Abstract
Background
Many blood glucose self-monitoring systems are privately and publicly used by people in Uganda and technical and human errors may occur during their operation. Many patients were referred to Kololo polyclinic laboratory to have their blood glucose checked because the values obtained on the patients' glucose meter systems did not tally with familiar clinical signs and symptoms. This prompted an experimental set up to check glucose meter systems using a larger number of patients.
Objective
The objective was to collate the technical conditions and standing operational procedures of four common glucose meter systems; observe the time, ambient temperature and humidity at which the meter systems operate locally; and compare the performance of three meter systems A, B, and C with the Sensorex glucose meter system on a number of capillary blood samples.
Setting
Kololo polyclinic laboratory-a privately run facility in Kampala, Uganda.
Design
An experimental set up to compare four glucose meter systems.
Methods
Instruction manuals of the four glucose monitoring systems were studied and used to familiarize with the meter operations. One hundred and fourteen capillary blood specimens were assayed for blood glucose. Blood glucose values were instantly read off the four randomly set meter systems A, B, C, and Sensorex, noting the time, ambient temperature and humidity. Results from meter systems A, B, and C were regressed against those of Sensorex using Epi-Info computer program.
Results
Blood glucose concentration levels on meter system A tallied with those on Sensorex meter system. However, those on meter system B and C were significantly lower and different. Temperature and humidity adversely affected the analytical performance of meter systems B and C in the Kampala environ.
Conclusion
Some of the blood glucose monitoring systems in Kampala, Uganda are poor performers and may lead to the mismanagement of patients. There is need for a system to ensure national quality control of blood glucose monitoring systems.
Introduction
Self monitoring of blood glucose is integral to the management of diabetes mellitus (DM)particularly in insulin dependent diabetes1. Clear benefits of stringent control of blood glucose concentration shown by the Diabetes Control and Complications Trial research group2 have increased the desirability of regular monitoring of blood glucose primarily for people with DM who need to check the level of glucose in their blood in order to adjust the dose of medication. Other aspects of self blood glucose monitoring have become conventional in fore-stalling hypoglycaemia at cot-side in the neonatal unit, patient glucose measurement in hospitals, general practitioner surgeries, nursing homes, hospices, and in the community3.
Originally these blood glucose meters were intended only for monitoring already conventionally diagnosed diabetes mellitus4. Meanwhile the American Diabetic Association recommended improvement in their technology to make them more robust and more accurate1. Subsequently, a number of modern meter systems are accurate enough to serve as diagnostic equipment. They include amperometric, reflectance, and colorimetric transduction devices5.
Although these monitoring systems were improved for use in temperate regions, they have hit global markets including hot and dry environments. This may affect their performance6. Consequently, physicians and professional organizations in Uganda and elsewhere have been cautioned about the limitations of the various systems resulting from the storage, extreme humidity, and temperatures7. Many different glucose meter systems are used in Uganda and are regarded by many patients as sources of solace and by health providers as reliable allies.
However, in routine clinical practice, cases of mismatch between glucose meter values of blood sugar and clinical signs and symptoms have been encountered and this has raised anxiety and concern among patients and clinicians. To gain insight into this issue, the authors compared the performance of four self-monitoring glucose meter systems commonly used in Kampala, Uganda.
Objectives
The objectives of the study were to collate the technical conditions and standing operational procedures of 4 common glucose meter systems; observe the time, ambient temperature, and humidity at which the meter systems operate locally; and compare the performance of three glucose meter systems A, B, and C with the performance of Sensorex meter system.
Methodology
Design: An experimental set up to compare the performance of four glucose meter systems.
Set up: Kololo polyclinic - a privately run institution in Kampala, Uganda
Glucose meter system working principles
Glucose meter systems are biosensors that operate on the following general scheme:
Glucose—Selective membrane—Enzyme layer—Transducer—Amplified, translated screen printing.
In this scheme, glucose passes through a selectively permeable membrane controlling the transport of analytes to a metabolizing enzyme system that generates a response on interaction with the glucose, leading to an electrical signal generated by the transducer and eventually amplified and translated into glucose concentration on the meter screen. Each of these components is briefly described.
The selective membrane
The selectively permeable membrane controls the flux of the solute by size and charge exclusion and thus helps to control the operating range and reduces the impact of sample matrix caused especially by the haematocrit. Data obtained by manipulation of selective membranes have shown a strong supportive evidence of how the same reaction chemistry such as that of glucose oxidase can be “refashioned” to extend measurement versatility8.
The enzyme layer
The enzymes used in glucose meters are oxidoreductases of which the lower-polarizing-potential oxidases (+650 mV) are preferred to the higher-polarizing-potential reductases (+800mV) which, as a result of high potential, are associated with surface fouling and free-radical side reactions 8,9.
Transducer
Four principal transducer types currently used in glucose meters are:
Thermal transducer - measuring calories liberated or consumed during a chemical reaction 10,11; optical transducer - measuring optical properties such as absorbance, fluorescence, reflectance, or light scatter 12; electrical transducer - measuring conductivity 13 and electrochemical transducer - measuring current out-put at fixed voltage, a technique known as amperometry14.
Amperometry has proved a decisive advantage in many comparative studies of modern glucose meter systems 5, especially when polymeric polyelectrolyte sugars have been used as an enzyme loader in the enzyme layer 14 to exclude background reducing species such as ascorbates, urates and others found in clinical specimens15. Moreover, inclusion of electron shuttle molecules in the enzyme layer have reduced the working electrode potential to <200mV thereby cutting off interferences such as tyrosine, paracetamol, ascorbate, and urate 8, 16, thus enabling the technique to be used both in vitro15 and in vivo 17.
Amplified, translated screen-printing
The measured signal is amplified, translated into glucose concentration and thereafter screen-printed on the meter dial. The screen-printing technology enables bulk manufacture with high precision and reproducibility resulting in low-cost electrodes for single use only 18.
The selective membrane and the enzyme layer are normally combined to make the glucose “strips” whereas the transducer and the amplifier screen-printer are integral parts of the meter. Proprietary glucose strips matched with their appropriate meter together make the glucose meter system each with its peculiar limitations in storage, longevity, humidity and temperature ranges. Collation of these limitations on the glucose meter systems used in this work was a cardinal goal.
Meter selection
Sensorex meter system had been earlier evaluated locally against standard spetrophotometric glucose oxidase method in which the value x obtained related to sensorex value as: Sensorex mg/dL = 0.97x−0.18, r2 = 0.98 which was close to successful evaluations of Sensorex meter system elsewhere5 and was thus in use routinely at the clinic. Three other meter systems A (One Touch™, Lifescan Inc. Milpitis, USA) B (Supreme Hypoguard™ Medsys Group Company, UK) and C (Bioscan™ Yeongdong Pharm Corp. Seoul) were brought by patients for evaluation.
Technical procedures
Instruction manuals of the 4 glucose monitoring systems were collated and used to familiarize with the systems operation between 1st and 24th March 2002. During this familiarization period it was established that all the meter systems use glucose oxidase enzyme but with a variety of transduction, amplification, translation and result exhibition.
In the Sensorex system, the test strips contain glucose oxidase, electron shuttle, and an enzyme protector on non reactive ingredients and are designed in such a way that when blood is applied to the reaction zone on the test strip, the glucose oxidase triggers the oxidation of glucose in the blood. The intensity of the electrons formed is chemically mediated with an electron shuttle and measured by the meter in correlation with the concentration of glucose in the blood sample. The test strips should be stored and used between 4°C and 30°C away from direct heat and sun light.
In meter system A, the test strips contain glucose oxidase, peroxidase, 3-methyl 2 benzothiozolinone hydrazone hydrochloride and 3-dimethylaminobenzoic acid on silica gel molecular sieve where glucose and oxygen react in presence of glucose oxidase yielding glucuronic acid and hydrogen peroxide which subsequently oxidizes the enzyme layer dyes in a reaction mediated by peroxidase to produce a blue colored form of the dye. The intensity of this blue color is by reflectance proportional to the glucose concentration in the sample and is screen printed on the meter dial. The test strips should be stored and used in cool, dry place at temperatures under 30°C.
In meter B, a disposable plastic strip containing a chemically treated test area is used to measure the amount of blood glucose. The test area is designated in such a way that when a drop of blood is placed on the top surface of the test area a color change occurs on the reverse side of the strip. The color change is determined either visually, by comparison to chart provided with the strips, or by the proprietary meter. The strips should be kept and used between 5°C and 25°C protected from contamination, humidity and direct sun light.
In meter system C, the reagent strip chemistry is based on the glucose/peroxide reaction in which glucose is oxidized in presence of atmospheric oxygen to glucuronic acid and the resulting hydrogen peroxide oxidizes o-tolidine/benzidine hydrochlorides to a color change catalyzed by peroxidase in the enzyme layer. The evaluations are achieved either visually with color charts supplied with the strips or by reading results screen printed on the proprietary meter. The strips should be stored away from moisture, light and heat at below 30°C.
From March to June 2002, one hundred and fourteen samples of capillary blood from consenting patients sent to the laboratory for routine glucose assays were each instantly analyzed on each of the four meters randomly in tandem according to manufacturers' instructions while noting the time, the temperature and humidity as read off Digital Satellite Television chronometer at the work bench.
Glucose results on meter systems A, B, and C were compared with those on Sensorex meter system according to the American National Committee for Clinical Laboratory Standards119, 20.
Data management
Data were analyzed using EPI-INFO computer programme. Glucose values on each of the three meter systems were regressed19 on the values obtained on Sensorex meter system used as a working gold-standard. Altman and Bland plots for comparing methods21 were used on glucose values of each of the three test meters against the values on Sensorex. Effects of time of the day, and humidity on the meter performance were tested using Kruskal-Wallis non parametric version of Chi square test for two groups19,22. The effects of temperature were studied using Levene's test for quality of variance at low and high temperatures.
Results
Technical data of the meter systems:
The technical data of the four meter systems collated as shown in Table 1.
Table 1.
Technical Data of the Glucose Meter Systems in this Study
| Meter System | Sensorex | A | B | C |
| Enzyme system | GOD/Shutt/Protector* | GOD/Perox/Perox/Dime Abe | GOD/Perox/Tolidine | GOD/Perox/Benz Citrate |
| Temp Range °C: | 04–30 | 4–30 | 05–25 | < 30 |
| Usual Fasting glucos range mg/dL: |
70–105 | 70–100 | Not given | 70–100 |
| Measuring glucose range mg/dL: |
30–550 | 0–600 | 38–450 | 20–800 |
| Humidity range % | 0–90% | 0–90 | 0–85 | Not given |
| Haematocrit range % | 20–60 | 25–60 | 35–55 | Not given |
| Reference method | YSI** | YSI** GOD | Hexokinase | Trinder's GOD |
| Transduction | Amperometry | Reflectance | Colorimetry | Colorimetry |
| Regression equation | y = 0.98x +8 y = 0.98x +8 Not r = 0.976 Where y = meter C value x = Trinder GOD value r = Deming regression coeff |
Not given | Not given | Y = x ™ 5 Y = x ™ 5 r = 0.993 Where y= Sensorex value X= YSI value Y= Deming regression coeff |
| Legend: *GOD/Perox/Benz/Citrate | = | Glucose oxidase 10µg. Peroxidase 1.24µL, O.tolidine HCl 31µg, Benzidine HCl 53µg, Sodium citrate 13.6µg Citric Acid 6.4 mg, non reactive support 0.2mg. |
| GOD/Perox/Tolid | = | Glucose oxidase 33.4µg. Peroxidase 0.75µg. Tolidine 8.5µg. |
| GOD/Perox/MeBenz/Dime A.Be | = | Glucose oxidase 14 IU, Peroxidase 11 IU, 3-ME-2-benzothiazolinonehydrazone HCl 0.06mg, 3 diMe aminobenzoate 0.12 mg. |
| God/shutt/Protect | = | Glucose oxidase 30 IU, Electron shuttle 1.5mg Enzyme protector 0.13mg, non reactive support 2.5 mg. |
| **YSI | = | a dedicated laboratory glucose analyser from Yellow Springs Instrument Co. Inc. Ohio, USA. |
where the temperature range recommended for all the meters is less than 30°C to cool (4°C )except for meter B whose range is 25°C–5°C. Humidity conditions for all the meters are up to at least 85% except for meter C which has no recommended numerical value. The operational glucose range of all the four meters covered the range clinically expected for hypo-, normo and hyper-glycaemia at least 0–450 mg/dL. The physicochemical principle for measuring glucose concentration was absorptiometry for both meters B and C while meter A depended on reflectance as Sensorex depends on amperometry both of which techniques have decisive advantage5. The reference methods of glucose analysis were all based on colorimetry but the enzyme systems for meter C and meter B incorporate the classical benzidine and tolidine respectively, whereas with meter A and Sensorex the enzyme systems incorporate more modern dyes of methyl benzothiazolinonehydrazone hydrochloride with dimethylaminobenzoic acid and electron shuttle, non reactive support with even an enzyme protector.
Capillary blood glucose concentration
Generally, glucose concentration meter readings on Sensorex and on meter A were higher than those on meter B and meter C. Specifically, individual meter readings show the same trend with samples from the same patient (table not shown). The mean values on Sensorex and on meter A were 142.27 and 140.27 respectively while the mean values on meter B and on meter C were as low as 102.21 and 82.99 respectively as summarized in Table 2.
Table 2.
Descriptive Summary Statistics of time temperature, humidity and glucose values on Sensorex, Meter A, Meter B and Meter C systems
| N | Minimum | Maximum | Mean | Std. Deviation | |
| Temp (°C) | 114 | 20 | 29 | 23.84 | 1.64 |
| Humidity (%) | 114 | 58 | 72 | 63.60 | 3.41 |
| Sensorex Glucose mg/dL | 114 | 42 | 530 | 142.27 | 89.29 |
| Meter A Glucose mg/dL | 114 | 44 | 540 | 140.27 | 3.73 |
| Meter B Glucose mg/dL | 114 | 36 | 400 | 102.21 | 82.99 |
| Meter C Glucose mg/dL | 114 | 20 | 359 | 76.34 | 46.02 |
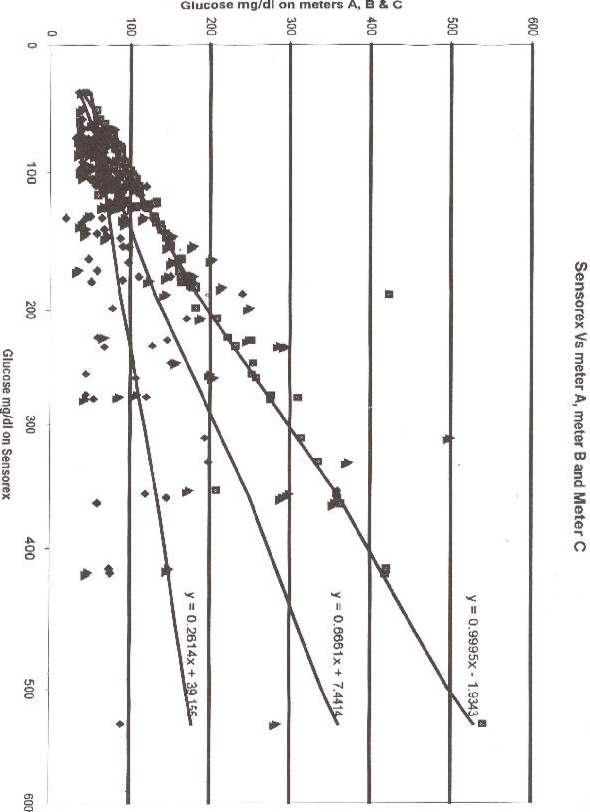
The glucose values on meter A , meter B and meter C regressed on values on Sensorex are Fig Figure 1 With the connecting equation on meter A y=0.995x–1.9343, there is near-perfect correlation between meter A and Sensorex values. As the values on meter B are generally lower than those of Sensorex , the regression equation of meter B is y=0.6661x + 7.4414. The lowest values are obtained on meter system C and the regression equation with Sensorex values on meter C is y =0.2614x + 39.156.
Error percentage of meter values at critical values on Sensorex
Schematically the regression line of meter A makes gradient of approximately 1 which indicates near perfect concurrence of Sensorex and meter A values. The regression line of meter B makes a gradient of 0.66 indicating divergence between meter B and Sensorex. Finally the regression line of meter C has a gradient of 0.23 a value suggesting discordance between meter C and Sensorex values.
With the usual reference range of blood glucose of 40–120 mg/dL, critical values of hypo and hyper glycaemia are 40 mg/dL and 120 mg/dL respectively. Using the regression equations obtained above, at hypoglycaemia borderline of 40mg/dL on Sensorex, meter A would read 38 mg/dL, an error of 5%,whereas meter B would read 34 mg/dL, an error of 15%, as meter C would read a mere 10 mg/dL, an error of 75%. At the upper border-line of 120 mg/dL on Sensorex, meter A would give 118 mg/dL, an error of 2% as meter B would give 87 mg/dL, an error of 28% whereas meter C would give 119 mg/dL, an error of 1%.
Therefore for the usual range of 40–120 mg/dL, meter A has an acceptable error range5 of 2–5%, meter B has an unacceptable error range of 15–20% while meter C has a grossly unacceptable error range of 1– 75%.
Altman and Bland plots of glucose values of the test meters and Sensorex
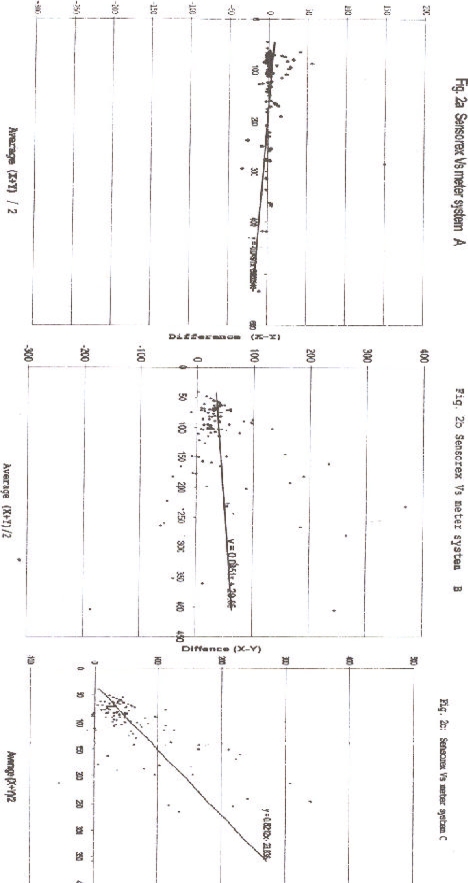
The difference between results on meters A, B, C, and Sensorex is studied by Altman-Bland plots given in Fig 2.
Figure 2.
Altman and Bland plots of glocuse values on meters A, B and C with glocuse values on Sensorex
The differences between the values on Sensorex method and meter A method plotted against the mean value of the two methods bestride the zero line, indicating that there is no big differences in accuracy between meter system A and Sensorex at a gradient of 0.049 (Fig 2a). The Altman and Bland plot of meter B values and those of Sensorex is shown in Fig 2b. The points are scattered on one side of the zero difference line and are more clustered towards the zero glucose value indicating that the meter B values are consistently lower than those of Sensorex at a gradient of 0.085 and that there is an absolute bias1,19. Similarly, the Altman and Bland plot between meter C and Sensorex values shows the points are all on one side of the zero difference at an acute angle with a gradient of 0.82, meaning that the meter C values are consistently lower than those of Sensorex and that the bias is proportionate with the absolute concentration 19 (Fig 2c). This makes meter C the most discordant with Sensorex.
Meter reliability based on Sensorex
When the glucose concentration results obtained on the three test meters are together regressed on Sensorex values as an indicator of reliability, the following equation emanates:
y = 0.83x1 +0.102x2 +0.059x3 + 10.9 (1), where x1 = glucose value on meter A, x2 = glucose values on meter B, while x3= the glucose values on meter C, and y = the glucose values on Sensorex meter. Meter A shows 83% reliability of the results based on Sensorex , meter B shows 10% reliability while meter C gives 6% reliability.
Time, Temperature, Humidity and Glucose values
Descriptive summary statistics of time, temperature, humidity, and glucose values on the meter systems are given in Table 2.
Effect of ambient temperature
Temperature was categorized into two: low ≤25°C, and high, > 25°C. Levene's test for equality of variance6 was then used to test the effect of low and high temperatures on the meter systems. Table 3 gives the results.
Table 3.
Levene's Test for Equality of variance of the meter systems on High and Low temperatures.
| Meter system | Variance | t-value | P | SE | 95% CI | Interpretation |
| Sensorex | Equal | 2.37 | 0.020 | 21.15 | 8.16–91.97 | Slight effect |
| Unequal | 3.47 | 0.001 | 14.42 | 21.21–78.92 | of temp. | |
| Meter A | Equal | 2.60 | 0.020 | 22.20 | 8.41–96.40 | Slight effect |
| Unequal | 3.54 | 0.001 | 14.80 | 22.80–81.998 | of temp | |
| Meter B | Equal | 2.35 | 0.021 | 19.17 | 7.17–85.11 | Slight effect |
| Unequal | 3.04 | 0.004 | 15.16 | 15.57–76.71 | of temp. | |
| Meter C | Equal | 2.02 | 0.46 | 10.97 | 0.39–43.88 | Considerable |
| Unequal | 3.12 | 0.003 | 7.10 | 7.97–36.30 | effect of temp. |
P = 2 tailed level of significance
CI = Confidence intervals
SE = Standard error
Whereas the effect of temperature on the performance of Sensorex, A, and B meter systems was slight, there was significant effect of temperature on the performance of meter system C.
i. Effect of humidity
The ambient humidity was divided into two categories: low humidity, ≤65% and high humidity, > 65%. Table 4 shows the effect of ambient humidity on the performance of the meter systems as studied using Ruskal-Wallis test, an appropriate variety of Chi square test22.
Whereas, the other meter systems were not affected, meter system B was slightly affected by humidity
ii. Effect of time on glucose meter systems
Time on military clock system was divided into two categories: early, £1430 hours, and late, > 1430 hours. The effect of time as a surrogate measure of light on the performance of the meter systems is shown in Table 5.
Table 5.
The effect of time as surrogate measure of light on the performance of the meter systems.
| Meter System | Kruskal-Wallis | P-Value | Interpretation |
| Sensorex | 3.971 | 0.046287 | Slight effect |
| Meter A | 2.851 | 0.091340 | No effect |
| Meter B | 2.782 | 0.095300 | No effect |
| Metet C | 1.828 | 0.176974 | No effect |
Whereas the other meter systems were not affected, Sensorex meter system performance was slightly affected by time.
Similarity of glucose values on the meters
Paired t-test results on the similarities of the glucose values on the different meter systems are given in Table 6.
Table 6.
Paired t-test on result similarity of each meter to Sensorex
| Meter System |
2-paired difference |
SD | SEM | t-value | P value | Interpretation |
| Meter A | −2.000 | 78.638 | 2.682 | −0.75 | > 0.05 | Same |
| Meter B | 40.0614 | 65.111 | 6.098 | 6.57 | < 0.05 | different |
| Meter C | −65.9298 | 76.956 | 7.208 | −9.15 | < 0.05 | different |
SD = Standard deviation
SEM = Standard error of the mean
P = 2 tailed level of significance
At the significance level of p<0.05, meter A values and those of Sensorex were not different while those on meters B and C were significantly different from those on Sensorex. This is not surprising because among other things, the physico-chemistry basis of their operation: reflectance for meter A and amperometry for Sensorex, have a decisive advantage over colorimetry5 of meters B and C.
Discussion
Addressing the concern of accuracy of glucose monitoring meter systems in Uganda, the results in this work have shown that meter A gave results close to those based on Sensorex, the working gold standard. This makes meter A a reliable appliance whose results can be safely used in designing a treatment regimen by the clinician. On the other hand, meter B and C gave unreliable results that can be a source of danger when used to treat diabetes as was the fatal case in Scotland23.
The values of B and C indicated false “meter hypoglycemia” as has been reported elsewhere24. This could lead a clinician or a patient to withhold insulin administration, even in the presence of hyperglycemia. This case involved only 3 experimental meter systems but there are many more types of meters in this country that were not studied in which cases there are possibilities of substandard meter reading values that indicate false glycaemia. Treatment of patients based on such results has led to patient death due to hypoglaceamia elsewhere23 and the same may happen in this country. Since self monitoring glucose meter systems are heavily relied upon and, by operation, replace both the laboratory scientist and the clinician, they deserve the highest order of quality assurance25, 26, 27.
Comparing the ambient temperature, humidity in Kampala with those at which each meter is recommended to operate (table 1), it is clear that meter systems B and C are not robust enough to operate at Kampala ambient conditions, which on the whole do not vary from those of the rest of the country where the mean annual maximum temperatures range between 25°C and 30°C as the maximum relative humidity is often high ranging between 70% and 100%28. Meter C is significantly affected by temperature in Kampala.
These results and those reported elsewhere 1,5,11,15 suggest that the kind of transduction of the amount of analyzed glucose plays a big role in the accuracy of the results. In this work, as has been reported elsewhere, amperometric15 and reflectance devices11 offered the most reliable results.
Conclusionand Recommendation
Some blood monitoring systems in Uganda perform poorly. Total quality management, such as documentary controls over point of care testing (POCT) in the USA and UK27 should be established for glucose meter systems in Uganda.
Sensorex Vs meter A, meter B and Meter C
Table 4.
The Effect of Humidity on Glucose meter systems
| Meter System | Kruskal-Wallis | P-Value | Interpretation |
| Sensorex | 0.402 | 0.526265 | No effect |
| Meter A | 0.010 | 0.918830 | No effect |
| Meter B | 4.092 | 0.043094 | Slight effect |
| Meter C | 0.439 | 0.507458 | No effect |
References
- 1.Johnson RN, Baker RJ. Accuracy of devices used for self-monitoring of blood glucose. Ann Clin Biochem. 1998;35:69–74. doi: 10.1177/000456329803500108. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group, author. The effects of Intensive treatment of diabetes on the development and progression of long term complications of insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Barlow IM, Beer SF. External glucose meter quality assurance in the UK: Are we doing enough? Ann. Clin Biochem. 2001;38:272–274. doi: 10.1258/0004563011900506. 2001. [DOI] [PubMed] [Google Scholar]
- 4.Basic™ One Touch ® Blood glucose monitoring system Owner's Booklet Life Scan Canada Ltd. 1996. [Google Scholar]
- 5.Johnson RJ, Baker JR. Analytical error of home glucose monitors: a comparison of 18 systems. Ann Clin Biochem. 1999;36:72–79. doi: 10.1177/000456329903600110. [DOI] [PubMed] [Google Scholar]
- 6.Nawawi H, Sazali BS, Kamaruzaman BH, Yazid TN, Jemain AA, Ismail F, Khalid BAD. Effect of ambient temperature on analytical and clinical performance of a blood glucose monitoring system: Omni test sensor glucose meter. Ann Clin Biochem. 2001;38:676–683. doi: 10.1258/0004563011901091. [DOI] [PubMed] [Google Scholar]
- 7.Sensorex® G, author. Blood Glucose test strip Insert. Hsinchu Taiwan R.O.C. P/N 65015010003: Apex Biotechnology Corp; [Google Scholar]
- 8.Pearson JE, Gill A, Valgama P. Analytic Aspects of Biosensors. Ann Clin Biochem. 2000:37119–37145. doi: 10.1258/0004563001899131. [DOI] [PubMed] [Google Scholar]
- 9.Katakis I, Domingez E. Catalytic Electrooxidation of NAD for Dehydrogenase Amperometric Biosensors. Microchin Acta. 1997;126:11–32. [Google Scholar]
- 10.Carsson T, Adamson U, Lins PE, Danielssn B. Use of aan enzyme Thermister for Semi-continous Blood Glucose Measurements. Clin Chem acta. 1996;251:187–200. doi: 10.1016/0009-8981(96)06306-1. [DOI] [PubMed] [Google Scholar]
- 11.Xie B, Hedberg U, Mecklenberg M, Danielsen B. Fast Determination of Whole Blood Glucose with Calorimetric Micro-Biosensor. Sensor Actvator B. 1993;15:141–144. [Google Scholar]
- 12.Blum LJ, Coulet PR. Making Light Work. Chem Br. 1994;30:300–302. [Google Scholar]
- 13.Cullen D, Sehti R, Lowe C. A Multi Analyte Miniature Conductance Biosensor. Anal Clin Acta. 1990;231:33–40. [Google Scholar]
- 14.Gibson TD, Hubert JN. Prevention of Shelf Life of Enzyme Based Analytical Systems using a Combination of Sugars, Sugar Alcohols and Cationic Polymers or Zinic Ions. Anal Chem Acta. 1993;279:185–192. [Google Scholar]
- 15.Liu BH, Hu RQ, Deng JQ. Characterization of immobilization of an enzyme in a Modified Y zeolite Matric and its Application to Amperometric Glucose Biosensor. Anal Chem. 1997;69:2342–2348. doi: 10.1021/ac960930u. [DOI] [PubMed] [Google Scholar]
- 16.Ohara TJ, Rajagopalan R, Heller A. Wired Enzyme electrodes for Amperometirc Determination of Glucose or Lactate in the Presence of Interfering Substances. Anal Chem. 1994;66:2451–2457. doi: 10.1021/ac00087a008. [DOI] [PubMed] [Google Scholar]
- 17.Atanasou P, Yang S, Sahli C, Ghindilis AL, Wilkins E, Schade D. Implantation of a Refillable Glucose Monitoring Telemetry Device. Biosens Bioelection. 1997;12:669–680. doi: 10.1016/s0956-5663(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 18.Kurabe I, Ikebukuro K, Murakami Y, Yokoyama K. Miccromachining Technology and Biosensors. Ann N Y Acas Sci. 1995;750:101–108. doi: 10.1111/j.1749-6632.1995.tb19935.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones RG, Payne BR. Analytical Methods: Control and comparison: Chapter 2 in Clinical Investigation and Statistics in Laboratory Medicine. CB Venture Publications; 1997. pp. 27–65. [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards, author. Method Comparison and Bias Estimation Using Patients Samples; Tentative guidelines. NCCLS 771 East Lancaster Avenue, Villanora Pennsylvania: 1993. ISBN 1-56238-189-X. [Google Scholar]
- 21.Bland JM, Altman DG. Statistical Methods for assessing agreement between measurements. Biochem Clin. 1987;11:399–404. [Google Scholar]
- 22.Munro BR, Batter EB. Chapter 5 in Statistical Methods for Health Care Research J B. Philadelphia: Lippincott Company; 1993. Selected nonparametric Techniques; pp. 81–89. [Google Scholar]
- 23.Hazard Notification CSA (SD)HAZ 16/1987 Blood Glucose Measurement, author. Reliability of Results Produced in Extra-Laboratory Areas. Edinburgh: Scottish Health Service CSA Supplies Division; 1987. [Google Scholar]
- 24.Gama R, Anderson NR, Marks V. Glucose meter hypoglycaemia: Often a non-disease. Ann Clin Biochem. 2000;37:731–732. doi: 10.1258/0004563001899825. [DOI] [PubMed] [Google Scholar]
- 25.Barlow IM, Beer SF. External glucose meter Quality Assurance in the UK: are we doing enough? Ann Clin Biochem. 2001;38:272–274. doi: 10.1258/0004563011900506. [DOI] [PubMed] [Google Scholar]
- 26.Bary Hill. Mark of approval, IVD directive 98/97/EC Health Care Equipment Supplies International (HESI) 2002 Mar–Apr;3(3):12.
- 27.Point of Care testing Guidelines. ACB News/Issue 433. 1999 May;:16–20. [Google Scholar]
- 28.Byaruhanga A, Kasoma P, Pomeroy D. Important Bird Areas in Uganda: Nature Uganda E A Natural History Society. 2001