Summary
In all eukaryotes chromatin physically restricts the accessibility of the genome to regulatory proteins such as transcription factors. Plant model systems have been instrumental in demonstrating that this restriction is dynamic and changes during development and in response to exogenous cues. Among the multiple epigenetic mechanisms that alter chromatin to regulate gene expression, histone modifications play a major role. Recent studies in Arabidopsis have provided the first genome-wide histone modification maps, revealed important biological roles for histone modifications, and advanced our understanding of stimulus-dependent changes in histone modifications.
Introduction
Within each cell, the genetic information encoded by DNA is compacted into chromatin. The fundamental unit of chromatin is the nucleosome, which is formed by wrapping 147 bp of DNA around a histone octamer (two copies each of histones H2A, H2B, H3 and H4). Nucleosomal DNA presents a barrier for proteins that need to contact the DNA, including those regulating gene expression. Constitutively expressed genes in plants and other organisms often have nucleosome free regions in their promoters[1,2]. Expression of many other genes is regulated by altering these chromatin constraints in response to endogenous and exogenous cues, which ultimately creates a cell- or stimulus-specific accessible genome. How is this achieved? Within the context of chromatin, three processes act in concert to regulate gene expression and thus should be considered together (Table 1). First, histone modifiers covalently alter amino acids primarily in the exposed N-terminal tails of histones, which changes the histone-DNA interaction and creates or blocks protein binding sites (Table 1)[3]. Second, chromatin remodeling ATPases use the energy derived from ATP hydrolysis to alter position or composition of nucleosomes (Table 1)[2]. Finally, methylation of cytosine residues in the DNA interferes with binding of some proteins (including transcription factors) and recruits other proteins (Table 1)[4,5].
Table 1.
Types of chromatin alterations that regulate gene expression
| Chromatin alteration1 | Subtype | Acts upon | Mechanism | Outcome for transcription |
|---|---|---|---|---|
| Histone Modifications | Ubiquitination | Lysine (K) | distances histones from DNA (bulky moiety) | Activation (H2B) or repression (H2A)2 |
| Methylation | Lysine, Arginine (R) | Recruitment of other chromatin regulators | Activation (H3K4, H3K363) or repression (H3K93, H3K27, H4K20, H4R3) | |
| Acetylation | Lysine | Charge neutralization4, recruitment of other chromatin regulators | Activation | |
| Phosphoryation | Serine (S), Threonine (T) | Charge neutralization4, recruitment of other chromatin regulators | Activation | |
| Chromatin5 remodeling | SWI/SNF | Nucleosome position and occupancy2 | Sliding to new position, histone octamer eviction2 | Actvation and repression |
| SWR1 | Histone exchange | H2A.Z histone variant incorporation | Activation | |
| DNA methylation | CG and non-CG | Promoter | Inhibition of transcription factor binding | Repression |
| CG | Gene (less at 5′ and 3′ ends) | May reduce transcription elongation | Repression |
These and additional chromatin alterations also play a role in heterochromatic silencing of repetitive DNA and transposons, genome integrity, and chromosome stability, which are reviewed elsewhere [24,49,51].
Not yet demonstrated for plants.
May activate transcription when localized in transcribed region, but repress transcription when localized in the promoter region.
Charge neutralization decreases the affinity of positively charged histones for negatively charged DNA.
For simplicity, only a subset of the chromatin remodeling complexes that regulate transcription are listed here.
Here we focus on the role of covalent histone modifications in regulation of gene expression in euchromatin. These modifications can activate or repress transcription by generating more ‘open’ or ‘closed’ chromatin configurations, respectively. Generally, open chromatin increases the accessibility of the genome to transcription factors and/or the general transcription machinery, thereby activating transcription, while closed chromatin represses transcription by limiting the accessibility of the genome to these proteins (Table 2). Within a histone, the amino acid modified, the type of modification and the degree of modification (for example mono-, di- or trimethylation) all affect whether a given modification is activating or repressive[3] (Table 2). The roles of the individual histone modifications are conserved between the plant and animal kingdoms.
Table 2.
Histone modifications in Arabidopsis
| Modification | Location | Effect | Transcription | Established by | Removed by |
|---|---|---|---|---|---|
| Ubiquitination | |||||
| H2BK143ub1 | Eu-chromatin | open | activation | Ring-Type E3 ligases
HUB1, HUB2[52,53] |
Deubiquitinases
SUP32/UBP26[54] |
| Methylation | |||||
| H3K4me* | Eu-chromatin | open | activation | trxG class of histone methyltransferases (HMTs)[55] | LSD1-type of histone demethylases (HDMs)[3,8]
FLD[28] |
| H3K4me1 | Eu-chromatin | open | |||
| H3K4me2 | Eu-chromatin | open | activation intron1[38] | ATX1[56] | |
| H3K4me3 | Eu-chromatin | open | activation proximal promoter[55]; 5′end gene[57] |
ATX1[56]
EFS/SDG8[57] |
|
| H3K9me* | Both | closed | Su(var) class of HMTs[55] | JmjC-domain and LSD1-type HDMs[3,8] | |
| H3K9me1 | Hetero-chromatin | closed |
SUVH2[58]
SUVH4, SUVH6[59] SUVH5[60] |
||
| H3K9me2 | Hetero-chromatin | closed |
SUVH2[58]
SUVH4, SUVH6[59] SUVR4[61] |
||
| H3K9me3 | Eu-hromatin | closed | repression proximal promoter, 5′ end gene, gene[11] | ||
| H3K27me* | Both | closed | repression | E(Z) class of HMTs (PRC2 complex)[55] | |
| H3K27me1 | Hetero-chromatin | closed | |||
| H3K27me2 | Hetero-chromatin | closed | |||
| H3K27me3 | Eu-chromatin | closed | repression promoter, 5′end gene, gene[11-13] | CLF, SWN, MEA [12,13] | |
| H3K36me* | Eu-chromatin | open | activation | Set domain HMTs | JmjC-domain HDMs.
Possibly REF6[28,62] |
| H3K36me1 | Eu-chromatin | open | |||
| H3K36me2 | Both | open | activation intron1[63] | EFS/SDG8[63] | |
| H3K36me3 | Eu-chromatin | open | |||
| H4K20me* | closed | JmjC domain HDMs | |||
| H4K20me1 | Hetero-chromatin | SUVH2[58] | |||
| H4K20me2 | Eu-chromatin | ||||
| H4K20me3 | Eu-chromatin | ||||
| H4R3sme2 | Eu-chromatin | closed | repression promoter[64] | Arginine methyltransferases
SKB1/AtPRMT5[64,65] |
Deimination[8] |
| Acetylation | |||||
| H3K* ac/H4K* ac | Both | open | activation promoter, 5′end gene[33,38,66] | Histone acetyltransferases (HATs)[25]
GNAT family: GCN5/HAG1[30,34,42] CBP/p300 family: HAC1, HAC5, HAC12[26,67,68] TAFII family: HAF2/TAF1[30] |
Histone Deacetylases (HDACs)[25]
RDP3 family: HDA19[27,30,37, 42,69] HDA6[70] HDA1 family: HDA18[71] HD2 family: HD2A, HD2B[72], HD2C[73] |
| Phosphorylation2 | |||||
| H3S10ph
H3S28ph H2T11ph |
Both | open | activation | Kinases[74] | Phosphatases[74] |
Histone modifications, their locations in the genome and their effects on chromatin (open/closed) as well as on transcription (where known) are listed. Also listed are the enzymes known to add and remove each modification. Plant histone modifiers are listed in bold type.
denotes all modifications of a certain type, for example, H3K9me* denotes mono, di, and trimethylation of lysine nine of histone H3, while H3*ac denotes general acetylation of lysines in histone H3.
These introns contain cis regulatory elements important for transcriptional regulation.
In this review we will discuss genome-wide elucidation of the distribution of histone modifications in plants, identification of the processes regulated by histone modifications, and how histone modifiers are recruited to their targets in plants. A unique advantage of studying chromatin regulation in plants is that functional and molecular studies are usually conducted in context of the multicellular organism, not in cell lines. While this can be technically challenging (see conclusions), it facilitates identification of biologically relevant changes in histone modifications. That very few chromatin regulators are essential in plants is a significant advantage for elucidating the in vivo role of these regulators.
Genome-wide distribution of covalent histone modifications
In addition to the type of histone modification present, the effect on gene expression depends on the spatial distribution of a given modification across a gene region (the histone modification landscape[6]) and the combinatorial presence of other modifications[3,7,8]. Two examples from yeast and mammalian cell lines highlight this point. Tri-methylation of histone H3 on lysines 9 and 36 (H3K9me3 and H3K36me3) is repressive when found in the promoter region but activating when found in gene proper (reviewed in[3,7,8]). These modifications close chromatin to prevent transcription initiation. Thus, when present within genes, this stimulates full-length transcript production by preventing internal initiation from cryptic start sites. Genome-wide binding and expression studies showed that presence of an activating histone modification such as H3K4me at the promoter alone is not sufficient to trigger transcriptional activation, additional histone modifications present at the same locus affect gene expression in a combinatorial fashion (reviewed in [3,6,7], see also[9,10]).
Recent studies in plants also address these topics. Genome-wide analyses of H3K27me3 were recently published for Arabidopsis[1,11]. This is a repressive histone modification established by SET-domain containing histone methyl transferases of the Polycomb Repressive Complex 2 (PRC2). H3K27me3 is required for proper expression of several important transcriptional regulators[12–18]. Chromatin immunoprecipitation studies with H3K27me3 antibodies followed by hybridization of the precipitated genomic DNA to whole genome Arabidopsis tiling arrays (ChIP on chip) revealed that H3K27me3 is associated with many individual genes (18% of all genes), especially transcription factors and developmental regulators that are not expressed at the developmental stage examined (ten-day-old seedlings)[1]. The modification is limited to the transcribed regions of genes, with a slight bias towards the 5′ end and the proximal promoter[1,11](Figure 1), suggesting that regulatory sequences in the DNA may be the primary targets of H3K27me3 repression. In metazoans H3K27me3 also represses expression of transcription factors and developmental regulators, but is generally associated with large multigene chromatin domains instead of single genes[19,20]. The reduced tendency of plants to form large compacted chromatin domains may be the basis for their high developmental plasticity and the ease with plant cells can change developmental programs and even de-differentiate.
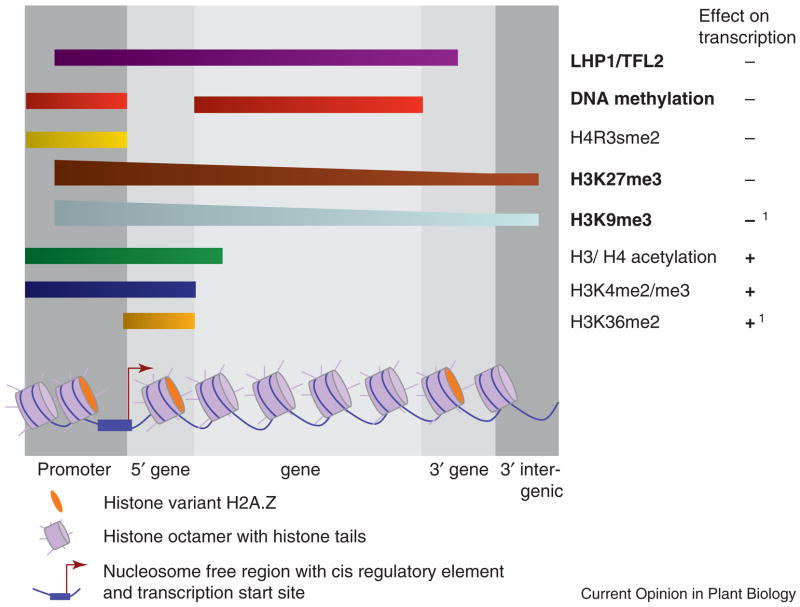
Figure 1. Spatial arrangement of chromatin modifications important for gene expression.
Shown is a generic euchromatic Arabidopsis thaliana gene in the context of chromatin modifications known to affect gene expression. Relevant gene regions are indicated. Histone octamers are shown as purple cylinders. The histone variant H2A.Z incorporated by the SWR1 chromatin remodeling complex[75–77] is shown as a green ellipse. The promoter is partially depleted of nucleosomes, which is observed in many constitutively expressed genes and in inducible genes after the combined activity of chromatin remodeling ATPases and histone modifiers. Above the graphic grey shading indicates the promoter region, the 5′ end of the gene, the central gene region, the 3′ end of the gene, and the 3′ intergenic region. The spatial distribution of covalent histone modifications is indicated relative to these five regions. Modifications for which distribution was determined by global binding studies are indicated in bold[1,11,47,48,50], to distinguish them from those for which information is only available from studies of a limited number of genes (see Table 2 for examples). Note that H3K27me3 and H3K9me3 do not overlap, genes repressed by H3K27me3 are devoid of H3K9me3 and vice versa[11]. Spatial distribution of DNA methylation and of the Heterochromatin Protein 1 homolog LHP1/TFL2, two other chromatin regulators whose distribution was mapped genome-wide, is also indicated.
1 H3K9me3 and H3K36me2 may have different roles on transcription dependent on their location[3].
H3K27me3 was found to be the in vivo binding site for the only Arabidopsis thaliana Heterochromatin Protein 1 (HP1) homolog, LHP1/TFL2[11], which plays a role in repressing gene expression in euchromatin[21,22]. This contrasts with the situation in metazoans, where instead of HP1, Polycomb Repressive Complex 1 (PRC1) recognizes H3K27me3[19,20] and generates large multigene silenced domains. Intriguingly, a homolog of PRC1 is not present in plants [18], thus LHP1/TFL2 may functionally substitute for PRC1-mediated repression in euchromatin[11]. The strong, pleiotropic phenotypes of lhp1/tfl2 mutants are consistent with a role for LHP1/TFL2 as an important repressor of gene expression [23].
A comprehensive analysis of multiple histone modifications was recently reported for H3K9me2, H3K9me3, and H3K27me3 using a chromosome 4 tiling array[11]. As previously described, H3K9me2 was exclusively associated with repetitive heterochromatic regions[24]. Both H3K27me3 and H3K9me3 were present in small dispersed but largely non-overlapping regions in euchromatin with a similar 5′ concentrated distribution over genes[11](Figure 1). The mutually exclusive presence of these two histone modifications suggests that they are part of distinct repressive mechanisms and perhaps regulate different groups of genes or respond to different types of cues.
Role of histone modifications in the organism
Histone modifications in plants regulate gene expression in response to diverse exogenous stimuli including stress, pathogen attack, temperature, and light[25]. For example, the histone acetyltransferase (HAT) HAC1 is required for transcriptional upregulation of the heat shock gene HSP17 and accumulation of HSP17 after heat shock[26]. The histone deacetylase (HDAC) HDA19 regulates expression of pathogenesis related genes and promotes resistance to a fungal pathogen[27]. Prolonged exposure to cold silences the flowering time repressor FLOWERING LOCUS C (FLC) in Arabidopsis winter annuals by histone deacetylation and repressive histone methylation (reviewed in [28,29]). HDA19 and two HATs (HAF2 and GCN5) have negative and positive roles, respectively, in light-responsive gene expression and in photomorphogenesis[30]. Thus, histone modifications control expression of important regulators in response to environmental signals in plants.
Histone modifications also play a central role in developmental regulation (reviewed in [17,18,25,28,31]). In a recent study, three-dimensional fluorescence in situ hybridization of the locus encoding the homeodomain transcription factor GLABRA2 (GL2) revealed cell-type-specific hybridization of a GL2 probe in atrichoblast cells (which express GL2 and do not form root hairs) but not in root-hair forming trichoblast cells[32]. This hybridization and hence accessibility of the locus changes dynamically when a cell perceives positional cues that cause a switch from trichoblast to atrichoblast fate, apparently by resetting of the responsible epigenetic marks during the cell cycle[32]. A second study showed that certain histone modifications (high levels of H3K9me2, low levels of H3K9me3, and low H3 acetylation) at the proximal promoter of GL2 are required for repression of this gene[33]. Because these histone modifications can also be reset during the cell cycle[33], it is possible that they cause the differential GL2 locus accessibility. It is intriguing that the H3K9me2 modification is readily reversible upon perception of altered positional cues. As described above, H3K9me2 is nearly exclusively heterochromatic[11,24], and thus likely associated with tightly compacted chromatin.
Recruitment and regulation of histone modifying activities
An important, largely unanswered, question is how histone-modifying activities are recruited to their targets. One mechanism is recruitment by transcription factors. As discussed below, transcription factors can directly bind and recruit histone-modifying complexes or recruit them indirectly, via co-regulators. In addition, existing histone modifications can affect recruitment of histone modifiers. Several plant transcription factors are known to interact with histone modifying complexes. The cold-inducible C-REPEAT BINDING FACTOR (CBF1) is proposed to recruit the GCN5 HAT complex via direct interaction with the HAT complex subunits ADA2a and ADA2b[34]. The C2H2 zinc-finger transcription factor SUPPRESSOR OF FRIGIDA4 (SUF4) is thought to recruit the histone methyl transferase EARLY FLOWERING IN SHORT DAYS (EFS) to the FLC promoter[35,36]. The APETALA2/EREBP-type transcription factor AtERF7, which mediates ABA responses, likely recruits HDA19 via its interaction with the HDAC complex subunit SIN3 [37]. The seed specific ABI3-like transcription factor ALF appears to recruit a HAT activity to the bean phaseolin promoter[38]. A steroid inducible system revealed that ALF binding causes promoter potentiation through increased histone acetylation. Full promoter activation after subsequent exposure to the hormone ABA correlates with altered histone acetylation, and increased H3K4 levels[38], indicating that a step-wise sequence of histone modifications is necessary for phaseolin activation. Although more recruiting factors need to be identified to better understand what types of transcription factors play this role, the available data suggests that expression and/or activity of the recruiting transcription factors is cell- or stimulus-specific.
The LEUNIG (LUG) co-repressor complex bridges interactions between transcription factors and chromatin regulators. LUG is recruited by certain MADS-domain transcription factors to the promoter of the floral homeotic regulator AGAMOUS(AG) and represses AG expression in the outer whorls of flowers via recruitment of the HDA19 HDAC[39–41]. TOPLESS (TPL), a protein with limited similarity to LUG, was recently identified as pivotal in embryo patterning in Arabidopsis. Among the earliest patterning events in the embryo are establishment of an apical (shoot) pole and a basal (root) pole. Dominant negative mutations in TPL inactivate all five TPL family members in Arabidopsis and convert the apical pole into a basal pole, which can lead to a dramatic double root phenotype[42]. Elegant genetic interaction studies indicate that the TPL family of proteins likely works in conjunction with HDA19 to repress genes required for basal fate in the apical half of transition stage embryos[42].
Once a histone modification has been added, it can serve to recruit or inhibit recruitment of additional histone modifying complexes. For example, GENERAL TRANCRIPTION FACTOR 6 (GTE6) contains a domain that binds acetylated lysines on histone tails. GTE6 is recruited to the promoter of the MYB transcription factor ASYMMETRIC LEAVES 2 (AS2) and is required for histone acetylation of the AS2 promoter via positive feedback, perhaps by recruiting a HAT complex[43].
Specific recruitment of histone modifying complexes enables them to regulate particular target genes in response to endogenous and exogenous cues. Formation of multiple histone modifier complexes also contributes to their target specificity. For example three distinct PRC2 complexes exist in Arabidopsis that regulate unique target genes through H3K27 trimethylation[18]. Specificity of histone modifiers can also be regulated posttranslationally, the maize HDAC Hda1 is activated by proteolytic cleavage during seed germination [44].
Conclusion
Ultimately many histone modifications are directed at coordinating gene expression. As summarized above the recent genome-wide studies in plants have provided tantalizing glimpses into how this coordination is actually achieved. These studies have revealed that in Arabidopsis repressive histone modifications (such as H3K27 me3) occupy smaller domains than in metazoans, likely making these modifications more readily reversible. The observation that factors binding this histone modification may also be distinct further underscores that fundamental differences may exist in chromatin-mediated regulation between the two kingdoms. These differences may reflect the higher developmental plasticity of plants and allow these sessile organisms to respond rapidly to fluctuating environmental conditions. It is the expectation that the coordinated gene expression enabled through these histone modifications patterns would be target of environmental or developmental cues. Indeed, as discussed above, histone modifications change rapidly in response to diverse exogenous cues and during patterning and differentiation to control expression of important regulators of the processes triggered by these cues. The precise patterns of histone modifications observed genome-wide as well as the dynamic changes in histone modifications observed at the single gene level suggest presence of an accurate recruitment mechanism for histone modifiers. As evidenced from the studies described here, this likely involves a combination of sequence specific DNA binding proteins, co-regulator complexes, feedback mechanisms as well as regulation of histone modifier activity.
The challenge for the future is to detect the global, dynamic changes in multiple histone modifications in the organism that occur within subpopulations of cells, to further delineate how these modifications are triggered by exogenous and endogenous cues at specific genes, and to determine how they correlate with gene expression. This will require new approaches and techniques, including inducible systems to dissect the responses to endogenous cues, better cell fractionation methods, and increased sensitivity of techniques such as ChIP. Genetic analyses will continue to be instrumental in deciphering the biological significance of the observed alterations in histone modifications.
Understanding chromatin-mediated regulation of genome accessibility will ultimately require expanding these studies to include other chromatin regulatory mechanisms such as chromatin remodeling and DNA methylation. The intricate epigenetic regulation of FLC [28,31] clearly demonstrates that histone modifications and chromatin remodeling act together to control expression of this master regulator. Other chromatin remodeling complexes play important roles in chromatin-mediated control of gene expression[45,46]. Similarly, genome-wide DNA methylation maps[47–51] suggest that in addition to its role in heterochromatin, DNA methylation may affect transcription in plants. Because of the viability of null mutants, plants are ideally suited to sort out how these processes coordinately regulate genome accessibility.
Acknowledgments
We apologize to all researches who contributed this field and whose work could not be cited here due to space limitations. We wish to thank John D. Wagner, Chang Seob Kwon, Kim Gallagher, Scott Poethig, Stewart Gillmor, and Nick Kaplinsky for critical comments on this manuscript. DW is supported by National Institutes of Health grant R01 GM64650-01 and National Science Foundation grant IBN 0516622, JP is supported by National Institutes of Health NSRA grant F32-GM076933.
Abbreviations
- ac
acetylation
- ChIP
chromatin immunoprecipitation
- H3K9
histone H3 Lysine 9
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
- HMT
histone methyl transferase
- HMD
histone demethylase
- HP1
Heterochromatin Protein 1
- me
methylation
- me2
demethylation
- me3
trimethylation
- LHP1
LIKE HETEROCHROMATIN PROTEIN 1 (also called TFL2); Arabidopsis HP1 ortholog
- ph
phosphorylation
- PRC1
Polycomb Repressive Complex 1
- PRC2
Polycomb Repressive Complex 2
- sme2
symmetric dimethylation
- SWR1
chromatin remodeling complex that mediates histone exchange of the histone variant H2A.Z for the canonical histone H2A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer Pfluger, Email: pfluger@sas.upenn.edu.
Doris Wagner, Email: wagnerdo@sas.upenn.edu.
References
- 1.Zhang X, Clarenz O, Cokus S, Bernatavichute YV, Pellegrini M, Goodrich J, Jacobsen SE. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007;5:e129. doi: 10.1371/journal.pbio.0050129. ** The first genome-wide analysis of a histone modification in plants. Trimethylation of lysine 27 of histone H3 (H3K27 me3) is localized throughout individual genes that are repressed at the developmental stage examined. A large fraction of the 4,400 genes marked by this histone modification are transcription factors and developmental regulators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rando OJ, Ahmad K. Rules and regulation in the primary structure of chromatin. Curr Opin Cell Biol. 2007 doi: 10.1016/j.ceb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 4.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Zemach A, Grafi G. Methyl-CpG-binding domain proteins in plants: interpreters of DNA methylation. Trends Plant Sci. 2007;12:80–85. doi: 10.1016/j.tplants.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V. Arabidopsis TFL2/LHP1 Specifically Associates with Genes Marked by Trimethylation of Histone H3 Lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. ** Comprehensive chromosome 4 binding study for the only Arabidopsis Heterochromatin Protein 1 homolog LHP1/TFL2 as well as for three repressive histone modifications. The study showed that in vivo LHP1 binds to one of these, H3K27me3. In addition, the repressive euchromatic histone modifications H3K9me3 and H3K27me3 are found in different genes and present throughout each gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Kohler C. Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 2006;7:947–952. doi: 10.1038/sj.embor.7400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. Embo J. 2006;25:4638–4649. doi: 10.1038/sj.emboj.7601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- 15.Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- 16.Schonrock N, Bouveret R, Leroy O, Borghi L, Kohler C, Gruissem W, Hennig L. Polycomb-group proteins repress the floral activator AGL19 in the FLC-independent vernalization pathway. Genes Dev. 2006;20:1667–1678. doi: 10.1101/gad.377206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler C, Makarevich G. Epigenetic mechanisms governing seed development in plants. EMBO Rep. 2006;7:1223–1227. doi: 10.1038/sj.embor.7400854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pien S, Grossniklaus U. Polycomb group and trithorax group proteins in Arabidopsis. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbaexp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 21.Libault M, Tessadori F, Germann S, Snijder B, Fransz P, Gaudin V. The Arabidopsis LHP1 protein is a component of euchromatin. Planta. 2005;222:910–925. doi: 10.1007/s00425-005-0129-4. [DOI] [PubMed] [Google Scholar]
- 22.Nakahigashi K, Jasencakova Z, Schubert I, Goto K. The Arabidopsis heterochromatin protein1 homolog (TERMINAL FLOWER2) silences genes within the euchromatic region but not genes positioned in heterochromatin. Plant Cell Physiol. 2005;46:1747–1756. doi: 10.1093/pcp/pci195. [DOI] [PubMed] [Google Scholar]
- 23.Kotake T, Takada S, Nakahigashi K, Ohto M, Goto K. Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 2003;44:555–564. doi: 10.1093/pcp/pcg091. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs J, Demidov D, Houben A, Schubert I. Chromosomal histone modification patterns--from conservation to diversity. Trends Plant Sci. 2006;11:199–208. doi: 10.1016/j.tplants.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZJ, Tian L. Roles of dynamic and reversible histone acetylation in plant development and polyploidy. Biochim Biophys Acta. 2007;1769:295–307. doi: 10.1016/j.bbaexp.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bharti K, Von Koskull-Doring P, Bharti S, Kumar P, Tintschl-Korbitzer A, Treuter E, Nover L. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell. 2004;16:1521–1535. doi: 10.1105/tpc.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Zhang L, Duan J, Miki B, Wu K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell. 2005;17:1196–1204. doi: 10.1105/tpc.104.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz RJ, Amasino RM. Vernalization: A model for investigating epigenetics and eukaryotic gene regulation in plants. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbaexp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Sung S, Amasino RM. Molecular genetic studies of the memory of winter. J Exp Bot. 2006;57:3369–3377. doi: 10.1093/jxb/erl105. [DOI] [PubMed] [Google Scholar]
- 30.Benhamed M, Bertrand C, Servet C, Zhou DX. Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell. 2006;18:2893–2903. doi: 10.1105/tpc.106.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes JC. Chromatin modifiers that control plant development. Curr Opin Plant Biol. 2006;9:21–27. doi: 10.1016/j.pbi.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Costa S, Shaw P. Chromatin organization and cell fate switch respond to positional information in Arabidopsis. Nature. 2006;439:493–496. doi: 10.1038/nature04269. ** Using 3D fluorescence in situ hybridization, the locus for GLABRA 2, a regulator of root epidermal cell fate, was shown to be accessible only in non hair-forming cells. Altered positional cues cause cell cycle linked resetting of this differential chromatin-mediated locus accessibility and altered cell fate. [DOI] [PubMed] [Google Scholar]
- 33.Caro E, Castellano MM, Gutierrez C. A chromatin link that couples cell division to root epidermis patterning in Arabidopsis. Nature. 2007;447:213–217. doi: 10.1038/nature05763. ** GEM, a new regulator of GLABRA 2(GL2) expression, represses GL2 by maintaining repressive H3 lysine 9 methylation at the GL2 promoter. The histone modifications and GL2 expression are cell-cycle regulated, providing a link between cell division and cell fate. [DOI] [PubMed] [Google Scholar]
- 34.Mao Y, Pavangadkar KA, Thomashow MF, Triezenberg SJ. Physical and functional interactions of Arabidopsis ADA2 transcriptional coactivator proteins with the acetyltransferase GCN5 and with the cold-induced transcription factor CBF1. Biochim Biophys Acta. 2006;1759:69–79. doi: 10.1016/j.bbaexp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Choi K, Park C, Hwang HJ, Lee I. SUPPRESSOR OF FRIGIDA4, encoding a C2H2-Type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis FLOWERING LOCUS C. Plant Cell. 2006;18:2985–2998. doi: 10.1105/tpc.106.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SY, Michaels SD. SUPPRESSOR OF FRI 4 encodes a nuclear-localized protein that is required for delayed flowering in winter-annual Arabidopsis. Development. 2006;133:4699–4707. doi: 10.1242/dev.02684. [DOI] [PubMed] [Google Scholar]
- 37.Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, Zhu JK. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell. 2005;17:2384–2396. doi: 10.1105/tpc.105.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng DW, Chandrasekharan MB, Hall TC. Ordered histone modifications are associated with transcriptional poising and activation of the phaseolin promoter. Plant Cell. 2006;18:119–132. doi: 10.1105/tpc.105.037010. * Steroid-inducible recruitment of the seed specific transcription factor ALF to its target promoter allowed identification of distinct promoter states that are correlated with unique chromatin modifications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sridhar VV, Surendrarao A, Gonzalez D, Conlan RS, Liu Z. Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc Natl Acad Sci U S A. 2004;101:11494–11499. doi: 10.1073/pnas.0403055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sridhar VV, Surendrarao A, Liu Z. APETALA1 and SEPALLATA3 interact with SEUSS to mediate transcription repression during flower development. Development. 2006;133:3159–3166. doi: 10.1242/dev.02498. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez D, Bowen AJ, Carroll TS, Conlan RS. The transcription corepressor LEUNIG interacts with the Histone Deacetylase HDA19 and Mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Mol Cell Biol. 2007 doi: 10.1128/MCB.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long JA, Ohno C, Smith ZR, Meyerowitz EM. TOPLESS regulates apical embryonic fate in Arabidopsis. Science. 2006;312:1520–1523. doi: 10.1126/science.1123841. ** Genetic characterization of the TOPLESS family as important regulators of apical-basal patterning in the embryo. TPL proteins act as transcriptional co-repressors together with histone deacetylases to repress basal fate in the apical embryo. [DOI] [PubMed] [Google Scholar]
- 43.Chua YL, Channeliere S, Mott E, Gray JC. The bromodomain protein GTE6 controls leaf development in Arabidopsis by histone acetylation at ASYMMETRIC LEAVES1. Genes Dev. 2005;19:2245–2254. doi: 10.1101/gad.352005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pipal A, Goralik-Schramel M, Lusser A, Lanzanova C, Sarg B, Loidl A, Lindner H, Rossi V, Loidl P. Regulation and processing of maize histone deacetylase Hda1 by limited proteolysis. Plant Cell. 2003;15:1904–1917. doi: 10.1105/tpc.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon CS, Wagner D. Unwinding chromatin for development and growth: a few genes at a time. Trends Genet. 2007 doi: 10.1016/j.tig.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Jerzmanowski A. SWI/SNF chromatin remodeling and linker histones in plants. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbaexp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. ** Genome-wide DNA methylation analysis revealed a possible role for DNA methylation in regulation transcription, in addition to its role in silencing repetetive DNA and transposons. Ubiquitously expressed genes are characterized by extensive CG methylation throughout the genes, while a small number of genes with a tissue specific expression pattern have methylated promoters. [DOI] [PubMed] [Google Scholar]
- 48.Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet. 2007;39:61–69. doi: 10.1038/ng1929. ** Genome-wide DNA methylation study revealed extensive CG methylation throughout moderately expressed genes with less methylation at the 5′ and 3′ ends. This methylation may be triggered by aberrant transcription initiation within the gene and may dampen transcription elongation. [DOI] [PubMed] [Google Scholar]
- 49.Gehring M, Henikoff S. DNA methylation dynamics in plant genomes. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbaexp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Penterman J, Zilberman D, Huh JH, Ballinger T, Henikoff S, Fischer RL. DNA demethylation in the Arabidopsis genome. Proc Natl Acad Sci U S A. 2007;104:6752–6757. doi: 10.1073/pnas.0701861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henderson IR, Jacobsen SE. Epigenetic inheritance in plants. Nature. 2007;447:418–424. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Koornneef M, Soppe WJ. The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell. 2007;19:433–444. doi: 10.1105/tpc.106.049221. * The enzyme required for monoubiquitination of lysine 143 of H2B (HUB1) was identified in a genetic screen for seed dormancy mutants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleury D, Himanen K, Cnops G, Nelissen H, Boccardi TM, Maere S, Beemster GT, Neyt P, Anami S, Robles P, et al. The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell. 2007;19:417–432. doi: 10.1105/tpc.106.041319. * The HUB1 E3 ubiquitin ligase monoubiquitinates lysine 143 of H2B in vitro. This study links H2BK143ub to activation of expression of cell cycle regulators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sridhar VV, Kapoor A, Zhang K, Zhu J, Zhou T, Hasegawa PM, Bressan RA, Zhu JK. Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature. 2007;447:735–738. doi: 10.1038/nature05864. ** Arabidopsis H2B was found to be ubiquitinated on lysine 143. A T-DNA mutagenesis in a sensitized background allowed identification of the H2BK143 deubiquitinase. This activity is required for silencing of transposons by promoting histone H3 lysine 9 dimethylation and DNA methylation. [DOI] [PubMed] [Google Scholar]
- 55.Ng DW, Wang T, Chandrasekharan MB, Aramayo R, Kertbundit S, Hall TC. Plant SET domain-containing proteins: Structure, function and regulation. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbaexp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez-Venegas R, Avramova Z. Methylation patterns of histone H3 Lys 4, Lys 9 and Lys 27 in transcriptionally active and inactive Arabidopsis genes and in atx1 mutants. Nucleic Acids Res. 2005;33:5199–5207. doi: 10.1093/nar/gki830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SY, He Y, Jacob Y, Noh YS, Michaels S, Amasino R. Establishment of the vernalization-responsive, winter-annual habit in Arabidopsis requires a putative histone H3 methyl transferase. Plant Cell. 2005;17:3301–3310. doi: 10.1105/tpc.105.034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naumann K, Fischer A, Hofmann I, Krauss V, Phalke S, Irmler K, Hause G, Aurich AC, Dorn R, Jenuwein T, et al. Pivotal role of AtSUVH2 in heterochromatic histone methylation and gene silencing in Arabidopsis. Embo J. 2005;24:1418–1429. doi: 10.1038/sj.emboj.7600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson JP, Johnson L, Jasencakova Z, Zhang X, PerezBurgos L, Singh PB, Cheng X, Schubert I, Jenuwein T, Jacobsen SE. Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma. 2004;112:308–315. doi: 10.1007/s00412-004-0275-7. [DOI] [PubMed] [Google Scholar]
- 60.Ebbs ML, Bender J. Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell. 2006;18:1166–1176. doi: 10.1105/tpc.106.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thorstensen T, Fischer A, Sandvik SV, Johnsen SS, Grini PE, Reuter G, Aalen RB. The Arabidopsis SUVR4 protein is a nucleolar histone methyltransferase with preference for monomethylated H3K9. Nucleic Acids Res. 2006;34:5461–5470. doi: 10.1093/nar/gkl687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noh B, Lee SH, Kim HJ, Yi G, Shin EA, Lee M, Jung KJ, Doyle MR, Amasino RM, Noh YS. Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell. 2004;16:2601–2613. doi: 10.1105/tpc.104.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Z, Yu Y, Meyer D, Wu C, Shen WH. Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat Cell Biol. 2005;7:1256–1260. doi: 10.1038/ncb1329. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Zhang Y, Ma Q, Zhang Z, Xue Y, Bao S, Chong K. SKB1-mediated symmetric dimethylation of histone H4R3 controls flowering time in Arabidopsis. Embo J. 2007;26:1934–1941. doi: 10.1038/sj.emboj.7601647. * SKB1, a novel histone modifier, represses FLC expression via symmetric dimethylation of arginine 3 on histone H4 (H4R3sme2). By contrast, this modification activates gene expression in metazoans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pei Y, Niu L, Lu F, Liu C, Zhai J, Kong X, Cao X. Mutations in the Type II Protein Arginine Methyltransferase AtPRMT5 Result in Pleiotropic Developmental Defects in Arabidopsis thaliana. Plant Physiol. 2007 doi: 10.1104/pp.107.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guyomarc’h S, Benhamed M, Lemonnier G, Renou JP, Zhou DX, Delarue M. MGOUN3: evidence for chromatin-mediated regulation of FLC expression. J Exp Bot. 2006;57:2111–2119. doi: 10.1093/jxb/erj169. [DOI] [PubMed] [Google Scholar]
- 67.Deng W, Liu C, Pei Y, Deng X, Niu L, Cao X. Involvement of the Histone Acetyltransferase AtHAC1 in the Regulation of Flowering Time via Repression of FLC in Arabidopsis. Plant Physiol. 2007 doi: 10.1104/pp.106.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han SK, Song JD, Noh YS, Noh B. Role of plant CBP/p300-like genes in the regulation of flowering time. Plant J. 2007;49:103–114. doi: 10.1111/j.1365-313X.2006.02939.x. [DOI] [PubMed] [Google Scholar]
- 69.Tian L, Fong MP, Wang JJ, Wei NE, Jiang H, Doerge RW, Chen ZJ. Reversible histone acetylation and deacetylation mediate genome-wide, promoter-dependent and locus-specific changes in gene expression during plant development. Genetics. 2005;169:337–345. doi: 10.1534/genetics.104.033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Earley K, Lawrence RJ, Pontes O, Reuther R, Enciso AJ, Silva M, Neves N, Gross M, Viegas W, Pikaard CS. Erasure of histone acetylation by Arabidopsis HDA6 mediates large-scale gene silencing in nucleolar dominance. Genes Dev. 2006;20:1283–1293. doi: 10.1101/gad.1417706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu CR, Liu C, Wang YL, Li LC, Chen WQ, Xu ZH, Bai SN. Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. Proc Natl Acad Sci U S A. 2005;102:14469–14474. doi: 10.1073/pnas.0503143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueno Y, Ishikawa T, Watanabe K, Terakura S, Iwakawa H, Okada K, Machida C, Machida Y. Histone deacetylases and ASYMMETRIC LEAVES2 are involved in the establishment of polarity in leaves of Arabidopsis. Plant Cell. 2007;19:445–457. doi: 10.1105/tpc.106.042325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sridha S, Wu K. Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J. 2006;46:124–133. doi: 10.1111/j.1365-313X.2006.02678.x. [DOI] [PubMed] [Google Scholar]
- 74.Houben A, Demidov D, Caperta AD, Karimi R, Agueci F, Vlasenko L. Phosphorylation of histone H3 in plants-A dynamic affair. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbaexp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Choi K, Park C, Lee J, Oh M, Noh B, Lee I. Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development. 2007;134:1931–1941. doi: 10.1242/dev.001891. [DOI] [PubMed] [Google Scholar]
- 76.Deal RB, Topp CN, McKinney EC, Meagher RB. Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell. 2007;19:74–83. doi: 10.1105/tpc.106.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.March-Diaz R, Garcia-Dominguez M, Florencio FJ, Reyes JC. SEF, a new protein required for flowering repression in Arabidopsis, interacts with PIE1 and ARP6. Plant Physiol. 2007;143:893–901. doi: 10.1104/pp.106.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]