Abstract
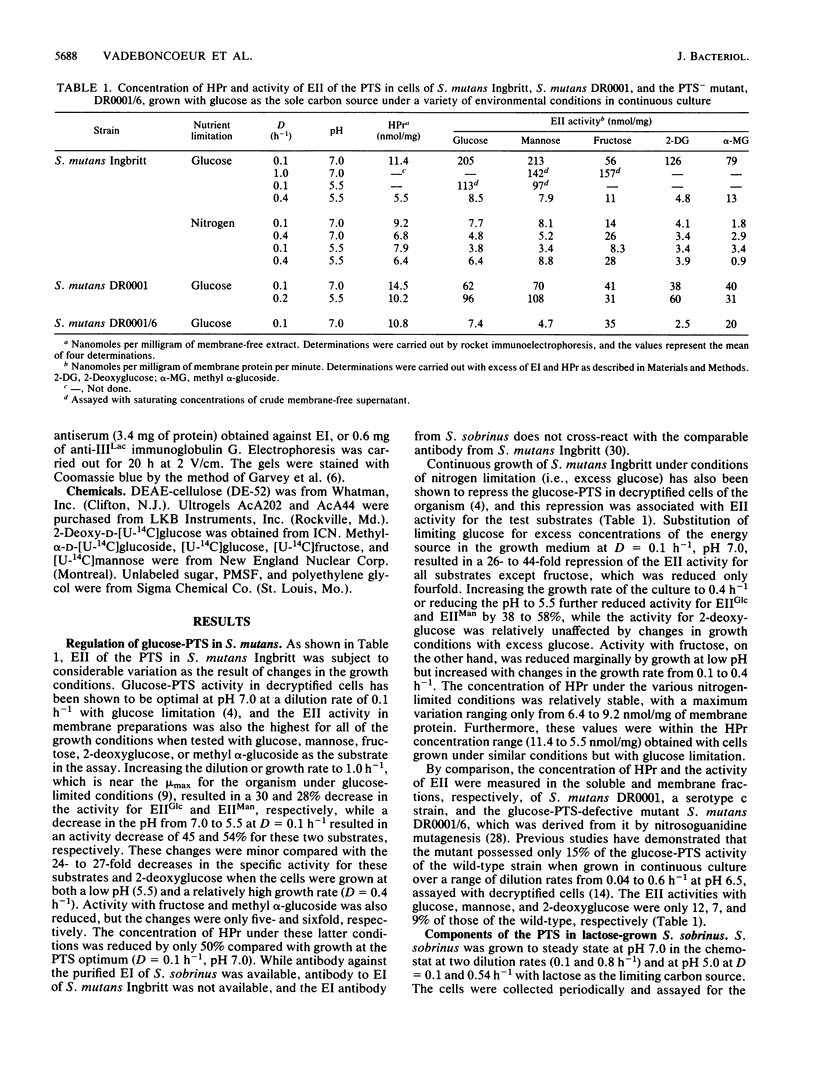
The membrane-bound, sugar-specific enzyme II (EII) component of the phosphoenolpyruvate:sugar phosphotransferase system (PTS) in Streptococcus mutans Ingbritt is repressed by growth on glucose under various conditions in continuous culture. Compared with optimal PTS conditions (i.e., glucose limitation, dilution rate [D] of 0.1 h-1, and pH 7.0), EII activity for glucose (EIIGlc) and mannose (EIIMan) in cells grown at a D of 0.4 h-1 and pH 5.5 with the same glucose concentration was reduced 24- to 27-fold. EII activity with methyl alpha-glucoside and 2-deoxyglucose was reduced 6- and 26-fold, respectively. Growth with excess glucose (i.e., nitrogen limitation) resulted in 26- to 88-fold repression of EII activity with these substrates. The above conditions of low pH, high dilution rate, and excess glucose also repressed EII activity for fructose (EIIFru), but to a lesser extent (two- to fivefold). Conversely, growth of S. mutans DR0001 at a D of 0.2 h-1 and pH 5.5 resulted in increased EIIGlc and EIIMan activity. Unlike the EII component, the HPr concentration in S. mutans Ingbritt varied only twofold (5.5 to 11.4 nmol/mg of protein) despite growth at pH 5.5 with limiting and excess glucose. The HPr concentrations in S. mutans DR0001 and the glucose-PTS-defective mutant DR0001/6 were similar. In a companion study, the soluble components of the PTS (i.e., HPr, EI, and EIIILac) in Streptococcus sobrinus grown on limiting lactose in a chemostat were not influenced significantly by growth at various pHs (7.0 and 5.0) and growth rates (D of 0.1, 0.54, and 0.8 h-1). However, growth on lactose resulted in repression of both EIIGlc and EIIFru, confirming earlier results with batch-grown cells. Thus, the glucose-PTS in some strains of S. mutans is regulated at the level of EII synthesis by certain environmental conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Hamilton I. R. Properties of Streptococcus mutans Ingbritt growing on limiting sucrose in a chemostat: repression of the phosphoenolpyruvate phosphotransferase transport system. Infect Immun. 1982 May;36(2):576–581. doi: 10.1128/iai.36.2.576-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Gauthier L., Mayrand D., Vadeboncoeur C. Isolation of a novel protein involved in the transport of fructose by an inducible phosphoenolpyruvate fructose phosphotransferase system in Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):755–763. doi: 10.1128/jb.160.2.755-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Bowden G. H. Response of freshly isolated strains of Streptococcus mutans and Streptococcus mitior to change in pH in the presence and absence of fluoride during growth in continuous culture. Infect Immun. 1982 Apr;36(1):255–262. doi: 10.1128/iai.36.1.255-262.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- Keevil C. W., Marsh P. D., Ellwood D. C. Regulation of glucose metabolism in oral streptococci through independent pathways of glucose 6-phosphate and glucose 1-phosphate formation. J Bacteriol. 1984 Feb;157(2):560–567. doi: 10.1128/jb.157.2.560-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., McDermid A. S., Marsh P. D., Ellwood D. C. Protonmotive force driven 6-deoxyglucose uptake by the oral pathogen, Streptococcus mutans Ingbritt. Arch Microbiol. 1986 Nov;146(2):118–124. doi: 10.1007/BF00402337. [DOI] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect Immun. 1984 Feb;43(2):536–542. doi: 10.1128/iai.43.2.536-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect Immun. 1984 Mar;43(3):1106–1109. doi: 10.1128/iai.43.3.1106-1109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P. D., Keevil C. W., McDermid A. S., Williamson M. I., Ellwood D. C. Inhibition by the antimicrobial agent chlorhexidine of acid production and sugar transport in oral streptococcal bacteria. Arch Oral Biol. 1983;28(3):233–240. doi: 10.1016/0003-9969(83)90152-8. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982 Nov 22;692(3):415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault L., Vadeboncoeur C. Phosphoenolpyruvate-sugar phosphotransferase transport system of Streptococcus mutans: purification of HPr and enzyme I and determination of their intracellular concentrations by rocket immunoelectrophoresis. Infect Immun. 1985 Dec;50(3):817–825. doi: 10.1128/iai.50.3.817-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Regulation of glycolysis and sugar phosphotransferase activities in Streptococcus lactis: growth in the presence of 2-deoxy-D-glucose. J Bacteriol. 1983 May;154(2):819–830. doi: 10.1128/jb.154.2.819-830.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M. Lactose transport in Streptococcus mutans: isolation and characterization of factor IIIlac, a specific protein component of the phosphoenolpyruvate-lactose phosphotransferase system. Infect Immun. 1984 Oct;46(1):213–219. doi: 10.1128/iai.46.1.213-219.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M., Trahan L. Purification of proteins similar to HPr and enzyme I from the oral bacterium Streptococcus salivarius. Biochemical and immunochemical properties. Can J Microbiol. 1983 Dec;29(12):1694–1705. doi: 10.1139/m83-260. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C. Structure and properties of the phosphoenolpyruvate: glucose phosphotransferase system of oral streptococci. Can J Microbiol. 1984 Apr;30(4):495–502. doi: 10.1139/m84-073. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Trahan L. Glucose transport in Streptococcus salivarius. Evidence for the presence of a distinct phosphoenolpyruvate: glucose phosphotransferase system which catalyses the phosphorylation of alpha-methyl glucoside. Can J Microbiol. 1982 Feb;28(2):190–199. doi: 10.1139/m82-025. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



