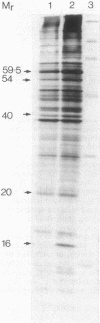
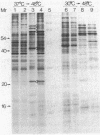
Abstract
Pretreatment of Bacillus subtilis with low concentrations of hydrogen peroxide protected the cells against the lethal effects of higher levels of oxidative stress. During the period of adaptation, eight proteins were induced, as detected by one-dimensional gel electrophoresis. Four of these proteins were the same size as four of the proteins induced by the temperature upshift. The range of proteins synthesized in response to an elevation in temperature depended both on the starting (lower) temperature and on the temperature to which the cells were shifted. Both catalase and superoxide dismutase were present at high levels in B. subtilis, but neither was induced by oxidative stress or temperature upshift. In fact, catalase activity was reduced after the temperature upshift.
Full text
PDF

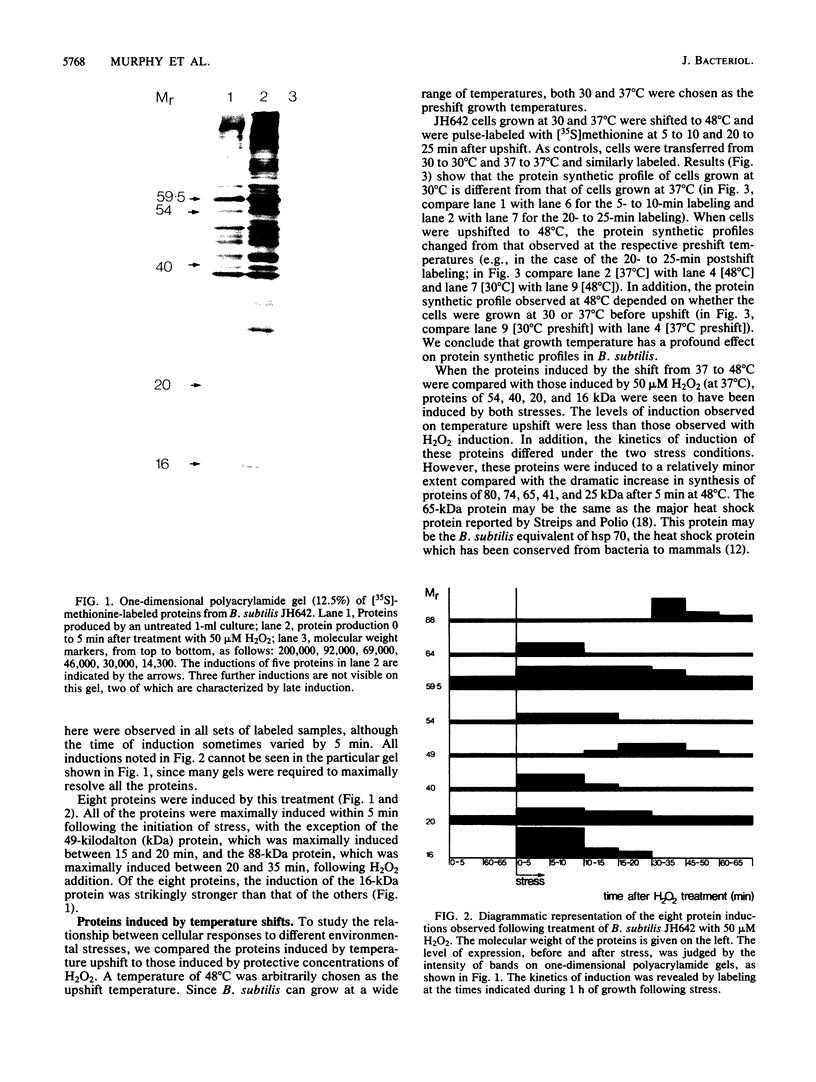


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Briat J. F., Gilman M. Z., Chamberlin M. J. Bacillus subtilis sigma 28 and Escherichia coli sigma 32 (htpR) are minor sigma factors that display an overlapping promoter specificity. J Biol Chem. 1985 Feb 25;260(4):2038–2041. [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Dowds B. C., Murphy P., McConnell D. J., Devine K. M. Relationship among oxidative stress, growth cycle, and sporulation in Bacillus subtilis. J Bacteriol. 1987 Dec;169(12):5771–5775. doi: 10.1128/jb.169.12.5771-5775.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Streips U. N., Polio F. W. Heat shock proteins in bacilli. J Bacteriol. 1985 Apr;162(1):434–437. doi: 10.1128/jb.162.1.434-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Hubbard T. J., Travers A. A., Ellar D. J. Heat-shock proteins during growth and sporulation of Bacillus subtilis. FEBS Lett. 1985 Sep 2;188(2):209–214. doi: 10.1016/0014-5793(85)80373-2. [DOI] [PubMed] [Google Scholar]
- Winquist L., Rannug U., Rannug A., Ramel C. Protection from toxic and mutagenic effects of H2O2 by catalase induction in Salmonella typhimurium. Mutat Res. 1984 Nov-Dec;141(3-4):145–147. doi: 10.1016/0165-7992(84)90087-3. [DOI] [PubMed] [Google Scholar]