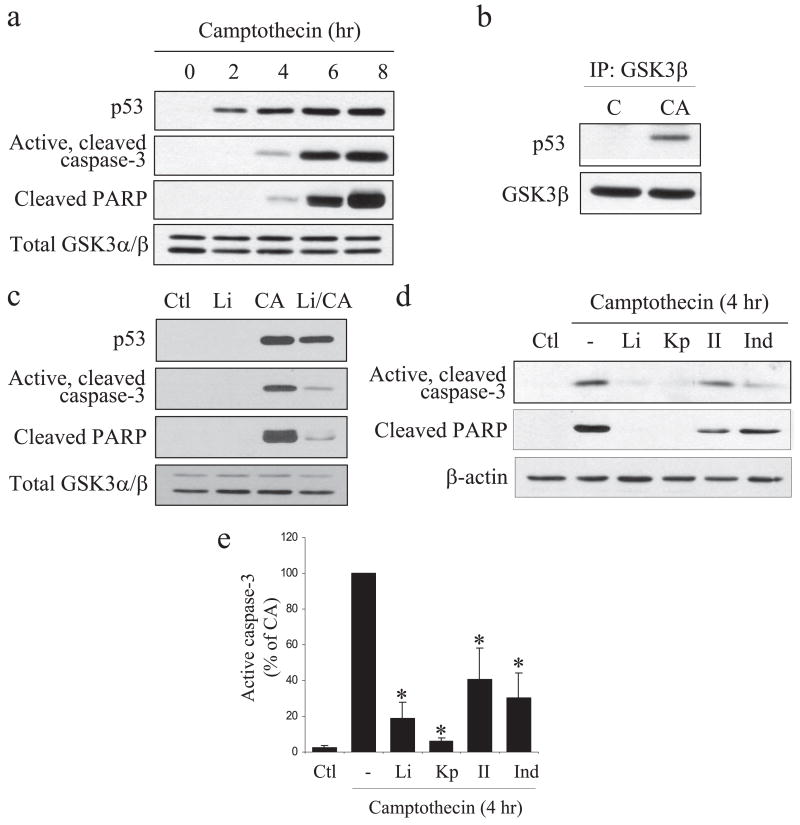
Apoptotic signaling in NPCs also was induced by the DNA-damaging agent camptothecin, a topoisomerase I inhibitor that induces apoptosis through a p53-dependent mechanism (38, 39). Camptothecin (10 μM) treatment of NPCs maintained in trophic factor-containing media caused a time-dependent increase in the level of p53 between 2 and 8 h after treatment, indicative of DNA damage (Fig. 6a). Active, cleaved caspase-3 and cleaved PARP were increased with an ~2 h delay after p53 accumulation (Fig. 6a). Immunocytochemistry confirmed the increased caspase-3 activation after camptothecin treatment (supplemental Fig. 3a). Furthermore, camptothecin-induced accumulated nuclear p53 co-immunoprecipitated with GSK3β (Fig. 6b). These results are in agreement with previous reports that after DNA damage NPCs undergo apoptosis by a mechanism dependent on p53 and caspase-3 (28, 31, 40) and that in other cell types GSK3β binds p53 in the nucleus after DNA damage to promote subsequent apoptotic signaling (42, 43).
FIGURE 6. Caspase-3 is activated in NPCs by camptothecin treatment and is blocked by GSK3 inhibitors.
a, NPCs in trophic factor-containing media were treated with 10 μM camptothecin for 0, 2, 4, 6, or 8 h, and extracts were immunoblotted for p53, active cleaved caspase-3, cleaved PARP, and total GSK3α/β as a loading control. b, p53 co-immunoprecipitated (IP) with GSK3β after camptothecin (CA) treatment. C, control. c, p53, active caspase-3, cleaved PARP, and total GSK3α/β were measured in NPCs 4 h after 10 μM camptothecin treatment with no pretreatment (Ctl) or with a 30-min pretreatment with 20 mM lithium. d, active caspase-3, cleaved PARP, and β-actin were immunoblotted in NPCs 4 h after 10 μM camptothecin treatment with no pretreatment (Ctl) or with a 30-min pretreatment with 20 mM lithium (Li), 5 μM kenpaullone (Kp), 5 μM GSK3 inhibitor II (II), or 20 μM indirubin-3′-monoxime (Ind). e, quantitative values of the effects of GSK3 inhibitors on camptothecin-induced caspase-3 activation were obtained by densitometric measurements of immunoblots of active caspase-3 (means ± S.E.; n = 3; *, p < 0.05).
Inhibition of GSK3 Protects NPCs from DNA Damage-induced Apoptosis
Inhibition of GSK3 with lithium modestly reduced the accumulation of p53, whereas it greatly attenuated camptothecin-induced activation of caspase-3 and cleavage of PARP (Fig. 6c). The effects of lithium were compared with three other inhibitors of GSK3, kenpaullone, GSK3 inhibitor II, and indirubin-3-monoxime, 4 h after camptothecin treatment (Fig. 6d). Quantitation of the results from three independent experiments showed that each of the GSK3 inhibitors greatly reduced caspase-3 activation induced by treatment with camptothecin (Fig. 6e). Thus, GSK3 promotes apoptotic signaling in NPCs after DNA damage as well as after trophic factor withdrawal.
To examine the role of Bax in DNA damage-induced apoptotic signaling in NPCs and its facilitation by GSK3, NPCs were prepared from wild-type and Bax-null embryos. Camptothecin treatment caused a large increase in active Bax immunoreactivity in NPCs from wild-type mice (supplemental Fig. 3b). This is in accordance with previous reports that Bax is an important intermediate in genotoxic stress-induced apoptosis in NPCs (28, 31, 40). Bax activation in response to camptothecin administration was attenuated by treatment with four different GSK3 inhibitors (Fig. 7a). In Bax-null NPCs, the camptothecin-induced increase of caspase-3 activation was retarded compared with wild-type NPCs (Fig. 7b). To examine if caspase-3 activation induced by DNA damage in bax−/− NPCs is also promoted by GSK3, we tested the effects of several GSK3 inhibitors. Pretreatment with lithium inhibited DNA damage-induced activation of caspase-3 and PARP cleavage between 4 and 8 h after treatment of Bax-null NPCs with camptothecin (Fig. 7c). Protection by GSK3 inhibition was confirmed by treatment with two other selective GSK3 inhibitors, indirubin-3′-monoxime and SB216763, which attenuated caspase-3 activation (Fig. 7d). These results indicate that GSK3 promotes DNA damage-induced apoptosis in NPCs, promoting both Bax activation and a Bax-independent mechanism leading to caspase-3 activation.
FIGURE 7. GSK3 inhibitors reduce camptothecin-induced apoptosis.
a, treatment with 20 mM lithium (Li), 5 μM kenpaullone (Kp), 5 μM GSK3 inhibitor II (II), or 20 μM indirubin-3′-monoxime (Ind) attenuated camptothecin (CA)-induced Bax activation (means ± S.E.; n = 3; *, p <0.05). Ctl, control. b, comparison of the time courses of caspase-3 activation and PARP proteolysis in NPCs from wild-type (WT) and Bax knock-out embryos after 10 μM camptothecin treatment. c, lithium treatment reduced camptothecin-induced caspase-3 activation and PARP proteolysis in NPCs from Bax knock-out embryos. β-Actin was used as loading control. d, caspase-3 activation in NPCs from Bax knock-out embryos was reduced by treatment with the GSK3 inhibitors 20 mM lithium (Li), 20 μM indirubin-3′-monoxime (Ind), or 5 μM SB216763 (SB) (means ± S.E.; n = 3; *, p < 0.05).
DISCUSSION
This investigation sought to identify signaling mechanisms regulating the balance between the survival and death of NPCs. NPCs were found to be vulnerable to trophic factor withdrawal and to genotoxic stress, each causing activation of apoptotic signaling involving Bax and caspase-3. Inhibition of GSK3 greatly diminished activation of apoptotic signaling caused by each of these conditions. Thus, GSK3 activity makes an important contribution to shifting the balance from survival to apoptosis in NPCs, and this facilitation of apoptotic signaling by GSK3 is amenable to pharmacological modulation to diminish apoptosis in NPCs.
Deficient trophic factor supply rapidly activated the apoptotic signaling cascade in NPCs, similarly to apoptosis in mature neurons after trophic factor withdrawal (43). Trophic factors support cell viability by activating a variety of signaling pathways, one of which is the phosphatidylinositol 3-kinase/Akt pathway (26, 32). This signaling activity was quickly arrested after trophic factor withdrawal from NPCs, as indicated by the rapid dephosphorylation of Akt that signifies its inactivation. GSK3 is one of the important targets of Akt (20), and trophic factor withdrawal reduced the inhibitory serine phosphorylation of GSK3 in parallel with the inactivation of Akt. Conversely, stimulating NPCs with IGF-1 (which activates the phosphatidylinositol 3-kinase/Akt pathway) reactivated Akt and inhibited GSK3, and IGF-1 treatment was sufficient to block activation of caspase-3. After the dephosphorylation of Akt and of GSK3 induced by trophic factor withdrawal, Bax was activated, and this was followed by activation of caspase-3, an executioner caspase. The finding that trophic factor withdrawal induced apoptosis in NPCs is in accordance with previous evidence that lack of trophic factor support impairs neurogenesis in vivo, although in vivo measurements of neurogenesis often entail the summation of proliferation and survival. For example, supplying exogenous growth factors increased neurogenesis in developing and adult rodent brain (44–50), and administration or overexpression of growth factors decreased NPC apoptosis in vivo (51–53).
In addition to finding that trophic factor withdrawal induced apoptotic signaling in NPCs, we identified GSK3 as an important modulator of this signaling, as a panel of GSK3 inhibitors effectively attenuated the activation of both Bax and caspase-3. Although each of these inhibitors is not entirely specific for GSK3, the only common target among them all is GSK3 (27). Furthermore, whereas lithium inhibits GSK3 by competing with magnesium, the others are structurally diverse inhibitors competitive with ATP. These inhibitors showed that the pro-apoptotic action of GSK3 was targeted partially upstream of Bax activation but was not entirely dependent on Bax since inhibition of GSK3 still provided protection from trophic factor withdrawal in Bax-null NPCs.
Genotoxic stress also activated apoptosis in NPCs, and this was attenuated by inhibitors of GSK3. A key protein activated by genotoxic stress is the tumor suppressor p53. Although normally a short-lived protein, DNA damage activates signaling pathways leading to the stabilization of p53, a transcription factor capable of inducing the expression of pro-apoptotic proteins (56). In accordance with these characteristics of p53 in other types of cells, DNA damage induced in NPCs by camptothecin caused a rapid increase in the level of p53 that preceded the activation of caspase-3. Stabilized p53 formed a complex with GSK3 in NPCs, and although inhibition of GSK3 had little effect on the accumulation of p53, it attenuated the progression of apoptotic signaling to caspase-3 activation, similar to reports in other cell types that GSK3 promotes p53-mediated apoptosis (41, 42, 57). Attenuation of the DNA damage-induced apoptotic signaling cascade by GSK3 inhibitors included suppression of the activation of Bax. That blocking Bax activation reduces apoptosis corroborates previous findings, also shown here, that Bax deficiency partially protects NPCs from genotoxic stress-induced apoptosis (28, 31, 40). However, additional actions of GSK3 were evident because caspase-3 activation was attenuated by GSK3 inhibitors in Bax-deficient NPCs.
The sites at which GSK3 acts to promote apoptosis still remain uncertain. Previous studies have indicated that GSK3 promotes apoptotic signaling both by enhancing signals that lead to the disruption of mitochondrial integrity and by regulating the expression of proteins that modulate apoptotic signaling (20). Bax activation is a key step in many apoptotic conditions, including apoptosis in cortical neurons after DNA damage (38, 58) and trophic factor deprivation (59) as well as in NPCs (60). Furthermore, GSK3 has been reported to activate Bax by direct phosphorylation (37). In NPCs, Bax was activated after both trophic factor withdrawal and camptothecin-induced DNA damage, and a portion of the pro-apoptotic signal of GSK3 was upstream of Bax activation and a portion downstream of Bax activation, as several selective GSK3 inhibitors reduced the activating conformational change of Bax by 50%, whereas they reduced caspase-3 activation by ~85%. We evaluated the significance of Bax for GSK3 to promote apoptosis induced by trophic factor withdrawal and DNA damage by using NPCs derived from Bax knock-out mice. Bax deficiency delayed caspase-3 activation in both conditions, but Bax activation was not absolutely required for NPC apoptosis after both types of insults as Bax deficiency did not fully block caspase-3 activation. Furthermore, inhibition of GSK3 still blocked Bax-independent caspase-3 activation, which indicates that promotion of apoptosis by GSK3 is not mediated exclusively by Bax. In summary, evidence obtained from the present investigation indicates that 1) Bax regulates NPC sensitivity to trophic factor withdrawal and genotoxic injury, 2) GSK3 is an upstream modulator of Bax activation, and 3) Bax is not absolutely essential for the promotion of apoptotic signaling by GSK3.
The identification of GSK3 as a promoter of apoptotic signaling induced in NPCs by either trophic factor withdrawal or genotoxic stress raises the possibility that inhibitors of GSK3 may provide support for shifting the balance toward survival for endogenous NPCs or for transplanted NPCs. The consistent results obtained with several GSK3 inhibitors and their equivalent effects in two apoptotic conditions verifies that GSK3 promotes apoptotic signaling in NPCs. The pro-survival effects of GSK3 inhibitors may underlie previous reports that neurogenesis is enhanced by lithium in vivo (61) and in cultured cells (62, 63). However, those studies did not identify the mechanism by which lithium enhanced neurogenesis, so other actions cannot be excluded, but the pro-apoptotic actions of GSK3 make it a prime target of lithium. Endogenous NPCs die by apoptosis, but the estimated death rates vary tremendously (12–14, 55). Apoptosis also underlies the elimination of most NPCs after transplantation (15). However, complete elimination of apoptosis of NPCs is detrimental, as indicated by studies of neurogenesis in transgenic mice in which apoptosis is greatly impaired, such as in caspase-3 (54) or Bax (59) knock-out mice. Caspase-3-deficient mice displayed embryonic or early postnatal lethality associated with a decrease in cell death, and Bax deficiency reduced naturally occurring developmental neuronal and progenitor cell death (28, 59). Therefore, agents that only shift the balance from death toward survival may provide a nondeleterious intervention for improving the survival rate of NPCs without overburdening the brain with excessive cells. The current results showing that GSK3 promotes apoptotic signaling and that inhibitors of GSK3 provide protection raises the possibility that inhibitors of GSK3 may be useful agents to promote the survival of NPCs.
Acknowledgments
We thank Drs. R. S. Akhtar and B. Clodfelder-Miller for help establishing NPC cultures.
Footnotes
This research was supported by National Institutes of Health Grants MH38752, AG021045, and NS41962.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
The abbreviations used are: NPCs, neural precursor cells; FGF2, fibroblast growth factor 2; GSK3, glycogen synthase kinase 3; IGF-1, insulin-like growth factor-1; PARP, poly(ADP-ribose) polymerase; BSA, bovine serum albumin; PBS, phosphate-buffered saline.
References
- 1.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 2.Abrous DN, Koehl M, Le Moal M. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 3.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 4.Imitola J, Snyder EY, Khoury SJ. Physiol Genomics. 2003;14:171–197. doi: 10.1152/physiolgenomics.00021.2002. [DOI] [PubMed] [Google Scholar]
- 5.Lazic SE, Grote H, Armstrong RJ, Blakemore C, Hannan AJ, van Dellen A, Barker RA. Neuroreport. 2004;15:811–813. doi: 10.1097/00001756-200404090-00014. [DOI] [PubMed] [Google Scholar]
- 6.Duman RS, Malberg J, Nakagawa S, D’Sa C. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs BL, Praag H, Gage FH. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 8.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manev H, Uz T, Smalheiser NR, Manev R. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- 10.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 11.Jin K, Lafevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM. Proc Natl Acad Sci U S A. 2005;102:18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaschke AJ, Staley K, Chun J. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 13.Thomaidou D, Mione MC, Cavanagh JF, Parnavelas JG. J Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- 15.Bakshi A, Keck CA, Koshkin VS, LeBold DG, Siman R, Snyder EY, McIntosh TK. Brain Res. 2005;1065:8–19. doi: 10.1016/j.brainres.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 16.Lossi L, Merighi A. Prog Neurobiol. 2003;69:287–312. doi: 10.1016/s0301-0082(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 17.Akhtar RS, Ness JM, Roth KA. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Pap M, Cooper GM. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 19.Bijur GN, De Sarno P, Jope RS. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 20.Beurel E, Jope RS. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jope RS, Johnson GVW. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Klein PS, Melton DA. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stambolic V, Ruel L, Woodgett JR. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 24.Jope RS. Mol Psychiatry. 1999;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- 25.Jope RS. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 26.Lawlor MA, Alessi DR. J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 27.Martinez A, Castro A, Medina A, editors. Glycogen Synthase Kinase 3 (GSK-3) and Its Inhibitors. John Wiley & Sons, Inc; Hoboken, NJ: 2006. [Google Scholar]
- 28.D’Sa-Eipper C, Leonard JR, Putcha G, Zheng TS, Flavell RA, Rakic P, Kuida K, Roth KA. Development. 2001;128:137–146. doi: 10.1242/dev.128.1.137. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Hsu YT, Youle RJ. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 31.D’Sa C, Klocke BJ, Cecconi F, Lindsten T, Thompson CB, Korsmeyer SJ, Flavell RA, Roth KA. J Neurosci Res. 2003;74:435–445. doi: 10.1002/jnr.10738. [DOI] [PubMed] [Google Scholar]
- 32.Woodgett JR. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee CL, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 34.Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- 35.Leclerc S, Garnier M, Hoessel R, Marko D, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, Eisenbrand G, Meijer L. J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 36.Cregan SP, MacLaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS. J Neurosci. 1999;19:7860–7869. doi: 10.1523/JNEUROSCI.19-18-07860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang H, Kinoshita Y, Knudson CM, Korsmeyer SJ, Schwartzkroin PA, Morrison RS. J Neurosci. 1998;18:1363–1373. doi: 10.1523/JNEUROSCI.18-04-01363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris EJ, Keramaris E, Rideout HJ, Slack RS, Dyson NJ, Stefanis L, Park DS. J Neurosci. 2001;21:5017–5026. doi: 10.1523/JNEUROSCI.21-14-05017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhtar RS, Geng Y, Klocke BJ, Roth KA. Cell Death Differ. 2006;13:1727–1739. doi: 10.1038/sj.cdd.4401879. [DOI] [PubMed] [Google Scholar]
- 41.Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GVW, Jope RS. Proc Natl Acad Sci U S A. 2002;99:7951–7955. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. J Biol Chem. 2003;278:48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oppenheim RW. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cameron HA, Hazel TG, McKay RD. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 46.Wagner JP, Black IB, DiCicco-Bloom E. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 49.Jin K, Mao XO, Sun Y, Xie L, Jin L, Nishi E, Klagsbrun M, Greenberg DA. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Dev Biol. 2006;289:329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O’Kusky JR, D’Ercole AJ. Eur J Neurosci. 2004;19:2056–2068. doi: 10.1111/j.0953-816X.2004.03320.x. [DOI] [PubMed] [Google Scholar]
- 52.Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Koll-mar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR. J Clin Investig. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 55.de la Rosa E, De Pablo F. Trends Neurosci. 2000;23:454–458. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- 56.Fridman JS, Lowe SW. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 57.Beurel E, Kornprobst M, Blivet-Van Eggelpoel MJ, Ruiz-Ruiz C, Cadoret A, Capeau J, Desbois-Mouthon C. Exp Cell Res. 2004;300:354–364. doi: 10.1016/j.yexcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Keramaris E, Stefanis L, MacLaurin J, Harada N, Takaku K, Ishikawa T, Taketo MM, Robertson GS, Nicholson DW, Slack RS, Park DS. Mol Cell Neurosci. 2000;15:368–379. doi: 10.1006/mcne.2000.0838. [DOI] [PubMed] [Google Scholar]
- 59.Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 60.Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW. J Neurosci. 2004;24:11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM. Neuroscience. 2003;117:55–61. doi: 10.1016/s0306-4522(02)00577-8. [DOI] [PubMed] [Google Scholar]
- 63.Shimomura A, Nomura R, Senda T. Neuroreport. 2003;14:1779–1782. doi: 10.1097/00001756-200310060-00004. [DOI] [PubMed] [Google Scholar]