Abstract
This study investigated the effects of three drying methods (open sun drying, visqueen-covered solar dryer and polyethylene-covered solar dryer) on b-carotene and vitamin C content of edible portions of mango fruit (Mangifera indica) and cowpea leaves (Vigna unguiculata). Commercial samples were analysed for vitamin C by titrimetry and b-carotene by spectrophotometery at 450nm. Differences in vitamin retention and loss associated with the three drying methods were assessed by analysis of variance and least significant difference (LSD) at (p<0.05. The fresh cowpea leaf b-carotene and vitamin C content was 140.9 and 164.3 mg/100g DM respectively and decreased (p<0.05) with drying. Open sun drying method caused the greatest b-carotene and vitamin C loss (58% and 84% respectively), while the visqueen-covered solar dryer caused the least loss (34.5% and 71% respectively). Blanching cowpea leaves improved b-carotene and vitamin C retention by 15% and 7.5% respectively. The b-carotene and vitamin C content of fresh ripe mango fruit was 5.9 and 164.3 mg/100g DM respectively. Similar to effects on cowpea leaves, the mango micronutrient content decreased (p<0.05) with drying. The open sun drying method caused the greatest b-carotene (94.2%) and vitamin C (84.5%) loss, while the visqueen-covered solar dryer caused the least (73 and 53% respectively). These results show that the three solar drying methods cause significant loss of pro-vitamin A and vitamin C in dried fruits and vegetables. However, open sun drying causes the most loss and the visqueen-covered solar dryer the least, making the later a probable better drying technology for fruit and vegetable preservation. The drying technologies should be improved to enhance vitamin retention.
Introduction
Vitamins A and C are essential nutrients mostly available from fruits and vegetables. Vitamin A is essential for vision, growth, cellular differentiation and proliferation, reproduction, immune system integrity and development of bone and teeth1. Vitamin C is essential for connective tissue formation and maintenance, immune system stimulation, works as anti-oxidant, and enhances iron utilization among other roles1,2. Annually, ¼ – ½ million children worldwide become blind due to vitamin A deficiency4. In Uganda, this deficiency approximates 28% among children5.
Vegetables and fruits are the main source of vitamins A and C in Uganda6. However, their post harvest loss is high (30 – 40%) resulting into economic and nutritional loss7. Therefore, appropriate methods of fruit and vegetable processing and preservation to bridge seasonal gaps in nutrient supply are required. The Uganda government promotes solar drying as a cheap and affordable procedure8 and traditional open-sun drying along with the visqueen-covered and polyethylene-covered solar dryers are used. However, the use of solar dryers as opposed to open sun drying is relatively new and studies on the effects of these technologies on food quality and their implications in human nutrition are lacking. Solar drying exposes fruits and vegetables to solar radiation, which, in the presence of oxygen might result into loss of vitamins A and C. This study investigated the effects of direct solar-driers and open-sun drying on vitamins A and C content of fruits and vegetables using edible ripe mango fruit (Mangifera indica) and cowpea leaves (Vigna unguiculata) as cheap and abundant sources of these vitamins in Uganda. A total of 6µg of β-carotene taken to equal 1 µg retinol (vitamin A) was adapted in the study.
Materials and Methods
i) Samples and study design
Fifteen samples of fresh ripe mango fruit (Mangifera indica) and fifteen samples of edible cowpea leaves (Vigna unguiculata), each weighing 1 and 2 kg respectively, were purchased from a local Kampala market. Mangoes were washed, peeled and sliced to 3–5mm thick slices and each sample divided into 4 portions that were subjected to either drying in the open sun, drying using a polyethylenecovered solar dryer, drying using a visqueen-covered solar dryer or retained and analysed fresh to serve as the control. Samples for open sun drying were spread on plastic trays and directly exposed to solar radiation until the moisture content reduced to 10–15%. The other samples were loaded into either the visqueen-covered dryer or the polyethylene covered solar dryer and thereafter directly exposed the dryers to the open sun. Both dryers were modelled as well ventilated wooden boxes of 1.5m long, 1m wide and 2m high, and differed only in the top cover transparent material of either visqueen or ordinary polyethylene sheets used for trapping the sun-rays7. The exposure to direct solar radiation was maintained until sample moisture content reduced to 10–15%. Moisture content was ascertained using the AOAC procedure9. Weighted fresh samples were oven dried to constant weight at 105°C and the % weight loss taken as the moisture content of the sample. The cowpea and mango fruit samples were treated in the same way except that cowpea samples were divided into two groups: the blanched (short time heat treatment in water or steam for ten minutes) before drying, and the unblanched group.
ii) Vitamin C assays
Vitamin C content was determined by titration following the AOAC protocol9. Standard ascorbic acid solution (Sigma, USA) was made by dissolving 0.05g of pure acid in 45ml of extraction solution in a volumetric flask and the result made up to the 50ml mark. The vitamin C extraction solution (5% trichloroacetic acid) was made by dissolving 50g of the pure acid in one litre of distilled water. Ten grams of the sample were macerated in a mortar containing 10mls extraction solution and the contents transferred into a volumetric flask. More extraction solution was added until the 100ml mark, the contents mixed thoroughly and filtered immediately. Aliquots (10mls) of the extract were titrated against standard 2,6 dichlorophenolindophenol (DCPIP) obtained by dissolving 0.05g of DCPIP in 100 ml distilled water and standardized by titration against 2ml of standard ascorbic acid. An equivalent amount of the extraction solution taken as the blank was titrated against standard DCPIP and a correction for it made in the final titre. The ascorbic acid content of the sample was calculated using the formula:
Where VE = vitamin C equivalent of 1 ml of DCPIP (mg/ml)
| V1 | = | total extract volume (ml) |
| V2 | = | titrated extract volume (ml) |
| S | = | sample weight |
| Y | = | sample dry matter (%) |
iii) b-Carotene assays
The De Ritter and Purcell procedures10 were followed. Mango fruit samples were extracted using hexane. To 1 g mango fruit (particle size, 1 mm) in a mortar, 15mls hexane was added, the mixture crashed into a paste and transferred immediately into an amber bottle to prevent destruction by light. More hexane (15mls) was added, the bottle shaken for 5–10 minutes, allowed to stand and the extract filtered into 50ml volumetric flask using Whatman filter paper No.1. The residue was treated with another 15mls hexane and the above procedure repeated to extract any remaining β-carotene pigments. All the extract was made up to 50ml mark with more hexane and stored in a plastic amber bottle under dark fridge conditions until absorbency reading by spectrophotometry.
Cowpea leaves were extracted using a 1:1 acetonehexane mixture and the extract cleaned by a chromatographic column (magnesium oxide column, BDH, UK). Cleaning involved first evaporating 50 ml of extract to dryness, dissolving the residue in 2 ml of the extracting solvent, and then applying 1ml of the resultant extract on the column. The extract was eluted from the column with excess acetone-hexane mixture and the deep coloured band of the carotenoids collected and made up to 50mls in a volumetric flask before taking spectrophotometric absorbency reading.
The standard curve for all spectrophotometric readings was derived using absorbency readings obtained for standard β-carotene capsules dissolved in hexane at concentrations of 0.005–0.02mg/ml. All β-carotene measurements were at 450 nm. The calculation of bβ-carotene was as follows:
| Where V = | total volume extract | |
| D | = | dilution factor |
| W | = | sample weight |
| Y | = | percentage dry matter content of the sample |
iv) Data analysis
The GENSTAT statistical program11 was used for all analyses. Data were subjected to analysis of variance (ANOVA) and differences among drying methods assessed by the least significant difference (LSD) at p<0.05. Results of micronutrient content are reported with standard error of means.
Results
I) Cowpea leaves
a) β-Carotene content and loss
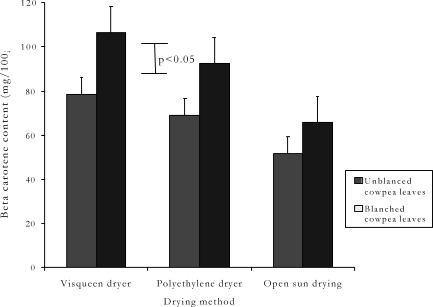
The results of cowpea leaf β-carotene content and loss under the three drying methods are summarized in Tables 1 and 2 and Figures 1 and 2. The β-carotene content was 140.9 mg / 100g fresh cowpea leaf DM, but decreased significantly (p<0.5) in dried samples regardless of the drying method. Open sun drying resulted into lower β-carotene content (p<0.5) than that of the visqueen-covered and polyethylene-covered solar dryers. The β-carotene content of samples dried by the polyethylene-covered and the visqueen-covered solar dryers did not differ (p>0.5), although, the values observed under the visqueen-covered dryer tended to be higher. The open sun drying method caused the greatest β-carotene loss. The visqueen-covered solar dryer caused the least (Table 2). For all drying methods, blanched cowpea leaf demonstrated higher (p<0.05) β-carotene content than the un-blanched samples. The β-carotene loss was 63.16% for un-blanched cowpea leaves and 53.37% for blanched cowpea leaves.
Table 1.
Average β-carotene and vitamin C content (mg/100g DM) of cowpea leaf and ripe mango fruit samples dried under solar radiation by three different methods in Uganda
| Fruit/ vegetable | Pro-vitamin / Vitamin |
Fresh sample (control) |
Visqueen-covered dryer |
Polyethylene-covered dryer |
Open sun drying |
| Mango fruit | β-Carotene | 5.90AA (0.6) | 1.58BB (0.3) | 0.94BC (0.13) | 0.34CD (0.1) |
| Vitamin C | 164.3AA (12.6) | 68.5BB (5.6) | 54.3BB (4.6) | 25.4CC (3.3) | |
| Cowpea leaves un-blanched) | |||||
| β-Carotene | 140.9AA (4.5) | 78.3BB (4.8) | 68.8BB (5.3) | 51.9CC (3.8) | |
| Vitamin C | 233.2AA (17.2) | 79.2BB (2.7) | 56.1BC (2.7) | 41.3CD (2.3) | |
| Cowpea leaves (blanched) | |||||
| β-Carotene | 140.9AA (4.5) | 106.4BB (5.0) | 92.4CC (4.1) | 65.7DD (4.8) | |
| Vitamin C | 233.2AA (17.2) | 56.5BB (2.9) | 41.9BB (1.6) | 32.3BB (0.9) | |
Table 2.
Percentage loss of β-carotene and vitamin C of cowpea leaf and mango fruit samples dried under solar radiation by three different methods in Uganda
| Fruit/ vegetable | Pro-vitamin / Vitamin |
Visqueen-covered solar dryer |
Polyethylene-covered solar dryer |
Open sun drying |
|
| Mango fruit | β-Carotene | 73.22 | 84.07 | 94.24 | |
| Vitamin C | 53.31 | 66.95 | 84.54 | ||
| Cowpea leaves | (un-blanched) | ||||
| b-Carotene | 44.43 | 51.17 | 63.16 | ||
| Vitamin C | 66.04 | 75.94 | 82.29 | ||
| Cowpea leaves (blanched) | β-Carotene | 24.49 | 34.42 | 53.37 | |
| Vitamin C | 75.77 | 82.03 | 86.15 | ||
Figure 1.
Comparison of average β-carotene content of blanched and un-blanched cowpea leaf samples dried under solar radiation by three different methods in Uganda
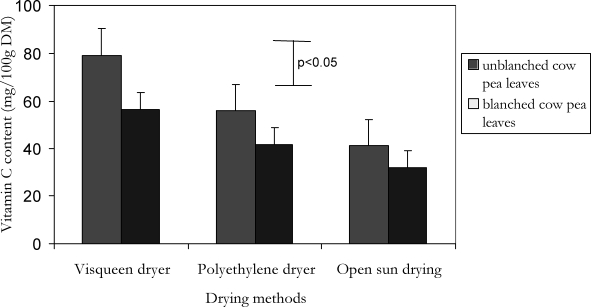
Figure 2.
Comparison of average vitamin C content of blanched and un-blanched cowpea leaf samples dried under solar radiation by three different methods in Uganda
Row means with a similar subscript between groups dot not differ (p>0.05). Row means with double subscripts differing between groups differ significantly (p<0.05). Figures in brackets are standard errors of mean. Recommended daily vitamin allowance for normal individuals21: (a) β-carotene: men (6000µg); women (4800µg); children (2400µg – 4200µg). (b) Vitamin C: 60 – 100g. (c) 6µg β-carotene = 1 µg retinol; 1mg=1000µg.
b) Vitamin C content and loss
The results of cowpea leaf vitamin C content and loss are also summarized in Tables 1 and 2 and Figures 1 and 2. The vitamin C content was 164.3 mg / 100g fresh cowpea leaf DM. The dried samples had lower (p<0.05) vitamin C content than fresh samples regardless of the drying method. Open sun drying resulted into significantly lower vitamin C content than that of the visqueen-covered and polyethylene-covered solar dryers. There was no difference (p>0.05) in vitamin C content of samples dried by the polyethylene-covered and the visqueen-covered solar dryers. The values observed under the visqueen-covered dryer tended to be higher than that of the polyethylene-covered dryer. Blanched and un-blanched samples dried by the visqueen-covered solar dryer differed (p<0.05) in vitamin C retention. The vitamin C content of blanched and un-blanched cowpea leaves dried under the open sun and polyethylene solar dryer were, however, not significantly different although the vitamin content tended to be lower in the blanched than un-blanched cowpea leaf samples.
II) Mango fruit
a) β-Carotene content and loss
The results of mango fruit b-carotene content and loss under the three drying methods are also summarized in Tables 1 and 2 and Figures 1 and 2. The b-carotene content of fresh ripe mango fruit was 5.9 mg/100g DM, but decreased with drying (Table 1). Similar to the results of cowpea leaf samples, the open sun drying method caused the greatest b-carotene loss (94.24%), while the visqueen-covered solar dryer caused the least (Table 2).
b) Vitamin C content and loss
The vitamin C content of fresh ripe mango fruit was 164.3 mg/100g DM (Table 1). Similar to cowpea leaf results, the vitamin C content decreased on mango fruit drying (Tables 1 and 2). The open sun drying method caused the greatest vitamin C loss (84.54%) while the visqueen-covered solar dryer caused the least (Table 2).
Discussion
This study investigated the suitability of three solar drying methods in retaining pro-vitamin A and vitamin C content of fresh ripe mango fruits and cowpea leaf vegetables for use in human diets. All drying methods studied caused significant (p<0.05) losses of the micronutrients (Tables 1 and 2; Figures 1 and 2). However, open sun drying caused the most loss indicating a probable exposure of the drying fruits and vegetables to greater solar radiation particularly ultra violet (UV) rays under this method. Pro-vitamin A and vitamin C are especially prone to oxidative destruction in the presence of heat, light, oxygen, enzymes, moisture and metal ions12. UV-radiation catalyses b-carotene oxidation leading to loss of vitamin activity.13, 14
During the study, fruits and vegetables drying under the open sun retained >15% moisture content for relatively longer time (6 days) compared to those under the visqueen-covered and polythene covered solar dryers that took 3 and 4 days respectively. Moisture content of > 15% promotes enzymatic reactions and interaction of other constituents of the drying product leading to loss of vitamins.15 This contributes to more vitamin loss under open-sun drying. On the other hand, the visqueen-covered solar dryer screens out more UV radiation7 and retains moisture content of > 15% for fewer days resulting into less vitamin loss. There was no significant difference, however, between the β-carotene content of the samples dried by the visqueencovered and the polyethylene-covered solar dryers suggesting a probable replacement role of the later in the construction of solar dryers. However, the costbenefit implication, of such a replacement design would have to be verified.
Blanching cowpea leaf samples improved β-carotene retention (Figure 1), strengthening the view that blanching reduces vegetable β-carotene predisposition to destruction.15, 16 However, the lower content of vitamin C in blanched samples compared to the un-blanched forms, suggests that blanching caused further vitamin C losses. Vitamin C is heat labile14 and susceptible to blanch-related nutrient leaching.15 Similar observations have been reported13 for blanched cabbages where vitamin C losses went up to 20%. Blanched bananas also demonstrated up to 75% vitamin C loss through leaching.17 However, the vitamin C and β-carotene content of blanched and un-blanched cowpea leaves dried under direct open sun and polyethylene-covered solar dryers during this study were not significantly different.
The observed β-carotene content of fresh cowpea leaves was higher than the reported18 values of 41667 – 55000µg/100g DM. That of fresh ripe mango fruit was lower than the reported19 7058.8µg/100g DM. On the other hand, the vitamin C content of fresh ripe mango fruit and edible cowpea leaves was lower than the reported20, 21 values of 176.7mg/100g DM and 373.3 mg/100g DM, respectively. The differences between the reported values and the values observed in the present study could be attributed to differences in variety, growth stage, geographical distribution and poor post-harvest handling of the fruits and vegetables.
This study revealed that ripe dried mango fruits could contain between 942 and 1580 µg β-carotene / 100g DM depending on the drying method. The recommended daily allowance (RDA) of β-carotene for a normal adult is in the range of 6000 µg for men, 4800 µg for women and between 2400 and 4200 µg for children.22 Given that 6 µg of β-carotene yields 1 µg retinol (vitamin A),22 a consumption of 100 to 400 g of ripe dried mango fruit would supply the RDA of retinol whereas 6 to 12g of dried cowpea leaves would be required for the same cause. However, processing may alter biological value of retinol and therefore, higher intake of these products may be essential. In case of vitamin C, the RDA is 60mg to 100g for an adult. Given that dried mango fruits and cowpea leaves in this study could supply 25 – 68.5mg/100g DM and 41.7 – 79.2mg/100g DM respectively (Table 1), a consumption of 100g to 300g of the dried products would be required to meet the RDA of vitamin C.
Despite the observed high vitamin C and β-carotene loss (Table 2), there is need to preserve these fruits and vegetables. The low cost drying methods would reduce the high fruits and vegetables post harvest loss (30–40%) in Uganda. The vitamin C and β-carotene quantities of dried mango fruits and cowpea leaf vegetable are sufficient to meet RDA. Eating dried products in relatively higher quantities may compensate for the loss of the vitamins incurred during processing and preservation. Further studies on the rate and extent of β-carotene and vitamin C loss and degradation during fruit and vegetable preservation and the factors involved under local conditions is recommended.
Acknowledgements
We are grateful to the Departments of Physiological Sciences, Faculty of Veterinary Medicine; Forest Products and Engineering; and Food science and Technology, Makerere University for the invaluable support.
References
- 1.Shrimpton DH. Nutritional aspects of Vitamins. In: Berry Ottaway P, editor. The technology of Vitamins in food. Kluwer Academic Publishers; 1993. pp. 22–25. [Google Scholar]
- 2.Mark H. Biological functions of vitamins. In: Berry Ottaway P, editor. The technology of vitamins in food. Kluwer Academic Publishers; 1993. pp. 1:1–1:18. [Google Scholar]
- 3.Elson J. [December 2002];Vitamin C. 2001 Health world online. http://www.healthy.net/asp/template.
- 4.FAO/WHO (Food and Agricultural Organization of the United Nations/World health organization of the United Nations), author International conference on Nutrition: Nutrition and Development FAO, Rome Italy. 1992
- 5.USAID (United States Agency for International Development), author Uganda Annual Report FY. 2002:7.
- 6.Ameny MA, Wilson PW. Relationship between Hunter colour values and b-carotene content in white-fleshed African sweet potatoes (Ipomoea batatas Lam) Journal of Science Food and Agriculture. 1997;73:301–306. [Google Scholar]
- 7.Byaruhanga YB, Kaaya AN, Bambona A, Mutyaba C. Training Manual Development Net for Staff of the East African Energy Technology Net-work (EAETDN) 2001. Processing and Preservation of Fruits and Vegetables using Solar Drying Technology; pp. 2–20. [Google Scholar]
- 8.Government of the Republic of Uganda, author. Government intervention to promote production, processing and marketing of selected strategic exports. Ministry of Trade and Industry. 2001
- 9.AOAC, author. Association of Official Analytical Chemists. Washington DC: AOAC; 1991. [Google Scholar]
- 10.De Ritter E, Purcell AE. Carotenoids Analytical methods. In: Bauernfeind JC, editor. Carotenoids as colorants and vitamin A precursors. Academic Inc London LTD; 1981. pp. 815–820. [Google Scholar]
- 11.Rothamsted Experiment Station, author. Lawes Agricultural Trust. United Kingdom: Rothamsted Experiment Station; 1995. Genstat 5 release 3.2 (PC/Windows 95) [Google Scholar]
- 12.McDowell RL. Vitamins in Animal Nutrition; comparative Aspects to human nutrition. New York: Academic Press, Inc; 1989. pp. 1–55.pp. 365–427. [Google Scholar]
- 13.Berry OP. Stability of Vitamins in food. In: Berry Ottaway P, editor. The Technology of Vitamins in Food. London: Chapman and Hall publishers; 1993. pp. 90–113. [Google Scholar]
- 14.Tannenbaum SR, Young VR, Archer MC. Vitamins and minerals. In: Fennema Owen., editor. Food chemistry. 1985. pp. 477–531. [Google Scholar]
- 15.Fellows PS. Food processing technology: Principles and practice. Wood-head publishing Ltd; 1997. pp. 65–69.pp. 197–208.pp. 304–310. [Google Scholar]
- 16.DeMan J. Principles of Food Chemistry. second edition. London, New Yolk, Tokyo: Chapman and Hall, International Thomson Publishing; 1990. pp. 222–232.pp. 241–247.pp. 334–353. [Google Scholar]
- 17.Atukwase A. Effects of solar drying on the nutritional composition of Apple-Bananas and their acceptability; a special project submitted for award of a BSc in Food Science and Technology. Kampala: Makerere University; 1999. pp. 22–27. [Google Scholar]
- 18.Bauernfeind JC, Klaui H. Carotenoids as food colours. In: Bauernfeind, editor. Carotenoids as Colorants and Vitamin A precursors. New York: Academic Press; 1981. pp. 143–173. [Google Scholar]
- 19.Kirk RS, Sawyer R. Pearson's composition and Analysis of foods. ninth edition. 1991. pp. 236–282.pp. 243–245.pp. 644–648.pp. 684–685. [Google Scholar]
- 20.Platt BS. Tables of representative values of Foods commonly used in Tropical countries. ninth edition. London: Medical Research Council; 1980. pp. 14–15.pp. 18–19. [PubMed] [Google Scholar]
- 21.Tindall HD. Vegetables in the tropics. London, Hong Kong: Macmillan Education LTD; 1988. pp. 303–304. [Google Scholar]
- 22.National Research Council (U.S.A), author Recommended Dietary Allowances. Tenth edition. Washington DC: National Academy Press; 1989. Subcommittee on the Tenth Edition of the RDAs; pp. 78–89.pp. 115–121. [Google Scholar]