Abstract
An epidemiological cross sectional study of Schistosoma mansoni was conducted in two hyper endemic fishing villages of Rhino Camp and Obongi both in West Nile district in northern Uganda in 1991 and 1992. People with various water contacts were registered. A small group of civil servants and clergies with less water contact in the river Nile were studied for control of infection and morbidity. An overall prevalence of 81.5% of the 1367 people studied in both fishing villages of Rhino Camp and Obongi were excreting from 100 to ≥ 500 Schistosoma mansoni eggs per gram (epg). 253 18.5% did not have Schistosoma mansoni eggs in their faeces. The influence of socioeconomic factors on infections in the study population was high among poorer illiterates who have frequent water contacts activities with River Nile.
The sonomorphological abnormalities of periportal thickening (PT) due to Schistosoma mansoni were performed using ultrasound.. 664 patients were found to have various stages of (PT stages 0, I, II and III). A total of 703 (51.4%) patients did not have any periportal thickening (PT 0) in their livers despite the fact that 450 (32.9%) of them had Schistosoma. mansoni eggs in their faeces. The gravities of schistosomiasis in the two villages were similar showing greater morbidity in the younger adults.
Keywords: epidemiology, sonomophological, morbidity, Schistosoma mansoni, infection, northern Uganda
Introduction
Previous studies on the morbidity of S. mansoni infection were based on clinical methods to describe the pathological sequelae induced by this parasite. However, the value, of clinical methods is limited 1, 2. Since the introduction of ultrasonography in applied field research of morbidity due to schistosomiasis on a larger scale, conflicting results have been reported from different endemic countries. Field studies in Sudan clearly showed high morbidity of S. mansoni infection 3, 4. The same is true for Zimbabwe 5. Other reports from Egypt, Mali and Senegal revealed intermediate or low morbidity 1, 6, 7.
These findings suggest that global descriptions of morbidity induced by S. mansoni, differ for each endemic setting. Thus the need to define the morbidity patterns in different foci. The present study describes the prevalence and morbidity of S. mansoni infection in two villages along Albert Nile in northern Uganda.
Methods
The study was conducted in the West Nile districts in northern Uganda in two fishing villages of Rhino Camp and Obongi. Each village has a population between 4,000 to 6,000 inhabitants. Rhino Camp is about 50 kilometers east of Arua town. Rhino Camp is located 30 north and 310 East. It formerly served as a harbour for steamer services on River Nile connecting Pakwach and Nimule at the Sudan Border. The village has two market days every week. Fish is the commonest commodity that is readily sold in this market and transported to neighbouring towns especially Arua. A total number of 636 (47%) people were studied in Rhino Camp.
Obongi is located north and 31.50 East and about 100 kilometers north of Arua town along the River Nile. The homesteads in Obongi are sparser than those in Rhino Camp. Transport is irregular in this village. Obongi is not readily accessible like Rhino Camp. Residents of Obongi therefore rely on water transport using canoes and boats. The main activity of the inhabitants of Obongi is fishing. They also cultivate cassava, sorghum and maize for domestic consumption. Here, 731 (53%) people were examined for S. mansoni infection. Residents at Rhino Camp and Obongi are Madi, Lugbara and Kakwa by tribe. These villages had boreholes drilled by the Water and sanitary department of United Nation International Children Education Found (UNICEF), but the residents of Rhino Camp do not use them 8. They prefer water from the River Nile. This is because the borehole water is hard and brackish. Resident of Obongi use their borehole water because the water is soft and insipid.
The stratification of the study groups in both villages was similar. First, all fishermen and women who were registered and licensed to maintain fishing boats within the local landing sites including their families were enrolled into the study. Second, pupils of a primary school situated near River Nile in Rhino Camp and Obongi were studied. Third, a small number of teachers, local administrators and priests who have little water contact with the water in River Nile were also studied. The fourth group was residents who voluntarily enrolled in the study each morning. This category constituted the most diverse group with various socioeconomic backgrounds. Some of these people travelled distances of up to 20 kilometer in order to participate in the study.
Participants in the study were given stool containers labeled with their registration numbers, names, and sex on the registration day. They were instructed to put about 5 gm. of their stool specimen in the containers and to return them to the study center the following day. Egg excretion in the stool was quantified by the classic Kato-Katz method processed using HelmR test kits from Brazil AK Industriae Commercio Ltd., Belo Horizonte, Brazil9. A minimum of 3 slides per stool sample containing 41.7 mg. of stool were examined for helminths eggs. The same individuals delivered another stool sample on the next day and another 3 slides were processed. A concentration technique modified by Magretha Saathoff, at the Institute of Medical Parasitology of the University in Bonn10, was used to control the accuracy of microscopic stool analysis in field. A measure of 0.5 gm. of each stool sample, which was negative for S. mansoni eggs in the field was concentrated and three slides re-examining microscopically.
Intensity of infection was classified as follows: low 1–99; medium 100–499; and high 500 or more eggs per gram stool.11
Ultrasound examination
Abdominal ultrasonography was performed with the Aloka machine (Hellige, Freiburg, Germany) fitted with a convex probe of 3.5 Mega Hertz. Sonomorphological abnormalities of periportal thickening were categorised according to the Managil score 12. Organomorphometry of the liver and spleen was done according to Dittrich et al.13
Ethical consideration
Informed consent was taken from adults. But in cases of children under eighteen years of age, their parents' consent was requested. Parents were explained the scope of the study in the local language and they were asked to decide the participation of their children.
Results
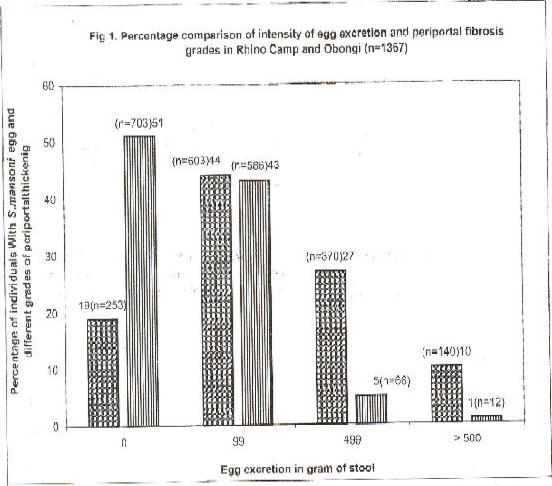
A total of 1367 individuals in Rhino Camp and Obongi were screened for Schistosoma mansoni egg excretion. Six hundred thirty six residents in Rhino Camp (47%) and in Obongi (n=731) 53% were investigated. The prevalence of S. mansoni egg excretion detected in the field was 1114 (81.5%) (Fig. 1.) Of the 139 negative specimens, re-examined in Germany using a concentration technique, 31 (2.3%) proved to be “positive” for S. mansoni eggs. The true prevalence of S. mansoni infection is therefore higher than 81.5% detected in the field. The intensity of egg excretion was classified according by Sliegh et al.11. It was found that 115 out of 1114 (10.3%) of the residents excreted high egg count ≥ 500 eggs per gram (epg) of stool.
Fig 1.
Percentage comparison of intesity of egg excretion and periportal fibrosis grades in Rhino Camp and Obongi (n=1367)
Percentage prevalence of Schistosoma mansoni infections in Rhino-Camp and Obongi in northern Uganda among people with different intensity of egg excretions and grade of periportal fibrosis of the liver. Percentage of infections, and percentage of different grades of periportal thickening.
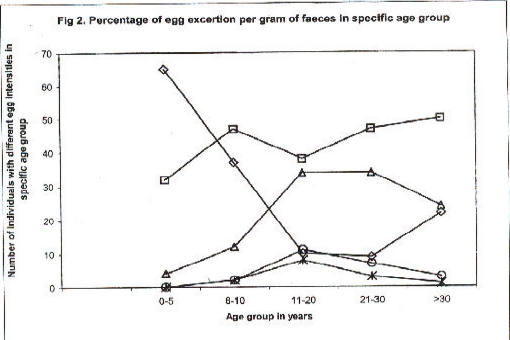
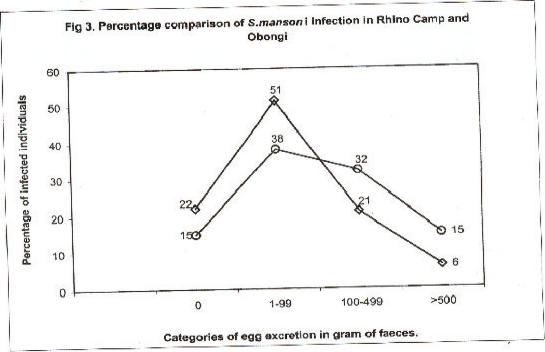
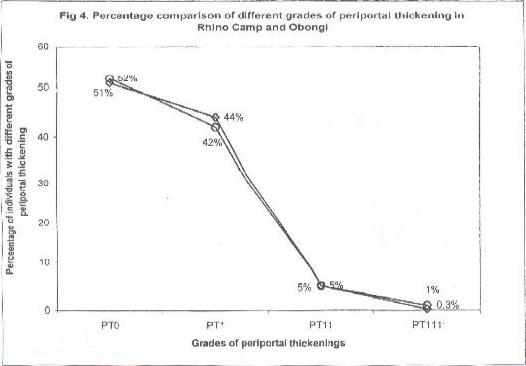
Fig. 2 compares prevalence and intensity of infection according to age group. There was an increasing prevalence of S. mansoni infection up to early adulthood. Therefater the prevalence declined slightly. Parallel to this, there was clear-cut evidence for higher intensity of infection following the same age pattern. A few patients excreted ≥ 1000 eggs per gram. The comparison between the two study sites Rhino Camp and Obongi indicated very similar patterns of prevalence and intensity of infection, Figs. 3, 4.
Fig 2.
Percentage of egg excertion per gram of faeces in specific age group
Percentage prevalence of different level of egg excretion of Schistosoma mansoni in different age groups in Rhino Camp and Obongi in north Uganda. Number of infected individuals with different level of egg excretions in specific age groups. 0–5 years (◇) n= 57; 6–10 years (□), n=130; 11–20years, (△) n=497; 21–30years (Î) n=245; and > 30 year(?)n=438.
Fig 3.
Percentage comparison of S.mansoni infection in Rhino Camp and Obongi
Percentage comparison of Schistosoma mansoni infections in different categories of egg excretion at Rhino Camp and Obongi in northern Uganda. Percentage of infection in Rhino Camp n=636 (◇) and Obongi n= 731 (Î)
Fig 4.
Percentage comparison of different grades of periportal thickening in Rhino Camp and Obongi
Percentage comparison of periportal thickening at Rhino Camp and Obongi in northern Uganda. Periportal thickening in Rhino Camp, (◇) n=636; periportal thickening in Obongi, (O) n=731
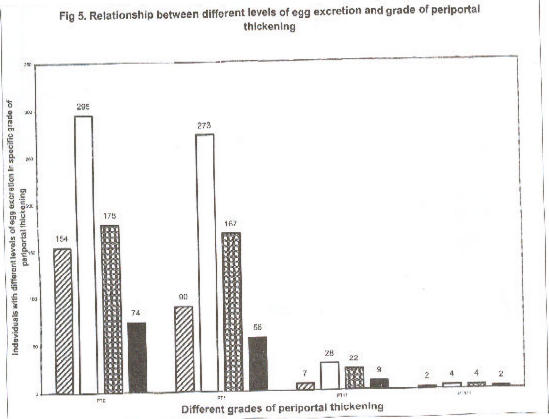
There was no correlation between intensity of infection and periportal thickening (PT) Fig.5. Periportal thickening of varying degrees were seen in 664 (49.4%), the majority constituting grade 1.
Fig 5.
Relationship between different levels of egg excretion and grade of periportal thickening
Relationship between different levels of intensity of infections and grades of periportal fibrosis (n= 1367) patients in Rhino Camp and Obongi in West Nile Province, northern Uganda. Number of individuals in different level of egg excretion; negative, 0 (?) n =253; 1–99 epg (?) n= 602; 100–499epg (?) n=371; ? 500 epg. (|) n=141.
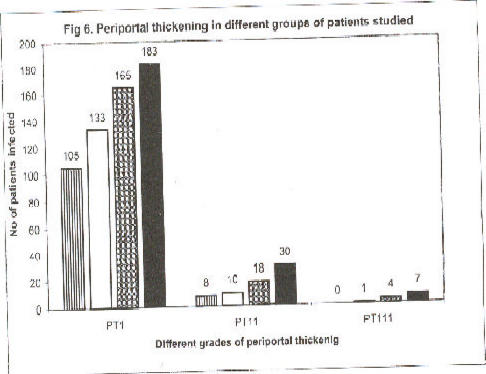
The distributions of (PT) among patients with different social and professional status, indicated that pupils of the primary schools were least affected with PT, followed by civil servants, volunteers, fishermen and their families in that order (Fig. 6). The fishermen with their families and the volunteers constituted groups with daily high water contact activities in the Nile. The majority of patients with grades 11 and 111 of (PT) of the livers occurred among these groups.
Fig 6.
Periportal thickening in different groups of patients studied
Periportal thickening in different categories of individuals studied at Rhino Camp and Obongi in north Uganda, (n=664). Periportal thickening, (PT); school pupils, (?); civil servants, (?); volunteers, (?); and fishermen, (?).
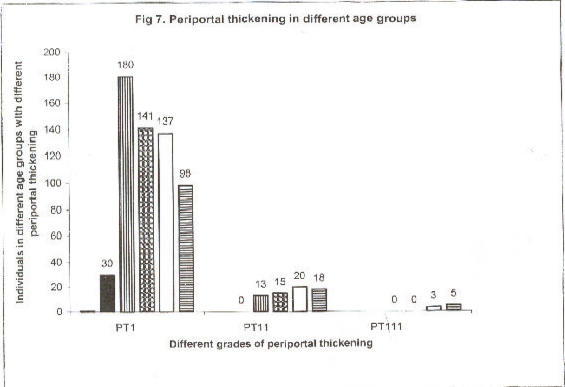
An age-dependent analysis of (PT) showed a clear trend of higher prevalence of grade 1 and 11 (PT) until early adulthood, paralleled by an increase of more severe in age group > 30 years (Figure 2).
Treatments
All patients with Schistosoma mansoni eggs in their stools were treated with praziquentel, 40 mg per kilogram (kg) body weight. Other intestinal nematode worms, Ascaris, lubricoides hookworms, and Trichuris trichiura were treated with mebendazole. Amoebiasis was treated with metronidazole. Pregnant women were advised to receive their treatment of praziquantel from their local health centers after giving birth. Minor ailments detected clinically or through ultrasound, were treated accordingly using local health facilities but severe conditions were referred to the district health facilities.
Discussion
Schistosomiasis was first described in Uganda 14 in 1904. A recent problem, which is drawing great epidemiological attention, is the association of the disease with rice paddies in Uganda 15. Prentise 16 and Kinoti 17 have provided reviews on the epidemiological situation.
In the West Nile region a number of epidemiological reports have been provided by Nelson 18. In Rhino Camp, Bukenya and Andama 19 reported a prevalence of 37% in 1986. Systematic epidemiological data on schistosomiasis in Obongi have, to the best of our , knowledge not yet been published.
The present investigation provides evidence of substantial prevalence and morbidity of Schistosoma mansoni infections in two fishing villages on the bank of River Nile in northern Uganda. The two study sites showed no variation in prevalence and morbidity patterns. There was no clear-cut relation between intensity of infection and periportal thickening in the liver on ultrasound, but a tendency towards higher prevalence of infection and more severe sonographical lesions with increasing age. Differences in the social and professional status of studied individuals were reflected in morbidity patterns and prevalence of infection.
The prevalence of Schistosoma mansoni infections in the area could be higher than 81.5% because on re-examination of 139 specimens, which were negative for S.mansoni by the Kato/Katz method, additional 31 (2.3%) positive were recorded when a concentration method was used. This finding is much higher than previously reported by Adama and Bukenya (37%) at Rhino Camp19.
This sharp increase in prevalence may be due to lack of control measures and civil wars over the years prior to the present investigations.
In 1973 a study from Panyagoro, 20south of the present study area provided proof for high morbidity by clinical observation. Thirty-one patients from this area were admitted to Mulago Hospital in Kampala and liver biopsies were performed 21. These showed occurrences of ascites in relation to palpable enlarged livers and spleens, and this was true for each organ, even when adults and children were considered separately.
Clinical evaluation and ultrasonography may provide a reasonable clinical approach to detect morbidity and provide data for pre-selection of groups of patients with severe liver pathology.
Fig 7.
Periportal thickening in different age groups
Periportal thickening in different age groups in years of 1367 subjects in Rhino Camp and Obongi, north Uganda. Periportal thickening, (PT) age group, 1–5 (|); age group, 6–10 (?); age group
Acknowledgements
This work received financial support from Edna MacConnell Clark Foundation, New York, grant number 05090 and UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases grant number ID 900 286. Ekkehard .Doehring. Was a recipient of a short-term stipend to Uganda by German Academic Exchange Service (Deutscher Akademischer Austauschdienst). Permission to perform the studies was given by the Ministry of Health, the Uganda National Council of Science and Technology and Arua District Medical Officer.
We are grateful to Mr. Patrick Okello Statistician, Uganda Bureau of Statistics for his invaluable work in data analysis, the administrators of the Local Councils of Rhino Camp and Obongi for their support. Reverend Father John Sabo of the Catholic Parish in Rhino Camp (Arua Diocese), Angwar Sector Setoro of Rhino Camp Dispensary, Rabindrath and Bipin Chawda provided logistical help. Anneliese Domke, Rana Lee-Schoeler and Ursula Massour at the Institute of Medical Parasitology assisted in technical work with great dedication in Germany.
References
- 1.Houston S, Munjoma M, Knyimbo K, et al. Use of ultrasound in a study of schistosomal periportal fibrosis in rural Zimbabwe. Acta Trop. 1993;53:51–58. doi: 10.1016/0001-706x(93)90005-v. [DOI] [PubMed] [Google Scholar]
- 2.Doehring Schwerdtfeger E, Kaiser Ch, Schlake J, et al. Ultrasound versus clinical examination as indication for Schistosoma mansoni associated morbidity in children. Trop Med Parasitol. 1992;43:245–248. [PubMed] [Google Scholar]
- 3.Homeida M, Ahmed S, Dafalla A, et al. Morbidity associated with Schistosoma mansoni infections as determined by ultrasound: a study in Gezira, Sudan. Am J Trop Med Hyg. 1988;39:196–201. doi: 10.4269/ajtmh.1988.39.196. [DOI] [PubMed] [Google Scholar]
- 4.Doehring-Schwerdtfeger E, Abdel-Rahim IM, Mohamed-Ali Q, et al. Ultrasonographical investigation of periportal fibrosis in children with Schistosoma mansoni infection: evaluation of morbidity. Am J Trop Med Hyg. 1990;42:581–586. doi: 10.4269/ajtmh.1990.42.581. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Wahab F, Esmat G, Narooz SI, et al. Sonographic studies of schoolchildren in a village endemic for Schistosoma mansoni. Trans Roy Soc Trop Med Hyg. 1990;84:69–73. doi: 10.1016/0035-9203(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 6.Rouquet PH, Verle P, Kongs A, Talla I, Niangn M. Hepatosplenic alterati determined by ultrasonography in a population recently infected with Schistosoma mansoni in Richard-Toll, Senegal. Trans Roy Soc Trop Med Hyg. 1993;87:190–193. doi: 10.1016/0035-9203(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 7.Franke D, Cante B, Diarra A, et al. Different morbidity pattern Schistosoma mansoni induced periportal fibrosis in Mali and Sudan; XIIIth International Congress for Tropical Medicine and Malaria in Pattaya; December 1992; Thailand. [Google Scholar]
- 8.Loroni-Lakwo T, Odongo Aginya EI, Schwelgmann U, Schickeling S, Lindner D, Doehring-Schwerdtfeger E. Transmission of Schistosoma mansoni in Rhino Camp - Uganda. East Africa Med J. 1994;71:165–166. [PubMed] [Google Scholar]
- 9.Katza N, Chaves A, Pellgrino J. A simple device for quantitative thick smear technique in Schistosoma mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:97–100. [PubMed] [Google Scholar]
- 10.Blagg W, Schloegel E, Mansour NS, et al. A new concentration technic for the demonstration of protozoa and helmith eggs in faeces. Am J Trop Med Hyg. 1955;4:23–28. doi: 10.4269/ajtmh.1955.4.23. [DOI] [PubMed] [Google Scholar]
- 11.Sleil A C, Mott KE, Hoff R, Barreto ML, Mota EA, Maguire JH, Weller TH. Three years prospective study of evaluation of mansoni's schistosomiasis in North-East Brazil. Lancet II. 1985;11:63–66. doi: 10.1016/s0140-6736(85)90177-1. [DOI] [PubMed] [Google Scholar]
- 12.Cairo working group, author. The use of diagnostic ultrasound in schistosomiasis-attempts at standardisation of methodology. Acta Trop. 1992;51:45–64. doi: 10.1016/0001-706x(92)90020-x. [DOI] [PubMed] [Google Scholar]
- 13.Doehring-Schwerdtfeger E, MohamedAli G, Abdel-Rahim IM, et al. Sonomorphological abnormalities in Sudanese children with Schistosoma mansoni infection: a proposed staging system for field diagnosis of periportal fibrosis. Am J Trop Med Hyg. 1989;41:63–69. [PubMed] [Google Scholar]
- 14.Castalani Dott Aldo. Osservazioni Sopra Alcuni Casi di Bilharziasis in Uganda Annali di Medicina Navale Anno IX Fasc I–II 1903. Luglid-Agostop - II. 1903:354–360. (Fre). [Google Scholar]
- 15.Bukenya GB, Nsugwa JL, Makanga B, Salvator A. Schistosomiasis mansoni and paddy-rice growing in Uganda an emerging new problem. Annals of Trop Med and Parasitology. 1994;88:379–384. doi: 10.1080/00034983.1994.11812880. [DOI] [PubMed] [Google Scholar]
- 16.Prentice MA. Distribution, Prevalence and transmission of Schistosomiasis in Uganda. Uganda Med J. 1972;1:136–140. [Google Scholar]
- 17.Kinoti GK. A report on Schistosomiasis in Uganda, Pamphlet. 1982. pp. 1–52. [Google Scholar]
- 18.Nelson GS. Schistosoma mansoni infection in the West Nile District of Uganda. Part (I–IV) East. Afr Med J. 1958;35:312–585. [PubMed] [Google Scholar]
- 19.Bukenya C, Andama S. Circumstantial epidemiology of Schistosoma mansoni in West Nile District Uganda, a result of a cross-sectional study in Rhino Camp area. J Trop Med Hyg. 1986;89:243–248. [PubMed] [Google Scholar]
- 20.Ongom VL, Bradly DJ. The epidemiology of consequences of Schistosoma mansoni infection in West Nile Uganda. I Field study of a community at Panyagoro. Tran Soc Trop Med Hygiene. 1972;66:835–851. doi: 10.1016/0035-9203(72)90118-6. [DOI] [PubMed] [Google Scholar]
- 21.Ongom VL, Owor R, Grundy R, Bradley DJ. The epidemiology and consequence of Schistosoma mansoni infection in West Nile, Uganda. II. Hospital investigation of a sample from the Panyagoro Community. Transaction of the Royal Society of Tropical medicine and Hygiene. 1972;66:852–863. doi: 10.1016/0035-9203(72)90119-8. [DOI] [PubMed] [Google Scholar]