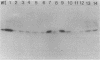
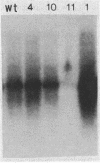
Abstract
The pilus-specific Pseudomonas aeruginosa bacteriophage P04 was used to select spontaneous mutants of strain PAK which have altered piliation. The largest class of phage-resistant mutants synthesized the pilin polypeptide, but did not assemble pili. These mutants are likely to contain mutations in genes required for pilus assembly and not mutations in the pilin structural gene, as they could not be complemented by a normal copy of the pilin gene. In addition, two alterations in pilin gene transcription were found among the mutants--hyperpiliated mutants which overproduce pilin mRNA, and a mutant with temperature-sensitive pilin gene transcription. We also present a model for the regulation of pilin gene transcription by a feedback mechanism sensitive to the relative rates of pilus assembly and disassembly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. J., Kristo C. L., Egerton J. R., Mattick J. S. Variation in the structural subunit and basal protein antigens of Bacteroides nodosus fimbriae. J Bacteriol. 1986 May;166(2):453–460. doi: 10.1128/jb.166.2.453-460.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology. 1973 Feb;51(2):489–492. doi: 10.1016/0042-6822(73)90448-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Basic characterization of a Pseudomonas aeruginosa pilus-dependent bacteriophage with a long noncontractile tail. J Virol. 1973 Nov;12(5):1139–1148. doi: 10.1128/jvi.12.5.1139-1148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J Gen Microbiol. 1972 Sep;72(2):303–319. doi: 10.1099/00221287-72-2-303. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Dixon R. The xylABC promoter from the Pseudomonas putida TOL plasmid is activated by nitrogen regulatory genes in Escherichia coli. Mol Gen Genet. 1986 Apr;203(1):129–136. doi: 10.1007/BF00330393. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Stewart D. J., McKern N. M., Peterson J. E. Expression of pili from Bacteroides nodosus in Pseudomonas aeruginosa. J Bacteriol. 1986 Nov;168(2):574–580. doi: 10.1128/jb.168.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froholm L. O., Sletten K. Purification and N-terminal sequence of a fimbrial protein from Moraxella nonliquefaciens. FEBS Lett. 1977 Jan 15;73(1):29–32. doi: 10.1016/0014-5793(77)80008-2. [DOI] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Johnson K., Parker M. L., Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986 Nov 25;261(33):15703–15708. [PubMed] [Google Scholar]
- Marrs C. F., Schoolnik G., Koomey J. M., Hardy J., Rothbard J., Falkow S. Cloning and sequencing of a Moraxella bovis pilin gene. J Bacteriol. 1985 Jul;163(1):132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKern N. M., O'Donnell I. J., Inglis A. S., Stewart D. J., Clark B. L. Amino acid sequence of pilin from Bacteroides nodosus (strain 198), the causative organism of ovine footrot. FEBS Lett. 1983 Nov 28;164(1):149–153. doi: 10.1016/0014-5793(83)80039-8. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafson R. W., McCarthy P. J., Bhatti A. R., Dooley J. S., Heckels J. E., Trust T. J. Structural and antigenic analysis of meningococcal piliation. Infect Immun. 1985 May;48(2):336–342. doi: 10.1128/iai.48.2.336-342.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Skerman T. M., Erasmuson S. K., Every D. Differentiation of Bacteroides nodosus biotypes and colony variants in relation to their virulence and immunoprotective properties in sheep. Infect Immun. 1981 May;32(2):788–795. doi: 10.1128/iai.32.2.788-795.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. J., Clark B. L., Peterson J. E., Griffiths D. A., Smith E. F. Importance of pilus-associated antigen in Bacteroides nodosus vaccines. Res Vet Sci. 1982 Mar;32(2):140–147. [PubMed] [Google Scholar]
- Strom M. S., Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J Bacteriol. 1986 Feb;165(2):367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Gonococcal pilus subunit size heterogeneity correlates with transitions in colony piliation phenotype, not with changes in colony opacity. J Exp Med. 1983 Nov 1;158(5):1459–1472. doi: 10.1084/jem.158.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Die I., Van Megen I., Zuidweg E., Hoekstra W., De Ree H., Van den Bosch H., Bergmans H. Functional relationship among the gene clusters encoding F7(1), F7(2), F9, and F11 fimbriae of human uropathogenic Escherichia coli. J Bacteriol. 1986 Jul;167(1):407–410. doi: 10.1128/jb.167.1.407-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Sastry P. A., Hodges R. S., Paranchych W. Mapping of the antigenic determinants of Pseudomonas aeruginosa PAK polar pili. Infect Immun. 1983 Oct;42(1):113–121. doi: 10.1128/iai.42.1.113-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]