Abstract
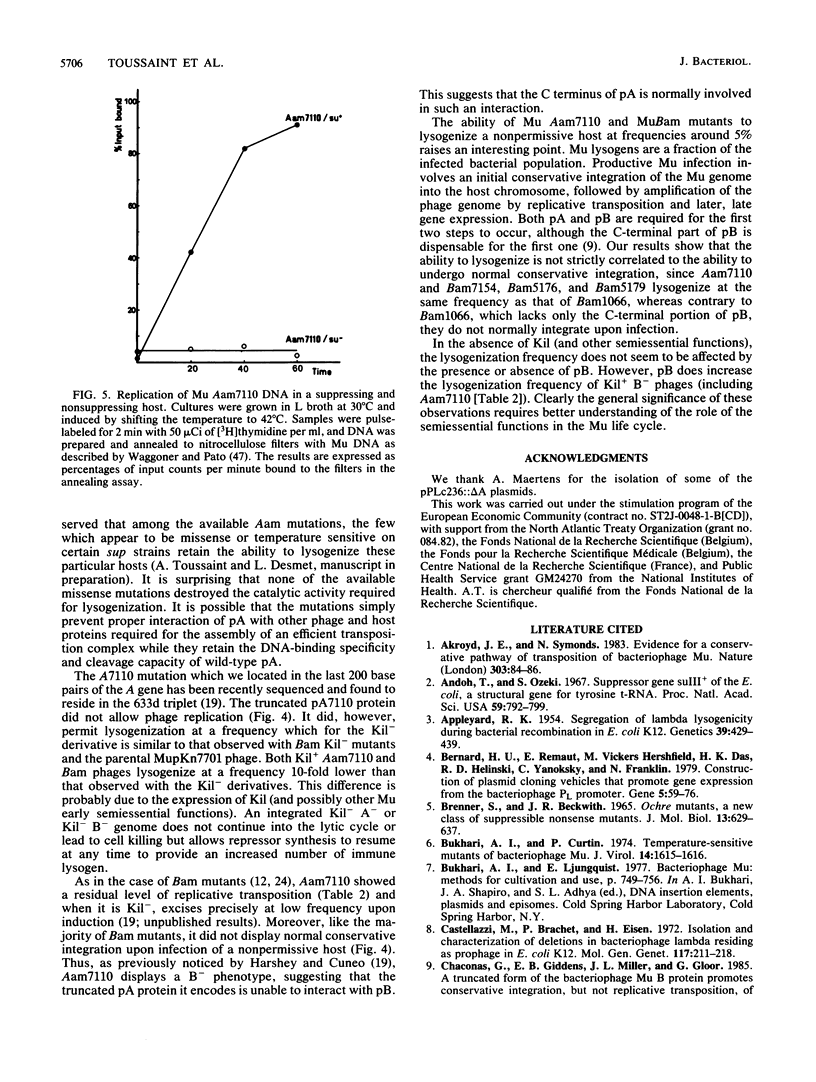
We devised a method for isolating mutations in the bacteriophage Mu A gene which encodes the phage transposase. Nine new conditional defective A mutations were isolated. These, as well as eight previously isolated mutations, were mapped with a set of defined deletions which divided the gene into 13 100- to 200-base-pair segments. Phages carrying these mutations were analyzed for their ability to lysogenize and to transpose in nonpermissive hosts. One Aam mutation, Aam7110, known to retain the capacity to support lysogenization of a sup0 host (M. M. Howe, K. J. O'Day, and D. W. Shultz, Virology 93:303-319, 1979) and to map 91 base pairs from the 3' end of the gene (R. M. Harshey and S. D. Cuneo, J. Genet. 65:159-174, 1987) was shown to be able to complement other A mutations for lysogenization, although it was incapable of catalyzing either the replication of Mu DNA or the massive conservative integration required for phage growth. Four Ats mutations which map at different positions in the gene were able to catalyze lysogenization but not phage growth at the nonpermissive temperature. Phages carrying mutations located at different positions in the Mu B gene (which encodes a product necessary for efficient integration and lytic replication) were all able to lysogenize at the same frequency. These results suggest that the ability of Mu to lysogenize is not strictly correlated with its ability to perform massive conservative and replicative transposition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akroyd J. E., Symonds N. Evidence for a conservative pathway of transposition of bacteriophage Mu. Nature. 1983 May 5;303(5912):84–86. doi: 10.1038/303084a0. [DOI] [PubMed] [Google Scholar]
- Andoh T., Ozeki H. Suppressor gene Su3+ of E. coli, a structural gene for tyrosine TRNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):792–799. doi: 10.1073/pnas.59.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Curtin P. Temperature-sensitive mutants of bacteriophage mu. J Virol. 1974 Dec;14(6):1615–1616. doi: 10.1128/jvi.14.6.1615-1616.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., Brachet P., Eisen H. Isolation and characterization of deletions in bacteriophage lambda residing as prophage in E. coli K 12. Mol Gen Genet. 1972;117(3):211–218. doi: 10.1007/BF00271648. [DOI] [PubMed] [Google Scholar]
- Chaconas G., Giddens E. B., Miller J. L., Gloor G. A truncated form of the bacteriophage Mu B protein promotes conservative integration, but not replicative transposition, of Mu DNA. Cell. 1985 Jul;41(3):857–865. doi: 10.1016/s0092-8674(85)80066-0. [DOI] [PubMed] [Google Scholar]
- Craigie R., Arndt-Jovin D. J., Mizuuchi K. A defined system for the DNA strand-transfer reaction at the initiation of bacteriophage Mu transposition: protein and DNA substrate requirements. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7570–7574. doi: 10.1073/pnas.82.22.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Mechanism of transposition of bacteriophage Mu: structure of a transposition intermediate. Cell. 1985 Jul;41(3):867–876. doi: 10.1016/s0092-8674(85)80067-2. [DOI] [PubMed] [Google Scholar]
- FRY B. A. Conditions for the infection of Escherichia coli with lambda phage and for the establishment of lysogeny. J Gen Microbiol. 1959 Dec;21:676–684. doi: 10.1099/00221287-21-3-676. [DOI] [PubMed] [Google Scholar]
- Faelen M., Huisman O., Toussaint A. Involvement of phage Mu-1 early functions in Mu-mediated chromosomal rearrangements. Nature. 1978 Feb 9;271(5645):580–582. doi: 10.1038/271580a0. [DOI] [PubMed] [Google Scholar]
- Faelen M., Toussaint A. Isolation of conditional defective mutants of temperate phage Mu-1 and deletion mapping of the Mu-1 prophage. Virology. 1973 Jul;54(1):117–124. doi: 10.1016/0042-6822(73)90121-9. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. The integration and excision of the bacteriophage lambda genome. Cold Spring Harb Symp Quant Biol. 1968;33:735–747. doi: 10.1101/sqb.1968.033.01.084. [DOI] [PubMed] [Google Scholar]
- Harshey R. M. Transposition without duplication of infecting bacteriophage Mu DNA. Nature. 1984 Oct 11;311(5986):580–581. doi: 10.1038/311580a0. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Howe M. M., O'Day K. J., Schultz D. W. Isolation of mutations defining five new cistrons essential for development of bacteriophage Mu. Virology. 1979 Mar;93(2):303–319. doi: 10.1016/0042-6822(79)90235-6. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- Khatoon H., Bukhari A. I. DNA rearrangements associated with reversion of bacteriophage Mu-induced mutations. Genetics. 1981 May;98(1):1–24. doi: 10.1093/genetics/98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D., Symonds N. The isolation and characterisation of a plaque-forming derivative of bacteriophage Mu carrying a fragment of Tn3 conferring ampicillin resistance. Mol Gen Genet. 1979 May 4;172(2):179–184. doi: 10.1007/BF00268280. [DOI] [PubMed] [Google Scholar]
- Liebart J. C., Ghelardini P., Paolozzi L. Conservative integration of bacteriophage Mu DNA into pBR322 plasmid. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4362–4366. doi: 10.1073/pnas.79.14.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder P., Churchward G., Caro L. Plasmid pSC101 replication mutants generated by insertion of the transposon Tn1000. J Mol Biol. 1983 Oct 25;170(2):287–303. doi: 10.1016/s0022-2836(83)80149-1. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Craigie R. Mechanism of bacteriophage mu transposition. Annu Rev Genet. 1986;20:385–429. doi: 10.1146/annurev.ge.20.120186.002125. [DOI] [PubMed] [Google Scholar]
- Nakayama C., Teplow D. B., Harshey R. M. Structural domains in phage Mu transposase: identification of the site-specific DNA-binding domain. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1809–1813. doi: 10.1073/pnas.84.7.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day K., Schultz D., Ericsen W., Rawluk L., Howe M. Correction and refinement of the genetic map of bacteriophage Mu. Virology. 1979 Mar;93(2):320–328. doi: 10.1016/0042-6822(79)90236-8. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Woods S. L. A temperature-sensitive suppressor enabling the manipulation of the level of individual proteins in intact cells. Cell. 1976 Feb;7(2):205–212. doi: 10.1016/0092-8674(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Reich C. Stoichiometric use of the transposase of bacteriophage Mu. Cell. 1984 Jan;36(1):197–202. doi: 10.1016/0092-8674(84)90089-8. [DOI] [PubMed] [Google Scholar]
- Rambach A., Brachet P. Sélection de mutants du bactériophage lambda incapables de se répliquer. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jan 4;272(1):149–152. [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Ross W., Shore S. H., Howe M. M. Mutants of Escherichia coli defective for replicative transposition of bacteriophage Mu. J Bacteriol. 1986 Sep;167(3):905–919. doi: 10.1128/jb.167.3.905-919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulet R., Allet B., Chandler M. Cloning and expression of the phage Mu A gene. Gene. 1984 Apr;28(1):65–72. doi: 10.1016/0378-1119(84)90088-x. [DOI] [PubMed] [Google Scholar]
- Résibois A., Toussaint A., van Gijsegem F., Faelen M. Physical characterization of mini-mu and mini-D108. Gene. 1981 Jun-Jul;14(1-2):103–113. doi: 10.1016/0378-1119(81)90152-9. [DOI] [PubMed] [Google Scholar]
- Surette M. G., Buch S. J., Chaconas G. Transpososomes: stable protein-DNA complexes involved in the in vitro transposition of bacteriophage Mu DNA. Cell. 1987 Apr 24;49(2):253–262. doi: 10.1016/0092-8674(87)90566-6. [DOI] [PubMed] [Google Scholar]
- Toussaint A., Faelen M., Résibois A. Chromosomal rearrangements induced by mini-Mu and mini-D108: mini review and new data. Gene. 1981 Jun-Jul;14(1-2):115–119. doi: 10.1016/0378-1119(81)90153-0. [DOI] [PubMed] [Google Scholar]
- Toussaint A., Lefebvre N., Scott J. R., Cowan J. A., de Bruijn F., Bukhari A. I. Relationships between temperate phages Mu and P1. Virology. 1978 Aug;89(1):146–161. doi: 10.1016/0042-6822(78)90048-x. [DOI] [PubMed] [Google Scholar]
- Waggoner B. T., Marrs C. F., Howe M. M., Pato M. L. Multiple factors and processes involved in host cell killing by bacteriophage Mu: characterization and mapping. Virology. 1984 Jul 15;136(1):168–185. doi: 10.1016/0042-6822(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Waggoner B. T., Pato M. L. Early events in the replication of Mu prophage DNA. J Virol. 1978 Sep;27(3):587–594. doi: 10.1128/jvi.27.3.587-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijffelman C., Lotterman B. Kinetics of Mu DNA synthesis. Mol Gen Genet. 1977 Mar 7;151(2):169–174. doi: 10.1007/BF00338691. [DOI] [PubMed] [Google Scholar]