Abstract
Rationale and Objectives
MR imaging of the restricted diffusion of laser-polarized 3He gas provides unique insights into the changes in lung microstructure in emphysema.
Results
We discuss measurements of ventilation (spin density), mean diffusivity, and the anisotropy of diffusion, which yields the mean acinar airway radius. In addition, the use of spatially modulated longitudinal magnetization allows diffusion to be measured over longer distances and times, with sensitivity to collateral ventilation paths. Early results are also presented for spin density and diffusivity maps made with a perfluorinated inert gas, C3F8.
Methods
Techniques for purging and imaging excised lungs are discussed.
Keywords: Lung, Diffusion MRI, Helium-3, anisotropic diffusion, gas MRI
The development of laser-polarized helium-3 (3He) technology (1,2) now allows for imaging of the gas spaces of human and animal lungs (3,4). Typical absolute polarizations of 30%–50% are approximately 100,000 times that available at thermal equilibrium in typical imaging fields of 1.5 T, with a corresponding increase in signal to noise (S/N) for a given quantity of gas. Many of the early applications of hyperpolarized 3He magnetic resonance (MR) imaging used ventilation images (or spin-density images) (3,4), which show the spatial distribution of the 3He after inhalation of a bolus followed by breathhold. In small animals with continuous breathing of 3He, exquisitely resolved microimages have been reported (5).
In recent years, the effort of our group at Washington University has focused on MR determinations of the changes in airways at the acinar level (airways lined with alveoli) using 3He diffusion MR. The following describes the approaches we have taken and presents some of the results at our institution and is not intended as a review of the field (for more comprehensive reviews, see references 3 and 4).
METHODS
All the images reported here were obtained on a 1.5 T Siemens Vision scanner. Three home-built polarizers and a General Electric (Durham, NC, formerly Amersham Health) polarizer were used, producing 0.5–1.0 L 3He gas at 30%–50% polarization (4). The studies involving human subjects were performed with the approval of the Washington University Human Studies Committee, under an Investigational New Drug Exemption of the US Food and Drug Administration
In vivo images were acquired using a homemade Helmholtz pair tuned to the 3He resonance frequency at 48.47 MHz. The coil is tunable to proton frequency without moving the patient, which allows coregistration of proton and 3He images for later comparison. Ex vivo images were acquired using a single-turn solenoid-like coil, built to take advantage of the lack of saline-induced loss and to achieve high Q and sensitivity. Because experimental details for each of the classes of experiments described here differ greatly, additional methods are discussed within each subsection that follows.
RESULTS AND DISCUSSION
Ventilation Imaging
Spin-density images provide visualization of gas during inspiration and after a breathhold to allow for some equilibration. Compared with diffusion maps, as discussed in the following section, their interpretation is straightforward. Spin-density images are useful in visually identifying the regional abnormalities in the distribution of ventilation, which are frequently marked in patients with severe emphysema. In our investigations, these patients are often imaged several days before lung volume reduction surgery (LVRS) (6,7).
Axial slice images of a female patient, pre-LVRS and on 6-month postsurgical follow-up, are presented in Fig 1a. These are gradient-echo images with small-angle radiofrequency (RF) pulses (typically 5–10°) with in-plane resolution of 7 mm × 7 mm; the slice thickness is 10 mm. The intensity of signal and hence the concentration of 3He after a single breath of polarized gas show improved uniformity after surgery. Some regions that received essentially no gas presurgery are ventilated after surgery. As expected, lung size decreases postoperatively as air trapping and hyperinflation decrease. This is a major mechanism for the relief of dyspnea noted in almost all patients undergoing LVRS at our institution. We note that the total 3He signal integrated across the lung only reflects the volume and polarization of the inhaled gas. Thus the important issue is the uniformity of such images.
Figure 1.

Transverse slice 3He images of two patients with severe emphysema, pre–lung volume reduction surgery (left) and 6 months after surgery (right). The images (a) and (c) are spin-density images to reveal the distribution of gas upon inhalation and breathhold; the central, color images (b) are ADC maps of the same patient and slices as (a). After surgery, the ventilation distribution is more uniform. The patient in (c) had a large bullous region presurgery in the left lung that is only partially rectified by the surgery.
Images pre- and post-LVRS for a male patient with a large bullous region in the left lung (right side of figure) are presented in Fig 1c. Successful matching of the slices is evident from the similar appearance of the two major bronchi (appear as two foci of high signal intensity between the lungs) in both axial images. Before surgery, essentially no gas is evident in the left lung; after surgery, ventilation is returned to the anterior regions of the left lung. In this patient, this pattern persists more than half the length (superior to inferior) of the lung (not shown).
Diffusion Imaging
Emphysema is defined as a disease of dilation of the acinar airways associated with destruction of the acinar airway and alveolar walls in the absence of significant fibrosis (8). The changes in the tissue microstructure are easily observed in an adequately inflated lung sampled and viewed under a microscope. The much larger alveolated airway “compartments” present in emphysema imply that the apparent diffusion coefficient of a gas will be larger (less restricted) than in healthy lung tissue (9,10). In particular, 3He has a small atomic mass, so a high thermal velocity, and has a small diameter, so a small collision cross-section; both result in a large free diffusivity, D0 (11). At 300 K and 1 atmosphere, D0 of the 3He is 2.2 cm2/second in pure 3He and 0.88 cm2/second dilute in N2 or air. Thus, in times t of 2–5 milliseconds, the root mean squared (RMS) free displacement of 3He dilute in air in one direction ( ) is 0.6 mm, showing that the gas atoms will thoroughly explore their gas spaces (normal alveolar diameters are about 0.3 mm).
Maps of 3He apparent diffusion coefficient (ADC or restricted diffusivity) are presented in Fig 2 for single 10-mm slices from a normal volunteer (a) and two patients (b) and (c) with severe emphysema. The ADC maps are made from the pixel-by-pixel ratio of intensities of two images, one with a diffusing-sensitizing gradient pulse (b > 0) and one with b = 0. The images are interlaced line-by-line in k-space (one b = 0, one b > 0, second b = 0, second b > 0) to minimize motion effects. We refer to these as 2-b ADC maps. “Diffusion” as used here is not related to the physiologic concept of gas crossing the thin alveolar-capillary membranes, but rather to the space available to gas molecules to diffuse by Brownian motion. In the normal lung in (a), the ADC (see color scale) is relatively uniform with values of about 0.2 cm2/second, a factor of 1/4.5 times the free diffusivity, showing the substantial restriction to diffusion from the airway and alveolar walls. In the emphysematous lungs (b) and (c), the ADC is much larger, reflecting airspace enlargement or tissue destruction, and is regionally nonuniform as is the disease process. Thus 3He ADC can be used to characterize the state of the lung tissue in each voxel. The ventilation and ADC maps address different issues: is the region ventilated and is its tissue destroyed or intact? For the patients with emphysema severe enough to be considered for LVRS, we find typical average ADC values of 0.55 cm2/second, an increase of 175% over normal volunteers (10).
Figure 2.

Transverse slice maps of 3He ADC from the 2-b value method in one healthy volunteer (a) and two patients with severe emphysema, (b) and (c). The color scale maximum of 0.88 cm2/seconds is the free diffusivity, D0. In the emphysema lungs, spatial variation of ADC shows spatial dependence of the severity of tissue destruction. The lung in (b) is nonuniformly ventilated, so parts of the lung do not appear in the ADC map.
The color images in Fig 1b are the pre- and post-LVRS ADC maps corresponding to the spin density images in Fig 1a. A region of tissue with lower ADC (appears as yellow) is evident after surgery, whereas no such comparatively healthy tissue appears in the pre-LVRS ADC map (including nearby slices). It is hard to understand how the gas ADC would be decreased by LVRS, because this is not microsurgery directed at improving the alveolar structure. The surgery, by removing volume occupying but nonfunctional lung, restores lung elastic recoil and improves chest wall function. This allows better function of previously compromised lung and improves chest wall and respiratory muscle mechanics. Regional improvement in 3He lung ventilation imaging coincides with the improved lung function. Previously nonvisualized lung appears in the post-LVRS ADC map, implying that this may be revealing lung that was previously anatomically compressed as well as physiologically impaired and is now returned to function.
Anisotropy of Diffusion
The free diffusion of 3He is sufficiently large that a 3He atom will explore in 2–5 milliseconds more than a single alveolus, which is a comparatively open structure in any case. The size of acinar airways (where 90+% of gas resides) is such that a 3He atom will traverse the interior cross-section of typical airways, with comparatively few atoms diffusing lengthwise far enough to enter a new airway. Thus one can approximate the lung as composed of (infinitely) long cylindrical tubes during the 2–5 millisecond diffusion measurement time (12). The tubes are not smooth-walled, but are sleeved with alveoli and so are called “alveolated airways.” In this geometry, the ADC of 3He will be much more restricted transverse to the airway axis than along it. Defining DL and DT as the longitudinal and transverse ADCs, we expect DT < DL < D0. The angular average of DL and DT is the mean diffusivity DM, DM = 1/3 DL + 2/3 DT; this is essentially the quantity measured in the 2-b ADC measurements discussed previously.
At first thought, it may appear impossible to measure the diffusion anisotropy in lung, because the airway axes are oriented in all directions in each voxel, more or less equally. However, for any orientation of the diffusion-sensitizing gradient G, the direction along which displacements are measured, some airways will be parallel to G, some will be perpendicular to G, and most will be in between. Thus, because the ADC depends on the orientation of the airway, there will be a distribution of effective diffusivities in the direction along G. The end result is that the signal amplitude will exhibit a nonexponential dependence on the diffusion sensitivity parameter b, with the airways parallel to G showing the fastest attenuation with b and the perpendicular airways displaying the lowest ADC and hence the slowest decay of signal as a function of b. The nonexponential decay is characterized by just three parameters (DL, DT, and signal amplitude at b = 0); with high S/N data, these three parameters can be reliably extracted. We note that the relative amplitudes of the signals from airways parallel, perpendicular, and in between are known, because of the assumption that the airway orientation distribution is uniform in all directions, for a given imaging voxel.
To characterize the decay, we use six equally spaced b values (one is b = 0), with all six scans interlaced line-by-line of k-space (12). To get the required additional S/N needed for extracting three parameters (instead of just two parameters in the 2-b ADC method), we double the slice thickness to 20 mm. The thicker slices also allow a larger fraction of the lung to be imaged in the limited breathhold time. We present in Fig 3 coronal images from a normal volunteer. The mean diffusivity DM is in good agreement (see color bar) with the 2-b ADC data of the healthy volunteer in Fig 2a. The transverse motion DT is extremely restricted, with DT less than 0.1 cm2/second. That DL is only about half of D0 (0.88 cm2/second) demonstrates that the airways are not smooth-walled, but rather are cylinders covered with a sleeve of alveoli.
Figure 3.

Coronal 3He diffusivity and mean radius maps from a healthy volunteer. DM is the mean diffusivity, DL is longitudinal diffusivity, and R is the mean acinar airway radius calculated from the transverse diffusivity DT. The color scale at right is in units of cm2/second or mm.
Calculations show (12) that DT is a very sensitive function of mean acinar airway major radius, R. Thus the DT data can be inverted to yield R, as in Fig 3. The value of R, 0.35 mm, is in excellent accord with values from microscopic measurements on normal lungs.
Both DT and DL become larger in the presence of emphysema. DL quickly saturates to nearly D0, so in severe disease DT is the more valuable measurement. We note that, in severe disease with development of large collateral pathways (see the following section), the model of long cylindrical tubes will break down. Simulations have been used to compare this model of lung airways to others (13).
Long-Range Diffusion
All of the methods discussed so far determine the ADC at diffusion times of 2–5 milliseconds, corresponding to free displacements of less than 1 mm. Thus most gas atoms start and end the diffusion-interval (determined by the gradient pulse) in the same acinar airway. Additional information about the lung airways is available from long-range ADC measurements.
Diffusion over longer distances (1–3 cm, for example) requires much longer diffusion time intervals (several seconds). This is much longer than the intravoxel T2* of approximately 20 milliseconds at 1.5 T, which determines the lifetime of transverse magnetization in gradient-echo images. The solution is to let the longitudinal spin magnetization MZ carry the position information, much as in a stimulated echo (14). At time zero, a sequence of two π/4 RF pulses is used, separated by a gradient pulse in the x-direction (15). The MZ is modulated from relative value 1 to 0 as a function of x,
where λ is determined by the area of the gradient pulse:
A small-flip angle inspection image taken afterwards in the x-y plane reveals a stripe pattern superimposed on the lung image, as in Fig 4, upper left.
Figure 4.
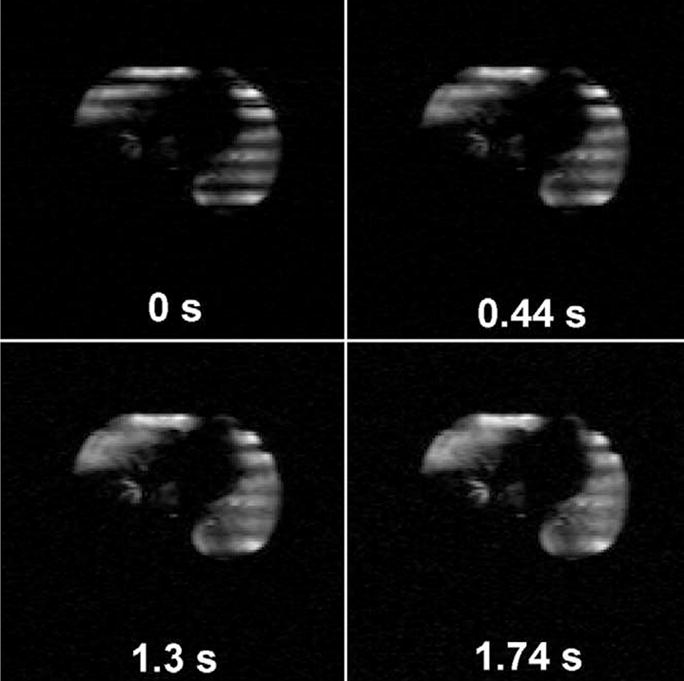
3He images of a single transverse slice from a patient with emphysema at the indicated times after the magnetization tagging (striping) is applied. The right lung contains a region that received negligible 3He gas. The rapid disappearance of the stripes in some regions indicates a very rapid long-range diffusivity, Dsec. The anterior portion of the left lung in the presented slice demonstrates comparatively low Dsec. The striping wavelength is 30 mm.
Brownian motion (diffusion) of the gas will mix the bright (magnetized) and dark (unmagnetized) spins, leading to a decay of the spatial modulation (16,17). That is, the stripes fade without losing average intensity, as in Fig 4. Nevertheless, we use a normalizing scheme that corrects for overall T1 decay of the signal, as well as consumption of MZ by the RF imaging pulses. A different approach that monitors filling in of magnetization from neighboring slices has been reported (18).
A succession of striped images from a patient with severe emphysema is presented in Fig 4. These are transverse slices of 20-mm thickness obtained just after striping and every 0.44 seconds thereafter. The right lung (toward left in the figure) is only partially ventilated. The stripe pattern fades most rapidly in the right lung and in the posterior portion of the left lung (at bottom), indicating that the long-range diffusivity (Dsec, diffusivity measured over seconds) is largest in those regions. The decay rate of the striping modulation allows a quantitative map of Dsec to be calculated from the data. We have found very large changes in Dsec, increasing from 0.02 cm2/second in normal canine and human lungs to greater than 0.5 cm2/second in the regions of worst emphysematous destruction.
The relevant length scale for diffusive motion is λ/2 or about 1–1.5 cm in our work. In healthy lungs, the airways form a network of bifurcating paths over about 23 levels (19). Thus the airways are singly connected with a unique path between any two points: a journey from one location at, for example, level 21 to level 22 at a point 1.5 cm away necessarily requires going from one acinus to another, because acini have maximum linear dimensions of about 7 mm. Thus the atom must move from level 21 to at least level 16 (the level where acini commence) and back to level 22. At each of many junctions, the atom must make the correct turn if it is to leave its starting acinus. This tortuous path will make the long-range ADC quite small (0.02 cm2/seconds, as mentioned previously, for normal lungs).
In emphysema, collateral ventilation pathways develop and can become as important for peripheral gas flow as the more usual routes (20 –22). This occurs as some collateral paths will have less resistance to gas flow than through diseased airways, allowing for ventilation and gas exchange to partially adapt to the structural and physiologic abnormalities imposed by the disease. Thus there are now an increased number of diffusion paths that are functioning routes for long-distance convective transport or diffusion of gas. In essence, collateral paths are short circuits (bypasses) and will result in substantial increase in the long-range diffusivity. Thus we believe that long-range ADC measurements provide information on the extent of collateral pathways.
Increased collateral ventilation will be required for the success of some (although not all) airway bypass procedures proposed to ameliorate emphysema. In one such scenario (23), an artificial airway is made by piercing an airway wall to connect a patent airway to a previously underventilated or gas-trapped region of lung parenchyma. The desired situation is that a large volume of hyperinflated lung that intercommunicates through collateral pathways could be emptied through a small number of artificial airways, producing many of the benefits of LVRS but with minimal invasiveness. Without collateral paths, this proposed procedure is unlikely to succeed.
Fluorinated Gases
A major obstacle to the widespread use of hyperpolarized 3He MRI remains the cost and required maintenance of the polarizer devices, as well as the special precautions needed with the non-renewable spin magnetization. Our group (24) and others (25,26) have explored the use of fully fluorinated inert gases: SF6, CF4, C2F6, and C3F8. We note the first gas images of lung were reported in canines by Lauterbur (27) using CF4. The potential advantage of 19F is that a polarizer is not involved. Signal is increased by four to six equivalent spins per molecule, and a short T1 allows for rapid signal averaging. The primary disadvantage is that the S/N will be inferior to that of 3He, even with signal averaging for 1 minute or more.
We have explored 19F gas imaging using C2F6 and C3F8 in explanted human lungs for proof of principle and to avoid any possible regulatory issues. Also, explanted emphysematous lungs are excellent models of the disease in vivo. A ventilation image (spin density) is presented in Fig 5a from C3F8 in an emphysematous lung removed at transplant surgery. Acquisition of the three-dimensional data set of 10 partitions of 32-mm thickness each with 5.5 mm × 5.5 mm in-plane resolution required 25 seconds. Additional averaging time can provide good quality images with better spatial resolution. We note that the lung imaging community has yet to decide the resolution required for useful lung images.
Figure 5.
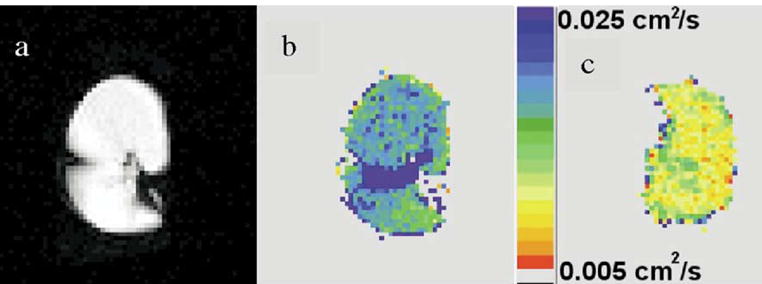
19F magnetic resonance images using C3F8 gas, perfluoropropane. The (a) and (b) images are spin-density and ADC (in color) from an excised lung with severe emphysema, respectively; an ADC map from an excised normal lung is shown (c) for comparison on the same color scale. Higher ADC because of the emphysema is evident in (b).
The free diffusivity of C3F8 has been measured by us to be only 0.022 cm2/seconds at 300 K and 1 atmosphere, much smaller than that of 3He. For structures with the characteristic sizes of alveoli and acinar airways, the diffusion of C3F8 will be restricted, but to a lesser extent than 3He. In fact, C3F8 ADC will be in a regime where the ADC can be used to determine the local surface to volume ratio, S/V. Mitra, Sen, and Schwartz showed that at short diffusion times t as apply here (28):
A measurement of S/V at each voxel should be an important and quantitative way to characterize the state of lung disease.
The image in Fig 5b is an ADC map of the same slice of the same lung as in Fig 5a. For comparison, the image of Fig 5c is an ADC map of an explanted normal lung that was unsuitable for use in a recipient for minor technical reasons. Clearly, diffusion in the normal lung is more restricted than in the emphysematous lung. Thus the ADC of C3F8 gas can be used to report on the local extent of emphysematous change in lung microstructure. This work, although preliminary, points to the potential of 19F imaging of perfluorinated gases as a possible alternative to use of hyperpolarized 3He.
Explanted Lung Imaging
Imaging lungs removed from transplant recipients or lobes removed from cancer patients offers some advantages over live subjects, when the goal is to develop MR imaging techniques. First, regulatory issues can largely be avoided. Second, experiments with long breathholds or no oxygen (which depolarizes 3He) are possible. Third, a given bolus of 3He gas can be used for many experiments, because T1 can be many minutes in the absence of oxygen (29). Fourth, the S/N can be improved by the use of a smaller RF coil (higher filling factor); the absence of a chest and its attendant RF losses results in a very high-Q RF coil. The coil can be a sideways-oriented solenoid, which delivers approximately 6 dB better RF performance (30) than a Helmholtz pair (as used with live subjects). Finally, the lung can be fixed in a state of inflation with formalin vapor (discussed in the following section), so the lung is available at a fixed volume for repeated measurements or new pulse sequences, long after the initial measurements.
Surgical preparation of the explanted lung is crucial to success. Leaks and tears from adhesions must be avoided and a leak-free connection to laboratory tubing is required. Various glues and patches have been found useful in repairing leaks. Strain relief (physical support) at the bronchus is important.
Purging of the lung with nitrogen in a bell jar apparatus ensures complete removal of oxygen. The lung is evacuated in a way that avoids creating a transpleural pressure differential. That is, the region external to the lung and the lung bronchus are both evacuated together. The lung and bell jar are then nitrogen backfilled in the same pressure-balanced way. The procedure is usually repeated three times. We have found only occasional, minor leaks that open in the pleural surface from this procedure, which also has somewhat of a drying effect. We note, however, that the worst gas-trapping lungs grow to large dimensions under reduced pressure before they begin to expire their trapped gas.
After purging with N2, the lung is placed in a plastic trough and covered with a plastic bag. A continuous N2 flow through the bag keeps any oxygen from contact with the lung. Thus, small leaks may allow 3He out but no oxygen into the lung. The covered trough is put into the RF coil and loaded into the center of the imaging magnet. After injection of typically 0.25–0.5 L 3He, imaging commences. The high sensitivity of the high-Q RF solenoid allows use of small RF tipping pulses (of order 3 degrees), so that many image sets can be obtained from a single bolus of 3He.
We have constructed an apparatus for fixing lungs with formalin-vapor (31,32). Formaldehyde-water solution is heated to 45–50°C and the vapor surrounds the lung. A diaphragm pump and solenoid valves are used to force the vapor alternately into and out of the lung, so that the formalin is distributed uniformly. The volume and rate of vapor respiration are adjustable. Generally, 4 – 6 hours in the vapor provide a thoroughly fixed lung that shows no evident changes over several months. Lungs fixed in this manner have been useful over days to weeks to months (depending on their source and need for clinical pathology) for testing new sequences and new measurement ideas.
CONCLUSIONS
The enhanced spin signals from laser-polarized 3He allow MR imaging of gas spaces in the lung. Ventilation (spin density) images permit underventilated lung regions to be identified. However, diffusion measurements with 2-b values provide an entirely new form of information. This technique provides maps of the extent of acinar airway expansion and alveolar destruction from emphysema. A more quantitative and detailed understanding is provided by 6-b value measurements that separately determine the longitudinal and transverse diffusivities, DL and DT. Both increase in emphysema, with DL reflecting the smoothness of the airway walls and DT being a measure of the mean acinar-airway major diameter. In healthy lungs, the diameters determined from DT are in excellent agreement with published results from microscopic examinations. Long-range diffusion measurements use sinusoidally modulated longitudinal magnetization. The diffusion Dsec over distances of 1–1.5 cm and times of seconds is found in normal human and canine lungs to be around 0.02 cm2/second, smaller by a factor of 10 than the ADC measured by the 2-b method over milliseconds and submillimeter distances. Exceptionally large increases of factors more than 25 are found in some lungs with severe emphysema.
19F MR imaging of perfluorinated gases may be an attractive, low-cost alternative to laser-polarized 3He. Spin-density images and measurements of restricted diffusion in excised healthy and emphysematous human lungs appear promising.
The use of explanted lungs benefits from careful surgical excision and preparation at lung transplant surgery. Thorough nitrogen purging can be realized in a bell jar apparatus, allowing for many MR measurements from a single bolus of 3He. Formalin fixation of the lungs allows repeated use with time and creation of local “standard reference lungs”.
Acknowledgments
Supported by awards from the NIH (NHLBI R01HL70037 and R01HL72369). Funding through the GEMI fund made the 19F work possible. A generous loan of a polarizer apparatus from General Electric is acknowledged. This article is based on a talk at the 2004 International Workshop on Pulmonary Imaging, Philadelphia, PA, November 2004. The authors are grateful to the patients and their families for making available their excised lungs. The lung transplant team is gratefully acknowledged for their assistance. The authors appreciate the efforts of the LVRS patients, the LVRS coordinators, and Nadia Blake, in arranging for 3He exams.
References
- 1.Happer W. Spin exchange, past, present, and future. Ann Phys Fr. 1985;10:645–657. [Google Scholar]
- 2.Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev Mod Phys. 1997;69:629–642. [Google Scholar]
- 3.Moller HE, Chen XJ, Saam B, et al. MRI of the lungs using hyperpolarized noble gases. Magn Reson Med. 2002;47:1029–1051. doi: 10.1002/mrm.10173. [DOI] [PubMed] [Google Scholar]
- 4.LeaWoods JC, Yablonskiy DA, Saam B, et al. Hyperpolarized 3He gas production and MR imaging of the lung. Concepts Magn Reson. 2001;13:277–293. [Google Scholar]
- 5.Moller HE, Chen XJ, Chawla MS, et al. Sensitivity and resolution in 3D NMR microscopy of the lung with hyperpolarized noble gases. Magn Reson Med. 1999;41:800–808. doi: 10.1002/(sici)1522-2594(199904)41:4<800::aid-mrm20>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JD, Patterson GA. Lung volume reduction surgery for severe emphysema. Semin Thor Cardiovasc Surg. 1996;8:52–60. [PubMed] [Google Scholar]
- 7.National Emphysema Treatment Trial Research Group. A randomized trial concerning lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 8.West JB. Pulmonary pathophysiology—the essentials. Baltimore: Williams and Wilkins; 1992. pp. 58–77. [Google Scholar]
- 9.Salerno M, de Lange EE, Altes TA, et al. Hyperpolarized 3He diffusion MRI of the lungs in emphysema: comparison with pulmonary function tests—initial experience. Radiology. 2001;22:252–260. [Google Scholar]
- 10.Saam BT, Yablonskiy DA, Kodibagkar VD, et al. MR imaging of diffusion of 3He gas in healthy and diseased lungs. Magn Reson Med. 2001;44:174–179. doi: 10.1002/1522-2594(200008)44:2<174::aid-mrm2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfelder JO, Curtiss CF, Bird RB. Molecular theory of gases and liquids. New York: Wiley; 1954. [Google Scholar]
- 12.Yablonskiy DA, Sukstanskii AL, Leawoods JC, et al. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Nat Acad Sci. 2002;99:3111–3116. doi: 10.1073/pnas.052594699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fichele S, Paley MNJ, Woodhouse N, et al. Finite differences simulations of 3He diffusion in 3D alveolar ducts: a comparison with the “cylinder-model. Magn Reson Med. 2004;52:917–920. doi: 10.1002/mrm.20213. [DOI] [PubMed] [Google Scholar]
- 14.Hahn EL. Spin echoes. Phys Rev. 1950;80:580–594. [Google Scholar]
- 15.Woods JC, Yablonskiy DA, Chino K, et al. Magnetization tagging decay to measure long-range 3He diffusion in healthy and emphysematous canine lungs. Magn Reson Med. 2004;51:1002–1008. doi: 10.1002/mrm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owers-Bradley JR, Fichele S, Bennattayalah A, et al. MR tagging of human lungs using hyperpolarized 3He gas. J Magn Reson Imaging. 2003;17:142–146. doi: 10.1002/jmri.10226. [DOI] [PubMed] [Google Scholar]
- 17.Owers-Bradley JR, Bennattayalah A, Fichele S, et al. Diffusion and tagging of hyperpolarized 3He in the lungs. Proceedings of the 10th Annual Meeting of ISMRM; Honolulu. 2002. p. 2016. [Google Scholar]
- 18.Fichele S, Paley MNJ, Woodhouse N, et al. Measurements and modeling of long range 3He diffusion in the lung using a “slice-washout” method. J Magn Reson. 2005;174:28–33. doi: 10.1016/j.jmr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 19.West JB. Respiratory physiology—the essentials. Baltimore: Williams and Wilkins; 1995. [Google Scholar]
- 20.Terry PB, Traystman RJ, Newball HH, et al. Collateral ventilation in man. N Engl J Med. 1978;298:10–15. doi: 10.1056/NEJM197801052980103. [DOI] [PubMed] [Google Scholar]
- 21.Macklem PT. Collateral ventilation. N Engl J Med. 1978;298:49–50. doi: 10.1056/NEJM197801052980112. [DOI] [PubMed] [Google Scholar]
- 22.Hogg W, Brunton J, Kryger M, et al. Gas diffusion across collateral channels. J Appl Phys. 1972;33:568–575. doi: 10.1152/jappl.1972.33.5.568. [DOI] [PubMed] [Google Scholar]
- 23.Lausberg HF, Chino K, Patterson A, et al. Bronchial fenestration improves expiratory flow in emphysematous lungs. Ann Thorac Surg. 2003;75:393–398. doi: 10.1016/s0003-4975(02)04553-8. [DOI] [PubMed] [Google Scholar]
- 24.Jacob RE, Chang YV, Choong CK, et al. 19F MR imaging of ventilation and diffusion in excised lungs. Magn Reson Med. 2005;54:577–585. doi: 10.1002/mrm.20632. [DOI] [PubMed] [Google Scholar]
- 25.Kuethe DO, Caprihan A, Fukushima E, et al. Imaging lungs using inert fluorinated gases. Magn Reson Med. 1998;39:85–88. doi: 10.1002/mrm.1910390114. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber WG, Eberle B, Laukemper-Ostendorf S, et al. Dynamic 19F-MRI of pulmonary ventilation using sulfur hexafluoride (SF6) gas. Magn Reson Med. 2001;45:605–613. doi: 10.1002/mrm.1082. [DOI] [PubMed] [Google Scholar]
- 27.Rinck PA, Petersen SB, Lauterbur PC. NMR-imaging von fluorhaltigen substanzen. Fortschr Roentgenstr. 1984;140:239–243. doi: 10.1055/s-2008-1052964. [DOI] [PubMed] [Google Scholar]
- 28.Mitra PP, Sen PN, Schwartz LM. Short-time behavior of the diffusion coefficient as a geometrical probe of porous media. Phys Rev B. 1993;47:8565–8574. doi: 10.1103/physrevb.47.8565. [DOI] [PubMed] [Google Scholar]
- 29.Saam BT, Happer W, Middleton H. Nuclear relaxation of 3He in the presence of O2. Phys Rev A. 1955;52:862–865. doi: 10.1103/physreva.52.862. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima E, Roeder SBW. Experimental pulse NMR, a nuts and bolts approach. Reading MA: Addison-Wesley; 1981. pp. 386–387. [Google Scholar]
- 31.Wright BM, Slavin G, Kreel K, et al. Postmortem inflation and fixation of human lungs. Thorax. 1974;29:189–194. doi: 10.1136/thx.29.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittermayer C, Wybitul K, Rau WS, et al. Standardized fixation of human lung for radiology and morphometry; description of a two-chamber system with formaldehyde vapor inflation. Pathol Res Pract. 1978;162:115–130. doi: 10.1016/S0344-0338(78)80134-4. [DOI] [PubMed] [Google Scholar]


