Abstract
Traumatic brain injury (TBI) results in both focal and diffuse brain pathologies that are exacerbated by the inflammatory response and progress from hours to days after the initial injury. Using a clinically relevant model of TBI, the parasagittal fluid-percussion brain injury (FPI) model, we found injury-induced impairments in the cyclic AMP (cAMP) signaling pathway. Levels of cAMP were depressed in the ipsilateral parietal cortex and hippocampus, as well as activation of its downstream target, protein kinase A, from 15 min to 48 hr after moderate FPI. To determine if preventing hydrolysis of cAMP by administration of a phosphodiesterase (PDE) IV inhibitor would improve outcome after TBI, we treated animals intraperitoneally with rolipram (0.3 or 3.0 mg/kg) 30 min prior to TBI, and then once per day for three days. Rolipram treatment restored cAMP to sham levels and significantly reduced cortical contusion volume and improved neuronal cell survival in the parietal cortex and CA3 region of the hippocampus. Traumatic axonal injury, characterized by β-amyloid precursor protein deposits in the external capsule, was also significantly reduced in rolipram-treated animals. Furthermore, levels of the pro-inflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), were significantly decreased with rolipram treatment. These results demonstrate that the cAMP-PKA signaling cascade is downregulated after TBI, and that treatment with a PDE IV inhibitor improves histopathological outcome and decreases inflammation after TBI.
Keywords: camp, Fluid-percussion, Inflammation, Interleukin-1β, PKA, Phosphodiesterase, Rolipram, TNF-α, Traumatic brain injury, TBI
Traumatic brain injury (TBI) is a prevalent, debilitating health problem, occurring in 1.4 million people each year and disabling 5 million people in the United States (Langlois et al., 2004). The subsequent progressive injury after brain trauma develops from hours to days after the initiating insult, providing an accessible time window for pharmacological therapies. Despite intense efforts, research in TBI has not yielded a therapy that has passed Phase III clinical trials (Doppenberg et al., 2004).
Brain trauma results in contusion formation, neuronal apoptosis, and axonal tract damage. These pathologies are worsened by the inflammatory cascade set into motion by the initial injury (Morganti-Kossmann et al., 2002, Dietrich et al., 2004). Two pro-inflammatory cytokines released after TBI are tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). Numerous studies have documented rapid increases in TNF-α and IL-1β levels after TBI (Taupin et al., 1993, Shohami et al., 1994, Fan et al., 1996, Kinoshita et al., 2002, Vitarbo et al., 2004).
IL-1β synergistically acts with TNF-α to induce cell death after TBI. These pro-inflammatory cytokines stimulate inflammatory cells to release damaging reactive oxygen and nitrogen species, raise glutamate levels to excitotoxic levels, impair the ability of glia cells to buffer extracellular potassium, compromise the blood-brain barrier, and attract more inflammatory cells into the brain (Tanaka et al., 1994, Meda et al., 1995, Soares et al., 1995, Hu et al., 1997, Keeling et al., 2000). Once initiated, the inflammatory cascade becomes a toxic positive-feedback loop, further exacerbating brain pathology.
In other models of CNS injury, several studies have demonstrated that restoration of cyclic AMP (cAMP) levels improves outcome. In spinal cord injury, application of rolipram to inhibit the degradation of cAMP promotes axon sparing and results in locomotor improvements (Nikulina et al., 2004, Pearse et al., 2004). Similarly, in transient global ischemia rolipram improves neuronal survival in the hippocampus and hippocampal-dependent learning (Kato et al., 1995, Block et al., 1997, Imanishi et al., 1997, Block et al., 2001).
The effects of cAMP are short-lived because phosphodiesterases (PDEs) rapidly degrade cAMP (Manganiello et al., 1995). Of the ten classes of PDEs, two isoforms are highly selective for degrading cAMP, PDE IV and VII. Rolipram, a selective inhibitor of PDE IV, reduces inflammation in a number of diseases including asthma, multiple sclerosis, septic shock, rheumatoid arthritis, and inflammatory bowel disease (Dal Piaz and Giovannoni, 2000, Castro et al., 2005). Consequently, PDE IV inhibitors are widely-utilized by the pharmaceutical industry as anti-inflammatory drugs.
A primary action of cAMP is activation of protein kinase A (PKA). PKA phosphorylates transcription factors, including cAMP-responsive element binding (CREB) protein and nuclear factor-κB (NF-κB) p50 (Montminy and Bilezikjian, 1987, Hou et al., 2003). Phosphorylation of CREB stimulates transcription of cell survival genes (Mayr and Montminy, 2001). Phosphorylation of NF-κB p50 subunit suppresses transcription of genes with IκB elements in their promoters; this includes the pro-inflammatory cytokines TNF-α and IL-1β (Cogswell et al., 1994, Verghese et al., 1995, Hou et al., 2003). Thus, we hypothesized that rolipram treatment may improve TBI outcome by decreasing pro-inflammatory cytokine production.
Materials and methods
Traumatic brain injury
All experimental procedures were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Miami Animal Care and Use Committee. Male Sprague-Dawley rats (270–320 g; Charles River Laboratories, Raleigh, NC, USA) were anesthetized with 3% halothane, 70% N2O, and 30% O2, then intubated endotracheally and mechanically ventilated (Harvard Apparatus, Holliston, MA, USA) with 1.5% halothane, 70% N2O, and 30% O2. To immobilize the animals and facilitate mechanical ventilation, pancuronium bromide (0.5 mg/kg) was intravenously administered through the femoral artery. On the day prior to TBI, animals received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) and a modified plastic 18 gauge syringe hub (8 mm length, PrecisionGlide needle, Becton Dickinson, Franklin Lakes, NJ, USA) was secured over the right parietal cortex. The next day, animals were anesthetized, intubated, and then placed under a fluid-percussion brain injury (FPI) device. A moderate fluid-percussion pulse (2.0±0.2 atmospheres) was delivered to the right parietal cortex. Sham-operated rats received all surgical manipulations, but without the fluid-percussion pulse, and were monitored under anesthesia for 15–30 min after the sham injury. Rectal and temporalis muscle thermistors were used to maintain core and brain temperatures at 36.8–37.3°C using self-adjusting feedback warming lamps. Blood gases (pO2 and pCO2), blood pH, and mean arterial pressure were monitored 15 min before TBI and up to 4 hr after TBI and maintained within normal physiological ranges.
cAMP Assays
Six experimental groups (n=47) were used to measure cAMP levels by ELISA. Animals received either sham surgery (n=11) or moderate parasagittal FPI followed by recovery for 15 min (n=8), 1 hr (n=6), 4 hr (n=7), 24 hr (n=5), or 48 hr (n=10). The right (injured) parietal cortex, hippocampus, and thalamus were rapidly dissected at 4°C and frozen on liquid nitrogen. The tissue was briefly sonicated on ice (10 s, setting 2, Branson sonifier 450, Danbury, CT, USA) in 20 volumes of 0.1 N HCl and 500 μM 3-isobutyl-1-methylxanthine (IBMX). cAMP levels were quantified using a cAMP low pH ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol for the nonacetylated method. Each sample was assayed in duplicate.
Immunohistochemistry
At 5 min, 4 hr, and 24 hr after TBI, animals were perfused with saline (2 min, 4°C), then with 4% paraformaldehyde in phosphate-buffered saline (PBS; 30 min, 4°C). The brains were sectioned in PBS (50 μm thick) using a Leica vibratome (Leica Microsystems, Inc., Exton, PA, USA). Free-floating sections were blocked for 1 hr at RT in blocking buffer (PBS containing 5% normal goat serum, 0.2% fish skin gelatin, 0.3% TX-100). Sections were then incubated overnight at 4°C in blocking buffer with anti-NeuN (1:400, Chemicon, Temecula, CA, USA, MAB377) and anti-cAMP antibodies (1:1000, Chemicon, AB306). After incubation with the primary antibodies, the sections were rinsed with PBS, and incubated 2 hr at RT in blocking buffer with anti-mouse and anti-rabbit secondary antibodies labeled with Alexa 488 and 546 (Invitrogen, Carlsbad, CA, USA), respectively. The sections were then rinsed with PBS, and mounted using ProLong Gold antifade mounting medium (Invitrogen).
Images were obtained with a LSM510 laser scanning confocal microscope (Carl Zeiss, Inc., Thornwood, NY, USA) using 25X 0.8 NA and 63X 1.2 NA water-immersion lenses. At least 3 different sections were prepared from each animal; all animals in each group yielded similar results.
Western blot analysis
To assess for changes in PKA after TBI, six experimental groups (n=39) were used. Animals received either sham surgery (n=8) or moderate TBI followed by recovery for 15 min (n=7), 1 hr (n=7), 4 hr (n=8), 24 hr (n=4), or 48 hr (n=5). At various times after the TBI surgery, the ipsilateral parietal cortex and hippocampus were dissected at 4°C in saline and frozen on liquid nitrogen within 2 min of decapitation. To specifically determine biochemical changes in PKA that occurred at the synaptic membrane, the tissue was fractionated (Hu et al., 1999). The tissue was homogenized with a Dounce homogenizer (35 strokes, 4°C) in 1 ml of Lysis Buffer: 15 mM Tris pH 7.6, 0.25 M sucrose, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1.25 μg/ml pepstatin A, 10 μg/ml leupeptin, 25 μg/ml aprotinin, 0.5 mM PMSF, 0.1 mM Na3VO4, 50 mM NaF, 2 mM Na4P2O7, and 1X phosphatase inhibitor cocktail set II (Calbiochem, San Diego, CA, USA). The samples were centrifuged (800xg, 10 min, 4°C). The supernatants were centrifuged again (10,000xg, 10 min 4°C) to generate a pellet containing synaptic membranes that was resuspended in lysis buffer with 0.1% Triton X-100. The samples were assayed for total protein using the Coomassie Plus assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples were boiled with 1X sample buffer for 7–9 min at 95°C. Equal amounts of protein (30 μg/lane) were electrophoresed (12.5% SDS-PAGE) and western blotted. The crude synaptic membrane fraction was western blotted for phospho-PKA Ser96 regulatory subunit II (RII; 1:1000, Upstate Cell Signaling Solutions, Lake Placid, NY, USA, 06-704), PKA RII (1:1000, Upstate Cell Signaling Solutions, 06-411), and β-tubulin (1:5000; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA, E7). For analysis of changes in phospho-CREB, total homogenates were western blotted and probed with antibodies against phospho-CREB Ser133 (1:1000, Cell Signaling Technology, 9191), anti-total CREB (1:1000, Cell Signaling Technology, 9192), and β-actin (1:5000, Sigma-Aldrich, AC-15). Epitopes were visualized with HRP-conjugated secondary antibodies (1:1000-1:5000; Cell Signaling Technology, Beverly, MA, USA) using the Phototope HRP Western blot detection system (Cell Signaling Technology) and developed on film (Phenix x-ray film BX; Phenix Research Products, Hayward, CA, USA). Films were developed to be in a linear range and densitized using LabWorks software (Ultra-Violet Products, Upland, CA, USA). Levels of phospho-protein immunoreactivity (e.g. phospho-PKA) were normalized to total protein immunoreactivity (e.g. PKA), then to β-tubulin immunoreactivity.
Rolipram administration
Rolipram (Sigma-Aldrich, St. Louis, MO, USA), was dissolved in 100% DMSO at 10 mg/ml, and then diluted with 0.9% NaCl for a final concentration of either 0.5 mg/ml or 0.05 mg/ml in 5% DMSO and 95% saline. The drug was administered intraperitoneally (i.p.) 30 min prior to TBI at 6 ml/kg. For each group, rolipram or vehicle (5% DMSO/95% saline) was administered once every 24 hr and on the final day of the experiment, 30 min prior to sacrifice.
Histopathological analysis
TBI- and sham-operated animals were anesthetized (3% halothane for 5 min) and perfused transcardially with isotonic saline for 2 min (75 mL) and then 30 min of 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4 (350 mL). The brains were embedded in paraffin and sectioned (10 μm thick). The sections were stained with hematoxylin and eosin (H&E) and alternative sections were immunostained with NeuN and β-APP. Cortical contusion volumes were determined by tracing the contused areas in H&E sections (150 μm apart) with a 20X objective on an Axiovert 200M microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) using the Neurolucida 7.50.1 software program (MicroBrightField Inc., Williston, VT, USA). The cortical contusion boundaries were well demarcated by pyknotic neurons, reactive astrocytes, hemorrhage, edema, and a shearing at the gray/white matter interface between the cortex and external capsule. Contusion areas were calculated for 5 coronal levels at and around the epicenter (-3.3, -4.3, -5.8, -6.8, and -7.3 mm posterior from bregma). To determine neuron survival and axonal tract pathology, serial sections (150 μm apart) from -3.3 to -5.8 mm posterior to bregma were incubated with NeuN antibody (1:500, Chemicon, MAB377) or β-APP antibody (1:500, Chemicon, MAB348), respectively. Immunostaining was developed with anti-mouse IgG (1:1000), ABC Elite (Vector Laboratories, Burlingame, CA, USA), and NiDAB (2.5% Nickle Ammonium Sulfate Acetate-Imidasole Buffer, 0.05% DAB, 0.001% H2O2, Vector Laboratories). NeuN-positive neurons were quantified in an unbiased, systematic manner using stereology with an Axiovert 200M microscope (Carl Zeiss MicroImaging, Inc.) by a blind observer (Suzuki et al., 2003, Suzuki et al., 2004). The parietal cortex overlying the contusion area and the CA3 region of the hippocampus were contoured at 20X, then a counting grid of 250×200 μm was placed in the parietal cortex or a grid of 140×70 μm was placed in the CA3 region. Using a 35×35 μm counting frame, NeuN-positive cells were counted in 25–40 randomly-placed sampling sites with Stereoinvestigator 7.50.1 software (MicroBrightField, Inc.) with a 63X, 1.4 NA objective. NeuN counts were measured from bregma levels -3.3 mm to -5.8 mm in sections spaced 150 μm apart. For cortical cell counts, Q values ranged from 446–715 and CE2/CV2 values were 0.04, 0.06, and 0.11 for the vehicle, 3.0 mg/kg rolipram and 0.3 mg/kg rolipram groups, respectively. For CA3 hippocampal cell counts, the Q range was 325–550 and CE2/CV2 values were 0.39, 0.11, and 0.11 for the vehicle, 3.0 mg/kg rolipram and 0.3 mg/kg rolipram groups, respectively. To quantify axonal pathology, the external capsule was traced at 20X magnification for 3 coronal levels, -3.3, -4.3, and -5.8 mm posterior to bregma. A counting grid of 120×300 μm (-3.3 mm bregma), 120×330 μm (-4.3 mm bregma), and 200×290 μm (-5.8 mm bregma) was placed over each tracing (Bramlett et al., 1997, Suzuki et al., 2004). Using a 35×35 μm counting frame, β-APP deposits in 40–50 randomly-placed sampling sites were counted in the external capsule at 63X magnification (NA 1.4) using Stereoinvestigator 7.50.1 software (MicroBrightField, Inc.). The Q values for β-APP counts ranged from 135–223 and the CE2/CV2 values were 0.10, 0.07, and 0.08 for the vehicle, 3.0 mg/kg rolipram and 0.3 mg/kg rolipram groups, respectively. The NeuN and β-APP counts at each bregma level were determined by averaging three consecutive sections at each specific bregma level.
Images were taken with a 40X objective on an Axiovert 200M microscope (Carl Zeiss MicroImaging, Inc.) and montaged using the virtual slice module in the Neurolucida 7.50.1 software program (MicroBrightField, Inc.).
IL-1β and TNF-α ELISAs
Three experimental groups (n=22) were used to assess IL-1β and TNF-α levels by ELISA. Animals received either sham surgery (n=5) or moderate parasagittal FPI and treatment with vehicle (n=8) or 0.3 mg/kg rolipram (n=9) 30 min prior to FPI and 30 min prior to sacrifice. At 3 hr after FPI, the animals were sacrificed and the ipsilateral parietal cortex, hippocampus and thalamus were rapidly dissected on ice in saline. The tissue was briefly sonicated on ice (10 s, setting 2, Branson sonifier 450, Danbury, CT, USA) in 10 volumes/weight of Lysis Buffer supplemented with 0.1% Igepal CA-630 (Sigma-Aldrich). Total protein was measured using the Coomassie Plus assay kit (Bio-Rad Laboratories). Each sample was assayed in duplicate according to the manufacturer’s protocol (R&D Systems, Inc.).
Statistical analysis
Data presented are mean±SEM. Statistical analyses are Student’s t test or one-way ANOVAs with post-hoc Tukey HSD t tests.
Results
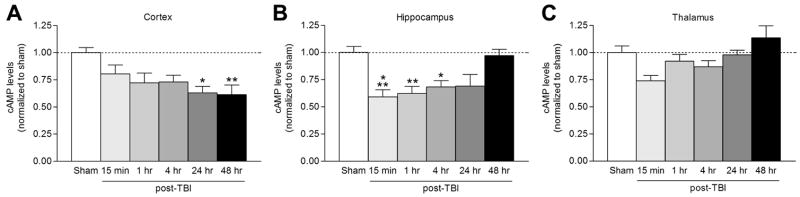
To ascertain if the cAMP-PKA pathway is a potential therapeutic target after TBI, we first determined if the cAMP-PKA pathway is modulated after TBI. At various times after sham or FPI surgery, the ipsilateral parietal cortex, hippocampus, and thalamus were assayed by ELISA for cAMP. Absolute levels of cAMP from cortices of sham animals were similar to levels previously reported in the literature (parietal cortex cAMP levels 184.1±5.6 pmol/mL, n=6) (Pearse et al., 2004). We found that cAMP levels decreased by 15 min after TBI in the ipsilateral hippocampus, and were depressed at 24 to 48 hr in the ipsilateral parietal cortex (Fig. 1). There were no changes in cAMP levels in the thalamus. To determine the cell types that were expressing cAMP, we performed immunohistochemistry of cAMP from animals after TBI. At 5 min after TBI, cAMP was predominantly localized in neurons, as identified by co-immunostaining with NeuN (Fig. 2). Similar results were obtained in animals at 4 and 24 hr after TBI.
Fig 1.
cAMP levels decrease after TBI. The ipsilateral parietal cortex (A; n=4–10), hippocampus (B; n=4–11), and thalamus (C; n=5–10) were assayed by ELISA for cAMP levels after moderate parasagittal FPI. cAMP levels were significantly decreased in the cortex at 24 hr (n=4, *p<0.05) and 48 hr (n=6, **p<0.01) after TBI, and at 15 min (n=6, ***p<0.001), 1 hr (n=6, **p<0.01), and 4 hr (n=5, *p<0.05) after TBI in the hippocampus as compared to sham levels. Data represent mean ± SEM.
Fig 2.
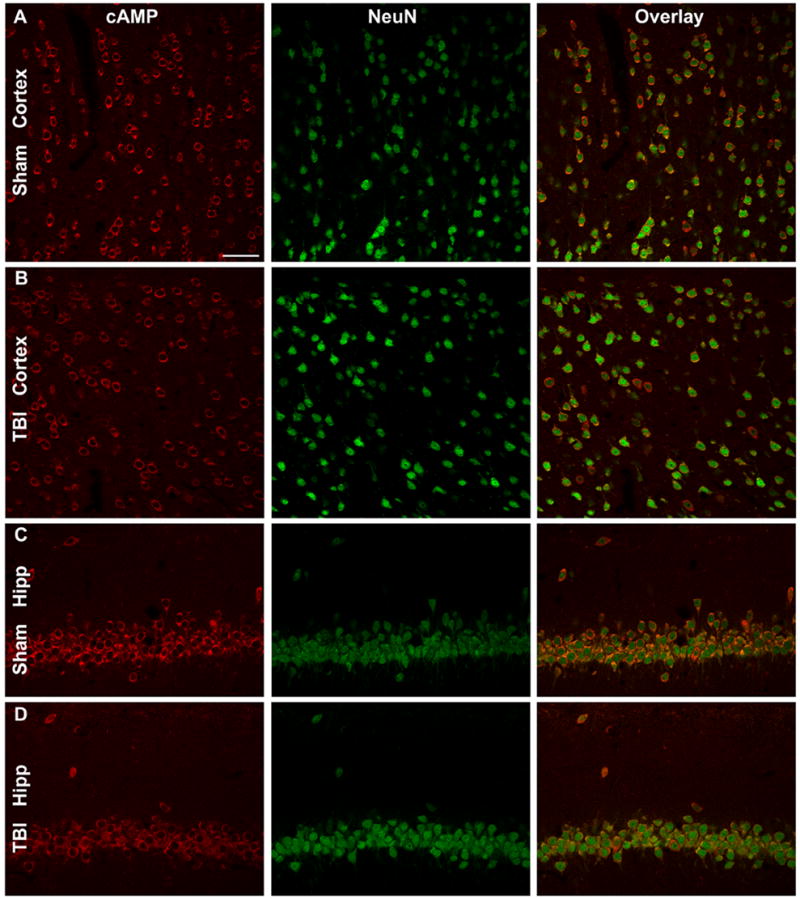
cAMP immunostaining after TBI. The ipsilateral parietal cortex of sham surgery animals (A) and TBI animals (B) were immunostained with cAMP (red) and NeuN (green). Images were from animals perfused 5 min after surgery. There was co-localization of cAMP with NeuN. The CA1 region of the hippocampus of sham surgery animals (C) and TBI animals 5 min after trauma (D) were immunostained for cAMP and NeuN. In TBI animals, cAMP levels were modestly reduced in NeuN-positive cells. Images are representative of 3 animals in each group. Scale bar, 50 μm.
cAMP primarily exerts its actions through PKA. When PKA is activated, the catalytic subunit autophosphorylates the regulatory subunit and this may facilitate dissociation and activation of the catalytic subunits (Keryer et al., 1998). To determine whether PKA activation is modulated after TBI, we performed western blot analysis with antibodies to phosphorylated, activated PKA regulatory subunit II. PKA autophosphorylation was downregulated in crude membrane fractions from the ipsilateral parietal cortex and hippocampus within 15 min after TBI, and this downregulation lasted for at least 48 hr (Fig. 3). Thus, the FPI model depresses both cAMP levels and PKA activation.
Fig 3.
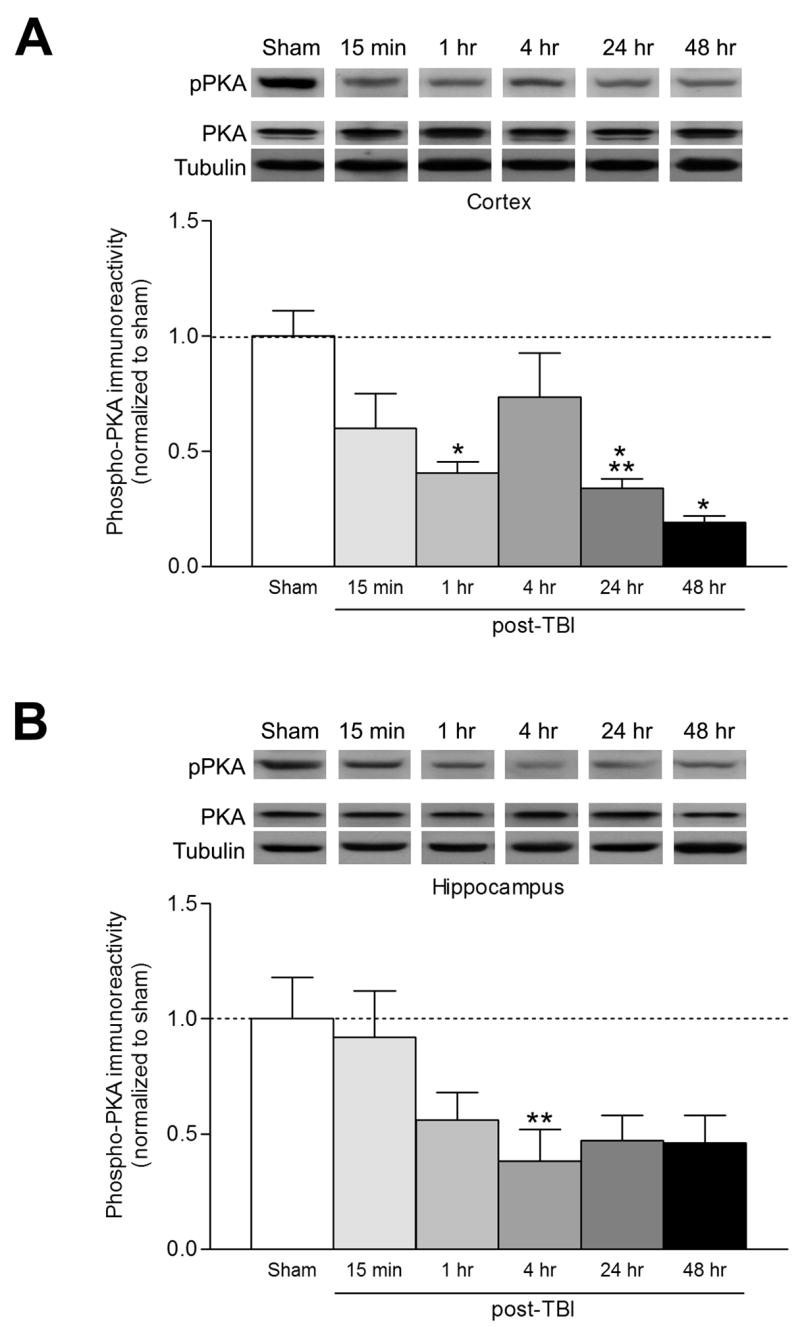
PKA activation is downregulated after TBI. The ipsilateral parietal cortex (A; n=3–8) and hippocampus (B; n=3–6) were western blotted at the indicated times after TBI for activated, phosphorylated PKA (pPKA). PKA activation was decreased significantly in the cortex at 1 hr (n=5, *p<0.05), 24 hr (n=3, ***p<0.001), and 48 hr (n=5, *p<0.05) after TBI as compared to sham animals. In the hippocampus, phosphorylated PKA levels were significantly decreased at 4 hr (n=7, **p<0.01) after TBI as compared to sham animals. Data represent mean ± SEM.
To determine whether inhibition of PDE IV would improve signaling through the cAMP-PKA pathway after TBI, we treated animals with vehicle (5% DMSO in saline, i.p.) or rolipram (0.3 mg/kg, i.p.), a selective PDE IV inhibitor, 30 min prior to moderate FPI, and 30 min prior to sacrifice at 24 hr after FPI, when cAMP levels are significantly depressed in the cortex and hippocampus. We found that rolipram restored cAMP levels in TBI animals to sham levels. Total CREB levels increased in the cortex and phosphorylated CREB levels increased in the hippocampus of TBI animals treated with rolipram as compared to TBI animals treated with vehicle (Fig. 4).
Fig. 4.
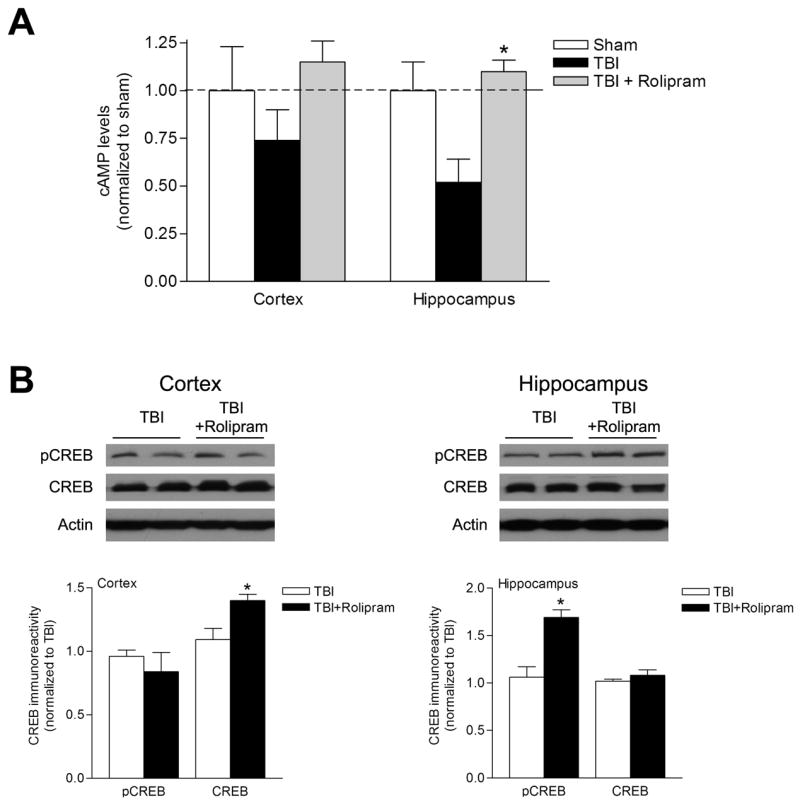
Rolipram treatment increased cAMP levels and phosphorylation of CREB. Animals were treated with vehicle (5% DMSO in saline) or rolipram (0.3 mg/kg, i.p.) 30 min prior to moderate parasagittal FPI. At 24 hr after injury, the animals were treated once more with vehicle or rolipram (0.3 mg/kg, i.p.), then sacrificed 30 min later. (A) cAMP levels were measured by ELISA in the ipsilateral parietal cortex and hippocampus. cAMP levels were increased to sham levels in the parietal cortex although this was not statistically significant (n=3 for each group). In the hippocampus, cAMP levels were significantly increased in TBI animals that received rolipram as compared to TBI animals that received vehicle (n=3 for each group, *p<0.05). (B) Phosphorylated CREB and total CREB levels were assayed by western blotting in the ipsilateral parietal cortex and hippocampus 24 hr after injury. Total CREB levels significantly increased in the parietal cortex in rolipram-treated animals as compared to vehicle-treated animals (n=3 for each group, *p<0.05). Phosphorylated CREB levels significantly increased in the hippocampus in rolipram-treated animals as compared to vehicle-treated animals (n=3 for each group, *p<0.05). Data represent mean ± SEM.
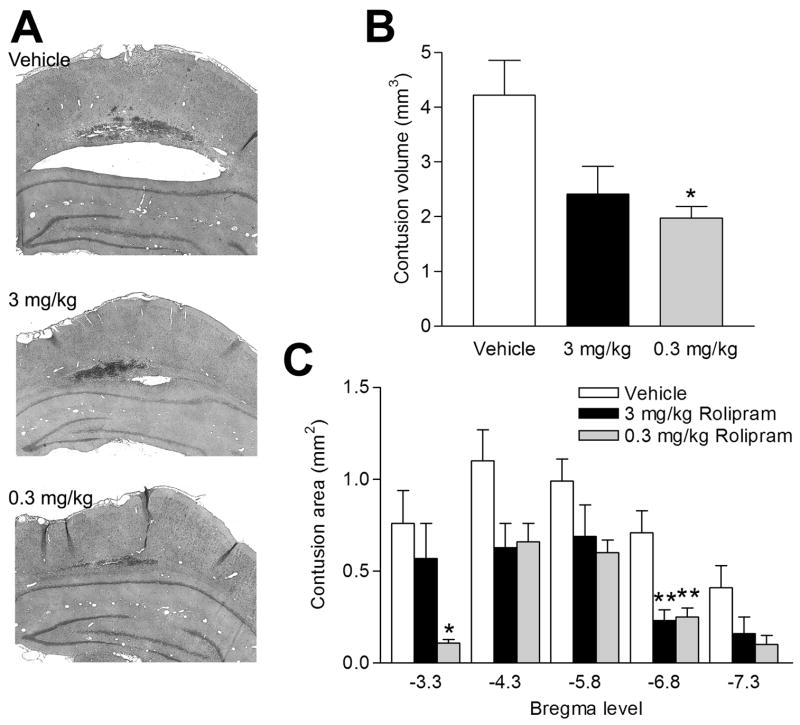
Next, to determine if rolipram would improve histopathology, we treated animals with vehicle or rolipram (0.3 mg/kg or 3 mg/kg) 30 min prior to moderate FPI, and then once per day for 3 days. We chose to pre-treat animals with rolipram to target the acute, rapid inflammatory signaling events initiated by trauma (Kinoshita et al., 2002, Vitarbo et al., 2004). Animals were assessed for histopathology at 3 days after TBI because there are reproducible, quantifiable histopathologies at this time point (Dietrich et al., 1994, Bramlett et al., 1997, Suzuki et al., 2003, Suzuki et al., 2004). Three days after moderate FPI and either vehicle or rolipram treatment, animals were perfused and the brains were stained with hematoxylin and eosin (H&E), a general nuclei and cytoplasmic stain to visualize the cortical contusions. We observed a significant decrease in cortical contusion size with 0.3 mg/kg rolipram treatment in comparison to vehicle treatment when quantified using unbiased stereology measurements (Fig. 5; contusion volume: vehicle 4.22±0.63 mm3, n=9; 3.0 mg/kg rolipram 2.41±0.51 mm3, n=8; 0.3 mg/kg rolipram 1.98±0.22 mm3, n=6, p<0.05). A comparison of contusion volume at the epicenter of injury (-3.8 mm bregma) and the surrounding bregma levels illustrated that 0.3 mg/kg rolipram treatment reduced cortical contusion areas significantly at bregma levels -3.3 and -6.8 mm.
Fig 5.
Rolipram treatment decreased cortical contusions. Rats received vehicle (5% DMSO in saline) or rolipram i.p. 30 min prior to moderate parasagittal FPI. After TBI, the animals received vehicle or rolipram for 3 days and were then perfused for analysis at 30 min after their final injection. The brains were sectioned and stained with H&E, and the cortical contusion area was imaged. Representative sections at bregma level -5.8 mm are shown (A). Cortical contusion volume (B) and contusion areas at specific bregma levels (C) were quantified by stereology. The epicenter of the injury was at -3.8 mm bregma. The lower dose of rolipram, 0.3 mg/kg, significantly reduced total cortical contusion volume (n=6, *p<0.05) as compared to vehicle-treated animals and contusion area near the epicenter of the injury (bregma -3.3 mm, *p<0.05) as well as in the penumbra (bregma -6.8 mm, **p<0.01) as compared to vehicle-treated animals (n=9). Although the higher dose of 3.0 mg/kg rolipram reduced contusion volume as compared to vehicle-treated animals, this was not statistically significant (n=8). Data represent mean ± SEM.
The parasagittal FPI model results in stereotypical neuronal death in the parietal cortex overlying the cortical contusion and in the CA3 region of the hippocampus (Grady et al., 2003, Witgen et al., 2005). Treatment of rolipram during TBI and for 3 days following moderate FPI improved neuronal survival in both the parietal cortex (Fig. 6) and the CA3 region of the hippocampus (Fig. 7) when assessed by counting cells positive for NeuN, a marker for neurons.
Fig 6.
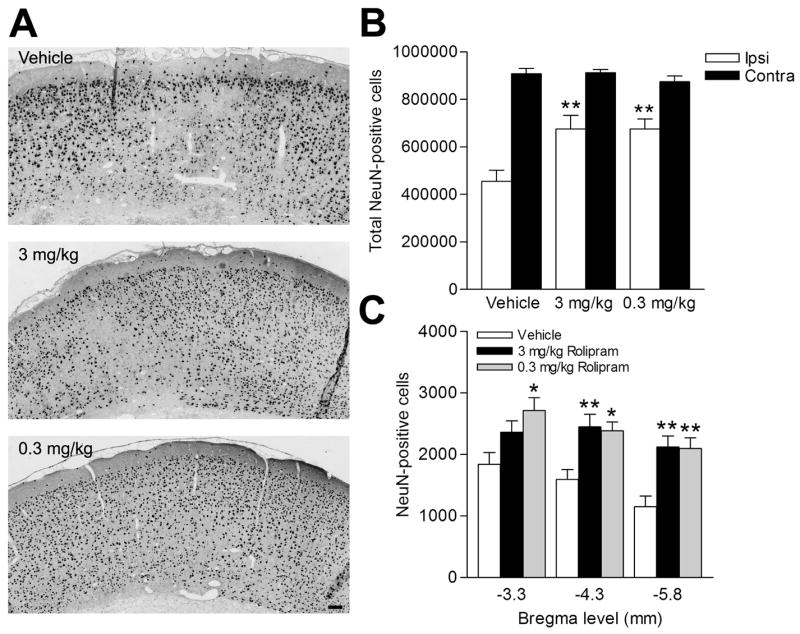
Cortical neuron survival was improved with rolipram treatment. The parietal cortex overlying the contusion area between bregma levels -3.3 mm and -6.8 mm was immunostained with NeuN to identify surviving neurons. Animals were treated with vehicle, 0.3 mg/kg rolipram, or 3.0 mg/kg rolipram i.p. 30 min prior to injury, followed by once per day for 3 days. (A) Shown are representative images at bregma level -5.8 mm. (B) Total cortical neuron survival on the ipsilateral side (Ipsi) was significantly improved with both 0.3 mg/kg rolipram (n=5, **p<0.01) and 3.0 mg/kg rolipram (n=8, **p<0.01) as compared to vehicle-treated animals (n=9). There were no significant differences in total numbers of neurons on the contralateral side with rolipram treatment (Contra). (C) Quantification of neuronal survival at specific bregma levels illustrates that 3.0 mg/kg rolipram increased neuronal survival at bregma levels -4.3 mm and -5.8 mm (n=8, **p<0.01) when compared to vehicle-treated animals (n=9). Rolipram at 0.3 mg/kg improved cortical neuron survival at all bregma levels tested (n=6; -3.3 mm, *p<0.05; -4.3 mm, *p<0.05; -5.8 mm, **p<0.01). Data represent mean ± SEM. Scale bar, 50 μm.
Fig 7.
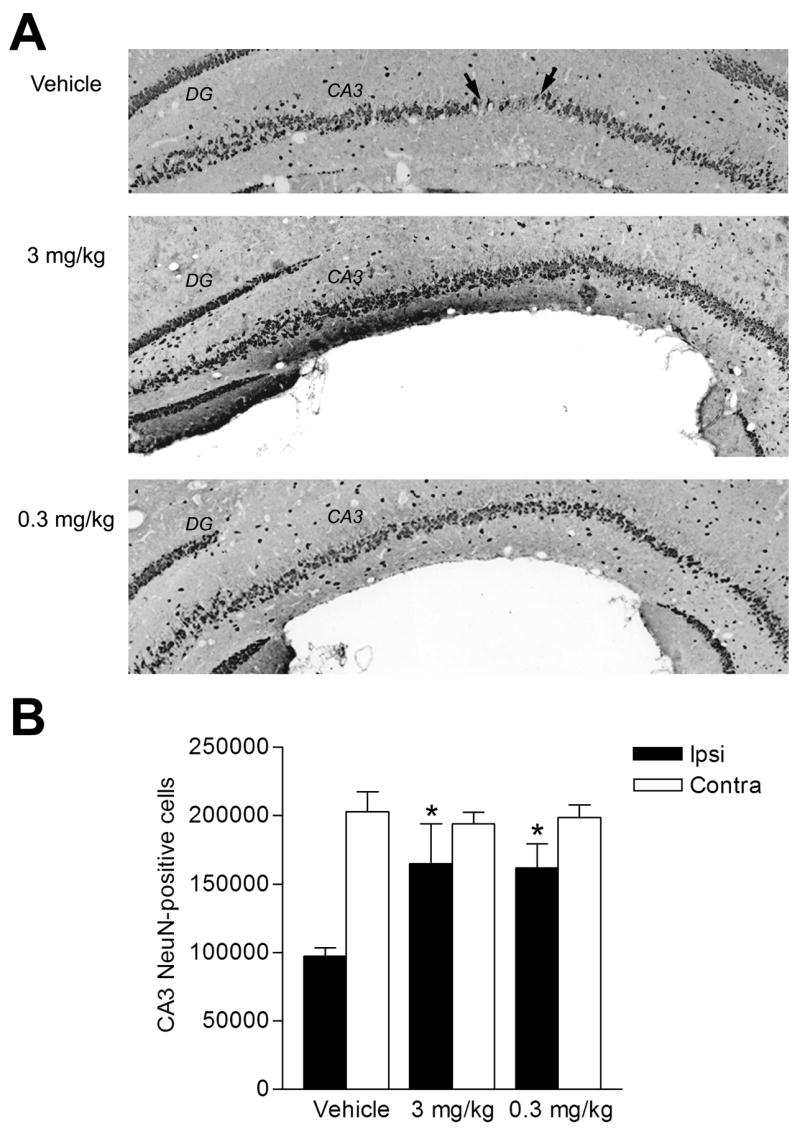
Survival of CA3 hippocampal neurons with rolipram. (A) Animals received rolipram or vehicle 30 min prior to TBI, then once per day for 3 days. Sections were immunostained with NeuN. Bregma level -5.8 mm is shown. Arrows denote boundaries of an area of neuronal dropout. (B) Significant survival of CA3 neurons on the ipsilateral side (Ipsi) was seen with 0.3 mg/kg rolipram (n=4, *p<0.05) and 3.0 mg/kg rolipram (n=8, *p<0.05) as compared to vehicle-treated animals (n=9). There were no significant differences in neuronal survival on the contralateral side (Contra). Data represent mean ± SEM.
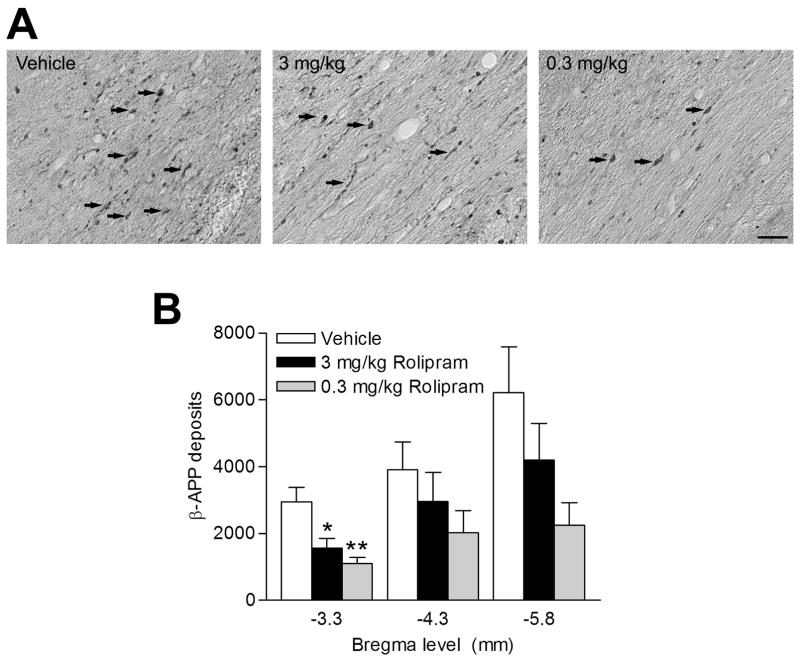
Another histopathological characteristic of TBI is traumatic axonal injury, which is exemplified by β-amyloid precursor protein (β-APP) deposits in white matter tracts (Bramlett et al., 1997, Ciallella et al., 2002, Suzuki et al., 2004). Traumatic axonal damage was assayed by quantifying the number of β-APP deposits in the external capsule the white matter tract between the hippocampus and parietal cortex. β-APP deposits were significantly reduced in animals treated with either 0.3 or 3.0 mg/kg rolipram at bregma level -3.3 mm, near the injury center (Fig. 8).
Fig 8.
The external capsule was stained with β-APP to assess axonal pathology. Animals were treated with vehicle, 0.3 mg/kg rolipram, or 3.0 mg/kg rolipram i.p. 30 min prior to injury and once per day for 3 days. (A) Shown is bregma level -5.8 mm. Arrows demarcate β-APP deposits in the external capsule. (B) Both 0.3 mg/kg (n=6, **p<0.01) and 3.0 mg/kg rolipram (n=8, *p<0.05) modestly reduced β-APP deposits at bregma level -3.3 mm as compared to vehicle-treated animals (n=8). Data represent mean ± SEM. Scale bar, 25 μm.
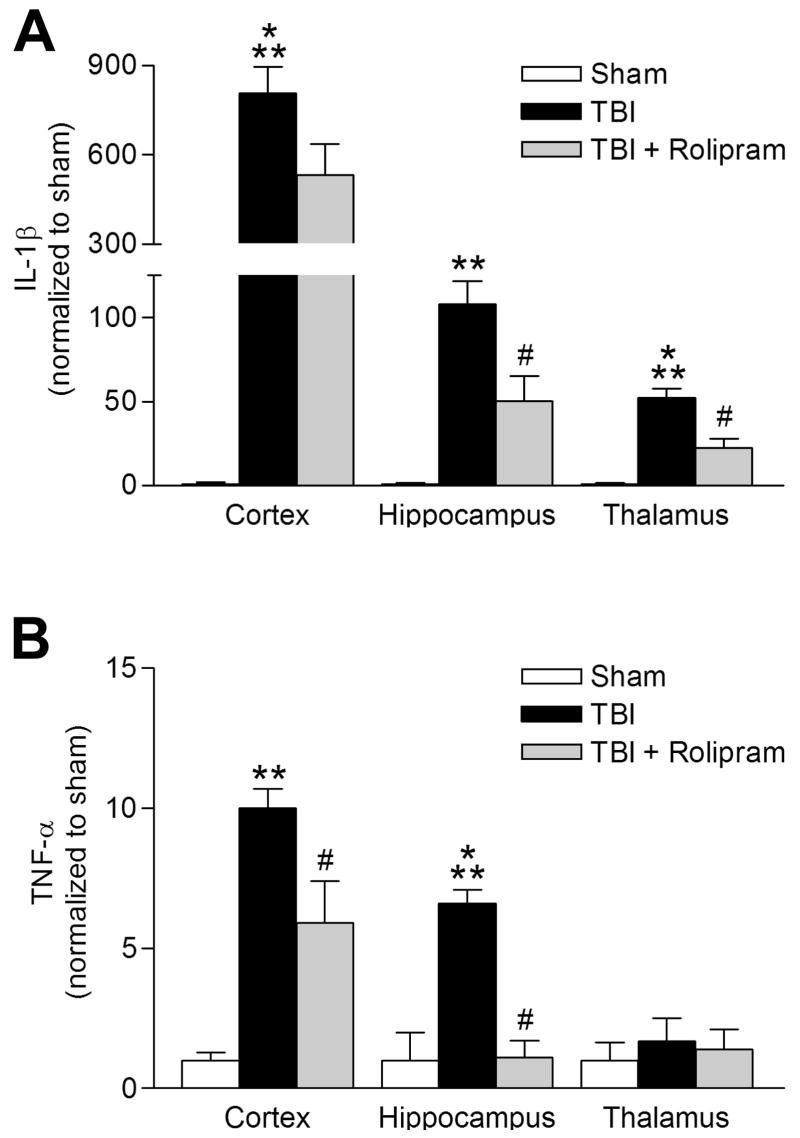
In other injury models, rolipram is well known to decrease the expression and release of the pro-inflammatory cytokines IL-1β and TNF-α (Prabhakar et al., 1994, Verghese et al., 1995, Griswold et al., 1998). To determine if rolipram treatment after TBI reduced the levels of IL-1β and TNF-α, animals were treated with vehicle or rolipram 30 min prior to moderate FPI, then treated with rolipram 30 min prior to sacrifice. This treatment regime was designed to be similar to the previous experiments assessing histopathological outcome in which the animals received a final rolipram injection 30 min prior to sacrifice. The animals were assayed 3 hr after FPI for IL-1β and TNF-α; a time point when these cytokines are significantly elevated after brain injury (Kinoshita et al., 2002, Vitarbo et al., 2004). Injury-induced increases in IL-1β levels were significantly reduced with rolipram treatment in the hippocampus and thalamus (Fig. 9). The increase in TNF-α after TBI was also significantly reduced in the cortex and hippocampus with rolipram treatment. These results demonstrate that rolipram treatment during TBI reduces the inflammatory response in the brain.
Fig 9.
Pro-inflammatory cytokine levels are attenuated with rolipram treatment. (A) IL-1β levels were assayed by ELISA at 3 hr after TBI. There was a significant increase in IL-1β levels in the ipsilateral parietal cortex (n=6, ***p<0.001), hippocampus (n=6, **p<0.01), and thalamus (n=8, ***p<0.001) as compared to sham animals (n=3–5). Rolipram treatment (0.3 mg/kg) 30 min prior to TBI and 30 min prior to sacrifice significantly reduced IL-1β levels in the hippocampus (n=7, #p<0.05) and thalamus (n=9, #p<0.001), but not in the parietal cortex (n=7), as compared to vehicle-treated TBI animals. (B) TNF-α levels significantly increased in the parietal cortex (n=6, **p<0.01) and hippocampus (n=6, ***p<0.001) at 3 hr after TBI as compared to sham animals. This increase in TNF-α was significantly reduced in rolipram-treated TBI animals (parietal cortex n=7, #p<0.05; hippocampus n=6, #p<0.001) as compared to vehicle-treated TBI animals. Data represent mean ± SEM.
Discussion
The parasagittal FPI model leads to reproducible histopathology in the brain, similar to the pathology typically seen in TBI patients (Dietrich et al., 1994, Gennarelli, 1994, Keane et al., 2001, Thompson et al., 2005). Accordingly, there are consistent, quantifiable focal and diffuse histopathologies that are all potential therapeutic targets (Dietrich et al., 1994, Bramlett et al., 1997, Ciallella et al., 2002, Grady et al., 2003, Suzuki et al., 2003, Suzuki et al., 2004, Witgen et al., 2005). In our studies, we found that rolipram, a selective PDE IV antagonist, improved histopathology at multiple levels. Rolipram treatment after TBI decreased cortical contusion size, neuronal death in the parietal cortex and CA3 region of the hippocampus, and β-APP deposits in the external capsule, the axonal tract between the hippocampus and parietal cortex. Of the two doses tested (0.3 and 3 mg/kg), we found that the lower dose trended towards more significance in reducing cortical contusion volume and β-APP deposits. These results are in accordance with previous results using rolipram as a therapeutic agent in spinal cord injury and transient global ischemia where lower doses were also more effective (Block et al., 1997, Nikulina et al., 2004). Rolipram has also been found to improve outcome in experimental allergic encephalomyelitis, Alzheimer disease, multiple sclerosis, ischemia, and striatal excitotoxicity (Genain et al., 1995, Navikas et al., 1998, Folcik et al., 1999, Gong et al., 2004, Demarch et al., 2007, Sasaki et al., 2007). Our results and the many studies assessing rolipram in models of neurological disorders suggest that use of a PDE IV antagonist may be a promising avenue of research as we search for a successful pharmacological therapy for TBI patients. However, the current studies utilized a pre-treatment paradigm to determine if rolipram would target relevant histopathology responses to brain trauma. These studies were proof of concept only to target the acute inflammatory response that occurs rapidly after TBI (Kinoshita et al., 2002, Vitarbo et al., 2004). It is important to determine the therapeutic time window of rolipram treatment after TBI to develop rolipram as a potential therapeutic intervention for TBI patients. Current studies are underway to determine if rolipram treatment attenuates histopathology and reduces inflammation when given after the TBI.
Initially, we had predicted that cAMP levels and PKA activation would rapidly and transiently increase after TBI. Type I and VIII adenylyl cyclases are activated by calcium, and there is a large influx of calcium into cells after TBI (Fineman et al., 1993, Cali et al., 1994, Matsushita et al., 2000, Osteen et al., 2004). Furthermore, in models of epilepsy and stroke, cAMP levels increase; thus it was surprising that we observed only a decrease in cAMP levels after brain injury (Ferrendelli et al., 1980, Prado et al., 1992). However, these results are in accordance with previous studies of TBI and spinal cord injury. In the FPI model of brain injury, cAMP levels have been found to decrease in the cortex, although in controlled cortical impact, one study has reported no change in cAMP levels after trauma (Dhillon et al., 1995, Armstead, 1997, Bell et al., 1998). In spinal cord injury, cAMP levels are chronically depressed from 1 day to 2 weeks (Pearse et al., 2004). Thus, unlike several other protein kinase cascades that are activated after TBI, the cAMP-PKA pathway is unique in that this signaling pathway is depressed after TBI (Hu et al., 2004, Atkins et al., 2006, Chen et al., 2006).
The decrease in cAMP levels after TBI could be due to either increased PDE activity or decreased adenylyl cyclase activity. In culture, PDE IV expression is upregulated when microglia, the endogenous inflammatory cells in the brain, are exposed to stimuli that induce their activation analogous to injury, such as lipopolysaccharide and the cytokine TNF-α (Jin and Conti, 2002, Sasaki and Manabe, 2004). Alternatively, one report has found that the pro-inflammatory cytokine TNF-α downregulates adenylyl cyclase activity in microglia (Patrizio, 2004). Further experiments are needed to determine whether the expression of PDE IV is upregulated after TBI and/or whether adenylyl cyclase activity is downregulated after TBI.
PDE IV selectively degrades cAMP (Km 4 μM) as compared to cGMP (Km >3000 μM), and is inhibited by the highly specific inhibitor, rolipram (Nemoz et al., 1985, Muller et al., 1996, Torphy, 1998). In the uninjured CNS, rolipram increases cAMP levels in the hippocampus (Barad et al., 1998, Van Staveren et al., 2001, Giorgi et al., 2004), and specifically in microglia and astrocytes as compared to neurons (Zhang et al., 2002). There are also very modest increases in cGMP levels with high concentrations of rolipram, suggesting that rolipram could work through cGMP although this is more unlikely (Van Staveren et al., 2001).
Activation of the classical cAMP-PKA signaling pathway by rolipram is a likely, though not only, mechanism that rolipram may have improved outcome after TBI. Rolipram can work through four mechanisms on PDE IV. First, rolipram inhibits cAMP hydrolysis by binding the cAMP catalytic site, the low affinity rolipram binding site. Secondly, rolipram also binds another region near the PDE IV catalytic site, the high affinity rolipram binding site, where it does not affect cAMP hydrolysis. The high affinity rolipram binding site is thought to elicit effects on PDE IV that are cAMP-independent and involve the MAPK signaling cascade (Souness and Rao, 1997, Martin et al., 2002, Zhao et al., 2003). Third, rolipram may inhibit PDE IV hydrolysis of cAMP and increase cAMP levels, but produce anti-inflammatory effects that occur independently of PKA, through Epac1, a cAMP-responsive guanine nucleotide exchange factor which activates the Ras family GTPases, or fourth, via the receptor for activated C kinase 1 and subsequent protein kinase C activation (De Rooij et al., 1998, Houslay and Adams, 2003). Further experiments are needed to determine the exact mechanism of how rolipram improved histopathology and reduced pro-inflammatory cytokine production after TBI.
Increasing activation of the cAMP-PKA pathway may improve the histopathology induced by TBI through a number of signaling pathways. Classically in neurons, PKA phosphorylates the transcription factor CREB to increase expression of cell survival genes such as BDNF and the anti-apoptotic protein bcl-2 (Freeland et al., 2001, Tabuchi et al., 2002, Deogracias et al., 2004, Meller et al., 2005). Previous studies have reported that CREB is activated after TBI and BDNF levels are elevated as well (Dash et al., 1995, Yang et al., 1996, Hicks et al., 1997, Truettner et al., 1999, Griesbach et al., 2004a, Griesbach et al., 2004b, Hu et al., 2004). Multiple protein kinases can phosphorylate CREB; a few of these include calcium/calmodulin-dependent protein kinase IV, ribosomal protein S6 kinase, mitogen- and stress-activated protein kinase, and MAP kinase-activated protein kinase-2 (Sun et al., 1994, Tan et al., 1996, Xing et al., 1996, Deak et al., 1998, Shaywitz and Greenberg, 1999, Bito and Takemoto-Kimura, 2003). Given the decreased levels of cAMP and PKA activation after TBI, it is likely that one of these other protein kinases phosphorylates CREB at 24 hr after TBI. Rolipram treatment increased CREB phosphorylation in the hippocampus and total CREB levels in the parietal cortex. The increase in total CREB levels may be a reflection of the increased neuronal survival with rolipram treatment. Together, these results suggest that the mechanism of rolipram’s action may be through CREB. This is supported in other injury models as well where rolipram significantly increased CREB phosphorylation (Nagakura et al., 2002, Hosoi et al., 2003, Lee et al., 2004, Demarch et al., 2007).
Another signaling pathway regulated by cAMP-PKA is through the transcription factor NF-κB p50 to reduce expression of pro-inflammatory cytokines such as IL-1β and TNF-α (Montminy and Bilezikjian, 1987, Hou et al., 2003). Transcription of the tnf-α gene is suppressed by the NF-κB p50 subunit which constitutively binds the IκB element in the promoter region (Kuprash et al., 1995, Jimenez et al., 2001, Takahashi et al., 2002, Foey et al., 2003). PKA phosphorylation of Ser337 on the NF-κB p50 subunit increases its binding and repression of transcription of IκB-containing gene promoter of the tnf-α gene (Ollivier et al., 1996, Baer et al., 1998, Hou et al., 2003). How the cAMP pathway regulates IL-1β expression is an active area of investigation. Several studies have shown that raising cAMP levels with either neurotransmitters or phosphodiesterase inhibitors reduce IL-1β levels, but the exact mechanism is still unclear (Cogswell et al., 1994, Verghese et al., 1995, Si et al., 1998, Caggiano and Kraig, 1999, Cho et al., 2001, Dello Russo et al., 2004).
Reducing pro-inflammatory cytokine levels after TBI to improve outcome has met with varying success. Administration of an inhibitor of IL-1β receptors, IL-1 receptor antagonist (IL-1ra), reduces contusion volume and transgenic mice overexpressing IL-1ra have improved behavioral recovery after TBI (Sanderson et al., 1999, Tehranian et al., 2002). Similarly, knockout mice of tnfα have improved behavior recovery one week after TBI, but worsened histopathology and behavioral outcome 2–4 weeks after injury (Scherbel et al., 1999). These studies indicate that inflammation is a complex, evolving series of biochemical events that can be both detrimental and beneficial for functional outcome after injury. Thus, targeting the inflammatory cascade as a therapeutic intervention requires careful consideration of the optimal time window, dosage, and mechanism of action.
Although these studies demonstrate an improvement in histopathology after TBI, in consideration of the many failed clinical trials of other neuroprotective agents for the treatment of TBI, these preliminary studies are only proof of concept for the FPI model. It is important to extend these observations to a post-injury treatment paradigm and determine the therapeutic window for rolipram treatment after TBI. Furthermore, whether these improvements in histopathology are accompanied by an improvement in behavioral deficits remains to be determined. Another important consideration is to understand the consequences of decreased cAMP levels after TBI: what cell types exhibit decreases in cAMP-PKA signaling and whether this can be rescued with rolipram treatment. And finally, understanding the mechanism of how rolipram leads to an improvement in functional outcome, possibly by increasing CREB-regulated gene expression and decreasing the inflammatory response, is necessary to develop PDE IV inhibition into a potential therapy.
Acknowledgments
This work was supported by NIH grants NS30291 and NS42133 (W.D.D.). We thank the Dietrich and Pearse labs for helpful discussions and Beata Frydel and Jarret Weinrich for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstead WM. Role of impaired cAMP and calcium-sensitive K+ channel function in altered cerebral hemodynamics following brain injury. Brain Res. 1997;768:177–184. doi: 10.1016/s0006-8993(97)00641-0. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu BR. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:1507–1518. doi: 10.1038/sj.jcbfm.9600301. [DOI] [PubMed] [Google Scholar]
- Baer M, Dillner A, Schwartz RC, Sedon C, Nedospasov S, Johnson PF. Tumor necrosis factor-α transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-κB p50. Mol Cell Biol. 1998;18:5678–5689. doi: 10.1128/mcb.18.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, Clark RS, Dixon CE, Marion DW, Jackson E. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma. 1998;15:163–170. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: A shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. doi: 10.1016/s0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- Block F, Bozdag I, Nolden-Koch M. Inflammation contributes to the postponed ischemic neuronal damage following treatment with a glutamate antagonist in rats. Neurosci Lett. 2001;298:103–106. doi: 10.1016/s0304-3940(00)01729-8. [DOI] [PubMed] [Google Scholar]
- Block F, Tondar A, Schmidt W, Schwarz M. Delayed treatment with rolipram protects against neuronal damage following global ischemia in rats. Neuroreport. 1997;8:3829–3832. doi: 10.1097/00001756-199712010-00033. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Kraydieh S, Green EJ, Dietrich WD. Temporal and regional patterns of axonal damage following traumatic brain injury: A β-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol. 1997;56:1132–1141. doi: 10.1097/00005072-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Kraig RP. Prostaglandin E receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1beta production. J Neurochem. 1999;72:565–575. doi: 10.1046/j.1471-4159.1999.0720565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994;269:12190–12195. [PubMed] [Google Scholar]
- Castro A, Jerez MJ, Gil C, Martinez A. Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: Advances in the development of specific phosphodiesterase inhibitors. Med Res Rev. 2005;25:229–244. doi: 10.1002/med.20020. [DOI] [PubMed] [Google Scholar]
- Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2006;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- Cho S, Kim Y, Cruz MO, Park EM, Chu CK, Song GY, Joh TH. Repression of proinflammatory cytokine and inducible nitric oxide synthase (NOS2) gene expression in activated microglia by N-acetyl-O-methyldopamine: Protein kinase A-dependent mechanism. Glia. 2001;33:324–333. doi: 10.1002/1098-1136(20010315)33:4<324::aid-glia1031>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ciallella JR, Ikonomovic MD, Paljug WR, Wilbur YI, Dixon CE, Kochanek PM, Marion DW, DeKosky ST. Changes in expression of amyloid precursor protein and interleukin-1β after experimental traumatic brain injury in rats. J Neurotrauma. 2002;19:1555–1567. doi: 10.1089/089771502762300229. [DOI] [PubMed] [Google Scholar]
- Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP, Gray JG. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol. 1994;153:712–723. [PubMed] [Google Scholar]
- Dal Piaz V, Giovannoni MP. Phosphodiesterase 4 inhibitors, structurally unrelated to rolipram, as promising agents for the treatment of asthma and other pathologies. Eur J Med Chem. 2000;35:463–480. doi: 10.1016/s0223-5234(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Dash PK, Moore AN, Dixon CE. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: Effects on nitric oxide and interleukin-1β production. J Neuroinflammation. 2004;1:9–24. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarch Z, Giampa C, Patassini S, Martorana A, Bernardi G, Fusco FR. Beneficial effects of rolipram in a quinolinic acid model of striatal excitotoxicity. Neurobiol Dis. 2007;25:266–273. doi: 10.1016/j.nbd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Deogracias R, Espliguero G, Iglesias T, Rodriguez-Pena A. Expression of the neurotrophin receptor trkB is regulated by the cAMP/CREB pathway in neurons. Mol Cell Neurosci. 2004;26:470–480. doi: 10.1016/j.mcn.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Dhillon HS, Yang L, Padmaperuma B, Dempsey RJ, Fiscus RR, Renuka Prasad M. Regional concentrations of cyclic nucleotides after experimental brain injury. J Neurotrauma. 1995;12:1035–1043. doi: 10.1089/neu.1995.12.1035. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Halley M. Early microvascular and neuronal consequences of traumatic brain injury: A light and electron microscopic study in rats. J Neurotrauma. 1994;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Chatzipanteli K, Vitarbo E, Wada K, Kinoshita K. The role of inflammatory processes in the pathophysiology and treatment of brain and spinal cord trauma. Acta Neurochir Suppl. 2004;89:69–74. doi: 10.1007/978-3-7091-0603-7_9. [DOI] [PubMed] [Google Scholar]
- Doppenberg EM, Choi SC, Bullock R. Clinical trials in traumatic brain injury: Lessons for the future. J Neurosurg Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-α mRNA in the CNS. Brain Res Mol Brain Res. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- Ferrendelli JA, Blank AC, Gross RA. Relationships between seizure activity and cyclic nucleotide levels in brain. Brain Res. 1980;200:93–103. doi: 10.1016/0006-8993(80)91097-5. [DOI] [PubMed] [Google Scholar]
- Fineman I, Hovda DA, Smith M, Yoshino A, Becker DP. Concussive brain injury is associated with a prolonged accumulation of calcium: A 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Foey AD, Field S, Ahmed S, Jain A, Feldmann M, Brennan FM, Williams R. Impact of VIP and cAMP on the regulation of TNF-α and IL-10 production: Implications for rheumatoid arthritis. Arthritis Res Ther. 2003;5:R317–328. doi: 10.1186/ar999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folcik VA, Smith T, O’Bryant S, Kawczak JA, Zhu B, Sakurai H, Kajiwara A, Staddon JM, Glabinski A, Chernosky AL, Tani M, Johnson JM, Tuohy VK, Rubin LL, Ransohoff RM. Treatment with BBB022A or rolipram stabilizes the blood-brain barrier in experimental autoimmune encephalomyelitis: An additional mechanism for the therapeutic effect of type IV phosphodiesterase inhibitors. J Neuroimmunol. 1999;97:119–128. doi: 10.1016/s0165-5728(99)00063-6. [DOI] [PubMed] [Google Scholar]
- Freeland K, Boxer LM, Latchman DS. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res Mol Brain Res. 2001;92:98–106. doi: 10.1016/s0169-328x(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Genain CP, Roberts T, Davis RL, Nguyen MH, Uccelli A, Faulds D, Li Y, Hedgpeth J, Hauser SL. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proc Natl Acad Sci USA. 1995;92:3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA. Animate models of human head injury. J Neurotrauma. 1994;11:357–368. doi: 10.1089/neu.1994.11.357. [DOI] [PubMed] [Google Scholar]
- Giorgi M, Modica A, Pompili A, Pacitti C, Gasbarri A. The induction of cyclic nucleotide phosphodiesterase 4 gene (PDE4D) impairs memory in a water maze task. Behav Brain Res. 2004;154:99–106. doi: 10.1016/j.bbr.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, Arancio O. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114:1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004a;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004b;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Griswold DE, Webb EF, Badger AM, Gorycki PD, Levandoski PA, Barnette MA, Grous M, Christensen S, Torphy TJ. SB 207499 (Ariflo), a second generation phosphodiesterase 4 inhibitor, reduces tumor necrosis factor α and interleukin-4 production in vivo. J Pharmacol Exp Ther. 1998;287:705–711. [PubMed] [Google Scholar]
- Hicks RR, Numan S, Dhillon HS, Prasad MR, Seroogy KB. Alterations in BDNF and NT-3 mRNAs in rat hippocampus after experimental brain trauma. Brain Res Mol Brain Res. 1997;48:401–406. doi: 10.1016/s0169-328x(97)00158-7. [DOI] [PubMed] [Google Scholar]
- Hosoi R, Ishikawa M, Kobayashi K, Gee A, Yamaguchi M, Inoue O. Effect of rolipram on muscarinic acetylcholine receptor binding in the intact mouse brain. J Neural Transm. 2003;110:363–372. doi: 10.1007/s00702-002-0797-1. [DOI] [PubMed] [Google Scholar]
- Hou S, Guan H, Ricciardi RP. Phosphorylation of serine 337 of NF-κB p50 is critical for DNA binding. J Biol Chem. 2003;278:45994–45998. doi: 10.1074/jbc.M307971200. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: Modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Liu C, Bramlett H, Sick TJ, Alonso OF, Chen S, Dietrich WD. Changes in trkB-ERK1/2-CREB/Elk-1 pathways in hippocampal mossy fiber organization after traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:934–943. doi: 10.1097/01.WCB.0000125888.56462.A1. [DOI] [PubMed] [Google Scholar]
- Hu BR, Fux CM, Martone ME, Zivin JA, Ellisman MH. Persistent phosphorylation of cyclic AMP responsive element-binding protein and activating transcription factor-2 transcription factors following transient cerebral ischemia in rat brain. Neuroscience. 1999;89:437–452. doi: 10.1016/s0306-4522(98)00352-2. [DOI] [PubMed] [Google Scholar]
- Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int. 1997;30:427–431. doi: 10.1016/s0197-0186(96)00078-2. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Sawa A, Ichimaru Y, Miyashiro M, Kato S, Yamamoto T, Ueki S. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur J Pharmacol. 1997;321:273–278. doi: 10.1016/s0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- Jimenez JL, Punzon C, Navarro J, Munoz-Fernandez MA, Fresno M. Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-κB and nuclear factor of activated T cells activation. J Pharmacol Exp Ther. 2001;299:753–759. [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-α responses. Proc Natl Acad Sci USA. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Araki T, Itoyama Y, Kogure K. Rolipram, a cyclic AMP-selective phosphodiesterase inhibitor, reduces neuronal damage following cerebral ischemia in the gerbil. Eur J Pharmacol. 1995;272:107–110. doi: 10.1016/0014-2999(94)00694-3. [DOI] [PubMed] [Google Scholar]
- Keane RW, Kraydieh S, Lotocki G, Alonso OF, Aldana P, Dietrich WD. Apoptotic and antiapoptotic mechanisms after traumatic brain injury. J Cereb Blood Flow Metab. 2001;21:1189–1198. doi: 10.1097/00004647-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Keeling KL, Hicks RR, Mahesh J, Billings BB, Kotwal GJ. Local neutrophil influx following lateral fluid-percussion brain injury in rats is associated with accumulation of complement activation fragments of the third component (C3) of the complement system. J Neuroimmunol. 2000;105:20–30. doi: 10.1016/s0165-5728(00)00183-1. [DOI] [PubMed] [Google Scholar]
- Keryer G, Yassenko M, Labbe JC, Castro A, Lohmann SM, Evain-Brion D, Tasken K. Mitosis-specific phosphorylation and subcellular redistribution of the RIIα regulatory subunit of cAMP-dependent protein kinase. J Biol Chem. 1998;273:34594–34602. doi: 10.1074/jbc.273.51.34594. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Chatzipanteli K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1β messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: Importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Udalova IA, Turetskaya RL, Rice NR, Nedospasov SA. Conserved κ B element located downstream of the tumor necrosis factor α gene: distinct NF-κ B binding pattern and enhancer activity in LPS activated murine macrophages. Oncogene. 1995;11:97–106. [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2004. pp. 1–68. [Google Scholar]
- Lee HT, Chang YC, Wang LY, Wang ST, Huang CC, Ho CJ. cAMP response element-binding protein activation in ligation preconditioning in neonatal brain. Ann Neurol. 2004;56:611–623. doi: 10.1002/ana.20259. [DOI] [PubMed] [Google Scholar]
- Manganiello VC, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys. 1995;322:1–13. doi: 10.1006/abbi.1995.1429. [DOI] [PubMed] [Google Scholar]
- Martin C, Goggel R, Dal Piaz V, Vergelli C, Giovannoni P, Ernst M, Uhlig S. Airway relaxant and anti-inflammatory properties of a PDE4 inhibitor with low affinity for the high-affinity rolipram binding site. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:284–289. doi: 10.1007/s00210-001-0525-7. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Shima K, Nawashiro H, Wada K, Tsuzuki N, Miyazawa T. Real time monitoring of glutamate following fluid percussion brain injury with hypoxia in the rat. Acta Neurochir Suppl. 2000;76:207–212. doi: 10.1007/978-3-7091-6346-7_42. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Muller T, Engels P, Fozard JR. Subtypes of the type 4 cAMP phosphodiesterases: Structure, regulation and selective inhibition. Trends Pharmacol Sci. 1996;17:294–298. doi: 10.1016/0165-6147(96)10035-3. [DOI] [PubMed] [Google Scholar]
- Nagakura A, Niimura M, Takeo S. Effects of a phosphodiesterase IV inhibitor rolipram on microsphere embolism-induced defects in memory function and cerebral cyclic AMP signal transduction system in rats. Br J Pharmacol. 2002;135:1783–1793. doi: 10.1038/sj.bjp.0704629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navikas V, Matusevicius D, Soderstrom M, Pirskanen R, Fredrikson S, Link H. The phosphodiesterase i.v inhibitor rolipram in vitro reduces the numbers of MBP-reactive IFN-gamma and TNF-alpha mRNA expressing blood mononuclear cells in patients with multiple sclerosis. Clin Neuropharmacol. 1998;21:236–244. [PubMed] [Google Scholar]
- Nemoz G, Prigent AF, Moueqqit M, Fougier S, Macovschi O, Pacheco H. Selective inhibition of one of the cyclic AMP phosphodiesterases from rat brain by the neurotropic compound rolipram. Biochem Pharmacol. 1985;34:2997–3000. doi: 10.1016/0006-2952(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-κB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305–322. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Patrizio M. Tumor necrosis factor reduces cAMP production in rat microglia. Glia. 2004;48:241–249. doi: 10.1002/glia.20074. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Lipshutz D, Bartus JO, Slivjak MJ, Smith EF, 3rd, Lee JC, Esser KM. Characterization of cAMP-dependent inhibition of LPS-induced TNF α production by rolipram, a specific phosphodiesterase IV (PDE IV) inhibitor. Int J Immunopharmacol. 1994;16:805–816. doi: 10.1016/0192-0561(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Prado R, Busto R, Globus MY. Ischemia-induced changes in extracellular levels of striatal cyclic AMP: Role of dopamine neurotransmission. J Neurochem. 1992;59:1581–1584. doi: 10.1111/j.1471-4159.1992.tb08480.x. [DOI] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Saatman KE, Martin D, Miller G, McIntosh TK. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Manabe H. KF19514, a phosphodiesterase 4 and 1 inhibitor, inhibits TNF-α-induced GM-CSF production by a human bronchial epithelial cell line via inhibition of PDE4. Inflamm Res. 2004;53:31–37. doi: 10.1007/s00011-003-1217-1. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kitagawa K, Omura-Matsuoka E, Todo K, Terasaki Y, Sugiura S, Hatazawa J, Yagita Y, Hori M. The phosphodiesterase inhibitor rolipram promotes survival of newborn hippocampal neurons after ischemia. Stroke. 2007;38:1597–1605. doi: 10.1161/STROKEAHA.106.476754. [DOI] [PubMed] [Google Scholar]
- Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW, McIntosh TK. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci USA. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF α and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Si Q, Nakamura Y, Ogata T, Kataoka K, Schubert P. Differential regulation of microglial activation by propentofylline via cAMP signaling. Brain Res. 1998;812:97–104. doi: 10.1016/s0006-8993(98)00954-8. [DOI] [PubMed] [Google Scholar]
- Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9:227–236. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinase type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Ruenes G, Dietrich WD. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002;277:35920–35931. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Tetsuka T, Uranishi H, Okamoto T. Inhibition of the NF-κB transcriptional activity by protein kinase A. Eur J Biochem. 2002;269:4559–4565. doi: 10.1046/j.1432-1033.2002.03157.x. [DOI] [PubMed] [Google Scholar]
- Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sotomatsu A, Yoshida T, Hirai S, Nishida A. Detection of superoxide production by activated microglia using a sensitive and specific chemiluminescence assay and microglia-mediated PC12h cell death. J Neurochem. 1994;63:266–270. doi: 10.1046/j.1471-4159.1994.63010266.x. [DOI] [PubMed] [Google Scholar]
- Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, Lundkvist J, Iverfeldt K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: A 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Torphy TJ. Phosphodiesterase isozymes: Molecular targets for novel antiasthma agents. Am J Respir Crit Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- Truettner J, Schmidt-Kastner R, Busto R, Alonso OF, Loor JY, Dietrich WD, Ginsberg MD. Expression of brain-derived neurotrophic factor, nerve growth factor, and heat shock protein HSP70 following fluid percussion brain injury in rats. J Neurotrauma. 1999;16:471–486. doi: 10.1089/neu.1999.16.471. [DOI] [PubMed] [Google Scholar]
- van Staveren WC, Markerink-van Ittersum M, Steinbusch HW, de Vente J. The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res. 2001;888:275–286. doi: 10.1016/s0006-8993(00)03081-x. [DOI] [PubMed] [Google Scholar]
- Verghese MW, McConnell RT, Strickland AB, Gooding RC, Stimpson SA, Yarnall DP, Taylor JD, Furdon PJ. Differential regulation of human monocyte-derived TNF α and IL-1 β by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J Pharmacol Exp Ther. 1995;272:1313–1320. [PubMed] [Google Scholar]
- Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor α expression and protein levels after fluid percussion injury in rats: The effect of injury severity and brain temperature. Neurosurgery. 2004;55:416–424. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Yang K, Perez-Polo JR, Mu XS, Yan HQ, Xue JJ, Iwamoto Y, Liu SJ, Dixon CE, Hayes RL. Increased expression of brain-derived neurotrophic factor but not neurotrophin-3 mRNA in rat brain after cortical impact injury. J Neurosci Res. 1996;44:157–164. doi: 10.1002/(SICI)1097-4547(19960415)44:2<157::AID-JNR8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yang L, Konishi Y, Maeda N, Sakanaka M, Tanaka J. Suppressive effects of phosphodiesterase type IV inhibitors on rat cultured microglial cells: Comparison with other types of cAMP-elevating agents. Neuropharm. 2002;42:262–269. doi: 10.1016/s0028-3908(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang HT, O’Donnell JM. Antidepressant-induced increase in high-affinity rolipram binding sites in rat brain: Dependence on noradrenergic and serotonergic function. J Pharmacol Exp Ther. 2003;307:246–253. doi: 10.1124/jpet.103.053215. [DOI] [PubMed] [Google Scholar]