Abstract
Background
Modified Giemsa staining has been favoured by many researchers because it is easy to perform but, like many other stains, demonstration of the bacteria depends on its morphology. It has been arged in some research circles that some of the organisms in the gastric mucosa may not be true H.pylori. Immunohistochemical techniques have been developed and make use of anti H.pylori antibody, which reacts, with somatic antigens of the whole bacteria and have been found to correlate well with the presence of the bacteria.
Objective
To ascertain the efficacy of modified Giemsa stain in an African setting where H.Pylori seems quite prevalent.
Study Design
A laboratory-based study of two diagnostic tests in which modified Giema stain was compared with immunohistochemistry.
Methods
A total of 48 consecutive autopsy cases with no upper gastro intestinal diseases had their gastric mucosa stained for demonstration of H.pylori using both modified Giemsa and immunohisto chemical staining techniques.
Results
Twenty-seven cases of H.pylori were demonstrated by both techniques and 14 cases were not identified by the two staining methods. In 2 cases immunostain could not demonstrate the bacteria but they were identified with modified Giemsa stain while in 5 cases the bacteria were identified by immunostain but not with modified Giemsa stain. The sensitivity of modified Giemsa stain was 85% (CI 66.5–98.8) while the specificity was 89% (CI 60.4–97.8). The positive predictive value of modified Giemsa stain was 93% CI 75–98.8%) while the negative predictive value was 74% (CI 48.6–89.9). The kappa statistic comparing the 2 stains was 0.69 (p value 0.00001) giving a good agreement between the two tests.
Conclusion
With the above results the modified Giemsa stain, which is readily available in most African laboratories, is recommenced for diagnosis of H.pylori, a prevalent infection in Africa.
Key words: H.pylori, modified Giemsa, immunohistochemical stains, CI - Confidence Interval
Introduction
H.pylori, formerly Campylobacter pylori, is now accepted as a major cause of chronic active antral gastritis and there is accumulating evidence to incriminate this microbe in the aetiology of duodenal ulcer and gastric carcinoma.1–2 It is therefore of paramount importance to determine the presence of the organism in surgical pathology specimens in order to manage these two common diseases of the upper gastrointestinal tract. Bacteriological methods would be the ideal confirmatory tests for H.pylori diagnosis but are difficult to perform as they require specialised enrichment media with complicated incubation techniques and characterisation of the microbe is time consuming. Antral biopsy specimens processed for histology would therefore provide an easier and more cost-effective alternative means of diagnosing H.pylori infection. Various special stains have been devised to detect H.pylori in these histological sections but their specificity and sensitivity vary greatly. The haematoxylin and eosin stain, the most frequently used stain in histology, has been found to be the most unreliable.3 The silver stain, though found to be more superior, is quite complicated to carry out and the granular appearance it gives the organisms may be confused with silver precipitate.4 Modified Giemsa stain described by Gray et al (1986)5 has been favoured by many researchers because of its easiness to perform and availability in most histopathology laboratories.
However all the above-mentioned stains depend on the morphology of the bacterium for identification and it is possible that there are other microbes in the gastric mucosa, which could resemble and become difficult to differentiate from H.pylori. It is also known that H.pylori may demonstrate pleomorphism so that depending on morphology alone may not be reliable for diagnosis. Immunohistochemical techniques have been developed and make use of anti H.pylori antibody which reacts with somatic antigens of the whole bacteria and have been found to correlate well with the presence of the bacteria.6 The aim of this study was therefore to ascertain the reliability of modified Giemsa stain in comparison with immunohistochemical technique in diagnosing H.pylori in an African setting where H.pylori seems to be quite prevalent.
Objective
To ascertain the effecicacy of modified Giesma stain in demonstration of H.pylori organisms in formal fixed paraffin embedded tissues.
Study Design
This was a laboratory-based study of two diagnostic tests in which modified Giesma stain was compared with immunohistochemistry.
Materials and Methods
Forty-eight antral gastric specimens were obtained from consecutive autopsies performed within 1 hour of death in the department of Pathology Makerere University Faculty of Medicine on persons who had had no upper gastrointestinal diseases. The specimens, 3 cm in thickness, were fixed in 10% formal saline for 24 hours and then dehydrated in increasing concentrations of isopropyl alcohol followed by clearing of alcohol by xylene before impregnating in paraffin wax. The specimens were subsequently embedded in paraffin wax in cassettes to facilitate tissue sectioning. Standard haemetoxylin and eosin stain was performed on 5 - µm - sections from each specimen block and examined for the degree of gastritis.
Modified Giemsa stain was used on subsequent levels of the same blocks by the method described by Gray et al (1996).5 Immunostains used on more levels of the same blocks were rabbit anti-pylori DAKO code No. BO 471 lot 076 edition 04.02.00, and the procedure of performing the technique was that as described by DAKO, the manufacturer.
Data Analysis
Data collected was entered into the using PC Epi-Info, was analyzed using the same package. The positive and negative predictive values were calculated to determine the sensitivity. The kappa statistic was employed to test statistical significant diffrence between the modified Giesma stain (test stain) and immunohistochemical stain (the Gold standard). The variable of interest was presence of H.pylori organisms.
Results
The 48 cases recruited in this study were all Africans aged between 16 and 74 (mean age of 36.6). There were 36 males and 12 females giving a male to female sex ratio of 3:1, which is the general sex distribution of autopsy cases in this department.
The overall H.pylori infection rate using immunostain was 67% with those dying of infections like tuberculosis, assault/accident and maternal death having H.pylori infection rate of 77, 75 and 67% respectively.
Various forms of antibiotics treatment appeared not to have had influence on the detection of H.pylori in this study as out of 4 patients who were on injectable crystalline penicillin 3(75%) had H.pylori infection. Of the 10 patients on other forms of antibiotics, 9(90%) had H. pylori infection and this was surprising particularly when it was found that 20(66%) of 30 patients who were not on any antibiotic treatment had H.pylori.
Table 1 summaries various forms of gastritis graded according to Sydney system and the frequency of H.pylori. All forms of gastritis including the normal histology had almost 50% H.pylori infection.
Table 1.
Histological findings with associated H. pylori infection
| No cases | H. pylori detection |
|
| Normal histology | 2 | 1 |
| Mild antral superficial gastritis | 1 | 1 |
| Moderatae antral superficial gastritis |
1 | 1 |
| Mild antral pangastritis | 19 | 10 |
| Moderate antral pangastritis | 18 | 11 |
| Severe antral pangastritis | 7 | 3 |
| Total | 48 | 27 |
Table 2 gives a summary analysis of comparing immunohistochemical stain and modified Giemsa stain. Both immonohistochemical stain and Giemsa stain were positive in 27 cases and negative in 14 cases. In 2 cases, they immunostain could not demonstrate the bacteria but were demonstrated by Giemsa while in 5 cases the bacteria were demonstrated by immunostain but not with Giemsa.
Table 2.
Summary of analysis comparing modified Giemsa and immunostain.
| Sensitivity 85% (CI 66.5–94.1) |
| Specificity 89% (CI 60.4–97.8) |
| Positive predictive value 93% (CI 75–98.8) |
| Negative predictive value 74% (CI 48.6–89.9) |
| Kappa 0.69 (p value = 0.00001) |
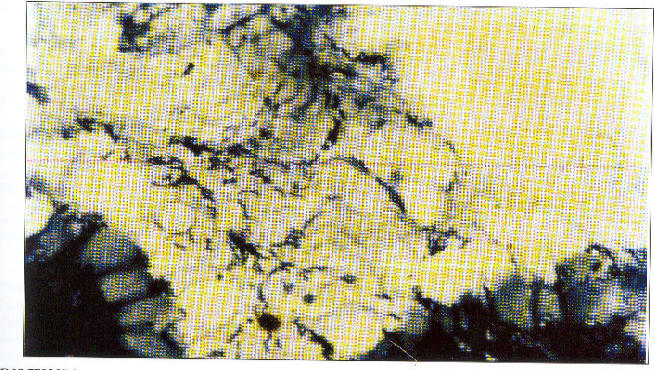
Figure 1 shows spiral bacilli in the gastric mucosa
Figure 1.
Gastric mucosa showing spiral bacilli consistent with H.pylori. Modified Giesma x×1000 (oil immersion)
Discussion
The modified Giemsa stain provided distinctive shape and uniform staining of the bacteria making their identification easy and this was reflected in a high positive predictive value. There were more false negative cases (5 cases) implying that these were probably not detected because of change of morphology from the usual spiral form. H.pylori changes its morphology in culture and during harsh conditions such as antibiotic treatment9 and it is possible that after death intragastric changes may also be harsh to the organism. It is therefore correct to assume that with endoscopic biopsy material, the negative predictive value improves.
It is remarkable to find that antibiotic treatment did not influence detection of H.pylori in this study. However a similar finding was reported by Dooley et al (1989) 8 in which antibiotic treatment before the study was a significant promoting factor.
It is most likely thatantibiotics may eradicate other competitive organisms from the stomach leading to over growth of H.plyori.
The study confirms earlier studies that H.pylori causes antral gastritis even in those who are assumed of being asymptomatic of upper gastro intestinal diseases. However it is also possible that in Africa the bacteria may cause no inflammatory response in some individuals as observed in one case in this study. Tsage et al (1996)7 also found 9 out of 25 Ethiopian patients with normal antral histology infected with H.pylori and this finding may explain the high seroprevalence of H.pylori accompanied by low prevalence of peptic ulcer and gastric cancer in most parts of Africa. More data on large number of health volunteers in Africa is required to understand the factors, which may enhance tissue response to this bacterium.
Conclusion
In conclusion the high positive predictive value of 93% and kappa of 0.69 (P value = 00001) of the modified Giemsa stain yields a good agreement and should be recommended in studying H.pylori infection in developing
Acknowledgement
This study was funded by Makerere University as M.D. programme Research Project No. 1334. I would also like to thank Mrs. Resty Ssemuwemba for the assistance in preparation of the manuscript.
References
- 1.Lamouliatte MF. Helicobacter pylori and duodenal ulcer. Evidence suggesting causation. Digesive Diseases Sciences. 1992;37:769–772. doi: 10.1007/BF01296437. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N. Is Helicobacter pylori a cause of gastric cancer? An appraisal of the seroepidemiological evidence. Cancer Epidemiology Biomarkers Prevention. 1994;3:445–451. [PubMed] [Google Scholar]
- 3.Molyneux AJ, Harris MD. Helicobacter pylori in gastric biopsies - should you trust the pathology report. Journal Royal College Physicians London. 1993;227:119–120. [PMC free article] [PubMed] [Google Scholar]
- 4.Madan E, Kemp J, Westblom TV, Subik M, Sexton S, Cook J. Evaluation of stain methods for identifying Camplobacter pylori. American Journal Clinical Pathology. 1988;90:450–454. doi: 10.1093/ajcp/90.4.450. [DOI] [PubMed] [Google Scholar]
- 5.Gray S, Wyatt JI, Ralhbone BJ. Simplified techniques for identifying Campylobacter pyloridis. Journal Clinical Pathology. 1986;39:1279. doi: 10.1136/jcp.39.11.1279-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson LP, Holck S, Povlsen CO. Campylobacter pylori detected by indirect immunohistochemical techniques. APMIS. 1988;96:559–564. [PubMed] [Google Scholar]
- 7.Tsega E, Giebric W, Hathaway AE, Kassa G. Helicobacter pylori infection in Ethiopian patients with dyspepsia. Ethiopian Medical Journal. 1996;34:145–151. [PubMed] [Google Scholar]
- 8.Dooley CP, Cohen H, Fitzgibbons PL, Baner M, Appleman MD, Perez-Perez GI. Prevalence of Helicobacter pylori infection and histologic gastritis in asymplomatic persons. New England Journal of Medicine. 1989;321:1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- 9.Nilius M, Strohle A, Bode G, Malfertheiner P. Coccoid like forms (CLF) of Helicobacter pylori. Enzyme activity and antigenicity. International Journal Medical Microbiology virology, Parasitology Infectious Disease. 1993;280:259–272. doi: 10.1016/s0934-8840(11)80964-3. [DOI] [PubMed] [Google Scholar]