Abstract
Background
Currently, there is renewed interest in the role community participation can play in Primary Health Care (PHC) programmes such as the delivery of effective anti-TB treatment to patients in high-burden settings.
Objectives
To explore the feasibility of community participation in a high-burden Tuberculosis Control Programme and to establish how supervision of treatment by lay volunteers compares with other methods of tuberculosis treatment delivery in the Northern Cape province of South Africa.
Methods
Prospective study involving 769 patients with confirmed pulmonary TB who were followed-up over a one-year period. Questionnaire interviews were also carried out with 135 lay volunteers participating in the TB programme.
Results
One-third of the TB patients in the study received their treatment from lay volunteers in the community. Treatment outcomes for new patients supervised from the community were found to be equivalent to those who received treatment through other modes of treatment delivery (RR=1.04[0.94–1.16], p=0.435). For the re-treatment patients, community-based treatment was found to be superior (RR=5.89[2.30–15.09], p<0.001), to self-administered therapy.
Conclusions
Health care planners should consider community participation as a viable way of ensuring accessibility and effectiveness in PHC programmes. There is need for more research into ways of achieving sustainability in resource-limited but high disease burden settings.
Keywords: community participation, tuberculosis, high-burden settings, resource limitations
Introduction
Community participation in health is a complex entity that has been extensively examined by a number of authors and continues to be of great interest even today.1–6 The genesis of the idea and its conceptual development are primarily attributed to large multinational organizations particularly the World Health Organization (WHO).6 This paper seeks to examine the concept of community participation in primary health care (PHC) activities with particular reference to tuberculosis (TB) control.
TB remains a global problem today despite the fact that effective treatment has been available for over 50 years.7 The greatest burden of the disease is in resource-limited, developing countries where the close association between TB and the HIV epidemic has resulted in an exponential rise in the number of TB cases seen over the past two decades.8–9
Poor patient adherence to prescribed medication has been recognised as one of the major hindrances to effective TB control.10 In response to this problem, the WHO Global Programme on Tuberculosis advocates for the use of Directly Observed Treatment (DOT) for all patients.11 While this approach results in improved cure rates,12–14 it is also labour intensive and over burdened health staff in high incidence areas often find it a daunting task to have to administer DOT to the large numbers of TB patients they see daily.15
Innovations with DOT have resulted in development of community-based TB treatment delivery whereby patients are offered ambulatory treatment at home. The need for community participation in TB control activities is not new: it was well recognised in the Ninth Report of the WHO Expert Committee on Tuberculosis in 1974.16 A decade earlier, the WHO had recognised the need to incorporate TB control into a comprehensive countrywide system, which is permanent, adapted to the needs of the population and integrated into the primary health care system.17
Today, the reality however is that in many developing countries, government services still reach only a proportion of the population. Some authors,18,19 think this is because of the limitations imposed by an inadequate health service infrastructure, an insufficient level of decentralization to ensure adequate access to health care, and a paucity of locally available human and financial resources.
Community participation in TB treatment delivery, as part of routine National Tuberculosis Programme activities has the potential to overcome at least some of these limitations. An ad-hoc Expert committee on Tuberculosis convened by WHO in 1998 concluded that “community involvement in TB care and a patient-centred approach need emphasis and promotion.” 20 Harnessing participation by the community could dramatically expand the provision of effective ambulatory TB treatment delivery and result in more widespread implementation of the internationally recommended Directly Observed Treatment - Short Course (DOTS) TB control strategy.
It is important to note, however, that until relatively recently, the focus of activities aimed at strengthening TB services in high incidence countries have been more towards improving the general health services rather than on harnessing community participation.18 It is the dramatic increase in the TB burden related to the HIV/AIDS epidemic in many countries in sub-Saharan Africa that has prompted fresh interest in evaluating the potential of local communities to contribute to TB care in this region and elsewhere.
Achieving community participation in health service delivery in resource-limited but a high disease burden setting is not a simple task. Here the perception of the general population is often that of a grossly inefficient formal health sector that has simply failed to deliver. Additionally, community members may seek remuneration for participation in health service delivery, a stance that is not often sustainable in poorly resourced developing countries. Alternatively, health service personnel may not be willing to involve laypersons in the execution of health programmes, an act that they may view as a dilution of their own expertise.
This paper explores the feasibility of community participation in a high-burden TB programme in a resource-limited setting and attempts to establish how supervision of TB treatment by lay volunteers compares with other methods of TB treatment delivery in the Northern Cape province of South Africa.
Methods
Setting
This study was conducted in the Northern Cape province, Republic of South Africa. South Africa is one of the 22 countries with the highest burden of tuberculosis in the world.21 The Northern Cape is the largest province in South Africa and had an estimated TB incidence of 547 cases per 100 000 population in 2000.22
Organization of TB Services
The introduction of a District Health Service (DHS) system in South Africa after 1994 meant that the responsibility of TB treatment was largely devolved to Primary Health Care units.23 Additionally, there has been a drive towards involving the community and non-governmental organizations (NGOs) in the National TB Control Programme. The aim is to recruit lay volunteers from the community to directly observe TB patients as they take their treatment.24
In 1997, the Northern Cape province adopted the guidelines of the WHO-recommended DOTS strategy for TB treatment. Direct Observation of treatment for TB patients (DOT) is one of the key components of this strategy. In order to achieve this, the TB programme in the Northern Cape province involves the participation of three major role players namely: the formal health services, NGOs and the community.
Treatment for uncomplicated pulmonary tuberculosis is ambulatory and is largely provided through Primary Health Care clinics, which are all managed under either the municipal or provincial governments. Treatment at these clinics is provided free of charge. NGOs participate in the TB programme by providing facilitation and training on community-based DOT provision to clinic staff and lay volunteers. The community provides non-paid volunteers who act as DOT supporters for the TB patients during the whole duration of treatment.
TB Treatment Delivery Options
After diagnosis of TB through sputum smear microscopy, the new case is then registered at the clinic and the attending nurse discusses the treatment options available with the patient.
The patient is normally given the options of clinic or community-based DOT and told to make a choice between these two with the advise of the clinic nurse. The choice of self-administered therapy (SAT) is also available to those patients who are unable to take up the supervised options.
Clinic-based DOT
Patients who opt for clinic-based DOT are asked to come to the clinic five days a week to be observed by the clinic nurse as they swallow their tablets. Nurses administering DOT at the clinic usually follow a set routine: patients are asked to fill a disposable cup with clean water, anti-TB tablets are dispensed into a small plastic cup and patients swallow their medication under the watchful eye of the nurse who then records their attendance and compliance on the patient treatment card.
Community-based DOT
Patients who opt for community-based DOT are assigned to a lay volunteer [DOT supporter] who is attached to that clinic and lives within reasonable proximity from the patient. The patient and DOT supporter then meet and work out the modalities of when it is convenient to supervise treatment for each day. DOT supporters are given secure boxes at the clinic in which to store the patients' drugs and are trained on how to administer and record TB treatment. Each DOT supporter can supervise up to 4 TB patients at any one time, for the duration of their treatment. The clinic nurse in charge of TB supervises the volunteers who normally return to the clinic every fort-night in order to replenish their supply of drugs and report any problems they may have encountered. Additionally, DOT supporters are expected to follow-up absent patients after one skip, remind patients of clinic appointments, and to refer those TB patients with other problems to the relevant services. DOT supporters do not receive any remuneration for providing these services.
Self-administered therapy
Patients receiving this option are given a monthly supply of anti-TB drugs to take home with them. They are also given a patient treatment card to record their compliance. These patients report to the clinic at monthly intervals to replenish their drug supply and to be evaluated.
Regimens
Short course chemotherapy is used for the treatment of uncomplicated pulmonary TB in the Northern Cape province. Treatment is provided 5 days per week and new patients are treated for 6 months while re-treatment patients receive treatment for 8 months. Drug dosages are calculated according to patient weight, which for adult patients is divided into 2 categories with a cut-off weight of 50 kilogrammes.
Re-treatment patients normally receive the first two months of the intensive phase of treatment at the clinic, as they have to get their daily streptomycin injections during this phase. New patients can start off with the supervision option of their choice as soon as they have been diagnosed.
Data Collection
This study was conducted during the period from October 1999 to October 2000. A prospective study involving patients with proven pulmonary TB who were started on anti-TB treatment within the first five months of the study period was undertaken. Patients who participated in the study were recruited from 45 Primary Health Care facilities in the Northern Cape province. Participating facilities were randomly selected from a sampling frame of 80 fixed clinics and day hospitals after secondary and tertiary health units had been excluded.
Sample size estimation
The total number of new TB patients to be followed up during the study period was estimated as follows:
n = number of patients required for follow-up.
r = number of patients who opt for community based TB treatment supervision.
p= proportion of patients who opt for community based TB treatment supervision.
Hence; p = r/n
We estimated this proportion of patients to be 0.5, Therefore, the confidence interval width, w at 95% level of confidence was calculated as:
Hence rearranging the equation to obtain the value of n:
Setting p=0.5 and w = 0.05 for the above equation; n was calculated as 384.
We took into consideration the observation that the treatment interruption rate in the Northern Cape Province during 1998 was about 19%. We therefore anticipated a total of 460 patients to be followed up at the selected clinics in the study.
In order to achieve the estimated sample size, all patients who fitted the selection criteria below in the selected clinics were approached to participate in this study.
Inclusion criteria
Patients with pulmonary tuberculosis - confirmed using a sputum smear examination
Patients 15 years of age and above of age
New TB patients who started treatment during the months of October 1999 – February 2000 inclusive
Re-treatment TB patients who started treatment in the months of November 1999 and February 2000
TB patients who were ordinarily residents of the regions where the study took place
At the end of the 5-month recruitment phase, 769 patients fitting the selection criteria had accepted to participate in the study.
DOT supporters supervising TB patients registered at 30 of the selected facilities were also interviewed as part of the study. Random sampling was achieved by use of a computer algorithm in SPSS 9.0 Statistical Package (SPSS Inc., Chicago, IL).
Data collection involved the administration of a largely pre-coded interviewer-administered questionnaire to eligible study participants [one for both patients and DOT supporters]. The structured questionnaires used in this study were developed with the help of staff from the provincial TB management unit and a specialist scientist from the Tuberculosis Research Programme of the Medical Research Council of South Africa. Initial drafts of the questionnaires were piloted to determine the most appropriate wording and structuring.
The questionnaires were translated into Afrikaans. The Afrikaans versions were then back translated into English by an independent person in order to determine whether the translated versions were similar to the original version and conveyed the questions the study sought to ask.
Consent was sought from each study participant before interview and for the patient interviews, only subjects of age 15 years and above were eligible to participate in the study. Subsequent followup of the TB patients was done through regular visits to the participating health facilities to collect information recorded about each study subject from the formal health records.
This paper reports only issues pertaining to community participation in TB treatment delivery programme in the Northern Cape province. Other aspects of the TB programme that were explored in the interviews have been described elsewhere.25,26
The study received ethical approval from the Department of Health, Northern Cape province.
Analysis
Data were entered into a portable computer, statistical analysis was done mainly using Epi Info 2000 version 1.0 (CDC, Atlanta, GA). Univariate analysis was done separately for new and re-treatment patient cohorts as recommended by WHO guidelines.27 The relative risk (RR) estimate and the Chi-squared test were used to analyse associations between variables. For this study, “statistically significant” was measured at the 0.05 level.
Results
769 patients with confirmed pulmonary tuberculosis were recruited for this study. The general characteristics of these patients are summarised in Table 1
Table 1.
General Characteristics of patients in the study (n=769)
| Characteristic | n(m/s) |
| Patient category | |
| New | 638 (83.0) |
| Re-treatment | 131 (17.0) |
| Sex | |
| Male | 451 (58.6) |
| Female | 318 (41.4) |
| Age category | |
| 16–29 | 278 (36.2) |
| 30–44 | 341 (44.3) |
| 45–59 | 110 (14.3) |
| > 60 | 40 (5.2) |
| Site of residence | |
| Urban | 661 (86.0) |
| Rural | 108 (14.0) |
| Interview status | |
| Interviewed | 698 (90.8) |
| Not Interviewed | 71 (9.2) |
| Place of first consultation* | |
| Government health unit | 614 (88.0) |
| Private Medical Practitioner | 81 (11.6) |
| Traditional healer | 3 (0.4) |
| Choice of TB treatment delivery | |
| Clinic-based | 388 (50.5) |
| Community-based | 280 (36.4) |
| Self-administered | 101 (13.1) |
| Education* | |
| None | 123 (17.6) |
| Primary | 338 (48.4) |
| Secondary | 225 (32.2) |
| Higher | 12 (1.8) |
| Occupation* | |
| Employed | 123 (17.6) |
| Unemployed | 501 (71.9) |
| Student | 45 (6.4) |
| Retired | 29 (4.2) |
| Treatment Outcome | |
| Cured | 453 (58.9) |
| Treatment completed | 31 (4.0) |
| Defaulted | 144 (18.7) |
| Transferred | 50 (6.5) |
| Died | 50 (6.5) |
| Treatment failure | 41 (5.3) |
| Successful§ | 484 (63.0) |
| Unsuccessful† | 235 (30.5) |
Data presented for 698 patients who were interviewed
“Successful” includes Cured and Treatment completed
“Unsuccessful” includes Defaulted, Died and Treatment failure
Table 1 shows that 71(9.2%) of the patients in this study were not interviewed. This was because these patients had transferred to a different region, defaulted from treatment or died by the time of the interviews. Outcomes for these patients where however obtained from the formal health records.
Fifty patients (6.5%) transferred to health facilities outside the study region. The treatment outcome of these patients was therefore indeterminable and hence they were excluded from further analysis. Of the remaining patients, 598 (83%) were new patients and 121 (17%) were re-treatment patients.
Only 54% (65/121) of re-treatment patients had a successful treatment outcome compared to 70% (419/598) of new patients. This difference was found to be statistically significant (p<0.001).
Overall, more than one-third of the patients chose community-based treatment supervision as their mode of TB treatment delivery. Table 2 shows the treatment outcome of both new and re-treatment patients according to choice of treatment delivery. It can be seen that there is no significant difference between community-based treatment supervision and the other options available for new patients. For the re-treatment patients however, community-based supervision was found to be superior to self-administered therapy (c2= 22.76, p<0.001).
Table 2.
Treatment outcome of both New and Re-treatment patients according to choice of treatment delivery
| Treatment Outcome | |||||
| Patient Category | TB treatment delivery option |
Successful (n) |
Unsuccessful (n) |
% Successful | p† |
| New patients | Community-based | 164 | 64 | 72 | |
| Clinic-based | 189 | 88 | 68 | 0.367 | |
| Self-administered | 66 | 27 | 71 | 0.862 | |
| Re-treatment patients |
Community-based | 19 | 6 | 76 | |
| Clinic-based | 42 | 23 | 65 | 0.301 | |
| Self-administered | 4 | 27 | 13 | <0.001* | |
Chi-Square test comparing community-based treatment supervision to other options
Statistically significant at the 0.05 level
Table 3 shows the general characteristics of the DOT Supporters who were interviewed in this study.
Table 3.
Characteristics of DOT supporters interviewed in the study (n=135)
| Characteristic | n(%) |
| Residence of DOT supporter | |
| Rural | 32 (23.7) |
| Urban | 103 (76.3) |
| Sex | |
| Male | 10 (7.4) |
| Female | 125 (92.6) |
| Age category | |
| 15–29 | 45 (33.3) |
| 30–44 | 59 (43.7) |
| 45–59 | 23 (17.0) |
| > 60 | 8 (6.0) |
| Education | |
| None | 4 (3.0) |
| Primary | 40 (29.6) |
| Secondary | 80 (59.3) |
| Higher | 11 (8.1) |
| Occupation | |
| Employed | 23 (17.0) |
| Unemployed | 101 (74.8) |
| Retired | 10 (7.4) |
| Student | 1 (0.8) |
| Place of DOT | |
| Volunteer's home | 94 (69.6) |
| Patient's home | 28 (20.8) |
| Clinic | 13 (9.6) |
| Responsibility for training | |
| Clinic Staff | 59 (43.5) |
| NGO Staff | 41 (30.6) |
| Both Clinic and NGO staff | 11 (8.1) |
| Other* | 24 (17.7) |
“Other” includes trainees from the Department of Health; notably HIV and nutritional advisors who have also obtained training in TB control
Table 3 shows that almost 93% of the DOT supporters were female, this contrasted sharply with the sex distribution of the TB patients in the study, where only 41% where female (χ2=120.65, p<0.001). 70% of DOT supporters were less than 45 years of age, which was almost similar to the number of TB patients in the study who were below 45 years of age (χ2= 0.86, p=0.355).
The occupation status of DOT supporters and TB patients in the study was found to be similar as shown in Figure 1.
Figure 1.
Occupation status of DOT supporters and TB patients
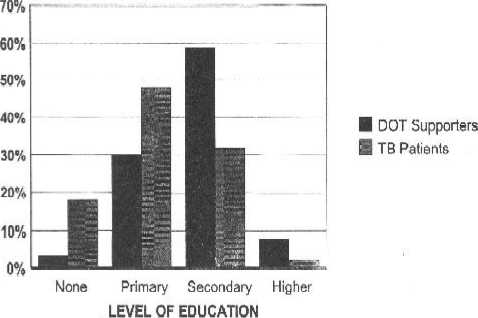
The majority of study participants in each case were found to be unemployed. The trend for ducation status however, differed between the patients and DOT supporters. A Chi-squared test for linear trend done between DOT supporters and TB patients in the study showed that the DOT supporters were significantly more educated than the TB patients (χ2=60.37, p<0.001). This trend is graphically represented by Figure 2.
Discussion
This study explored the role lay community members can play in a TB programme run at the Primary Health Care level in a high-burden but resource-limited setting. One-third of the TB patients in this study were found to receive their treatment from DOT supporters in the community.
The finding that community-based DOT produced outcomes that were equivalent to the other treatment options for new patients and was superior to self-administration of drugs for re-treatment patients suggests that lay volunteers can effectively dispense anti-TB medication and community participation should be encouraged.
The implication of this in high TB burden settings is that community-based TB treatment is an effective and viable option that can supplement other modes of treatment delivery. Furthermore, community-based TB treatment delivery has been found to be cost-effective,28 and it is a low cost technology that can easily be adapted to diverse areas of need and appropriate lay volunteers recruited according to availability in each contextual setting.
Additionally, community participation in health can be extended to involve other high-burden diseases, notably HIV/AIDS. The World Health Organization currently advocates for the home-based care and integrated management of dually infected TB/AIDS patients. DOT supporters can be further trained to carry out this additional task and pilot studies are underway to evaluate the feasibility of this approach.22
A major hindrance to community participation in developing countries is the desire for remuneration by the lay volunteers. There is evidence to suggest that in the absence of appropriate incentives, attrition rates in lay worker programmes tend to be high after the initial novelty wears off.26 Economic realities in developing countries often dictate that people expect some kind of remuneration for work done. As this study reveals, TB patients and those who volunteered to support them during their treatment often come from the same socio-economic background and in areas where unemployment is high, expectation of payment for any kind of work performed is also high.
In the Northern Cape province, the combination of young, reasonably well educated but unemployed DOT supporters in the TB programme posed a serious threat to the sustainability of the programme because volunteers started to drop out as soon as they realised that they would not be paid for their work. This study also revealed that the majority of lay participants in the TB programme in the Northern Cape province were female, similar findings have been reported in Community Health Worker programmes elsewhere29 and is perhaps a reflection of the fact that in many developing countries, women tend to stay at home [and hence can volunteer for health programmes] whilst the men are expected to go off to look for work to support the family.
The onus is on health planners to devise appropriate and context-specific ways in which to achieve sustainability by keeping lay people motivated in community participation in health programmes while at the same time keeping in mind the limitations of cost-containment. More research is required on exactly how this can be achieved in specific settings with a high burden of disease.
Acknowledgements
The authors would like to thank all the patients and DOT supporters who participated in this study. Financial assistance for this study was obtained through grants from the Sir Halley Stewart Trust and the Department for International Development, UK and is gratefully acknowledged.
References
- 1.Muller F. Participation in Primary Health Care Programmes in Latin America. Medillin: Colombia: University of Antioquia, National School of Public Health; 1980. [Google Scholar]
- 2.Oakley P. Community Involvement in Health Development: An examination of the critical issues. Geneva: WHO; 1989. [Google Scholar]
- 3.MacDonald J. Primary Health Care. London: Earthscan Publications; 1993. [Google Scholar]
- 4.Rifkin S. Community participation in MCH/FP programmes: An analysis based on case study materials. Geneva: World Health Organization; 1990. [Google Scholar]
- 5.Rifkin S. Paradigms lost: towards a new understanding of community participation in health programmes. Acta Tropica. 1996;61:79–92. doi: 10.1016/0001-706x(95)00105-n. [DOI] [PubMed] [Google Scholar]
- 6.Zakus JD, Lysack CL. Revisiting community participation. Health Policy Plan. 1998;13:1–12. doi: 10.1093/heapol/13.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Dolin PJ, Raviglione MC, Kochi A. Global Tuberculosis Incidence and Mortality during 1990–2000. Bull World Health Organ. 1994;72(2):212–220. [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CJL, Styblo K, Rouillon A. Tuberculosis in developing countries; burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:2–20. [PubMed] [Google Scholar]
- 9.Narain JP, Raviglione MC, Kochi A. HIV associated tuberculosis in developing countries; epidemiology and strategies for prevention. Tubercle Lung Dis. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 10.Fox W. Compliance of patients and physicians. Experience and lessons from tuberculosis. BMJ. 1983;287:33–37. doi: 10.1136/bmj.287.6384.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher D, Hausler HP, Raviglione MC, et al. Tuberculosis care in community care organizations in sub-Saharan Africa. Practise and potential. Int J Tuberc Lung Dis. 1997;1(3):276–283. [PubMed] [Google Scholar]
- 12.Chowdhury AMR, Chowdhury S, Islam MN, Islam A, Vaughan P. Control of tuberculosis by community health workers in Bangladesh. Lancet. 1997;350:169–172. doi: 10.1016/S0140-6736(96)11311-8. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson D. High compliance tuberculosis treatment programme in a rural community. Lancet. 1994;343:647–648. doi: 10.1016/s0140-6736(94)92640-9. [DOI] [PubMed] [Google Scholar]
- 14.China Tuberculosis Control Collaboration, author. Results of directly observed intermittent treatment short course chemotherapy in 112 842 Chinese patients with smear positive tuberculosis. Lancet. 1996;347:358–362. [PubMed] [Google Scholar]
- 15.Zwarenstein M, Schoeman JH, Vundule C, Lombard CJ, Tatley M. Randomised controlled trial of self supervised and directly observed treatment for tuberculosis. Lancet. 1998;352:1340–1343. doi: 10.1016/S0140-6736(98)04022-7. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization Expert Committee on Tuberculosis, author. Ninth Report. Technical Series 552. Geneva: WHO; 1974. [PubMed] [Google Scholar]
- 17.World Health Organization Expert Committee on Tuberculosis, author. Eighth Report. Technical Series 290. Geneva: WHO; 1964. [PubMed] [Google Scholar]
- 18.Maher D, Van Gorkom JLC, Gondrie PCFM, Raviglione M. Community contribution to tuberculosis care in countries with high tuberculosis prevalence: past, present and future. Int J Tuberc Lung Dis. 1999;3(9):762–768. [PubMed] [Google Scholar]
- 19.Ogden J. Improving tuberculosis control - Social Science Inputs. Trans R Soc Trop Med Hyg. 2000;94:135–140. doi: 10.1016/s0035-9203(00)90249-9. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization, author. Report of the Ad-hoc Committee on the Tuberculosis epidemic. Geneva: WHO/TB/98.245; 1998. [Google Scholar]
- 21.World Health Organization, author. Global Tuberculosis Programme, Global Tuberculosis Control - WHO Report 2000. Geneva: WHO/CDS/TB/2000.275; [Google Scholar]
- 22.Kironde S. Tuberculosis. In: Ntuli A, Crisp N, Clarke E, Baron P, editors. South African Health Review 2000. Durban: Health Systems Trust; pp. 335–349. [Google Scholar]
- 23.African National Congress, author. A Policy Framework. Johannesburg: ANC; 1994. The Reconstruction and Development Programme. [Google Scholar]
- 24.Cameron N, Matsha N, Matji R, editors. Faces of TB 1998–99. South Africa: TB Advocacy Publication; 1999. [Google Scholar]
- 25.Kironde S. Implementing community-based tuberculosis treatment in the Northern Cape province of South Africa - An emerging model for high incidence areas in Africa? Oxford University; 2001. [Google Scholar]
- 26.Kironde S, Klaasen S. What motivates lay volunteers in high burden but resource-limited tuberculosis programmes? Perceptions from the Northern Cape province, South Africa. Int J Tuberc Lung Dis. 2001 [PubMed] [Google Scholar]
- 27.World Health Organization, author. Tuberculosis Programme Framework for Tuberculosis Control. Geneva: WHO/TB/94.179; 1994. [Google Scholar]
- 28.Dick J, Henchie S. A cost analysis of the tuberculosis control programme in Elsies River, Cape Town. S Afr Med J. 1998;88:380–383. [PubMed] [Google Scholar]
- 29.Kahssay MH, Taylor ME, Berman PA. Community Health Workers: The way forward. Geneva: WHO; 1998. [Google Scholar]