Abstract
Introduction
Over 2 million children globally are HIV positive. More than 90% are infected in utero from their mothers. Current pharmacological methods to reduce the rate of vertical transmission are too expensive for the developing world. Chloroquine, a cheap, widely available drug, has anti-HIV properties. We conducted a pilot study to determine if chloroquine can reduce HIV vertical transmission.
Methods
287 samples of stored, frozen cord blood from a cohort of Ugandan infants born to HIV positive mothers were analyzed for concentrations of chloroquine and its two major metabolites, monodesethylchloroquine and didesethylchloroquine. The HIV status of each infant was determined by ELISA with Western Blot confirmation at 15 and 18 months of age.
Results
49% of samples had measurable chloroquine or metabolite. Of those with measurable drug, the higher concentrations of chloroquine and its metabolites were more frequently associated with HIV negative infants. However, only the median concentration of didesethylochloroquine was significantly higher in HIV negative infants vs. HIV positive infants (1.6ng/ml vs. 0.9ng/ml, p=0.05).
Conclusions
Nearly half of all infants in a Ugandan cohort are exposed to chloroquine in the last trimester of pregnancy. Such random maternal chloroquine use may be associated with a decreased rate of HIV vertical transmission. The issue of maternal chloroquine use requires controlled study before any clinical conclusions may be drawn.
Funding
This work was supported by an AIDS International Training and Research Program at Case Western Reserve University (TW00011) Fogarty Grant
Keywords: human immunodeficiency virus, chloroquine, vertical transmission
Introduction
The World Health Organization (WHO) estimated that by the end of 1998, over two million children globally were HIV positive, and that there were 590,000 new perinatal infections that year, with most of them in the developing world.1 In wealthy countries children and their mothers have access to a growing pharmacopoeia of reverse transcriptase and protease inhibitors for HIV treatment. However, precise dosing regimens dependent on a steady supply of both drugs and money leave children in poor countries without treatment to either reduce vertical transmission, or retard the progression from HIV infection to AIDS and, ultimately, death.
Pediatric HIV researchers are well aware of the economic and political factors that affect treatment options for children in the developing world. The most cost-effective way to reduce the morbidity and mortality of HIV-related disease is to prevent infection. Accordingly, research has focused on reducing vertical transmission from mother to child. The ACTG 076 protocol of prenatal, intrapartum, and postnatal AZT that reduces vertical transmission in selected women from 25% to 8%2 is now the benchmark by which other trials are measured. In 1998, a trial from the CDC in Thailand showed that a short course of AZT twice a day orally beginning at 36 weeks gestation, and continued through labor and delivery reduced transmission by 51%, from 18.6% to 9.2%.3 However, Mansergh et al concluded that although nationwide use of such a short course of AZT could provide long-term savings to a society with high HIV prevalence, it would require substantial short-term investment to implement.4 Due to limited budgets, pressing economic demands for allocation of those budgets, and frequently unstable political climates that make long-term investments especially risky, it is doubtful whether such widespread implementation of AZT protocols can occur.
Most recently, in 1999, researchers in Uganda have shown that the non-nucleoside reverse-transcriptase inhibitor nevirapine decreases vertical transmission by 50% when given as a single dose to a laboring mother and subsequently to the infant within the first 72 hours of life.5 This is the most promising pharmacological intervention yet, because it is simple, cheap, and effective. However, experience with AZT and the other anti-HIV drugs has shown that single-drug therapy against the virus rapidly induces resistance. Although nevirapine in this setting is to be given as a single dose, with widespread use and misuse, retroviral resistance is a real possibility.
Therefore, another safe, extremely cheap drug, efficacious against HIV and already widely available in developing nations would be a boon. For the last decade there has been slowly accumulating biochemical and clinical evidence that chloroquine (CQ), long a mainstay in the treatment of malaria, may be such a drug. We conducted a pilot retrospective study to measure cord blood concentrations of CQ taken from a cohort of infants born to HIV positive Ugandan mothers. Our hypothesis was that the average concentration of CQ in cord blood would be higher in HIV negative infants.
Methods
A collaborative study of HIV infection in Ugandan women and their children was established in 1988 between the Ministry of Health, Makerere University, Kampala, and Case Western Reserve University in Cleveland, Ohio. A cohort of children born to HIV-positive mothers was generated and followed in a fashion described elsewhere.6 Of the 390 children in this cohort, cord blood samples for 322 remained in freezers at an average temperature of −70°C at New Mulago Hospital in Kampala, Uganda.
These samples were shipped on dry ice to Case Western Reserve University for measurement of concentration of CQ and its two major metabolites, monodesethylchloroquine (MCQ) and didesethylchloroquine (DCQ). We used the high performance liquid chromatographic (HPLC) method of Chaulet et al7, but modified it for a smaller sample volume, greater sensitivity, and shorter analysis time. MCQ and DCQ were generously provided by Dr. C. G. Caillard at Rhone-Poulenc Rorer (Vitry, France). CQ diphosphate and imipramine HCl (IM) were purchased from Sigma (St. Louis, MO). Sample extraction was performed using a Gilson ASPEC XL sample processor (Middletown, WI). The 1 ml C8 Bondelute cartridge (Varian, Harbor City, CA) was conditioned with 2 ml methanol and 2 ml 0.1 M ammonium formate, pH 9.2 (AF). A sample containing 0.5 ml plasma plus 0.1 ml of internal standard (50 mcg/ml IM) was added and the extraction cartridge then washed with 4 ml AF followed by 1 ml of methanol: AF (1:1). The sample was eluted with 2 ml of methanol:AF (99:1). The eluate was dried under nitrogen in a 16×100 mm silanized glass tube (Eale Picher, Miami, OK) at 35°C, and redissolved in 0.1 ml mobile phase.
Equipment from Varian Analytical Instruments, including the model 5060 HPLC (San Fernando, CA), was used for the analysis. The 50 mcl sample was injected onto a 300×4 mm MCH Si-10 silica column using a model 9090 autosampler with a Valco C6U switching valve. Mobile phase was acetonitrile: 7.5% ammonium hydroxide in methanol (55:45) and was pumped at 1 ml/min. A model 9070 fluorescence detector was set at 326 nm excitation and 376 nm emission and chromatography peak areas were integrated and printed on a model 401 printer integrator. Peak elution times for IM, CQ, DCQ, and MCQ were 4.3, 8.3, 11.7, and 14.3 minutes, respectively.
Standards between 2 and 1000 ng/ml were analyzed with each set of unknowns. A linear equation was derived from log-transformed data of the area ratios of standards to internal standard using Delta Graph software on the Macintosh IIsi (Apple Computer, Cupertino, CA). The mean recoveries for IM, CQ, DCQ, and MCQ were 77%, 85%, 78%, and 78%, respectively. The mean day-to-day percent coefficients of variation (precision) for CQ, DCQ, and MCQ were 2.0%, 1.4%, and 2.4%. The mean percent deviations from linearity (accuracy) were 2.3%, 2.4%, and 2.7%, respectively.
Measurement of enzyme-linked immunosorbent assay (ELISA) at 15 and 18 months of age, with Western Blot confirmation of positives determined HIV status. The 35 unknowns (11.5%) either died or were lost to follow-up before HIV status could be established.
Results
Of the original cohort of 390 children born to HIV positive Ugandan mothers, 322 samples remained in the freezers. The remaining 68 samples were lost or used for other studies. Over the ten years since the samples had been collected, some of them had degraded and were no longer analyzable. Table 1 shows the distribution by HIV category of the remaining 322 samples adequate for CQ assay.
Table 1.
Samples (percent) adequate for analysis of CQ and metabolites
| Adequate Sample |
Inadequate Sample |
Total | |
| HIV+ | 74 (90.2) | 8 (9.8) | 82 |
| HIV− | 193 (94.2) | 12 (5.8) | 205 |
| Unknown | 35 (100) | 0 (0) | 35 |
| Total | 302 (93.8) | 20 (6.2) | 322 |
Table 2 shows the distribution of these analyzed samples with regard to the original 390 samples. The 35 children who died (97%) or were lost to follow-up (3%) before determination of HIV status were included in the calculation of in utero exposure to CQ, but not included in the analysis of the relationship between prenatal CQ use and HIV transmission. Of the 287 children with known HIV status (regardless of adequacy of sample for analysis), 82 (28.5%) were HIV positive by ELISA with Western Blot confirmation at 15 or 18 months of age. This estimate of baseline vertical transmission is similar to that reported in the ACTG 076 trial.2
Table 2.
Distribution of analyzable samples with respect to original cohort.
| Original Cohort |
Present Cohort |
|
| HIV+ | 89 | 74 |
| HIV− | 259 | 193 |
| Unknown | 42 | 35 |
There is no significant difference in the number of samples lost from each group, i.e. the distribution of the original cohort is preserved in the present cohort (p=0.87, Chi-Square)
Of the analyzed samples of cord blood from children with known HIV status, 130 (49%) had some CQ and/or metabolites. Since the terminal half-life of CQ in adults is approximately 30–60 days,8 this indicates that nearly half the mothers took CQ during the last trimester of the pregnancy.
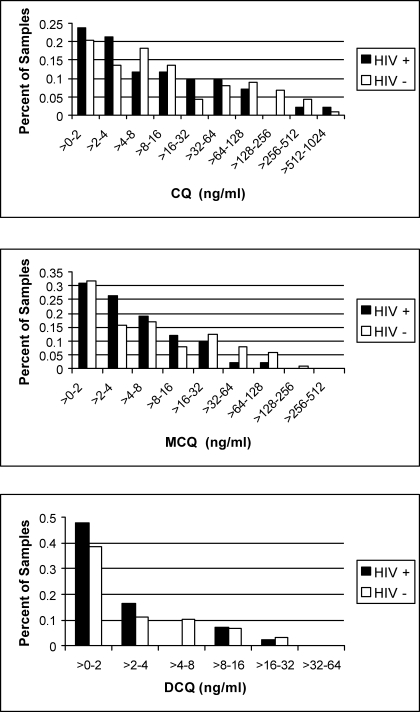
Two samples with supraphysiological CQ concentrations were judged to be contaminated and excluded, as were the 35 unknowns, leaving 265 samples matched with children of known HIV status. Of the 265 samples, 125 (47%) had no CQ or metabolites. Figure 1 shows the distribution of concentrations of CQ, MCQ, and DCQ, with respect to HIV status (excluding those without drug present). We used Wilcoxan rank analysis for all comparisons to test statistical significance. Simply testing the relationship between presence of CQ or either of its metabolites and negative HIV status revealed trends, but no significance (p=0.17, 0.10, 0.20 for CQ, MCQ, DCQ, respectively). When we looked only at cord blood samples that had some drug present, in each case again the median concentration was higher in the HIV negative group (Table 3), and was significantly so for DCQ (p=0.05).
Figure 1.
Concentration of parent drug (CQ) and its two major metabolites (MCQ, DCQ) in cord blood plasma with respect to HIV status. Samples without any drug present have been excluded.
Table 3.
Median drug concentrations (ng/ml) by HIV category, excluding all samples without any drug present
| HIV+ | HIV− | pvalue | |
| CQ | 4.85 | 7.90 | 0.23 |
| MCQ | 2.8 | 4.5 | 0.17 |
| DCQ | 0.9 | 1.6 | 0.05 |
Discussion
In 1989, French researchers reported that human monocytes incubated with HIV virus in the presence of CQ demonstrated intracellular vacuoles filled with virions in various stages of degradation. Untreated cells showed no such viral degradation. Because they did not find viral particles in monocyte cytoplasm, they hypothesized that CQ may have interfered with the release of HIV virions from vacuoles into cytoplasm.9 The following year Tsai and colleagues showed that CQ reduced the size of HIV-1 infected cells, the expression of viral gp120 membrane proteins, and that total viral progeny yield as well as individual virion infectivity was reduced.10 In 1993, Sperber et al demonstrated that hydroxychloroquine (HCQ), an analogue of CQ, inhibited HIV-1 replication by more than 75% (as measured by reverse transcriptase activity) in T cells and monocytes.11 In contrast to the earlier French study, Sperber did not attribute decreased viral replication to vacuolar degradation, because he found evidence for cytoplasmic virus as measured by p24 antigen staining. Rather, Sperber found that although there was no difference in the total amount of extracellular HIV-1 mRNA produced from treated and untreated cells, the mRNA particles from HCQ-treated cells were not infectious.
Chiang et al published a study in 1996 further explaining the mechanism of HCQ's anti-HIV-1 activity.12 The drug raises cellular endosomal pH and interferes with the enzymatic glycosylation of viral gp120 that is required for infectivity. When they compared the anti-HIV-1 effect of HCQ to zidovudine, they found that HCQ suppressed viral replication in a dose-dependent manner in both newly and chronically infected T-cells and monocytes. In contrast, zidovudine only inhibited HIV-1 replication in newly infected cells, although to a much greater degree than HCQ. An additive effect of the two drugs was noted for newly, but not chronically infected cells. They concluded that although the overall anti-HIV-1 effect of HCQ was less than that of zidovudine, HCQ may be a useful alternative or adjunctive therapy.
In 1996, a Chinese group published a report of another mechanism for CQ-mediated interference with HIV replication. CQ was found to inhibit Tat-mediated LTR-regulated viral gene expression necessary for replication. Quinine, another anti-malarial drug, did not have such inhibitory activity.13
Considering yet another effect of CQ, enhancement of lymphocyte apoptosis, might highlight the difference between CQ and quinine. Macfarlane et al published a report in 1998 documenting that CQ and quinacrine effectively inhibited the anti-apoptotic effects of an immune activator on murine B-lymphocytes. Both compounds also inhibited IL-6 synthesis. Quinine induced apoptosis much less effectively than the other two drugs.14 Meng et al demonstrated that HCQ induced apoptosis in all T lymphocyte lines.15 Potvin et al showed that CQ increased apoptosis in vitro, as well as suppressing IL-6 synthetic burst.16 No studies have been done to demonstrate that CQ-mediated enhancement of lymphocyte apoptosis has an anti-HIV effect, but it is conceivable that by hastening destruction of infected lymphocytes, the spread of HIV from cell to cell can be retarded.
The distinction between quinine and CQ also becomes apparent when two studies from 1996 and 1991 are considered together. The former, a small cooperative study between groups in Cincinnati and China, reported that eight HIV-positive men, all anti-retroviral naïve, were intentionally infected with Plasmodium vivax, allowed to develop malaria, and cured with CQ three weeks later. At the time of the report, two of the men had been followed for two years, and the remaining six men for six months. The authors documented a sustained increase in CD4 counts in the first two men, and in two of the remaining six, and noted that all of them remained clinically well. They concluded that malariotherapy, long a treatment for neurosyphillis, can be helpful in retarding the progression of HIV to clinical AIDS.17 However, in addition to the small sample size and short follow-up times, the authors failed to recognize that it might have been the CQ conferring benefit.
The earlier report was a large prospective, longitudinal cohort study in Kinshasa, Zaire (now Congo) examining the relationship between falciparum malaria and HIV. The authors monitored 260 children born to HIV-positive mothers, and 327 age-matched controls born to HIV-negative mothers on a monthly basis for febrile episodes. Any fever more than 37.3°C resulted in a malaria smear, and if positive, treatment with either oral or IV quinine, but not CQ. No statistical difference in incidence or severity of malaria, or response to therapy was noted between those with AIDS (WHO criteria), HIV-positive (but not meeting WHO criteria for AIDS), seroreverters (initially positive by ELISA from transferred maternal antibody, but not truly infected), or seronegatives. They concluded that “malaria was not more frequent or more severe in children with progressive HIV-1 infection, and malaria did not appear to accelerate the rate of progression of HIV-1 disease.”18 In contrast to the malariotherapy study, they did not note that malaria retarded progression from HIV infection to AIDS. It is possible that the crucial difference between the two studies was the use of CQ, which has an anti-HIV effect, or quinine, which does not.
Several other trials have documented clinical effects of CQ or HCQ on HIV consistent with biochemical effects. In 1995, Sperber conducted a randomized, double-blinded, placebo-controlled study of 40 asymptomatic HIV-positive patients with CD4 counts between 200 and 500 cells/mm3. Half of the patients received HCQ, the other half served as controls. The placebo group showed an increase in viral RNA load, no change in IL-6 levels, and a decline in both the CD4 percentage and T-cell proliferation to Candida. In contrast, the HCQ-treated group experienced a decline in HIV-1 RNA and IL-6, and maintained CD4 percentages and mitogen and antigen-specific T-cell proliferative responses.19
Sperber extended his study by comparing HCQ to zidovudine in the same group of patients. There was no statistical difference in the magnitude of RNA viral load decline, levels of cultured virus, p24 antigen, or CD4 counts. IL-6 and serum IgG levels were significantly reduced in the HCQ group, but not the zidovudine group. They concluded that “HCQ may be potentially useful in the treatment of patients with HIV-1 infection.”20
A year later, Ornstein and Sperber administered HCQ to two patients with AIDS and inflammatory arthritis. Both patients had a dramatic decrease in arthritic activity, and neither developed an opportunistic infection or required additional immunosuppressive therapy. One of the patients had RNA load, mitogen- and antigen-specific T-cell proliferation, and IL-6 levels measured before therapy and after a year of therapy, and showed improvement in all parameters.21
In 1997, Kalyesubula et al published a study on the effects of malaria and CQ use on HIV in a cohort of four hundred fifty-eight children born to HIV-1-positive Ugandan mothers. They found a significant association between time to clinical AIDS and absence of malaria infection. Their conclusion was that “the results suggest a possibility that malaria may offer some protection against HIV-1 progression or that CQ used to treat malaria may have a direct effect against the HIV-1 virus.”6
In summary, there is biochemical data to suggest that CQ inhibits the post-translational modification of HIV virions, rendering them non-infectious. This appears to have the clinical effect of improving immunological parameters of HIV-1 infected individuals and delaying progression to AIDS. Given the importance of preventing vertical transmission of HIV from mother to child, and on the basis of such biochemical and clinical evidence, we conducted a preliminary study to answer the question: Does random maternal CQ use during the last trimester of pregnancy lower the rate of HIV-1 vertical transmission?
In this cohort, 49% of the infants had detectable CQ and/or one of its two major metabolites present in cord blood. The FDA has evaluated CQ as Pregnancy Category A for prophylactic doses and C for treatment doses. This means that the drug is safe for use during pregnancy when taken as prophylaxis to prevent malaria infection, but although no teratogenic effect has been demonstrated, CQ should be used at treatment doses with caution only if the benefits outweigh the risks.
There is concern about fetal exposure to CQ because, as mentioned in the introduction, there is data that CQ acts as an immunosuppressive. This study, however, only documents that a significant portion of infants in a non-industrial setting is exposed to CQ, a readily available, cheap drug. Based on our findings a study to examine the effects of such exposure on frequency and type of perinatal infections is warranted.
With respect to HIV, we were able to document an interesting effect of CQ on the passage of the virus from mother to fetus. Because just over half of our samples were drug-free, any effect of CQ was diluted, and only revealed when we examined those samples that contained parent drug or metabolite. The fraction of samples from HIV negative children at high concentrations (CQ > 64 ng/ml; MCQ > 16 ng/ml; DCQ > 4 ng/ml) was twice that of samples from HIV positive children as seen in Table 4, although the difference was not statistically significant. This might suggest a therapeutic target for efficacy. Although the median concentrations of CQ and each of its metabolites were greater in the cord blood of children who were subsequently found to be HIV negative, this was only significantly so for DCQ. It is not possible to discern whether the larger concentrations of the terminal metabolite of CQ reflect larger cumulative maternal doses or that mothers who ultimately do not transmit HIV to their infants excrete DCQ more slowly.
Table 4.
Number (percent) of cord blood samples with concentration of CQ, MCQ, or DCQ above threshold values with regard to HIV status.
| HIV+ (n=42) |
HIV− (n=88) |
p | |
| CQ > 64 ng/ml | 5 (12) | 19 (22) | 0.18 |
| MCQ > 16 ng/ml | 6 (14) | 24 (27) | 0.10 |
| DCQ > 4 ng/ml | 4 (10) | 18 (20) | 0.12 |
Previous studies on the original cohort revealed that the only other variable to affect the rate of transmission was maternal CD4 count less than 100 cells/mm3. Within this cohort, only 3 women had such a count. Therefore, this variable should not confound the results. At the time that the original cohort was generated, RNA PCR was not available.
There are several limitations to this exploratory study. First, the freezers at the laboratory have been plagued by frequent power outages, often daily. While there are backup generators, it is possible that some thawing occurred and samples degraded over time. Second, this study was done on stored samples from a cohort nearly 10 years old. Since the time of collection and storage, Sperber's studies have shown that HCQ's clinical anti-HIV effect takes several weeks to build, so a random sampling reflecting sporadic use is not enough to adequately assess CQ's potential benefit. Furthermore, although most HIV transmission from mother to fetus likely occurs during the time of delivery, earlier transplacental infection has been documented, and a more sustained, regular use of CQ may be beneficial. Breastfeeding is universal in this culture, and post-partum transmission of HIV to the baby confuses our analysis since HIV status could not be reliably assigned until at least 15 months of age. Finally, although the data is very limited, the therapeutic serum concentration of CQ plus DCQ against susceptible strains of Plasmodia is thought to be 100 ng/ml,22 but there is no data to suggest what a therapeutic anti-HIV serum concentration may be, i.e. there are no dose response or dose finding studies. Most of our samples were well below 100 ng/ml (Figure 1).
This is the first study to examine chloroquine levels in cord blood. The frequent exposure of Ugandan newborns to chloroquine in utero is not surprising, and may also exist in many other countries. Given the anti-HIV and immunosuppressive effects of CQ, and its widespread use in this cohort of pregnant women in the developing world, this preliminary study suggests the need for a more controlled look at the effect of neonatal exposure to CQ on congenital infections in general, and HIV in particular.
Ideally, CQ should not play a central role in anti-HIV therapy; rather, it could enhance currently used drugs. However, in the developing world, with its limited resources, CQ could potentially play an important role in providing some measure of protection to infants of HIV positive mothers both antenatally, and for the duration of breastfeeding.
Acknowledgements
This work was supported by an AIDS International Training and Research Program at Case Western Reserve University (TW00011) Fogarty Grant. The authors are grateful to MaryAnn O'Riordan for her statistical analysis.
References
- 1.World Health Organization, author. Weekly Epidemiological Report. 1998;48:373–389.
- 2.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [see comments] [DOI] [PubMed] [Google Scholar]
- 3.Administration of zidovudine during late pregnancy and delivery to prevent perinatal HIV transmission—Thailand, 1996–1998. MMWR Morb Mortal Wkly Rep. 1998;47:151–154. [PubMed] [Google Scholar]
- 4.Mansergh G, Haddix AC, Steketee RW, et al. Cost-effectiveness of short-course zidovudine to prevent perinatal HIV type 1 infection in a sub-Saharan African Developing country setting. Jama. 1996;276:139–145. [see comments] [PubMed] [Google Scholar]
- 5.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [see comments] [DOI] [PubMed] [Google Scholar]
- 6.Kalyesubula I, Musoke-Mudido P, Marum L, et al. Effects of malaria infection in human immunodeficiency virus type 1- infected Ugandan children. Pediatr Infect Dis J. 1997;16:876–881. doi: 10.1097/00006454-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Chaulet JF, Robet Y, Prevosto JM, Soares O, Brazier JL. Simultaneous determination of chloroquine and quinine in human biological fluids by high-performance liquid chromatography. J Chromatogr. 1993;613:303–310. doi: 10.1016/0378-4347(93)80146-u. [DOI] [PubMed] [Google Scholar]
- 8.White NJ. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985;10:187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]
- 9.Desportes I. Effect of chloroquine onf HIV1 infection of monocytes. Int Conf AIDS. 1996 [Google Scholar]
- 10.Tsai WP, Nara PL, Kung HF, Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res Hum Retroviruses. 1990;6:481–489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- 11.Sperber K, Kalb TH, Stecher VJ, Banerjee R, Mayer L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res Hum Retroviruses. 1993;9:91–98. doi: 10.1089/aid.1993.9.91. [DOI] [PubMed] [Google Scholar]
- 12.Chiang G, Sassaroli M, Louie M, Chen H, Stecher VJ, Sperber K. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin Ther. 1996;18:1080–1092. doi: 10.1016/s0149-2918(96)80063-4. [DOI] [PubMed] [Google Scholar]
- 13.Jiang MC, Lin JK, Chen SS. Inhibition of HIV-1 Tat-mediated transactivation by quinacrine and chloroquine. Biochem Biophys Res Commun. 1996;226:1–7. doi: 10.1006/bbrc.1996.1302. [DOI] [PubMed] [Google Scholar]
- 14.Macfarlane DE, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol. 1998;160:1122–1131. [PubMed] [Google Scholar]
- 15.Meng XW, Feller JM, Ziegler JB, Pittman SM, Ireland CM. Induction of apoptosis in peripheral blood lymphocytes following treatment in vitro with hydroxychloroquine. Arthritis Rheum. 1997;40:927–935. doi: 10.1002/art.1780400522. [DOI] [PubMed] [Google Scholar]
- 16.Potvin F, Petitclerc E, Marceau F, Poubelle PE. Mechanisms of action of antimalarials in inflammation: induction of apoptosis in human endothelial cells. J Immunol. 1997;158:1872–1879. [PubMed] [Google Scholar]
- 17.Heimlich HJ, Chen XP, Xiao BQ, et al. Malariotherapy for HIV patients. Mech Ageing Dev. 1997;93:79–85. doi: 10.1016/s0047-6374(96)01813-1. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg AE, Nsa W, Ryder RW, et al. Plasmodium Falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325:105–109. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 19.Sperber K, Louie M, Kraus T, et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther. 1995;17:622–636. doi: 10.1016/0149-2918(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 20.Sperber K, Chiang G, Chen H, et al. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin Ther. 1997;19:913–923. doi: 10.1016/s0149-2918(97)80045-8. [DOI] [PubMed] [Google Scholar]
- 21.Ornstein MH, Sperber K. The antiinflammatory and antiviral effects of hydroxychloroquine in two patients with acquired immunodeficiency syndrome and active inflammatory arthritis. Arthritis Rheum. 1996;39:157–161. doi: 10.1002/art.1780390122. [DOI] [PubMed] [Google Scholar]
- 22.Baird JK, Leksana B, Masbar S, et al. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–626. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]