Abstract
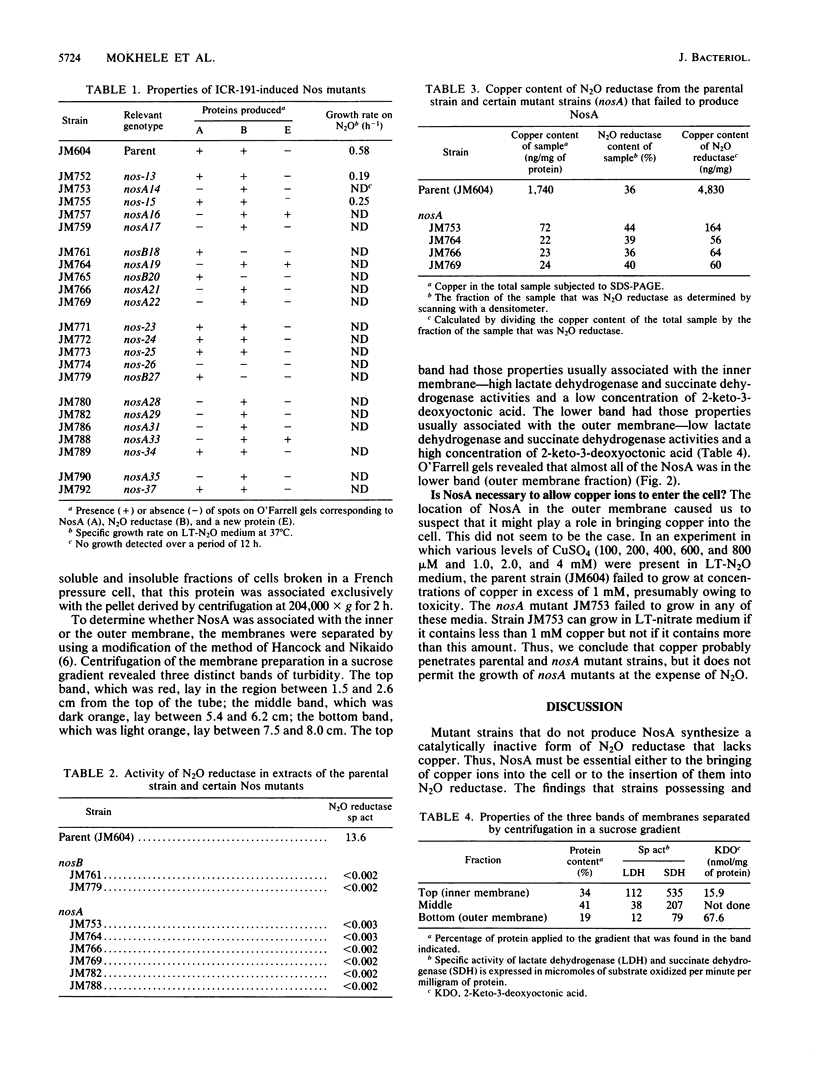
Among a set of frameshift mutagen (ICR-191; Polysciences, Inc.)-induced mutations that confer inability to grow anaerobically with N2O as the sole electron acceptor, one class was found that produced an inactive N2O reductase which lacked copper. All of these mutant strains failed to produce a 61,000-Mr protein located in the outer membrane. This protein, termed NosA, seems not to be responsible for bringing copper into the cell because the mutant strains and their parent were similarly sensitive to the copper content of the growth medium and no intermediate copper concentration in the medium permitted the mutant strains (nosA) to grow anaerobically with N2O as the sole electron acceptor. We conclude that NosA is necessary to insert copper into N2O reductase or to maintain it there.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boogerd F. C., Van Verseveld H. W., Stouthamer A. H. Respiration-driven proton translocation with nitrite and nitrous oxide in Paracoccus denitrificans. Biochim Biophys Acta. 1981 Dec 14;638(2):181–191. doi: 10.1016/0005-2728(81)90226-7. [DOI] [PubMed] [Google Scholar]
- Booth B. R., Curtis N. A. Separation of the cytoplasmic and outer membrane of Pseudomonas aeruginosa PAQ. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1168–1176. doi: 10.1016/0006-291x(77)91641-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Pierson L. S., Rosen J. J., Ingraham J. L. Pseudomonas stutzeri and related species undergo natural transformation. J Bacteriol. 1983 Jan;153(1):93–99. doi: 10.1128/jb.153.1.93-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C. L., Zumft W. G., Kroneck P. M., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985 Dec 16;153(3):459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J Biol Chem. 1980 Jan 25;255(2):704–707. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. W., Hollocher T. C. Purification and some characteristics of nitrous oxide reductase from Paracoccus denitrificans. J Biol Chem. 1987 May 15;262(14):6515–6525. [PubMed] [Google Scholar]
- Stewart G. J., Carlson C. A., Ingraham J. L. Evidence for an active role of donor cells in natural transformation of Pseudomonas stutzeri. J Bacteriol. 1983 Oct;156(1):30–35. doi: 10.1128/jb.156.1.30-35.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Döhler K., Körner H. Isolation and characterization of transposon Tn5-induced mutants of Pseudomonas perfectomarina defective in nitrous oxide respiration. J Bacteriol. 1985 Sep;163(3):918–924. doi: 10.1128/jb.163.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]





