Abstract
Human Immunodeficiency Virus Type I (HIV-1) establishes a latent reservoir early in infection that is resistant to the host immune response and treatment with highly active antiretroviral therapy (HAART). The best understood of these reservoirs forms in resting CD4+ T cells. While it remains unclear how reservoirs form, a popular model holds that the virus can only integrate in activated CD4+ T cells. Contrary to this model, our previous results suggest that HIV-1 can integrate directly into the genomes of resting CD4+ T cells. However, a limitation of our previous studies was that they were conducted at high viral inoculum and these conditions may lead to cellular activation or saturation of restriction factors. In the present study, we tested if our previous findings were an artifact of high inoculum. To do this, we enhanced the sensitivity of our integration assay by incorporating a repetitive sampling technique that allowed us to capture rare integration events that occur near an Alu repeat. The new technique represents a significant advance as it enabled us to measure integration accurately down to 1 provirus/well in 15,000 genomes – a 40-fold enhancement over our prior assay. Using this assay, we demonstrate that HIV can integrate into resting CD4+ T cells in vitro even at low viral inoculum. These findings suggest there is no threshold number of virions required for HIV to integrate into resting CD4+ T cells.
Introduction
Human Immunodeficiency Virus-1 (HIV-1) establishes a latent infection that effectively evades immune surveillance and drug therapy (Blankson, Persaud, and Siliciano, 2002; Chun et al., 1997b; Lassen et al., 2004; Lehrman et al., 2005; Pierson, McArthur, and Siliciano, 2000; Stevenson, 2003). The best characterized latently infected cells are resting CD4+ T cells. It is thought that latently infected cells form when HIV DNA integrates into an activated cell just before that cell returns to a resting state (Han et al., 2007). This hypothesis is supported by early findings in vitro (Bukrinsky et al., 1991; Korin and Zack, 1998; Stevenson et al., 1990; Sun and Clark, 1999; Unutmaz et al., 1999; Yamashita and Emerman, 2006; Zack et al., 1990; Zack et al., 1992) and in HIV infected individuals (Chun et al., 1997a; Chun et al., 1995; Ostrowski et al., 1999). However, this hypothesis must be reconciled with studies showing that HIV (and SIV) is produced from CD45RA+ naïve CD4+ T cells in HIV infected organ cultures (Eckstein et al., 2001) and in HIV (and SIV) infected individuals (Kinter et al., 2003a; Nishimura et al., 2005; Zhang et al., 1999). The fact that HIV is produced from naïve cells suggests that an activation step may not be required for HIV integration, although it is also possible that all the CD45RA+ cells that are producing HIV are not truly naïve (De Rosa, Herzenberg, and Roederer, 2001) or that naïve cells receive an activating stimuli that allows productive infection to occur without causing the cell to switch to the CD45RO isoform.
At odds with the early in vitro findings (Yamashita and Emerman, 2006), we recently reported that integration does occur in resting CD4+ T cells inoculated with HIV in vitro (Swiggard et al., 2005). In this study, we used high viral inoculum conditions in order to obtain a measurable transduction frequency. To achieve high viral inoculum conditions, we used a technique called spinoculation which deposits many virions to each resting CD4+ T cell (O'Doherty, Swiggard, and Malim, 2000). Thus, it was possible that the observed integration in resting T cells was due to these artificial conditions. It was possible that the high inoculum conditions may have activated the cells without inducing upregulation of activation markers. Consistent with this idea, it had been reported that productive infection of inoculated PBMCs occurs when these cells are exposed to high concentrations of gp120 (Briant et al., 1996), presumbably by inducing cross-linking of CD4 (Briant et al., 1999; Kinter et al., 2003b; Kornfeld et al., 1988). Alternatively, it was possible that depositing many virions per cell could saturate restriction factors in a manner similar to cross-species saturation of TRIM5α (Besnier, Takeuchi, and Towers, 2002; Best et al., 1996; Keckesova, Ylinen, and Towers, 2004; Luban, 2006; Stremlau et al., 2004; Towers et al., 2000; Towers, Collins, and Takeuchi, 2002; Towers et al., 2003). Notably, the saturable nature of HIV-1 restriction in resting CD4+ T cells has not been directly investigated. To address these possibilities, we set out to test whether a threshold number of virions are required to bind to resting CD4+ T cells in order for integration to occur. In other words, we sought to determine if HIV could transduce resting CD4+ T cells under low virus inoculum conditions. To approach these questions, we found it necessary to improve our original integration assay because it lacked the sensitivity required to detect low levels of provirus (O'Doherty et al., 2002).
In the present study, we increase the sensitivity of our original integration assay, which used Alu-gag PCR to distinguish integrated from unintegrated DNA (O'Doherty et al., 2002). We demonstrate that an inherent limitation of Alu-gag PCR is variable amplification at low proviral copy number. As a result, integration is only detectable when HIV inserts close to an Alu repeat limiting the sensitivity of the Alu-gag PCR. We go on to show that the sensitivity of our method is enhanced ∼40-fold by testing samples repetitively. Sampling repetitively not only reduces the standard deviation, but, more importantly, it enables us to capture rare integration events where HIV integrates close to an Alu element. Using the more sensitive integration assay, based on repetitive sampling, we demonstrate that integration can occur in resting CD4+ T cells even under low viral inoculum conditions. These findings suggest that there is no threshold number of virions required for integration to occur and that the interaction of a single viral particle with a resting CD4+ T cell and any stimulus that this interaction may cause are sufficient for integration of HIV.
Results
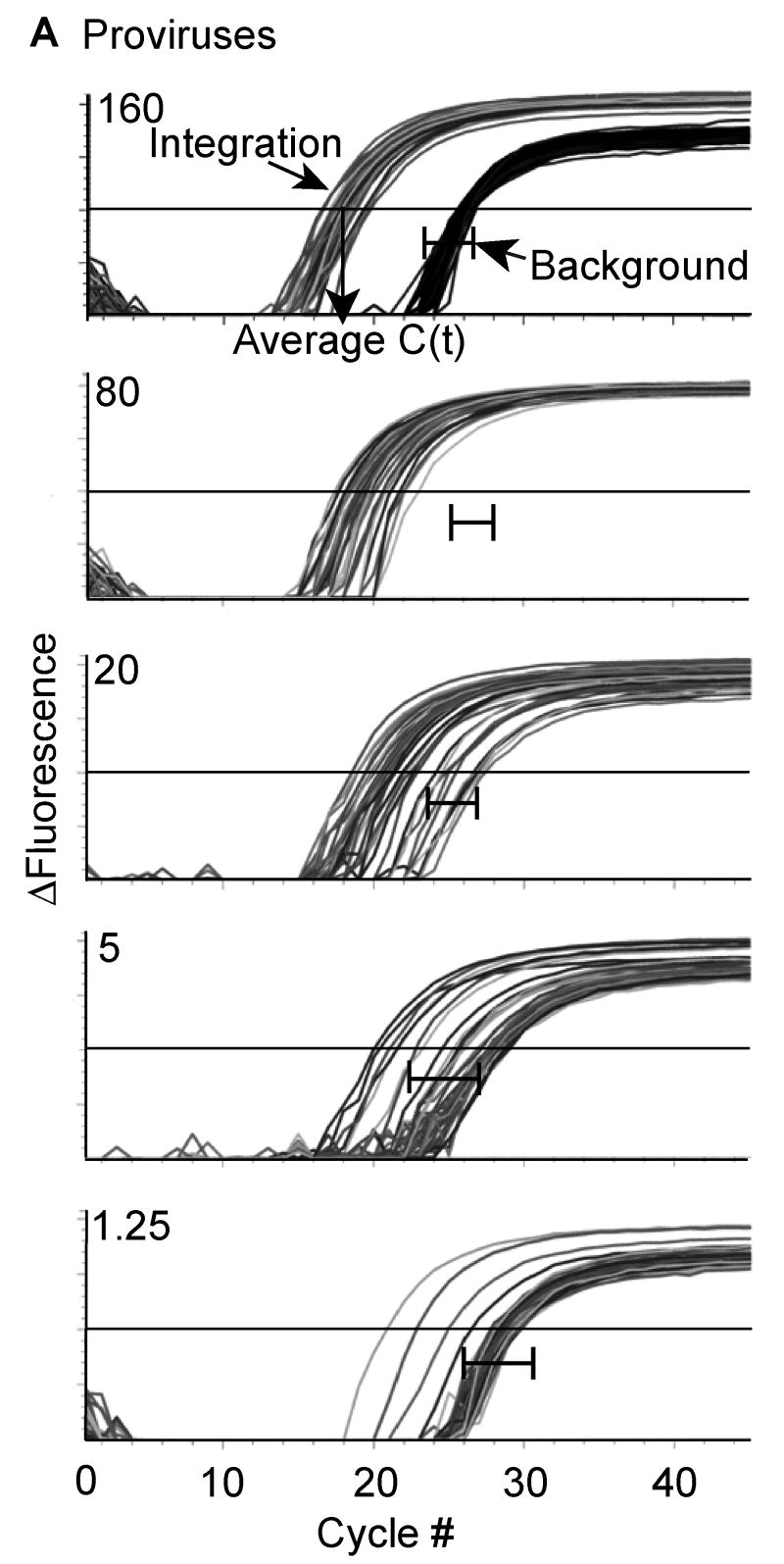
Generation of a standard curve demonstrates enhanced sensitivity with repetitive sampling. (Figure 1)
Figure 1. Repetitive sampling increases the sensitivity of the integration assay.

1A. DNA from the polyclonal IS was diluted into a constant amount of uninfected human genomic DNA and subjected to our 2-step Alu-PCR. The first PCR reaction used primers to Alu and gag or only primers to gag. The second PCR reaction used HIV-1 specific primers to the LTR elements, R and U5. For each dilution sample, Alu-gag and gag-only (background) amplification was measured 40 times and the provirus number was determined. At low proviral copy number, integration is detected at a low frequency as demonstrated by the Alu-gag amplification. For clarity, we did not show the gag-only signals at the lower dilutions, but instead show the bracketed black lines ( |―| ) which represent where the gag-only signal (background) was detected at each proviral number.
1B. A log-log relationship exists between the average cycle threshold value and the provirus number. Given the provirus number in our integration standard, we were able to determine the relationship between the average cycle threshold (CT) value and the provirus number. The average cycle threshold is obtained by averaging the cycle numbers where each PCR amplification curve crosses the y = -2.5. After calculating the average CT for IS at several provirus counts, a linear regression was performed on the natural logarithm of the average CT and the natural logarithm of the average provirus count to obtain the following formula: ln(n)≅ −9.6999 × ln(Ct) + 33.079. Each point represents 40 measurements performed at each IS concentration and the error bars represent the standard deviation.
To calculate HIV integration events, we needed to compare the level of integration in our unknown samples to a standard that contained diverse integration sites to accurately reflect integration in vivo (Butler, Hansen, and Bushman, 2001). We generated an integration standard cell line (IS) with diverse integration sites at ∼1 integration event per cell (not shown) using a similar strategy as our previous work (O'Doherty et al., 2002). The strategy that we used to determine that there was one integration event per cell and that all the HIV DNA was integrated is detailed in Supplement 1 and in the Materials and methods. Our integration assay is based on Alu-PCR, which uses primers for the repetitive element Alu of the human genome and for the gag gene of HIV. We then measured the level of Alu-gag signal generated with the IS and compared it to the signal generated using only the gag primer, which provides the signal from unintegrated DNA. We reasoned that we could enhance the sensitivity of our assay by repetitive sampling to capture single integration events on the rare occasion that HIV integrated near to an Alu site. To test this hypothesis and to determine the sensitivity of our assay when performed repetitively, we serially diluted our IS DNA in DNA of uninfected PBMCs and then assayed each dilution 40 times (Fig. 1A). We consistently assayed total DNA concentrations equivalent to 1.5×104 cells per reaction (IS cells + uninfected PBMC) to maintain a constant number of Alu sites (see the PCR conditions section of Materials and methods for explanation). When 160 proviruses in 1.5×104 genomes were assayed repetitively, the standard deviation was small and the Alu-gag and gag-only signals did not overlap. When 80 proviruses per 1.5×104 genomes were assayed, the standard deviation increased, but integration was detectable 100% of the time. When less than 40 proviruses per 1.5×104 genomes were assayed, only a fraction of the samples gave a positive signal, i.e. several Alu-gag signals overlapped with the gag-only signal. Nonetheless, a reproducible relationship existed between the number of proviruses at low copy number and the average of the Alu–gag signals. With this assay, we could detect down to ∼1 provirus per 15,000 genomes 10% of the time since 4 out of 40 Alu-gag signals were statistically different from the gag-only signal (p<0.02 by the Student t-test). Thus, the sensitivity of our assay was increased by about 40-fold since the detection limit of our prior integration assay was about ∼40-80 proviruses per reaction.
Finally, we determined how the cycle threshold varied with the number of proviruses by calculating the average Alu-gag cycle threshold (including the signals that overlapped with the gag-only signal) and plotting it against the proviral number for IS. We observed a linear relationship between the natural logarithm of the proviral number (n) and the natural logarithm of the average cycle threshold (μ): ln n = −9.6999 ln μ + 33.079 with R2= 0.975 (Fig. 1B).
Integration is not detected when HIV inserts far from an Alu sequence. (Figure 2)
Figure 2. Alu-gag amplification depends on the distance between Alu and integrated virus.

Alu-gag amplification efficiency was related to the distance between the HIV-1 integration site and the nearest Alu sequence. Alu-gag amplification, and hence integration, was undetectable when HIV-1 integrated >5000 bp from the nearest Alu site.
2A. Four clones (A, B, C, and D) were derived from polyclonal IS-293. The clones were generated by culturing IS-293 at ∼1 cell/well. For each clone, the distance from the nearest Alu to gag was determined as described in Materials and methods.
2B. Alu-gag amplification was more efficient when the nearest Alu was close to the integration site and undetectable when Alu was far from the integration site. DNA from each clone was subjected to 2-step PCR. Alu-gag and gag-only were measured three times and the response curves for each clone are shown. In every experiment, a horizontal threshold line was drawn at y = -2.5. The smaller the distance between HIV-1 gag and Alu, the more efficient the amplification and the lower the CT (compare A and D). For each clone, the Alu-gag response curves were compared to the gag-only response curves. When the distance between HIV-1 gag and Alu was >5000 bp, as in clone D, there was no difference between the Alu-gag signal (integration) and the gag-only signal (background).
We presumed that the undetectable integration events in Figure 1 occurred when HIV inserted distantly from the nearest Alu. We also presumed that the amplification was variable due to the variable distance between the provirus and the Alu site. To measure what is the minimum distance between the site of integration and Alu required for detection in our system, we compared the efficiency of amplification in four clones that contained unique integration sites at different distances from Alu. The four clones were isolated by inoculating 293 cells (IS-293) with VSV-G pseudotyped HIVΔenv at a low multiplicity of infection to reduce the chance of individual cells containing multiple integration sites (in Fig. 1, CEMss cells were infected while in Fig. 2, 293 cells were infected with VSV-G pseudotyped HIVΔenv). The polyclonal IS-293 cells were grown in hygromycin and clones were subsequently isolated by limiting dilution. After 28 days in culture, clones were FACS sorted to be uniformly GFP positive. First, we confirmed that each clone had one copy of HIV DNA per cell by quantitative PCR (data not shown). Next, for each clone, we employed inverse PCR to clone the HIV integration site as described (Han et al., 2004) and sequenced it. We then aligned the obtained sequence to the human genome to find the exact location of the integration site. We also determined the location of the nearest Alu (in the correct orientation, with less than 2 mismatches to our Alu primer and no mismatches in the 3′ end) by sequence alignment. From these data, we calculated the distance between the nearest Alu and the HIV integration site (Fig. 2A).
We found that the smaller the distance between the integration site and the Alu sequence, the greater the Alu-gag amplification (Fig. 2B). However, when the distance between the integration site and the nearest Alu sequence was large, integration was not detected, in other words, there was no difference between the Alu–gag amplification and the gag-only primer amplification (Fig. 2B). We did not detect integration when HIV inserted > ∼5,000 bp from the nearest Alu using a 2.5 minute extension. Thus, the limit for detecting an Alu-gag amplicon occurs when HIV inserts between 2,000 and 5,000 bps from the nearest Alu. While longer extension times would allow amplification of larger Alu-gag amplicons, the overall amplification efficiency was not enhanced when we extended the amplification time beyond 2.5 minutes (data not shown).
Isolation of highly enriched resting CD4+ T cells. (Figure 3)
Figure 3. Purification of resting CD4+ T cells.

PBMC (enriched by leukapheresis) were negatively depleted by RBC rosette using antibodies against: Glycophorin A, CD8, 16, 19, 36, 56, 66b and TCRγδ to yield >96% partially purified CD4+ T cells (ppCD4). ppCD4 were then stained with PE-labeled antibodies with specificities for HLA-DR, CD25 and CD69 in order to deplete activated cells with anti-PE magnetic beads. Purified resting CD4+ T cells (rCD4) contain less than 1% activated T cells. The gates were placed based on fluorescence minus one (FMO) controls, which were only labeled with anti-CD4. The gates were set conservatively such that 1% of unstained cells were in the upper quadrants. The above figure represents a typical purification of resting CD4+ T cells.
With this improved assay, we tested if HIV-1 could integrate in resting CD4+ T cells when delivered at low inoculum. Partially purified CD4+ T cells (ppCD4) were obtained from leukapheresis-enriched PBMC by negative selection rosette and successfully depleted cells of other lineages (CD8, 16, 19, 36, 56, 66b, TCRγδ) yielding >96% CD4+ T cells (Fig. 3). However, a significant percentage of these cells expressed activation markers. The contaminating activated cells were depleted with magnetic beads and antibodies recognizing markers for T cell activation: HLA-DR, CD25 and CD69 as described in Materials and methods. After the second purification step, less than 1% of the cells expressed activation markers (Fig. 3, compare rCD4 with the fluorescence minus one [FMO] control).
Integration of HIV-1 in CD4+ T cells can be detected at low viral inoculum. (Figure 4 and 5)
Figure 4. Integration of HIV-1 in CD4+ T cells is detected at low viral inoculum.
A dose-dependent decrease in viral integration was observed with serial dilution of the viral inoculum (A, B). ppCD4 cells were inoculated with three-fold dilutions of supernatant containing VSV-G pseudotyped pNL4-3. The dark bars indicated by an asterisk represent the number of virions bound per cell. The top and bottom viral dilutions correspond to 16 and 0.36 virions/cell respectively based on p24 ELISA (O'Doherty, Swiggard, and Malim, 2000). Cells were inoculated by routine inoculation for 2hr at 25°C. A dose-dependent decrease in RU5 reverse transcripts was also observed with serial dilution of the viral inoculum. The results are presented in provirus per cell (A), log(3) of provirus per cell (B), RU5 per cell (C, D) and log(3) RU5 per cell (D). The level of viral integration and reverse transcripts are shown as log(3) to convert the exponential relationship (A,C) to a linear relationship (B,D). Error bars represent the standard deviation of 5-51 measurements of integration and 4-8 measurements of reverse transcripts, respectively, for each virus dilution depending on proviral number (and sample availability). More repeats were required to capture integration events at low proviral numbers. The number of proviruses/cell and RU5/cell are written above each point (B, D).
Figure 5. Integration of wild type HIV-1 in pure resting CD4+ T cells exhibits a dose-dependent decrease in integration and reverse transcription with each dilution.
A dose-dependent decrease in viral integration (A) and reverse transcription (B) was observed with serial dilution of the viral inoculum. Resting CD4+ T cells were inoculated with two-fold dilutions of supernatant containing pNL4-3. Cells were inoculated by routine inoculation for 2hr at 25°C. After infection, the cells were cultured for 72hr before measuring integration. The results are presented in log(2) of provirus per cell (A) and log(2) of RU5 per cell (B). For integration, the error bar at the top dilution represents the standard error of 5 independent inoculations. Each inoculation at the top dilution was measured for viral integration 10-12 times depending on sample availability. The error bars of the lower dilutions represent the standard deviation of 12-42 measurements of integration for each virus dilution depending on sample availability. In general more replicates were performed at the lower dilutions. For reverse transcription, the error bar at the top dilution represents the standard error of 5 independent inoculations. Each inoculation at the top dilution was measured for reverse transcription 2-6 times depending on sample availability. The error bars of the lower dilutions represent the standard deviation of 4-6 measurements of reverse transcription for each virus dilution depending on sample availability. The number of proviruses/cell and RU5/cell are written above each point.
We previously demonstrated that HIV-1 can integrate in resting CD4+ T cells under high inoculum conditions. To test if HIV-1 could integrate at low inoculum, we serially diluted our viral stocks and then inoculated T cells under routine conditions. We first tested the ability of a VSV-G pseudotyped HIV-1 to integrate into CD4+ T cells when delivered at low inoculum. ppCD4 (Fig. 3) were infected with 3-fold serial dilutions of pNL4-3 VSV-G by standard inoculation for 2hr at 25°C. After inoculation, samples from high and low inoculation conditions were collected to assess the level of viral binding by measuring the p24 antigen level with an ELISA (O'Doherty, Swiggard, and Malim, 2000). The cells were subsequently cultured for 3 days to allow integration to occur in the presence of 1.25 μM of saquinavir to ensure the infection was limited to a single round. The cells were then harvested and genomic DNA was purified to measure the total number of cells based on the number of β-globin genes present (O'Doherty, Swiggard, and Malim, 2000). We then measured the level of integration in 1.5×104 cells with our improved Alu-PCR assay. As shown in Figure 4, integration occurred under low inoculum conditions and decreased in a dose-dependent manner. In the same way, the number of reverse transcripts per cell (RU5/cell) decreased in a dose-dependent manner (Fig. 4C, D). Similarly, a dose-dependent decrease in reverse transcription and integration were observed when cells were spinoculated with the same stock of diluted virus (Supplement 2).
In order to determine the validity of the dose response, we plotted the logarithm of the proviral count and the dilution. The logarithmic conversion of the data revealed a linear relationship as expected with a dose-response and allowed us to determine the R2 value (R2=0.99). In addition, we detected, on average, less than 1 virion/cell (0.36 virions/cell) at the end of the inoculation with the most dilute virus (based on p24) and still detected integration (0.0005 proviruses/cell) (Fig. 4A, dark bars with asterisks). This suggests that there is no requirement for a threshold number of virions to be bound per cell for successful integration. In summary, a dose dependent decrease was obtained over a 3 log range of viral inoculums, ranging from 800 virions bound per cell down to ∼1 virion bound per 3 cells at the end of the inoculation (Fig. 4 and Supplement 2).
We next tested if a threshold effect exists using purer resting CD4+ T cells (rCD4) inoculated with wild type HIV-1 (Fig. 5). It was important to determine if we obtained the same results with wildtype HIV-1 since VSV-G-mediated entry differs significantly from gp120-mediated entry. It was also important to confirm that we detected integration in the resting fraction of circulating CD4+ T cells as circulating blood CD4+ T cells (ppCD4) represent a range of activation states. Consistent with the observations made for pseudotyped HIV-1, a dose-dependent decrease in integration and reverse transcription occurred in resting CD4+ T cells inoculated with pNL4-3. Integration could be inhibited by 98% and reverse transcription could be inhibited by 92% in the presence of AZT (added at the top dose). We estimated that ∼3 virions were bound per cell at the top dilution based on p24 ELISA (not shown) which corresponds to a multiplicity of infection (MOI) of 0.02 as assessed by infection of CEMss-GFP indicator cells. We also tested the inoculated resting CD4+ T cells for upregulation of CD69, CD25 and HLA-DR before and 3 days after inoculation and found no detectable staining at either time point (not shown, but consistent with our previous study (Swiggard et al., 2005)). In our prior report, no activation was detected by three distinct methods. There was no BrdU incoporation over a 72 hr incubation period, no change in cell cycle by DNA/RNA stain and no change in activation markers. These studies were performed at higher inoculums compared to the current study and so we presume that there is less activation in the current study.
Both resting and intermediately activated CD4+ T cells are equally susceptible to HIV-1 integration at low inoculum conditions (Figure 6)
Figure 6. Sorting strategy to obtain highly pure resting and intermediately activated CD4+ T cells.

Resting and intermediately activated CD4+ T cells were sort-purified from PBMC by FACS Aria. PBMC were stained with FITC-labeled antibodies against lineage markers (CD8, CD14, CD16, CD20, CD56) and PE-labeled antibodies against activation markers (CD25, CD69, HLA-DR)(pre-sort). Gates were set around the double negative and activation intermediate populations to obtain 98% and 94% pure resting and intermediately activated CD4+ T cells respectively (post-sort).
Circulating CD4+ T cells have a range of activation states. In our prior publication, we showed that the integration signal we obtained in resting CD4+ T cells could not be due to rare contaminating strongly activated CD4+ T cells. However, low level to intermediately activated CD4+ T cells are the predominant contaminants in our purified resting CD4+ T cells. To test if the signal we obtained in our resting CD4+ T cells could be attributed to contaminating intermediately activated cells we compared the susceptibility of intermediately activated CD4+ T cells relative to resting cells. Resting and intermediately activated cells were sort purified from PBMC using the FACS Aria (Fig. 6). Conservative gates were set around the lineage (FITC) and activation (PE) double negative population to separate resting cells from the lineage negative and activation intermediate population. Post-sort analysis of these populations showed that resting cells were 98% pure and that intermediately activated cells were 94% pure. Immediately after sorting, both populations of cells were infected with serial dilutions of pNL4-3. We estimated, by p24 binding assay (O'Doherty, Swiggard, and Malim, 2000), that approximately 3 virions were bound per cell (not shown) when the resting and intermediately activated cells were inoculated with 1:9 dilution of the viral stock, demonstrating that our experiments were conducted at low inoculum. Reverse transcription and integration were measured at the peak of reverse transcription and integration which occurred 24hr after inoculation in stimulated cells and 48-72hrs after incoculation in resting and intermediately activated cells.
Reverse transcription is more efficient in stimulated cells than in resting or intermediately activated cells (Fig. 7). Reverse transcription occurred 10 times more efficiently in the CD3 and CD28 stimulated cells. In this experiment, integration occurred with similar efficiency in resting, intermediately activated and stimulated cells since approximately 1 in 5 reverse transcripts integrated. Our data overall however (including prior work (Swiggard et al., 2005)) suggest that integration is slightly more efficient in activated cells as we find between 1 in 2 to 1 in 5 reverse transcripts integrate in activated cells and between 1 in 5 and 1 in 13 reverse transcripts integrate in resting CD4+ T cells.
Figure 7. Both resting and intermediately activated CD4+ T cells have similar susceptibility to HIV-1 integration.
Resting, intermediately activated and stimulated CD4+ T cells were infected with 3-fold dilutions of the same pNL4-3 stock, but a different stock of virus than in Figure 5. After infection, the cells were cultured for 48 (resting and intermediately activated) or 24hr (stimulated) before measuring integration (A) and reverse transcription (B). The level of reverse transcription was determined using primers that detect the second strand transfer step (SST/cell). Both resting and intermediately activated cells had similar susceptibility to HIV-1 integration and reverse transcription while stimulated cells were more susceptible. Error bars represent the standard deviation of 8-15 measurements of integration and duplicate measurements of reverse transcription depending on sample availability. The number of proviruses/cell and SST/cell are written above each point.
Discussion
In the present study, we increase the sensitivity of our original, Alu-gag PCR-based, HIV integration assay (O'Doherty et al., 2002) by applying a repetitive sampling technique. Using the new assay, we demonstrate that HIV integrates into resting CD4+ T cells even under low viral inoculum conditions. We find a dose-dependent decrease in the level of integration suggesting that there is no threshold number of virions required to bind to a CD4+ T cell for integration to occur. A threshold effect seemed likely since high viral inoculums might activate CD4+ T cells (Briant et al., 1996; Kornfeld et al., 1988) or possibly saturate a restriction factor that might be present in resting CD4+ T cells.
We used the new, more sensitive integration assay to test if integration can occur under low inoculum conditions. Integration occurs (albeit at low levels, e.g. ∼5 in 10,000 CD4+ T cells, Fig. 4) even when, on average, less than one virion was bound per T cell at the end of the inoculation. Similar observations were noted when we inoculated pure resting CD4+ T cells with wild type HIV-1 (∼2 in 10,000 cells, Fig. 5). It could be argued that the rare integration events that we detect under low inoculum are due to integration events in rare activated T cells. However, we demonstrated that intermediately activated cells, which are the predominant contaminants among our purified resting cells, have the same susceptibility to integration as resting cells and so contaminating intermediately activated cells could not account for the signal. In addition, our prior work (Swiggard et al., 2005) demonstrated that rare activated cells contributed less than 5% to the integration signal detected in resting T cells when infected at high viral inoculum. Finally, while it can be argued that circulating CD4+ T cells represent a continuum of activation states, it has been shown that resting CD4+ T cells in blood represent the low end of that continuum (Stefanova, Dorfman, and Germain, 2002) and so represent the least activated cell that HIV encounters. Our results suggest that resting and intermediately activated CD4+ T cells - the majority of circulating CD4+ T cells -, are transduced with similar susceptibility (Figure 7).
Our observation that HIV can integrate into resting CD4+ T cells under low virus inoculum conditions suggests that there is no threshold number of virions required to bind to a CD4+ T cell for integration to occur. Instead of a threshold, there is a dose-dependent decrease in the level of integration (as well as viral binding and reverse transcription when tested). If a threshold number of virions were required for integration to occur, we would expect that at the threshold dilution there would be a greater decrease in the level of integration. While our data suggest that there is no threshold effect, our data are still consistent with the possibility that non-saturable restriction factors, possibly APOBEC3G/F, play a role during infection of resting cells (Chiu et al., 2005). For example, the kinetics of reverse transcription and integration are slower in resting T cells than in activated T cells (Chiu et al., 2005; Pierson et al., 2002; Spina, Guatelli, and Richman, 1995; Swiggard et al., 2004; Zack et al., 1990; Zack et al., 1992) consistent with restriction of an early step in the HIV life cycle.
Our study demonstrating that HIV integration occurs in resting CD4+ T cells is at odds with many prior studies that suggest an activation step is required (Bukrinsky et al., 1992; Ducrey-Rundquist, Guyader, and Trono, 2002; Korin and Zack, 1998; Oswald-Richter et al., 2004; Stevenson et al., 1990; Sun and Clark, 1999; Swingler et al., 2003; Unutmaz et al., 1999; Verhoeyen et al., 2003; Yamashita and Emerman, 2006; Zack et al., 1990; Zack et al., 1992; Zhou et al., 2005). Prior studies show that reverse transcription (Zack et al., 1990; Zack et al., 1992), nuclear import (Bukrinsky et al., 1992; Sun and Clark, 1999) and integration (Stevenson et al., 1990) are blocked in resting T cells. In addition, the fact that integrated HIV DNA is enriched in memory over naïve CD4+ T cells in HIV infected individuals suggests that a prior activation step allowed integration to occur (Chun et al., 1997a; Chun et al., 1995; Ostrowski et al., 1999). Thus, in the current study we tested if HIV integration into resting CD4+ T cells was an artifact of spinoculation or high inoculum. To the contrary, we establish that integration still occurs in resting CD4+ T cells in the absence of spinoculation and under low inoculum conditions.
The discrepancy between our results and the literature may be explained by considering two issues: delayed kinetics and assay sensitivity. The early studies that demonstrate a block in HIV reverse transcription (Zack et al., 1990; Zack et al., 1992), nuclear import (Bukrinsky et al., 1992; Sun and Clark, 1999) and integration (Stevenson et al., 1990) in resting CD4+ T cells were all performed within 24 hrs of inoculation, but the kinetics are delayed in resting CD4+ T cells and viral intermediates are detected more easily after 2-3 days of culture (Chiu et al., 2005; Pierson et al., 2002; Spina, Guatelli, and Richman, 1995; Swiggard et al., 2004). In addition, the prior integration assays were probably not sufficiently sensitive to detect integration under routine inoculation conditions. For example, we would not be able to detect integration in any of the samples inoculated under routine conditions from Figure 5 using our previous standard integration assay (O'Doherty et al., 2002). The infection frequency in resting CD4+ T cells in our hands (Swiggard et al., 2005; Swiggard et al., 2004) and others (reviewed by (Yamashita and Emerman, 2006)) is lower than in fully activated T cells such as the CEMss cell line. Thus, it would be possible to detect integration in fully activated T cells, but not in resting T cells.
Notably, our study is in agreement with in vivo studies showing that naïve CD4+ T cells are productively infected in vivo (Schacker et al., 2001; Zhang et al., 1999) and in lymphoid organ cultures (Eckstein et al., 2001). In addition, the predominant infection of “apparently resting” memory CD4+ T cells during acute SIV infection also argues for a role of direct infection of resting CD4+ T cells (Li et al., 2005; Zhang et al., 2004). Recently, a second study also reported that integration occurs in resting CD4+ T cells (Vatakis et al., 2007) albeit at lower levels. In our study, the fraction of reverse transcripts that integrate in resting CD4+ T cells vary from about 1 in 5 to about 1 in 10, which is higher than the fraction reported by Vatakis et al. The difference between our findings and those reported by Vatakis et al., may be related to differences in viral DNA stability. Although we and Vatakis et al. reported that viral DNA can be stable in resting T cells (Swiggard et al., 2004; Vatakis et al., 2007), we have found the stability of viral DNA to be variable, depending on the blood donor, day of donation and virus used (unpublished observations). In addition, the discrepancy in the results may also be related to slight differences in the assays for integration that were used. Nonetheless, the two studies are similar in two important ways. One, both studies found that integration occurs in resting CD4+ T cells. Two, both studies found that the proportion of reverse transcripts that integrate in resting and activated T cells are comparable or only slightly less efficient in resting CD4+ T cells.
Our original Alu-gag PCR-based HIV integration assay had an inherent limitation: it lacked the ability to detect all integration events due to the fact that exponential amplification cannot occur when HIV integrates far from an Alu repeat. We overcame this limitation by applying a repetitive sampling technique in combination with a proper integration standard. By repetitively sampling an integration standard we control for undetectable integration events and variable amplification efficiency. In addition, by repetitively sampling our unknowns, we enhance our ability to capture rare integration events. From Figure 1A, we estimate that we detect a single provirus in 15,000 genomes approximately 10% of the time (i.e. 4 out of 40 Alu–gag replicates are above the gag-only signal when we assay on average ∼1 provirus per well repetitively). This is a reasonable result since HIV will only occasionally insert near the Alu sequence (Schroder et al., 2002). We estimate that repetitive sampling enhances the sensitivity of our assay ∼40-fold since we can detect 40-80 proviruses per well in 100% of the wells (with 15,000 cells per well) without repetitive sampling and 1 provirus per well with repetitive sampling (Fig. 1A). This represents a significant advance over prior techniques (Kumar et al., 2002): linker ligation PCR (Vandegraaff et al., 2001), Alu PCR (Brussel, Delelis, and Sonigo, 2005; Brussel and Sonigo, 2003; Butler, Hansen, and Bushman, 2001; O'Doherty et al., 2002; Ostrowski et al., 1999; Sonza et al., 1996; Suzuki et al., 2003; Yamamoto et al., 2006) and inverse PCR (Chun et al., 1997a; Chun et al., 1995), which are similar conceptually and have the same inherent limitation – that only a fraction of integration events should be detectable due to variable amplicon size. This study is the first to show how genomic anchor based integration assays perform at low proviral copy with multiple repeats. Some studies, including our own (O'Doherty et al., 2002), claimed high sensitivities without showing repetitive sampling at low copy number. For example, in our prior study (O'Doherty et al., 2002), we overestimated the sensitivity of our assay by not recognizing the importance of multiple repeats at low copy number (and by not diluting our standard in a background of genomic DNA). Our current study demonstrates that multiple repeats are absolutely necessary at low proviral numbers to determine the true sensitivity of an integration assay.
The evidence presented here suggests that there is no threshold number of bound virions required for HIV integration to occur in CD4+ T cells. Thus, a single viral particle can interact with a resting CD4+ T cell and result in integration. In other words, the direct interaction of virus with a resting CD4+ T cell may be sufficient for latent infection to occur. We propose that repetitive sampling as exploited in our new method to measure HIV integration may provide a step toward measuring integration in patient samples more accurately. We are currently applying this technique to patient samples in an attempt to measure more accurately the relative susceptibility of different cell types.
Materials and methods
Cell lines, plasmids, and viruses
The CD4+ T-lymphoblastoid cell line CEM-ss was maintained at 1-5×105 cells per ml. The culture medium used was RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 25 mM HEPES, with 100 μg/ml penicillin-streptomycin (Invitrogen Life Technologies). VSVg pseudotyped (Blumenthal et al., 1987; Emi, Friedmann, and Yee, 1991) virions were collected 24 hours after Ca3(PO4)2 transfection of 293T cells with pVSVg (pHIT) (Fouchier et al., 1997) and pNL4-3Δenv/GFP/HygR. The reporter virus was derived from pNL4-3, an X4-tropic molecular clone of HIV (Adachi et al., 1986). The nef open reading frame (orf) was replaced with a GFP and hygromycin cassette which contained an internal ribosome entry site between the two orfs. The transfection supernatants were treated with 30 μg/ml of DNase I (Roche) and 10 mM MgCl2 at 37°C, 1 hr prior to collection. For the infections at low inoculum, pNL4-3 and pNL4-3 VSV-G were used from 293T cell Ca3(PO4)2 transfection supernatant.
Preparation of the Integration Standard (IS) cell line
CEM-ss cells were infected with pseudotyped virions (described above) by spinoculation. Briefly, 2×107 CEM-ss cells were mixed with 1ml of viral stock (VSVg pseudotyped pNL4-3Δenv/GFP/HygR containing 1 μg/ml p24 Gag protein) and placed into one well of a flat-bottom six-well tissue culture plate. The plates were sealed in plastic bags and centrifuged in microplate carriers at 1,200×g for 2 hours at 25°C. Cells were collected and washed once with 50 ml of ice-cold culture medium (RPMI1640 with 10% heat-inactivated fetal calf serum, 10 mM HEPES, 1% penicillin-streptomycin (Gibco)). Infected cells were cultured for 2 days and then weaned into 800 μg/ml of hygromycin. The acutely infected cells contained unintegrated HIV-1 DNA and integrated proviral DNA. With every cell division, the level of unintegrated DNA per cell decreased while the integrated proviral DNA replicated with cellular DNA. After 28 days in culture, the level of unintegrated DNA became undetectable by Southern blot as demonstrated in our previous work (O'Doherty et al., 2002) and the GFP positive transduced cells were selected by FACS sorting.
Preparation of clones with unique HIV integration sites
293 cells were infected with VSVg pseudotyped reporter virus pNL4-3Δenv/GFP/HygR at a low multiplicity of infection to minimize the chance of multiple transductions within one cell. The 293 cells were grown for 2 days and then weaned into hygromycin at 800 μg/ml. After hygromycin selection and expansion, the transduced 293T cells were sorted for expression of GFP. Clonal populations with unique HIV integrations sites were prepared by culturing cells at <1 cell/well. Hygromycin was added to the wells after single cell cloning. Clones which grew in hygromycin were again sorted for expression of GFP. After sorting the GFP positive cells, we measured the level of HIV DNA per cell using quantitative PCR and found ∼1 copy of HIV per cell consistent with one transduction event per cell.
Identification of unique integration sites within clonal populations
Inverse PCR was used to clone the integration sites as described (Han et al., 2004). Briefly DNA was prepared and cleaved by Pst1 which cuts genomic DNA frequently, but cleaves pNL4-3Δenv/GFP/HygR once at position 1419 (HXB2R coordinates). Digested DNA was then ligated under dilute conditions to favor intramolecular ligation. Outwardly directed primers to LTR and gag are designed to exponentially amplify circular DNA. Thus, host genomic DNA adjacent to the HIV-1 insertion site was amplified along with the 5′end of the HIV genome. The amplified DNA was cloned and sequenced after a nested reaction again with outward primers. The genomic insertion site was determined by aligning the sequence with the human genome sequence. The nearest Alu sequence that matched the Alu primer with less than 2 base pair mismatches was located.
PCR conditions to measure proviral number
Two step PCR amplification was performed as described (O'Doherty et al., 2002) with some modifications. Briefly, the first amplification was performed on dilutions of the IS cells as well as samples from infected CD4+ T cells. The sequence of the first step amplification primers were: genomic Alu forward 5′ GCC TCC CAA ACT GCT GGG ATT ACA G-3′ and HIV gag reverse 5′ GTT CCT GCT ATG TCA CTT CC-3′. The gag primer is different from the previously described primer (O'Doherty et al., 2002). It provides a better consensus sequence than the original primer, based on Los Alamos HIV Sequence Database, and provides more efficient Alu-gag amplification. DNA from the Integration Standard cell line (IS) was diluted in uninfected peripheral blood mononuclear cells (PBMC) DNA at 2 μg/ml to keep the number of Alu sites per reaction constant. We kept the number of Alu sites constant because the first-step PCR amplification efficiency increased with decreasing number of Alu sites. If we diluted the IS DNA in buffer our assay would inaccurately appear to be more sensitive. By diluting IS DNA in uninfected PBMC DNA, we mimic the conditions of low infection frequency. For similar reasons, we cannot simply add more cellular DNA to enhance the sensitivity of our assay since the sensitivity decreased as the number of cells per sample increased. In other words, increased numbers of Alu sites decreased the amplification efficiency. We also reasoned that keeping the number of genomes low minimizes PCR inhibition which occurred more frequently at higher concentrations of genomes.
Reactions were carried out in a volume of 50 μl containing: 10 mM Tris-HCl, pH 8.3; 3 mM MgCl2; 1 mM mixed dNTPs; 50mM KCl; 100 nM Alu forward primer; 600 nM gag reverse primer; and 0.05 units of Platinum Taq DNA polymerase (Invitrogen Life Technologies). The thermal cycler (DNA Engine Opticon, MJResearch) was programmed to perform a two-minute hot start at 94°C, followed by 20 cycles: denaturation at 93°C for 15s, annealing at 50°C for 15s and extension at 70°C for 2.5 minutes. The Alu primer binds only to cellular genomic DNA, not to unintegrated HIV DNA; the gag primer binds to one strand of HIV DNA. When both Alu and gag primers are used in the amplification, the original template strands and every copy are duplicated in each round, so amplification becomes exponential. Linear, one-strand amplification (i.e. primer extension) was also monitored by performing the first amplification PCR with the gag primer alone. When only the gag primer is used, only one strand of the original template DNA is copied so amplification is linear. We used the gag-only primer amplification reaction as a control because it provides the signal expected from unintegrated HIV DNA. While increased extension times increases the ability of Taq polymerase to make longer amplicons, the overall amplification efficiency was maximal at 2.5 minutes of extension.
The second round real-time quantitative PCR was performed using 25 μl of the material from the first amplification. These were run with an HIV-1 copy number standard. The sequences of the primers were: LTR (R) forward, 5′-GCC TCA ATA AAG CTT GCC TTG A-3′; LTR (U5) reverse, 5′-TCC ACA CTG ACT AAA AGG GTC TGA-3′. The LTR molecular beacon probe, labeled at its 5′ terminus with the reporter fluorophore 6-carboxyfluorescein (FAM) and at its 3′ terminus with the quencher 4-(4′-dimethylamino-phenylazo)-benzene (DABCYL), had the following sequence: 5′-FAM-GCG AGT GCC CGT CTG TTG TGT GAC TCT GGT AAC TAG CTC GC-DABCYL-3′. Reactions were carried out in a volume of 50 μl containing: 10 mM Tris-HCl, pH 8.3; 75 mM KCl; 5.5 mM MgCl2; 500 nM carboxy-X-rhodamine (ROX, Molecular Probes) as a passive reference; 1.2 mM freshly added dNTPs, 250 nM LTR forward and reverse primers; 200 nM molecular beacon probe; and 0.025 units of Platinum Taq DNA polymerase. The reactions were performed on a DNA Engine Opticon (MJResearch) instrument running Opticon Monitor v1.1 software with the following thermal program: 2 min hot start at 95°C, 4 cycles of denaturation at 95°C for 15 sec, annealing at 50°C for 15 sec, and extension at 72°C for 1 min followed by 44 cycles of denaturation for 15 sec, annealing at 50°C for 15 sec, plate read, and lastly extension at 72°C for 1 min.
To express integration as a ratio of proviruses per target cell, a kinetic PCR assay for β-globin DNA was used as described (O'Doherty, Swiggard, and Malim, 2000). A standard curve is made using purified DNA from PBMC (Qiagen). The quantity of DNA is determined by OD260/280. The number of genomes is calculated given that ∼3.18×109 bp are present per genome. Minus strand strong stop DNA (RU5/cell) and the level of DNA that has completed the second strand transfer step of reverse transcription (SST/cell) were measured as previously described (O'Doherty et al., 2002; Swiggard et al., 2005).
Repetitive sampling to measure integration at low proviral copy number
Alu–gag PCR and gag-only PCR were performed repetitively on 25 μl aliquots of DNA at 2 μg/ml from the IS line and samples from infected CD4+ T cells. Then HIV-1 specific kinetic PCR is performed on 25 μl aliquot of the first reaction. After the kinetic PCR reaction, the cycle thresholds (Ct) for each well are determined, i.e. the cycle value where the accumulating fluorescence (from fluorescently labeled amplicons) crosses a specified fluorescent threshold value. In all of the experiments described here, the fluorescent threshold value was set at -2.5 for data analysis. The cycle threshold values from each concentration of IS and for each sample were then averaged. The average cycle thresholds from the IS with known proviral count were then used to determine the relationship between the average cycle threshold and the proviral count (Fig. 1B). The proviral number from samples was then calculated by inputting the average cycle threshold into the equation described in Figure 1B.
Purification of resting and intermediately activated CD4+ T cells
For the studies testing if a threshold number of virions is required for integration, CD4+ T cells were isolated by negative selection from leukapheresis-enriched PBMC by rosette (RosetteSep™ kit, StemCell Technologies, Inc.) as recommended by the manufacturer. Briefly, leukapheresis-enriched PBMC were labeled with antibodies recognizing Glycophorin A, CD8, CD16, CD19, CD36, CD56, CD66b and TCRγδ. A secondary anti-mouse antibody was added to cross-link labeled PBMC to labeled red blood cells. Following these labeling steps, the cells were applied to a Ficoll-Paque® (GE Healthcare) density gradient to remove the cross-linked cell complexes. Resting CD4+ T cells were then isolated by negative selection with saturating concentrations of PE-labeled antibodies specific for HLA-DR, CD25, and CD69 (BD Pharmingen™) and anti-PE magnetic beads (Miltenyi Biotec) as recommended by the manufacturers. After purification, the cells were cultured overnight in RPMI 1640 culture medium supplemented with 10% heat inactivated human serum and 1% penicillin-streptomycin (Gibco).
For the studies comparing the susceptibility of resting and intermediately activated CD4+ T cells, the cells were sort purified as described (Swiggard et al., 2005) but using a FACS Aria instrument (BD Biosciences). Briefly, PBMC were isolated from whole blood by Ficoll-Paque® (GE Healthcare) density gradient. Cells were subsequently labeled with saturating amounts of FITC-labeled antibodies recognizing lineage markers (CD8, CD14, CD16, CD20, CD56) and PE-labeled antibodies recognizing activation markers (CD25, CD69, HLA-DR). The double negative population, corresponding to resting cells, and the cells expressing intermediate levels of activation markers were selected. Immediately after sorting the cells were washed once with PBS containing 10% human serum and resuspended in the appropriate viral supernatant for immediate infection.
Flow cytometry
To determine the level of purity and activation, the cells were stained in FACS buffer (PBS with 1%BSA and 2mM EDTA) with 10% human serum and mouse IgG to block non-specific binding, and antibodies recognizing CD3, CD4, HLA-DR, CD25 and CD69 (BD Pharmingen™). The labeled cells were then screened by flow cytometry with a FACScalibur instrument (BD Biosciences). The results were later analyzed with FlowJo software (Treestar). Dot plot gates were set based on an unlabeled control, single positive controls, and fluorescence minus one controls (FMO) missing one of the labels. We set our quadrants so that 0.5 – 1% of the events in the unstained sample gave a positive signal in order to detect low levels of staining.
Stimulation of CD4+ T cells
A portion of the sort-purified cells were selected to be stimulated prior to infection. Cells were cultured for 72hr in the presence of CD3/CD28 beads at 3 beads per cell, and 10U/ml of recombinant human IL-2. After incubation, the beads were removed with a magnet, the cells were washed once with PBS containing 10% human serum and resuspended in the appropriate viral supernatant.
Inoculation of primary CD4+ T cells
Purified CD4+ T cells were resuspended at 1×107 cells/ml in 100 μl of serially diluted viral supernatants. Cells were then inoculated at 1×g or spinoculated at 1,200×g (O'Doherty, Swiggard, and Malim, 2000) for 2-3hr at 25°C. Following inoculation, the cells were washed twice with PBS containing 10% human serum and cultured in RPMI 1640 culture medium supplemented with 10% human serum, 1% penicillin-streptomycin (Gibco), and 1.25 μM saquinavir (Roche US Pharmaceuticals) for 24, 48 or 72hr. Selected samples were treated with 100 μg/ml zidovudine (AZT) (NIH AIDS Reagents Program) to inhibit reverse transcription as a negative control.
Assessment of viral binding
Viral binding was assessed by measuring the level of p24Gag associated with the samples collected immediately after inoculation as previously described (O'Doherty, Swiggard, and Malim, 2000). p24Gag levels were measured by p24-specific enzyme-linked immunosorbent assay (ELISA; Coulter Corporation, Miami, FL) with little cross-reactivity to other viral proteins. Binding was subsequently calculated based on the estimation that 15,800 virions are present per 1 pg of p24 (O'Doherty, Swiggard, and Malim, 2000; Vogt and Simon, 1999). This method provides a good estimate of viral binding, although it may overestimate the number of infectious virions bound per cell due to the presence of virus-like particles without genomes or inactive virions. The limit of sensitivity of our p24 assay is 8pg/ml and so to detect viral binding at our lowest inoculum (∼1 virion per 3 cells), 400,000 cells/ml would be required, but 1×106 cells/ml were generally used at the lowest inoculum to ensure that the signal fell in the range of detection.
Supplementary Material
Supplement 1: Generation of an integration standard (IS) cell line.
To calculate HIV integration events, we needed to compare the level of integration in our unknown samples to a standard. The standard needed to contain diverse integration sites to accurately reflect integration in vivo (Butler, Hansen, and Bushman, 2001). To generate a standard with diverse integration sites, we used the strategy outlined in Supplement 1. pNL4-3Δenv/EGFP/HygR (Supplement 1A) and pVSVG, an expression plasmid for vesicular stomatitis virus glycoprotein (VSV-G), were co-transfected into 293T cells to generate pseudotyped virions bearing the HIVΔenv genome and VSV-G glycoprotein. CEMss T cells (or 293 cells) were inoculated by spinoculation with transfection supernatant containing pseudotyped virions. The pseudotyped virions lacked HIV env, so infection was limited to a single cycle, and contained the coding sequence of EGFP and hygromycin resistance in place of the nef gene. The infected cells were grown for 28 days under hygromycin selection and sorted for EGFP expression. Soon after infection, both integrated and unintegrated DNA were detectable; however after 28 days only integrated DNA was detectable by Southern blot (not shown but previously described (O'Doherty et al., 2002)).
We then measured the level of HIV DNA/cell in this Integration Standard (IS) cell line using kinetic PCR (Swiggard et al., 2005) and detected ∼1 copy/cell (Supplement 1B). Given that all of the DNA was integrated, we thus concluded that there is ∼1 integration event per cell present in the IS cell line. We then used the same DNA in our two-step PCR assay to detect integration and generate a standard curve by plotting the number of proviruses vs the cycle threshold. In the first step of the PCR assay, we amplified DNA using either Alu and gag primers or only the gag primer (see the PCR conditions section of Materials and methods for explanation). In the second step of the PCR assay, we used HIV-1 specific primers, which detect Alu-gag and gag-only amplicons, but do not detect Alu-Alu amplicons.
Supplement 2: Integration of HIV-1 in CD4+ T cells after spinoculation also exhibits a dose dependent decrease in integration with each dilution.
ppCD4+ T cells were inoculated with three-fold dilutions of supernatant containing VSV-G pseudotyped pNL4-3. Cells were inoculated by spinoculation for 2hr at 25°C, then washed and the level of cell associated virus (viral binding) was determined at the most concentrated and most dilute doses by p24 ELISA. The cells were cultured for 3 days, DNA was prepared, and reverse transcription and integration were measured. Similar to Figure 4, a dose-dependent decrease in integration and RU5 reverse transcripts was observed with serial dilution of the viral inoculum. The results are presented in provirus per cell (A), log (3) of provirus per cell (B), RU5 per cell (C) and log(3) RU5 per cell (D). AZT inhibited reverse transcription (78%) and integration (99%) (added at the top dose). Error bars represent the standard deviation of 16 measurements of integration and 2-4 measurements of reverse transcripts, for each virus dilution depending on sample availability. The number of proviruses/cell and RU5/cell are written above each point (B, D).
Acknowledgments
This study was supported by NIH grants R01 A058862 (U.O.) and R21 AI64031 (U.O.). Additional support came from Merck Research Laboratories, West Point PA.
The pNL4-3Δenv/EGFP/HygR plasmid was a generous gift from Robert Siliciano. We are grateful to Frederic Bushman, Drew Weissman, Terri Finkel, Peter Wang, and Elizabeth Colston for their insightful suggestions and critical reading of the manuscript. We are also grateful to the Penn-CFAR Immunology and Molecular Cores for providing ppCD4+ T cells and for p24 ELISA testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59(2):284–91. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnier C, Takeuchi Y, Towers G. Restriction of lentivirus in monkeys. Proc Natl Acad Sci U S A. 2002;99(18):11920–5. doi: 10.1073/pnas.172384599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best S, Le Tissier P, Towers G, Stoye JP. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annual Review of Medicine. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- Blumenthal R, Bali-Puri A, Walter A, Covell D, Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987;262(28):13614–9. [PubMed] [Google Scholar]
- Briant L, Coudronniere N, Robert-Hebmann V, Benkirane M, Devaux C. Binding of HIV-1 virions or gp120-anti-gp120 immune complexes to HIV-1-infected quiescent peripheral blood mononuclear cells reveals latent infection. J Immunol. 1996;156(10):3994–4004. [PubMed] [Google Scholar]
- Briant L, Reynes J, Coudronniere N, Benezech JP, Devaux C. HIV-1 reactivation in resting peripheral blood mononuclear cells of infected adults upon in vitro CD4 cross-linking by ligands of the CDR2-loop in extracellular domain 1. J Acquir Immune Defic Syndr. 1999;21(1):9–19. doi: 10.1097/00126334-199905010-00002. [DOI] [PubMed] [Google Scholar]
- Brussel A, Delelis O, Sonigo P. Alu-LTR real-time nested PCR assay for quantifying integrated HIV-1 DNA. Methods Mol Biol. 2005;304:139–54. doi: 10.1385/1-59259-907-9:139. [DOI] [PubMed] [Google Scholar]
- Brussel A, Sonigo P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J Virol. 2003;77(18):10119–24. doi: 10.1128/JVI.77.18.10119-10124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proceedings of the National Academy of Sciences USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Hansen MST, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nature Medicine. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435(7038):108–14. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997a;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1(12):1284–90. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences USA. 1997b;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa SC, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7(2):245–8. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- Ducrey-Rundquist O, Guyader M, Trono D. Modalities of Interleukin-7-Induced Human Immunodeficiency Virus permissiveness in quiescent T lymphocytes. Journal of Virology. 2002;76(18):9103–9111. doi: 10.1128/JVI.76.18.9103-9111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein DA, Penn ML, Korin YD, Scripture-Adams DD, Zack JA, Kreisberg JF, Roederer M, Sherman MP, Chin PS, Goldsmith MA. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15(4):671–82. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- Emi N, Friedmann T, Yee JK. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65(3):1202–7. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RAM, Meyer BE, Simon JHM, Fischer U, Malim MH. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. European Molecular Biology Organization Journal. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lassen K, Monie D, Sedaghat AR, Shimoji S, Liu X, Pierson TC, Margolick JB, Siliciano RF, Siliciano JD. Resting CD4+ T cells from HIV-1 infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78(12):6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wind-Rotolo M, Yang HC, Siliciano JD, Siliciano RF. Experimental approaches to the study of HIV-1 latency. Nat Rev Microbiol. 2007;5(2):95–106. doi: 10.1038/nrmicro1580. [DOI] [PubMed] [Google Scholar]
- Keckesova Z, Ylinen LM, Towers GJ. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci U S A. 2004;101(29):10780–5. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A, Moorthy A, Jackson R, Fauci AS. Productive HIV infection of resting CD4+ T cells: role of lymphoid tissue microenvironment and effect of immunomodulating agents. AIDS Res Hum Retroviruses. 2003a;19(10):847–56. doi: 10.1089/088922203322493012. [DOI] [PubMed] [Google Scholar]
- Kinter AL, Umscheid CA, Arthos J, Cicala C, Lin Y, Jackson R, Donoghue E, Ehler L, Adelsberger J, Rabin RL, Fauci AS. HIV envelope induces virus expression from resting CD4+ T cells isolated from HIV-infected individuals in the absence of markers of cellular activation or apoptosis. J Immunol. 2003b;170(5):2449–55. doi: 10.4049/jimmunol.170.5.2449. [DOI] [PubMed] [Google Scholar]
- Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. Journal of Virology. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld H, Cruikshank WW, Pyle SW, Berman JS, Center DM. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988;335(6189):445–8. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- Kumar R, Vandegraaff N, Mundy L, Burrell CJ, Li P. Evaluation of PCR-based methods for the quantitation of integrated HIV-1 DNA. J Virol Methods. 2002;105(2):233–46. doi: 10.1016/s0166-0934(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10(11):525–31. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, Coffin JM, Bosch RJ, Margolis DM. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–55. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434(7037):1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Luban J. Cyclophilin A, TRIM5, and Resistance to HIV-1 Infection. J Virol. 2006 doi: 10.1128/JVI.01519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Brown CR, Mattapallil JJ, Igarashi T, Buckler-White A, Lafont BA, Hirsch VM, Roederer M, Martin MA. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc Natl Acad Sci U S A. 2005;102(22):8000–5. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U, Swiggard WJ, Jeyakumar D, McGain D, Malim MH. A sensitive, quantitative, assay for Human Immunodeficiency Virus type 1 integration. Journal of Virology. 2002;76(21):10,942–10,950. doi: 10.1128/JVI.76.21.10942-10950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. Journal of Virology. 2000;74(21):10074–80. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski MA, Chun TW, Justement SJ, Motola I, Spinelli MA, Adelsberger J, Ehler LA, Mizell SB, Hallahan CW, Fauci AS. Both memory and CD45RA(+)/CD62L(+) naive CD4(+) T cells are infected in human immunodeficiency type 1-infected individuals. Journal of Virology. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald-Richter K, Grill SM, Leelawong M, Unutmaz D. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur J Immunol. 2004;34(6):1705–14. doi: 10.1002/eji.200424892. [DOI] [PubMed] [Google Scholar]
- Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: Mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annual Review of Immunology. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C, Siliciano RF. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J Virol. 2002;76(17):8518–31. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacker TW, Little S, Connick E, Gebhard K, Zhang Z, Krieger J, Pryor J, Havlir D, Wong J, Schooley RT, Richman D, Corey L, Haase AT. Productive infection of T cells in lymphoid tissue during primary and early HIV infection. Journal of Infectious Diseases. 2001;183:555–562. doi: 10.1086/318524. [DOI] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–9. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. Journal of Virology. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina CA, Guatelli JC, Richman DD. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. Journal of Virology. 1995;69:2877–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420(6914):429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Stevenson M. HIV-1 pathogenesis. Nat Med. 2003;9(7):853–60. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. European Molecular Biology Organization Journal. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–53. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Sun Y, Clark EA. Expression of the c-myc proto-oncogene is essential for HIV-1 infection in activated T cells. Journal of Experimental Medicine. 1999;9:1391–1397. doi: 10.1084/jem.189.9.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Misawa N, Sato C, Ebina H, Masuda T, Yamamoto N, Koyanagi Y. Quantitative analysis of human immunodeficiency virus type 1 DNA dynamics by real-time PCR: integration efficiency in stimulated and unstimulated peripheral blood mononuclear cells. Virus Genes. 2003;27(2):177–88. doi: 10.1023/a:1025732728195. [DOI] [PubMed] [Google Scholar]
- Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, Theodosopoulos T, O'Doherty U. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol. 2005;79(22):14179–88. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiggard WJ, O'Doherty U, McGain D, Jeyakumar D, Malim MH. Long HIV Type 1 reverse transcripts can accumulate stably within resting CD4+ T cells while short ones are degraded. AIDS Research and Human Retroviruses. 2004;20(3):285–295. doi: 10.1089/088922204322996527. [DOI] [PubMed] [Google Scholar]
- Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 2003;424(6945):213–9. doi: 10.1038/nature01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers G, Bock M, Martin S, Takeuchi Y, Stoye JP, Danos O. A conserved mechanism of retrovirus restriction in mammals. Proc Natl Acad Sci U S A. 2000;97(22):12295–9. doi: 10.1073/pnas.200286297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers G, Collins M, Takeuchi Y. Abrogation of Ref1 retrovirus restriction in human cells. J Virol. 2002;76(5):2548–50. doi: 10.1128/jvi.76.5.2548-2550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers GJ, Hatziioannou T, Cowan S, Goff SP, Luban J, Bieniasz PD. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat Med. 2003;9(9):1138–43. doi: 10.1038/nm910. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. Journal of Experimental Medicine. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegraaff N, Kumar R, Burrell CJ, Li P. Kinetics of Human Immunodeficiency Virus type 1 DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. Journal of Virology. 2001;75:11253–11260. doi: 10.1128/JVI.75.22.11253-11260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis DN, Bristol G, Wilkinson TA, Chow SA, Zack JA. Immediate Activation Fails to Rescue Efficient Hiv Replication in Quiescent Cd4+ T Cells. J Virol. 2007 doi: 10.1128/JVI.02569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N, Cosset FL. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood. 2003;101(6):2167–74. doi: 10.1182/blood-2002-07-2224. [DOI] [PubMed] [Google Scholar]
- Vogt VM, Simon MN. Mass Determination of Rous Sarcoma Virus Virions by Scanning Transmission Electron Microscopy. Journal of Virology. 1999;73:7050–7055. doi: 10.1128/jvi.73.8.7050-7055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Tanaka C, Wu Y, Chang MO, Inagaki Y, Saito Y, Naito T, Ogasawara H, Sekigawa I, Hayashida Y. Analysis of human immunodeficiency virus type 1 integration by using a specific, sensitive and quantitative assay based on real-time polymerase chain reaction. Virus Genes. 2006;32(1):105–13. doi: 10.1007/s11262-005-5851-2. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344(1):88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen ISY. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack JA, Haislip AM, Krogstad P, Chen ISY. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. Journal of Virology. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZQ, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Wietgrefe SW, Li Q, Shore MD, Duan L, Reilly C, Lifson JD, Haase AT. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101(15):5640–5. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol. 2005;79(4):2199–210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1: Generation of an integration standard (IS) cell line.
To calculate HIV integration events, we needed to compare the level of integration in our unknown samples to a standard. The standard needed to contain diverse integration sites to accurately reflect integration in vivo (Butler, Hansen, and Bushman, 2001). To generate a standard with diverse integration sites, we used the strategy outlined in Supplement 1. pNL4-3Δenv/EGFP/HygR (Supplement 1A) and pVSVG, an expression plasmid for vesicular stomatitis virus glycoprotein (VSV-G), were co-transfected into 293T cells to generate pseudotyped virions bearing the HIVΔenv genome and VSV-G glycoprotein. CEMss T cells (or 293 cells) were inoculated by spinoculation with transfection supernatant containing pseudotyped virions. The pseudotyped virions lacked HIV env, so infection was limited to a single cycle, and contained the coding sequence of EGFP and hygromycin resistance in place of the nef gene. The infected cells were grown for 28 days under hygromycin selection and sorted for EGFP expression. Soon after infection, both integrated and unintegrated DNA were detectable; however after 28 days only integrated DNA was detectable by Southern blot (not shown but previously described (O'Doherty et al., 2002)).
We then measured the level of HIV DNA/cell in this Integration Standard (IS) cell line using kinetic PCR (Swiggard et al., 2005) and detected ∼1 copy/cell (Supplement 1B). Given that all of the DNA was integrated, we thus concluded that there is ∼1 integration event per cell present in the IS cell line. We then used the same DNA in our two-step PCR assay to detect integration and generate a standard curve by plotting the number of proviruses vs the cycle threshold. In the first step of the PCR assay, we amplified DNA using either Alu and gag primers or only the gag primer (see the PCR conditions section of Materials and methods for explanation). In the second step of the PCR assay, we used HIV-1 specific primers, which detect Alu-gag and gag-only amplicons, but do not detect Alu-Alu amplicons.
Supplement 2: Integration of HIV-1 in CD4+ T cells after spinoculation also exhibits a dose dependent decrease in integration with each dilution.
ppCD4+ T cells were inoculated with three-fold dilutions of supernatant containing VSV-G pseudotyped pNL4-3. Cells were inoculated by spinoculation for 2hr at 25°C, then washed and the level of cell associated virus (viral binding) was determined at the most concentrated and most dilute doses by p24 ELISA. The cells were cultured for 3 days, DNA was prepared, and reverse transcription and integration were measured. Similar to Figure 4, a dose-dependent decrease in integration and RU5 reverse transcripts was observed with serial dilution of the viral inoculum. The results are presented in provirus per cell (A), log (3) of provirus per cell (B), RU5 per cell (C) and log(3) RU5 per cell (D). AZT inhibited reverse transcription (78%) and integration (99%) (added at the top dose). Error bars represent the standard deviation of 16 measurements of integration and 2-4 measurements of reverse transcripts, for each virus dilution depending on sample availability. The number of proviruses/cell and RU5/cell are written above each point (B, D).