Abstract
Mutational analyses of genes that encode components of the anchoring complex underlying the basolateral surface of external epithelia indicate that this structure is the major element providing for resistance to external friction. Ultrastructurally, laminin 5 (α3β3γ2; a component of the anchoring filament) appears as a thin filament bridging the hemidesmosome with the anchoring fibrils. Laminin 5 binds the cell surface through hemidesmosomal integrin α6β4. However, the interaction of laminin 5 with the anchoring fibril (type VII collagen) has not been elucidated. In this study we demonstrate that monomeric laminin 5 binds the NH2-terminal NC-1 domain of type VII collagen. The binding is dependent upon the native conformation of both laminin 5 and type VII collagen NC-1. Laminin 6 (α3β1γ1) has no detectable affinity for type VII collagen NC-1, indicating that the binding is mediated by the β3 and/or γ2 chains of laminin 5. Approximately half of the laminin 5 solubilized from human amnion or skin is covalently complexed with laminins 6 or 7 (α3β2γ1). The adduction occurs between the NH2 terminus of laminin 5 and the branch point of the short arms of laminins 6 or 7. The results are consistent with the presumed orientation of laminin 5, having the COOH-terminal G domain apposed to the hemidesmosomal integrin, and the NH2-terminal domains within the lamina densa. The results also support a model predicting that monomeric laminin 5 constitutes the anchoring filaments and bridges integrin α6β4 with type VII collagen, and the laminin 5–6/7 complexes are present within the interhemidesmosomal spaces bound at least by integrin α3β1 where they may mediate basement membrane assembly or stability, but contribute less significantly to epithelial friction resistance.
Electron microscopic evaluation of the epithelial– stromal junction of many external tissues after conventional fixation and dehydration protocols shows a lamina densa and lamina lucida much like that seen for other basement membranes. In addition, spaced along the length of the basal lamina are structures termed the adhesion complex (for reviews see Burgeson, 1993 a,b; Gerecke et al., 1994a ). These complexes consist of electron dense thickenings of the basolateral plasma membrane called hemidesmosomes, due to their resemblance to half of the desmosomal plaques present on the plasma membranes at sites of cell–cell contact. Cytoskeletal keratin filaments insert into both the hemidesmosomes and the desmosomes bridging all aspects of the cellular plasma membranes within a given cell, and provide a continuous intercellular network throughout the entire epidermis.
Thin filaments, termed “anchoring filaments,” span the lamina lucida and insert into the lamina densa. At high resolution, the anchoring filaments appear to be continuous with centrosymmetrically banded structures, termed “anchoring fibrils” (Ellison and Garrod, 1984). The anchoring fibrils originate within the lamina densa and project into the upper regions of the papillary dermis. About 800 nm in length, the anchoring fibrils either loop back to reinsert into the lamina densa, or insert into structures termed “anchoring plaques” (Keene et al., 1987) that appear as junctions between two or more anchoring fibrils. Additional anchoring fibrils originate in the plaques and extend further into the papillary dermis. The resulting network engulfs banded collagen fibers and other fibrous elements of the papillary dermis, thus assuring the strength of the union of the basement membrane and the upper dermis.
The molecules contained in these structures have been partly identified and characterized. Laminin 5 localizes to the anchoring filaments (Rousselle et al., 1991); it contains three unique subunits, α3 (Ryan et al., 1994), β3 (Gerecke et al., 1994b ), and γ2 (Kallunki et al., 1992). In skin and amnion, laminin 5 is proteolytically processed after secretion (Marinkovich et al., 1992a ). The 200-kD α3 chain is cleaved first to 165 kD, and finally to 145-kD. The γ2 chain is processed from 155 kD to 105 kD, while the β3 chain remains intact. Thus, three forms of laminin 5 are seen: an intracellular form (α3, 200 kD; β3, 140 kD; γ2, 155 kD), and two tissue forms (α3, 165 kD; β3, 140 kD; γ2, 105 kD) and (α3, 145 kD; β3, 140 kD; γ2, 105 kD). The γ2 chain cannot bind nidogen under physiological conditions (Mayer et al., 1995), and therefore laminin 5 alone cannot associate with perlecan or the collagen IV network (Yurchenco et al., 1985). Laminin 5 also lacks the short arm domains believed to be required to promote assembly into laminin networks (Schittny and Yurchenco, 1990; Yurchenco et al., 1992). Laminin can be incorporated into the basement membrane through a unique mechanism involving cross-linking of laminin 5 with laminin 6 (α3β1γ1) or laminin 7 (α3β2γ1) (Champliaud et al., 1996).
Anchoring fibrils are disulfide bond–stabilized dimers of type VII collagen (Morris et al., 1986; Sakai et al., 1986). The biosynthetic product is a monomer containing a large NH2-terminal globular domain (Parente et al., 1991), NC-1 (Lunstrum et al., 1986), an unusually long and interrupted triple-helical domain (Bentz et al., 1983), and a relatively small COOH-terminal globular domain, NC-2, that is proteolytically removed during maturation of the anchoring fibrils, and is probably involved in the dimerization process (Morris et al., 1986; Lunstrum et al., 1987; Bruckner-Tuderman et al., 1994). Immunoelectron microscopic studies of the location of well-characterized epitopes within type VII collagen indicate that the NC-1 domain is contained within the lamina densa (Keene et al., 1987). Therefore, a model of the interactions that stabilize the epithelial–stromal attachment has evolved that postulates epithelial cell binding to the laminin 5–6/7 complex α3 G domains, binding of the laminin 5 complex to the collagen network via nidogen, and binding of the type VII collagen NC-1 domain to the basement membrane through interactions of NC-1 with type IV collagen (Burgeson et al., 1990).
Recently, the gene defects underlying Herlitz's junctional epidermolysis bullosa have been identified as mutations in genes encoding laminin 5 chains that result in premature termination codons in both alleles. The resulting laminin 5 null phenotype is characterized by extensive blistering of the skin and the mucosal surfaces of the respiratory and digestive tracts, leading to death during the early months of life. The Herlitz phenotype can be due to mutations in the genes encoding α3 (Kivirikko et al., 1995), β3 (Pulkkinen et al., 1994b ; Pulkkinen et al., 1995; Vailly et al., 1995), or γ2 (Pulkkinen et al., 1994a ). This was surprising since the model predicted that laminin 6 or 7 could partially substitute for laminin 5 since it can bind both epithelial cells (through the α3 chains) and basement membranes (through γ1-mediated interactions with nidogen). Therefore, only α3 mutations were predicted to cause Herlitz's junctional epidermolysis bullosa. These findings indicate that additional and uncharacterized interactions must be required for epithelial–stromal stability. The ultrastructure of the anchoring complex (Ellison and Garrod, 1984) suggests a direct interaction of the anchoring filaments with the anchoring fibrils. Hence, we evaluated the binding of type VII collagen to laminin 5.
Materials and Methods
Antibodies
Preparation and characterization of laminin 5 and 6 antibodies mAb BM165 (anti-α3 chain), mAb SF12 (anti-γ2), mAb 545 (anti-β1), and polyclonal antibody (pAb)1 anti-laminin 5 have been described elsewhere (Rousselle et al., 1991; Marinkovich et al., 1992b ). mAb NP32 to the NC-I domain of type VIl procollagen, and pAb made to the whole type VII procollagen molecule have been previously described (Sakai et al., 1986; Lunstrum et al., 1986). pAb anti-laminin 1 was purchased from Sigma Chimie (St. Quentin Fallavier, France).
Cell Culture and Immunopurification of Type VII Procollagen, Laminin 5, and Laminin 6
WISH cells derived from human amnion (certified cell line [CCL] 25; American Type Culture Collection, Rockville, MD) were grown in DME (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FCS, 2 mM glutamine, 1 mM sodium pyruvate, 50 U/ml penicillin, 50 ng/ml streptomycin. Squamous carcinoma cells (SCC) 25 (CCL 25; American Type Culture Collection) were grown in 50% Ham's F-12 and 50% DME-F12 (GIBCO BRL), supplemented with 10% FCS 2 mM glutamine, hydrocortisone (0.4 μg/ml), and the same cocktail of antibiotics. Normal human keratinocytes (NHK) were isolated from neonatal foreskin (Rousselle et al., 1991) and grown in monolayer culture in synthetic serum-free medium (keratinocyte-SFM; GIBCO BRL).
For type VII procollagen purification, proteins secreted from confluent WISH monolayers were collected during a 24-h incubation in serum-free medium containing 100 ng/ml ascorbic acid as previously described (Lunstrum et al., 1986). For laminin 5 and laminin 6 purification, culture media were regularly harvested from confluent SCC25 culture. In all cases, after collection, unattached cells were removed by centrifugation (2,000 g/min), and EDTA, N-methylmaleimide, and phenylmethanesulfonyl fluoride were added to final concentration of 5 mM, 50 mM, and 50 mM, respectively. Spent culture media (500 ml) from WISH cells was passed sequentially over 25 ml of gelatin–Sepharose (Pharmacia Fine Chemicals, Piscataway, NJ) and 2 ml of NP32–Sepharose (Pharmacia Fine Chemicals). Spent culture media from SCC25 cells was passed sequentially over 25 ml of gelatin-Sepharose, and then over 5 ml of 545–Sepharose (anti-laminin β1) for laminin 6 purification, and then over 10 ml of BM165–Sepharose (anti-laminin α3) for laminin 5 purification. Affinity chromatography on gelatin Sepharose was necessary and sufficient to remove fibronectin from the conditioned media as indicated by immunoblotting with a pAb against fibronectin (not shown). The antigens were eluted with 1M acetic acid, immediately neutralized, and fractions were monitored for absorbance at 280 nm. Pooled fractions were dialyzed against PBS and kept frozen at −20°C. Protein concentration was determined by microprotein assay using bincinchoninic acid (Pierce Interchim, Montlucon Cedex, France).
Extraction of NC1 (Type VII Collagen) and Laminin 5–Laminin 6 Complex from Amniotic Membranes
Extraction and purification of NC-1 (type VII collagen) and laminin 5–laminin 6 complex from collagenase extract of amniotic membrane have been described previously (Bachinger et al., 1990; Marinkovich et al., 1992b ). Antibodies used during the affinity chromatography step include mAbs NP32, 545, and 6F12. Bound material was eluted with 1 M acetic acid and immediately neutralized. Eluted fractions were analyzed by electrophoresis, pooled, and dialyzed against PBS and kept at −20°C.
Extraction of the Laminin 5–NC1 Complex from Amniotic Membranes
The type VII collagen–NC1 preparation immunopurified from collagenase extracts of amniotic membranes was dialyzed against PBS and passed over an mAb 6F12 (anti-laminin β3) affinity chromatography column. Bound material was eluted with 1 M acetic acid, immediately neutralized, and eluted fractions were ethanol precipitated and analyzed by Western blotting with polyclonal anti-laminin 5 and with mAb NP-32 (anti-VII NC1).
Sources of Other Proteins
Collagens XII and XIV were extracted from bovine tendon (Aubert-Foucher et al., 1992) and kindly donated by Dr. E. Aubert-Foucher (Institut de Biologie et Chimie des Proteines [IBCP], Lyon, France). Laminin 1–nidogen complex and collagen IV extracted from the Engelbreth-Holm-Swarm tumor of the mouse were kindly provided by Dr. M. Aumailley (IBCP), and purchased from Collaborative Biomedical Products (TEBU, Perray en Yvelines, France), respectively. Bovine collagen I was a generous gift of Dr. F. Ruggiero (IBCP).
Collagenase and Pepsin Treatment of Type VII Collagen
Incubations with bacterial collagenase (10 μg/ml; CLSPA; Worthington Biochemical Co., Freehold, NJ) were for 8 h at 37°C in 50 mM Tris-HCl, pH 7.5, 500 mM NaCl, and 5 mM CaCI2. Pepsin digestion (100 μg/ml) was for 45 min at 4°C in 0.5 M acetic acid, 0.1 M NaCl. After enzymatic treatments, samples were dialyzed against PBS before interaction studies.
Protein Binding and Cell Adhesion Assays
The assay used was based on proteins immobilized on a plastic surface that were then exposed to varying concentrations of dissolved ligands followed by an enzyme immunoassay detection system. Multiwell plates (96 wells; Greiner, Dutscher, France) were incubated with solutions of the first ligand (10 μg/ml, 100 μl per well) dissolved in 20 mM Na2CO3, pH 9.2, for 18 h at 4°C. The wells were then blocked with 2% milk powder dissolved in 0.05 M Tris-HCl, pH 7.2, 0.2 M NaCl (TBS). All further steps were carried out in 2% milk powder in TBS, 0.02% Tween 20, and washes between steps were with TBS, 0.02% Tween 20. Wells were incubated with soluble ligand (0.6–40 μg/ml) for 2 h at 22°C and exposed (1 h, 22°C) to a constant amount of diluted pAb against the soluble ligand followed by a typical enzyme–immunoassay reaction with peroxidase conjugate of goat anti–rabbit immunoglobulin (DAKOPATTS, Copenhagen, Denmark) as second antibody and 2,2′-azino-bis(3-ethylbenthiazoline-6-sulfonic acid) as the chromogenic substrate. Color yields were determined at 405 nm in an ELISA reader (MR5000; Dynatech Labs., Ltd., Guernsey, Channel Islands, UK). Negative control values, from wells in which the soluble ligands were omitted, were subtracted from the binding data. For inhibition assays, soluble ligands were incubated with inhibitors (0.2–60 μg/ml) for 6 h at 4°C before being added to the immobilized ligand. For experiments testing effects of cations, 1% BSA dissolved in TBS was used instead of 2% milk powder.
Measurements of cell adhesion to test protein-coated 96-well plates were performed using colorimetric detection assays as have been previously described (Aumailley et al., 1989). Adhesion of NHK to laminin 5 (0.5 μg/well) was prevented by preincubation of the substrate with various concentrations of mAb BM165 (0–10 μg/ml) as previously described (Rousselle and Aumailley, 1994).
Formation of the Laminin 5-NC1 (Type VII Collagen) Complex In Vitro and Immunoprecipitation
Preparations of purified laminin 5 (20 μg/ml), purified NC-1 type VII collagen (10 μg/ml), a mixture of laminin 5 (20 μg/ml) and NC-1 (10 μg/ml), and a mixture of laminin 5 (20 μg/ml) and thermal denatured NC-1 (10 μg/ml) were obtained after dilution in PBS to a final volume of 300 μl. To perform thermal denaturation of NC-1, the protein was heat denaturated for 10 min at 80°C, immediately cooled and mixed with the laminin 5. All the samples were incubated 16 h at 4°C under gentle agitation. Each sample was then incubated 1 h at room temperature with a specific immunobead complex prepared as follows: 200 μl GammaBind G Sepharose (Pharmacia Biotech S.A., St. Quentin Yvelines, France) were incubated overnight with 2 μg of mAb BM165 specific to α3 chain of laminin 5, washed four times with PBS, and then incubated with each of the samples described above. The beads were collected by centrifugation, washed five times with PBS containing 0.05% Tween, and two times with PBS. The beads were resuspended in SDS–lysis buffer and boiled for 3 min. The released materials were analyzed by SDS-PAGE and immunoblotting.
Rotary Shadowing
Solutions of single proteins and of protein complexes were diluted to 10 μg/ml with 0.2 M ammonium bicarbonate, and after addition of an equal volume of glycerol, the solutions were sprayed onto freshly cleaved mica sheets. The samples were immediately placed on the holder of a MED 010 evaporator (Balzers S.p.A., Milan, Italy) and rotary shadowing was carried out as previously described (Ruggiero et al., 1993). Observations of replicas were performed with a Philips CM120 microscope (Phillips Technologies, Cheshire, CT) at the Centre de Microscople Electronique Appliquée à la Biologie et à la Géologie, Université Claude Bernard, Lyon, France).
Enbloc (Diffusion) Immunolabeling
Fresh neonatal foreskin was immunolabeled as previously described (Sakai and Keene, 1994). Briefly, fresh tissue was rinsed in PBS and then submersed overnight in mAb BM-165 (anti-laminin α3) diluted 1:5 in PBS or a mixture of BM-165 and rabbit pAb 3959 (anti-type VII collagen) at a ratio of 1:1:4 parts PBS. After an extensive rinse in PBS, the tissue was submersed overnight in secondary antibodies conjugated to different size gold particles (Amersham International, Little Chalfont, UK) as described in the legend for Fig. 9. Silver enhancement was performed on samples labeled with 1 nm gold. The samples were rinsed extensively in PBS and 0.1 M cacodylate buffer and then fixed, dehydrated, and embedded in Spurr's epoxy resin. 90-nm thick sections, which included the epithelium, papillary, and reticular dermis, were stained in uranyl acetate and lead citrate and examined using a Philips EM 410 transmission electron microscope.
Figure 9.
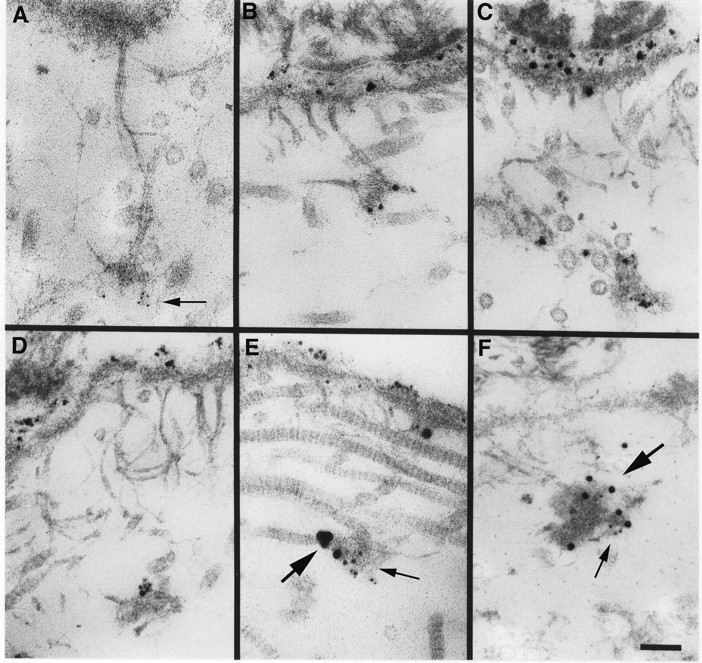
Localization of laminin 5 to anchoring plaques. Laminin 5 is localized to anchoring plaques in human skin using mAb BM165 and 5-nm (A, arrow) or 1 nm silver-enhanced colloidal gold (B–D). Labeling of the lamina lucida and anchoring plaques is intense using silver-enhanced 1 nm colloidal gold in comparison to 5 nm colloidal gold. Using a mixture of mAb BM165 (anti-laminin α3) and pAb 3959 (anti-type VII collagen NC-1), laminin 5 is seen to colocalize with type VII collagen in anchoring plaques (E, 1-nm silver-enhanced particles specific for mAb BM165 [small arrow] and 10-nm silver-enhanced particles are specific for pAb 3959 [large arrow]; F, 5-nm particles specific for mAb BM165 (small arrow) and 15-nm particles specific for pAb 3959 [large arrow]. Bar, 100 nm.
Results
Laminin 5 was purifed from the culture medium of SCC25 cells. The preparation was homogeneous as judged by SDS-PAGE (Fig. 1, lane 1), and contained the proteolytically processed α3 chain at 165 kD, the unprocessed β3 chain (140 kD), and unprocessed γ2 chain (155 kD), which fail to resolve on this gel, and the fully processed γ2 chain (105 kD). Solid phase protein binding assays were first optimized for signal strength and specificity using the documented affinity of collagen IV for the laminin 1–nidogen complex (Aumailley et al., 1989; and data not shown). The assay demonstrated saturable binding of collagen IV to laminin–nidogen, four- to fivefold stronger than to collagen I. Under these conditions, laminin 5, collagens XII and XIV, and the NC-1 domain of collagen VII showed minimal binding.
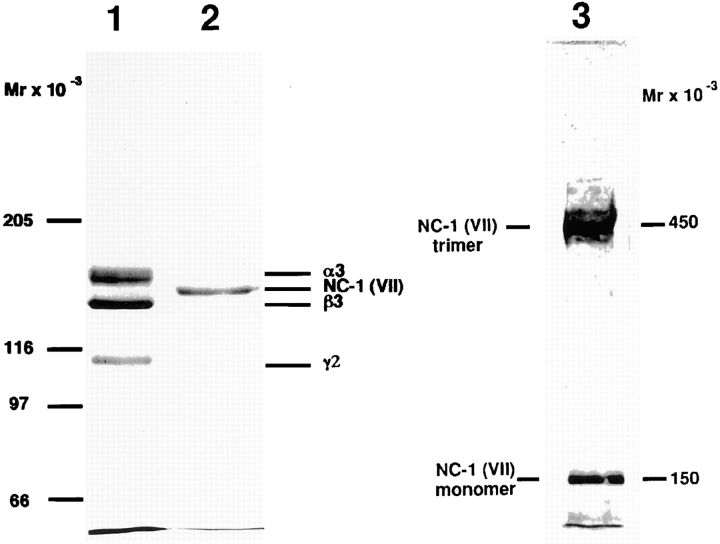
Figure 1.
Electrophoretic analysis of immunoaffinity-purified laminin 5 from cell culture media and type VII collagen–NC-1 from collagenase extracts of amniotic membranes. 2 μg of materials affinity purified on mAb 6/F12 from SCC25 cell culture medium (lane 1), and 2 μg of materials affinity purified on mAb NP-32 from collagenase extracts of amniotic membranes (lane 2) were separated by SDS-PAGE after reduction on a 3–7.5% gradient polyacrylamide gel and visualized by Coomassie blue staining. Consistent with previous results, the reduced bands representing subunits of laminin 5 (α3, 165 kD; β3, 140 kD; γ2, 155 and 105 kD) and type VII collagen–NC-1 (150 kD). The electrophoretic migration position of purified NC-1 before disulfide-bond reduction is shown in lane 3. Migration positions of the molecular weight markers are indicated to the left of each gel.
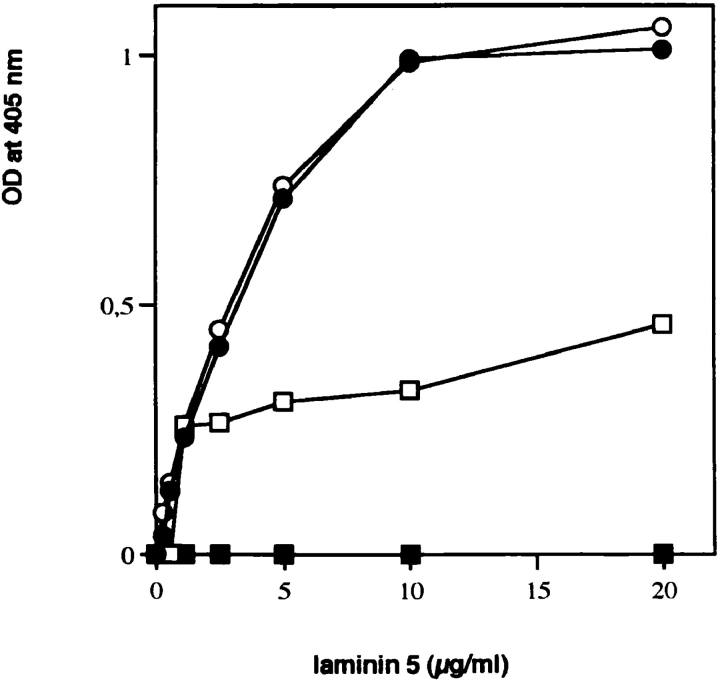
To test the potential binding of laminin 5 to type VII collagen, type VII procollagen was partially purified from WISH cell (spontaneously transformed amniotic epithelial cell line) culture medium by immunoaffinity chromatography using NP-32–Sepharose. Solutions of 0–20 μg/ml of laminin 5 were incubated with multiwell plates precoated with partially purified type VII procollagen, a clostridial collagenase digest of the type VII procollagen preparation, a pepsin digest of the type VII procollagen preparation, and purified type VII collagen NC-1. Saturable binding of type VII procollagen to laminin 5 was observed (Fig. 2), indicating that the two molecules did interact either directly or indirectly. To localize the binding activity within type VII procollagen to the triple-helical or nontriple-helical domains, the procollagen solution was digested separately with pepsin or collagenase before coating multichamber wells. Incubation with pepsin completely eliminated binding of laminin 5, while removal of the triple helix augmented the interaction. These observations suggest that the laminin 5–type VII collagen interaction occurs through either NC-1 or NC-2 of type VII procollagen. Since NC-2 is proteolytically removed during anchoring fibril formation (Lunstrum et al., 1987; Bruckner-Tuderman et al., 1994), if the interaction has physiological relevance, it is most likely an interaction of NC-1 with laminin 5. Therefore, NC-1 was purified from human amnion using established methods. The product was >95% pure as judged by SDS-PAGE (Fig. 1, lane 2) and by rotary shadowing (Bachinger et al., 1990) (not shown). The majority of the purified NC-1 remained trimeric as shown by electrophoretic separation without disulfide bond reduction (Fig. 1, lane 3). NC-1 showed comparable binding to laminin 5 as was seen with collagenase-digested type VII procollagen. The results show a direct interaction of laminin 5 with type VII collagen mediated by the large NC-1 domain. We believe that the difference in binding seen between pure NC-1 or collagenase-treated type VII procollagen and intact type VII procollagen is not significant as it most likely results from removal of collagenous contaminants (primarily type VI collagen aggregates) from the procollagen preparation by collagenase digestion thereby increasing the effective NC-1 concentration.
Figure 2.
Binding of soluble laminin 5 to immobilized type VII procollagen. Microtiter wells were coated with 1 μg of type VII–NC-1 purified from amnion (•) or intact type VII procollagen immunopurified from WISH cell culture medium (□), or type VII procollagen digested with either bacterial collagenase (○) or pepsin (▪). pAb to laminin 5 were used for detecting the amount of bound substrate.
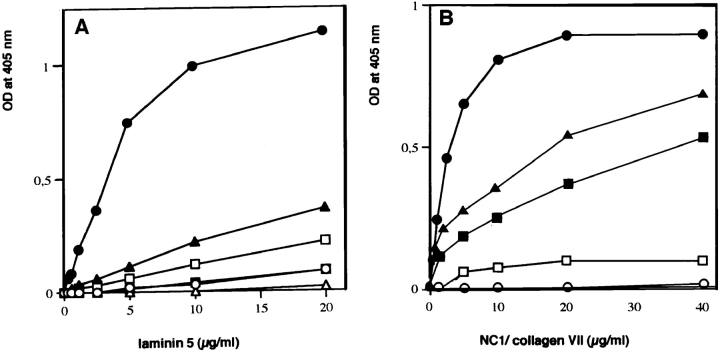
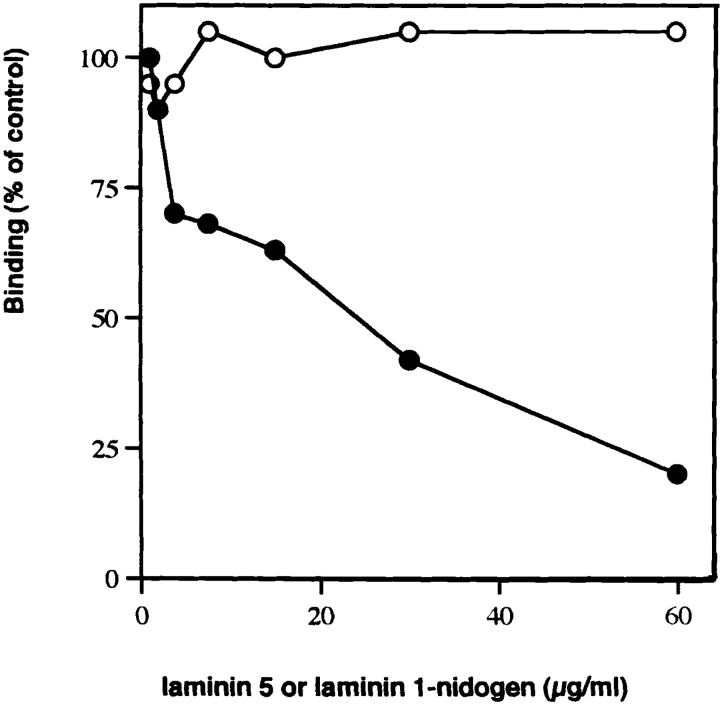
The specificity of this interaction was tested by evaluating the relative binding of type VII–NC-1, laminin 1–nidogen, and collagens I-, IV-, XII-, and XIV-coated wells to soluble laminin 5. As illustrated in Fig. 3 A, only type VII– NC-1 strongly bound laminin 5. The laminin 1–nidogen complex showed background affinity for laminin 5. When type VII–NC-1 was added in solution to laminin 5–coated wells (Fig. 3 B), again NC-1 bound most strongly to laminin 5. Under these conditions, NC-1 showed no affinity for laminin 1–nidogen, but type VII–NC-1 did bind collagen IV as previously shown (Burgeson et al., 1990), but only to about half the extent of the binding to laminin 5. Of particular interest, laminin 6 showed little binding to type VII– NC-1 relative to laminin 5 even though both laminins share the same α3 subunit (Champliaud et al., 1996). Consistent with this finding, NC-1 showed diminished binding to laminin 5 when complexed with laminin 6. However, the molar amounts of laminin 5 present in the complex solutions are ∼50% the molar concentration of laminin 5 in solutions of laminin 5 alone. Since laminin 6 does not bind NC-1, the laminin 5–6 complex binds NC-1 with the same efficiency or only slightly diminished efficiency relative to laminin 5 alone. The binding of 5 μg of NC-1 to laminin 5 was inhibited by preincubation of NC-1 with increasing concentrations of laminin 5, but not by preincubation with laminin 1–nidogen (Fig. 4).
Figure 3.
(A) Interaction of laminin 5 with various immobilized extracellular matrix components Binding of soluble laminin 5 to immobilized collagen I (□), collagen IV (▪), collagen XII (▴), collagen XIV (▵), NC-1-VII (•), and laminin 1–nidogen complex (○) at a concentration of 1 μg per well was determined by a pAb in an enzyme-coupled second antibody reaction as described in Materials and Methods. (B) Interaction of type VII–NC-1 with type IV collagen and with various laminin isoforms. Binding of soluble type VII–NC-1 to immobilized collagen IV (▪), laminin 1–nidogen complex (○), laminin 5 (•), laminin 6 (□), and laminin 5–laminin 6 complex (▴) at the concentration of 1 μg per well was determined by a pAb in an enzyme-coupled second antibody reaction as described in Materials and Methods.
Figure 4.
Inhibition of type VII–NC-1 binding to immobilized laminin 5 by preincubation with various competitors. The test system consisted of laminin 5 as the immobilized phase at the concentration of 1 μg per well, and a fixed amount of soluble type VII collagen– NC-1 (5 μg/ml). Type VII– NC1 was incubated at 4°C for 8 h with varying concentration of laminin 5 (•) or laminin 1–nidogen complex (○) before testing on the insoluble ligand.
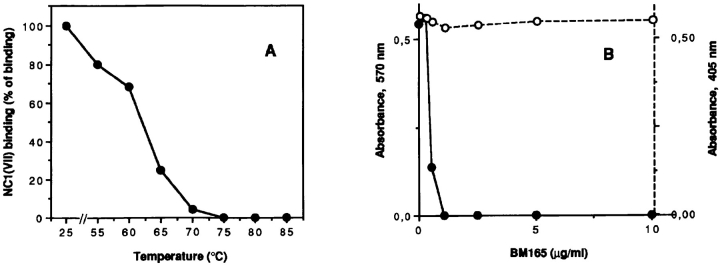
The dependence of binding upon the native conformation of either laminin 5 or NC-1 was tested by separately heating each of the ligands to 85°C for 10 min, and then rapidly cooling them before the solid phase binding assay. The denaturation products were not degraded during the heat treatment (data not shown). Denaturation of either ligand completely abolished binding (data not shown). The temperature at which the laminin 5 conformation necessary for binding to NC-1 is lost was then examined. The midpoint of the temperature where loss of active conformation occurs was ∼65°C (Fig. 5 A). mAb BM165 inhibits the binding of epithelial cells to laminin 5 (Rousselle et al., 1991). Therefore, the ability of BM165 to disrupt the binding of laminin 5 to NC-1 was evaluated. As shown in Fig. 5 B, BM165 very effectively inhibits cell binding, but has no effect upon the laminin 5–NC-1 interaction. Divalent cations are also required for the binding of type VII collagen and laminin 5, as the addition of 10 mM EDTA completely inhibits the interaction (data not shown).
Figure 5.
(A) Effect of heat denaturation on laminin 5 interaction with type VII–NC-1. Multiwell plates were coated with laminin 5 (•) at concentration of 1 μg before or after heating at the indicated temperatures. Soluble type VII–NC-1 was then added at concentration of 10 μg/ml and bound material was determined by a pAb in an enzyme-coupled second antibody reaction as described in Materials and Methods. (B) Effects of mAb BM165 on adhesion of normal human keratinocytes and on binding of type VII–NC-1 to laminin 5. Multiwell plates were coated with laminin 5 at 10 μg/ml. After saturation, the wells were incubated with indicated concentrations of mAb BM165 for 60 min before the cell adhesion experiment (•), or together with soluble type VII–NC-1 (10 μg/ml) for the binding assay (○). Extent of cell adhesion was measured with a colorimetric reaction at 570 nm. Each point represents the average of triplicate wells. In the ELISA assay, bound type VII–NC-1 was determined by a pAb in an enzyme-coupled second antibody reaction as described in Materials and Methods. Because the OD values in both systems were in the same range, the data were presented on a single graph.
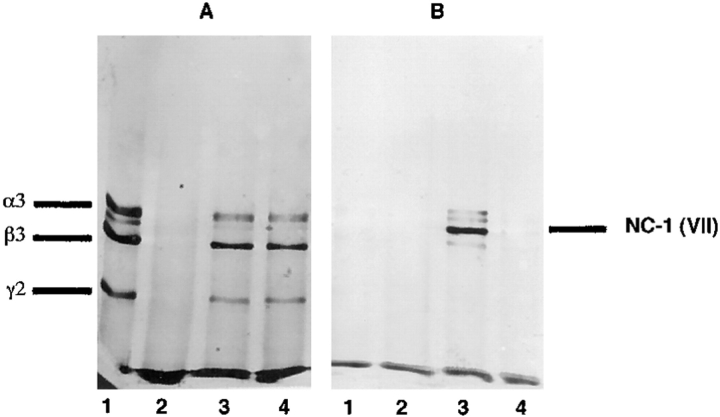
To be certain that the binding of laminin 5 to type VII collagen NC-1 was not artifactually influenced by the solid-phase conditions, purified laminin 5 was coincubated in solution with and without NC-1, and with heat-denatured NC-1, and then complexed and uncomplexed laminin 5 was isolated by immunoprecipitation using anti-laminin α3 (BM-165). The immunoprecipitation products were separated by SDS-PAGE after disulfide bond reduction. Laminin 5 and NC-1 were identified by Western blot analysis using polyclonal anti-laminin 5 and monoclonal anti-NC-1 (NP32). Immunoprecipitated laminin 5 in the absence of NC-1 gave the expected pattern by Western analysis (Fig. 6, lane A1) and did not react with anti-NC-1 (Fig. 6, lane B1). Immunoprecipitation of NC-1 in the absence of laminin 5 produced no product (Fig. 6, lanes A2 and B2). Both laminin 5 (Fig. 6, lane A3) and NC-1 (Fig. 6, lane B3) were immunoprecipitated from the mixture of the two, while denatured NC-1 did not precipitate with laminin 5 (Fig. 6, lanes A4 and B4). The results obtained using these conditions confirm the solid-phase binding data and again emphasize the importance of the native conformation of NC-1.
Figure 6.
Western blot analysis of the complex of laminin 5 and NC-1 produced in solution. Laminin 5 alone (lanes A1 and B1), NC-1 alone (lanes A2 and B2), a mixture of laminin 5 and NC-1 (lanes A3 and B3), or a mixture of laminin 5 and heat denatured NC-1 (lanes A4 and B4) were immunoprecipitated with anti-α3 mAb (BM-165). The contents of the precipitates were separated by SDS-PAGE after disulfide bond reduction and visualized by Western analysis using (A) polyclonal anti-laminin 5 or (B) monoclonal anti-NC-1 (mAb NP-32).
Images of NC-1–laminin 5 adduct produced in solution as described above were examined by transmission electron microscopy after rotary shadowing. Three types of images were seen (Fig. 7). One was identical to the images previously reported for laminin 5 (Rousselle et al., 1991). The second image appeared as a somewhat irregular spheroid, with its shortest diameter being ∼41 nm (41 ± 9 nm; n = 53). Occasionally spheroids with contiguous single rod-like structures resembling collagen triple helices were seen. We interpret these structures to be trimeric NC-1 with some partial digestion products, consistent with the electrophoretic band pattern (Fig. 1). The third image seen appears as complexes of the first two forms. The images are consistent with visualization of the NC-1 and laminin 5 monomers, and of the complex of one laminin 5 molecule with each NC-1 fragment.
Figure 7.
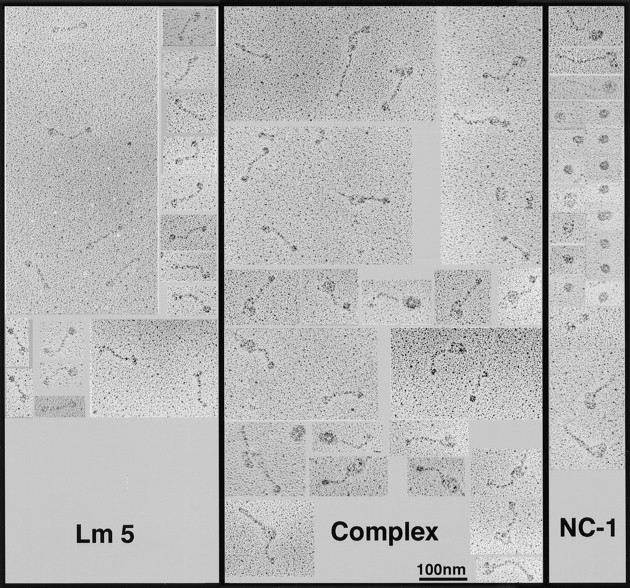
Visualization of the laminin 5–NC-1 complex formed in solution by transmission electron microscopy after rotary shadowing. Collagen VII–NC-1, laminin 5, and a mixture of both molecules were dialyzed against 0.2 M ammonium bicarbonate, diluted 1:1 with glycerol, and rotary shadowed. (Lm5) The images visualized are characteristic of laminin 5 molecules. The 107-nm molecules appear as extended rods with globular knobs at each terminus. (NC1) Under the conditions used to maintain the stability of the complex, NC-1 appears as an asymmetrical spheroid, ∼41 nm in narrowest diameter. Due to incomplete digestion, helical domains of type VII collagen can be seen on some NC-1 molecules. (Complex) Images of the laminin 5–type VII collagen–NC-1 complex preparation contains uncomplexed laminin 5 and NC-1, and images that appear as a complex of one laminin 5 molecule with one NC-1 molecule, in which the long arm may correspond to laminin 5 and the big globular domain to NC-1.
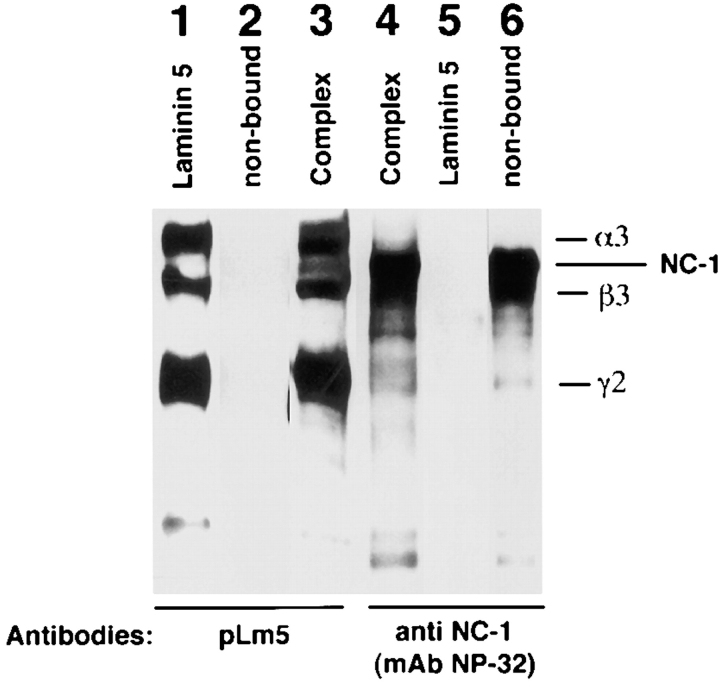
If laminin 5 and type VII collagen NC-1 interact in vivo, it may be possible to solubilize the complex if the tissue is solubilized under nondenaturing conditions. NC-1 was prepared as a collagenase digest of amnion and partially purified by NP32 (anti-NC-1) immunoaffinity chromatography. If a complex exists in this mixture, it should be retained by 6F12 (anti-laminin β3) immunoaffinity columns. Thus, partially purified NC-1 eluted from the anti-NC-1 column was applied to a column containing anti-β3. The flow-through (Fig. 8, lanes 2 and 6, nonbound) is Western blotted by anti-NC-1 (Fig. 8, lane 6) but not by anti-laminin 5 (Fig. 8, lane 2) indicating that the nonbound fraction contains only NC-1 and is equivalent to the NC-1 used in the binding studies described above. If complexed laminin 5 and NC-1 are present in the extract, they should be bound by the anti-β3 column. As seen in Fig. 8, lanes 3 and 4, the materials eluted from the anti-β3 column are Western blotted by both anti-laminin 5 (Fig. 8, lane 3) and anti-NC-1 (Fig. 8, lane 4) indicating that the bound fraction contains both NC-1 and laminin 5.
Figure 8.
Western blot analysis of the laminin 5–type VII collagen–NC-1 complex immunoaffinity purified from collagenase extracts of amniotic membranes. Type VII collagen–NC-1 was first affinity purified on mAb NP-32 from collagenase extracts of amniotic membranes. Eluted fractions of NC-1 were pooled, dialyzed against PBS, and passed over a mAb 6F12 affinity chromatography column. Bound materials were immunoblotted with polyclonal anti-laminin 5 (pLm5; lane 3), and anti-NC-1 (mAb NP-32, lane 4). The bound material shows the pattern obtained for purified laminin 5 (lane 1), which does not contain NC-1 (lane 2) and for purified NC-1 (not shown, but identical to lane 6). The nonbound fraction contains NC-1 only (lane 6) but no laminin 5 (lane 2).
In previous studies, Keene et al. (1987) showed that the anchoring fibrils in the papillary dermis of skin terminated in junctions, the anchoring plaques. These plaques showed the inclusion of type IV collagen by ultrastructural immunolocalization. Studies conducted at the same time, but not included in the publication, showed the absence of anchoring plaque labeling with mAbs to laminin α1, but minor labeling with polyclonal anti-laminin antibodies. At that time we were unsure how to interpret the result. We have repeated the immunolocalization of laminin 5 in skin with particular attention to the anchoring plaques. As shown in Fig. 9, A–D, the plaques are clearly recognized by anti-α3 antibodies. Further, in double-labeling experiments, the reactivity of monoclonal anti-α3 is colocalized to the plaques with the reactivity of polyclonal anti-NC-1 (Fig. 9, E and F).
Discussion
The data presented here suggest that at least two mechanisms promote epithelial–stromal attachment. The first is the previously suggested (Champliaud et al., 1996) binding of the laminin 5–6/7 complex to lamina densa components of the epithelial basement membrane. That model suggests that the presence of γ1 chains in laminin 6 or 7 provides a nidogen binding site, and the VI domains of both β1/β2 and γ1 allow for assembly of the complex with the laminin 1 network. The model also suggests that null phenotypes resulting from mutations in laminin α3 would result in significant destabilization of epithelial–stromal adhesion, as these mutations would be null for laminin 5, 6, and 7. However, the model also predicts that mutations in β3 or γ2 would only affect the presence of laminin 5, and not either laminin 6 or 7. Therefore, since both laminins 6 and 7 contain an α3 chain and promote adhesion of keratinocytes, laminin 6 or 7 should at least partially substitute for the absence of laminin 5. This prediction is incorrect, as mutations in genes encoding α3, β3, or γ2 all result in Herlitz's junctional epidermolysis bullosa. Therefore, a second and equally (or more) important mechanism by which laminin 5 interacts with a basement membrane or stromal component must exist.
The present study indicates a second mechanism involving the binding of monomeric laminin 5 to type VII collagen NC-1 domain. These findings suggest a model (Fig. 10) that predicts that monomeric laminin 5 is the primary bridge between hemidesmosomal α6β4 and type VII collagen, and the laminin 5–6/7 complex is present within the interhemidesmosomal spaces. This model is consistent with the following observations: (a) Monomeric laminin 5 binds integrin α6β4 (Niessen et al., 1994) and type VII collagen strongly, but has minimal affinity for other basement membrane components; (b) approximately half of the extractable laminin 5 is complexed with laminins 6 and 7 (Champliaud et al., 1996); (c) the laminin 5–6/7 complex binds collagen IV strongly through nidogen (Mayer et al., 1995); (d) laminin 5 is uniformly distributed along the epithelial basement membrane (Rousselle et al., 1991), while anchoring fibrils (Ellison and Garrod, 1984) and type VII collagen (Sakai et al., 1986) are concentrated under hemidesmosomes. This model is also consistent with (e) the ultrastructure of the anchoring complex and the spatial orientation of the involved molecules (Ellison and Garrod, 1984); and (f) with the phenotypes of null mutations in BPAG2 (McGrath et al., 1995; Jonkman et al., 1995), integrin β4 (Vidal et al., 1995; Dowling et al., 1996; van der Neut et al., 1996) or α6 (Georges-Labouesse et al., 1996), laminin 5 chains, and type VII collagen (Uitto et al., 1994; Bruckner-Tuderman, 1994), as the absence of laminin 5 results in the lethal Herlitz's form of epidermolysis bullosa. The absence of type VII collagen results in severe generalized dystrophic epidermolysis bullosa, and the lack of α6β4 integrin causes lethal blistering in the mouse (Dowling et al., 1996; van der Neut et al., 1996; Georges-Labouesse et al., 1996) although the absence of integrin α6β4 in humans appears to be less severe (Vidal et al., 1995). Absent mouse BPAG1 (Guo et al., 1995), BPAG2 (McGrath et al., 1995, 1996), or plectin (Gache et al., 1996; McLean et al., 1996; Smith et al., 1996) results in relatively mild blistering, suggesting that their roles in epidermal adhesion can be partially compensated by other hemidesmosomal components.
Figure 10.
Model for the presence of monomeric laminin 5 and complexed laminin 5 within an epithelial–stromal junction. The cartoon depicts monomeric laminin 5 as the bridge between hemidesmosomal integrin α6β4 and the type VII collagen NC-1 domain. The tight binding of laminin 5 to α6β4 and to type VII collagen provides the primary resistance to frictional forces. The laminin 5–6/7 complex is shown within the basement membrane between hemidesmosomes, bound by integrin α3β1 where it potentially functions to maintain basement membrane stability and contact with the epithelial basolateral surface.
The precise role of laminin 5–6/7 complex–nidogen binding is now less clear. As the presence of laminins 6 or 7 cannot substitute for laminin 5 in stabilizing epithelial attachment to the basement membrane, the characteristics of monomeric laminin 5 best fit its direct role in the anchoring complex. Since monomeric laminin 5 has no known mechanism for binding to components of the lamina densa other than to type VII collagen, it is difficult to imagine how it might function outside the hemidesmosome, given our current level of understanding. It is likely that the laminin 5 detected within the basement membrane between hemidesmosomes is the laminin 5–6/7 complex. Within this interhemidesmosomal space it is capable of associations with the basolateral plasma membrane through integrin α3β1 (Delwel et al., 1994; DiPersio et al., 1995). Its presence there may stabilize the association of the basement membrane with the basolateral keratinocyte surface. The laminin 5–6/7 complex may play additional important roles in basement membrane assembly and stability.
We have previously shown that NC-1 can bind collagen IV and, to a considerably lesser extent, laminin 1 (Burgeson et al., 1990). As shown in Fig. 3 B, in this assay the relative binding of NC-1 to the laminin 1–nidogen complex is minimal, the binding to type IV collagen is moderate, and NC-1 binds laminin 5 most strongly. The failure of NC-1 to bind laminin 6 was informative. As laminin 5 and laminin 6 share the α3 chain, this observation suggests that either β3 or γ2 mediate the interaction with type VII collagen. Binding to β3 or γ2 could occur within the α-helical long arm domains I/II, or within the short arms. In this regard, the measurements of conformational stability of the NC-1 binding domain are informative. NC-1 binding is lost if laminin 5 is subjected to temperatures above 65°C. As this is well below the midpoint transition temperature for denaturation of the coiled-coil long arm (72°C) (Rousselle et al., 1995), the data suggest that binding of laminin 5 to NC-1 occurs within the β3 or γ2 short arms. Of interest, recently a case of junctional epidermolysis bullosa has been described that is due to a homozygous inframe exon skip resulting in the deletion of part of domains III and VI of the γ2 chain (Pulkkinen et al., 1994a ). The processing cleavage site is retained within domain III, but lies only six residues from the deletion, and therefore the authors speculate that the phenotype may result from failure of γ2 chain processing. If only processed γ2 interacts with NC-1, this would be consistent with the observed phenotype. The laminin 5 used in this present study consists of a mixture of molecules containing both full-length, unprocessed γ2 (155 kD), and processed 105 kD γ2. Therefore, we are unable to determine if γ2 processing influences NC-1 binding.
The rotary-shadowed images of the NC-1–laminin 5 complex are consistent with the proposed interaction of NC-1 with an end of laminin 5, but not with the coiled-coil domain. We were unable to unambiguously identify the globule at the extended end of laminin 5 within the complex as the α3G domain since the monomeric laminin 5 in the preparation is very heterogeneous at one end, consistent with the electrophoretic pattern indicating the presence of a mixture of processing intermediates, and consistent with the observed heterogeneity being at the NH2-terminus. However, our impression is that the globule seen at the free end of laminin 5 in the complex is less heterogeneous than seen for the NH2 terminus of monomeric laminin 5, and is therefore likely to be the G domain. It is interesting that under the lower glycerol concentrations used in the spreading buffer NC-1 appears as a spheroid rather than in an extended trident shape as we have previously reported (Lunstrum et al., 1986). It is possible that the observed globular structure is due to noncovalent intrachain interactions of the von Willebrand factor domains (Fretto et al., 1986). The lower glycerol concentrations were needed to visualize the complex, suggesting either that glycerol at high concentration destabilizes the complex, or that spreading of the short arms disrupts the complex. It is also surprising that all the complexes visualized appeared to be between a single molecule of trimeric NC-1, and a single laminin 5 molecule. Since NC-1 is a homotrimer, we expected that up to three laminin 5 molecules might be bound by each NC-1 domain.
These studies do not directly address the subdomain(s) within NC-1 to which laminin 5 binds. However, type XII and XIV collagens were selected as candidates for the solid-phase binding studies since they share both fibronectin type III repeats and von Willebrand factor A repeats with NC-1. Therefore, since laminin 5 does not bind either of these collagens, the subdomain responsible for binding must contain sequences that are unique to type VII collagen.
If laminin 5 binds type VII collagen NC-1 domains with high affinity, then laminin 5 could mediate the interaction of anchoring fibrils and would be expected within anchoring plaques. Previous attempts to localize laminin to the anchoring plaques with monoclonal anti-α1 or polyclonal anti-laminin 1 were ambiguous. The present study shows the localization of anti-α3 reactivity to the anchoring plaques, colocalized with anti-type VII collagen NC-1. This strongly suggests that the tight binding of NC-1 and laminin 5 seen in vitro is physiologically significant.
Acknowledgments
We thank Dr. E. Aubert-Foucher for helpful discussions, and Ms. C. Ridgeway for technical assistance.
Abbreviations used in this paper
- NHK
normal human keratinocytes
- pAb
polyclonal antibody
- SCC
squamous carcinoma cells
Footnotes
Part of this work was supported by a grant (3056) from the Association pour la Recherche sur le Cancer (to P. Rousselle); from a Fondation René Touraine pour la Recherche en Dermatologie award (to P. Rouselle); a Journal of Cell Science Traveling Fellowship to P. Rouselle, U.S. Public Health Service grants AR35689 and AR38923 (R.E. Burgeson); and from the Cutaneous Biology Research Center, Massachusetts General Hospital.
Please address all correspondence to Robert E. Burgeson, Ph.D., MGH-East-CBRC, Building 149, 13th Street, Charlestown, MA 02129. Tel.: (617) 726-4186. Fax: (617) 726-4187. e-mail: BBurgeson@CBRC.MGH.Harvard.edu
References
- Aubert-Foucher E, Font B, Eichenberger D, Goldschmidt D, Lethias C, van der Rest M. Purification and partial characterization of native type XIV collagen. J Biol Chem. 1992;267:15759–15764. [PubMed] [Google Scholar]
- Aumailley M, Wiedemann H, Mann K, Timpl R. Binding of nidogen and the laminin-nidogen complex to basement membrane collagen type IV. Eur J Biochem. 1989;184:241–248. doi: 10.1111/j.1432-1033.1989.tb15013.x. [DOI] [PubMed] [Google Scholar]
- Bachinger HP, Morris NP, Lunstrum GP, Keene DR, Rosenbaum LM, Compton LA, Burgeson RE. The relationship of the biophysical and biochemical characteristics of type VII collagen to the function of anchoring fibrils. J Biol Chem. 1990;265:10095–10101. [PubMed] [Google Scholar]
- Bentz H, Morris NP, Murray L, Sakai LY, Hollister DW, Burgeson RE. Isolation and partial characterization of a new human collagen with an extended triple-helical structural domain. Proc Natl Acad Sci USA. 1983;80:3168–3172. doi: 10.1073/pnas.80.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner-Tuderman L. Collagen VII and bullous disorders of the skin. Dermatology. 1994;189:16–20. doi: 10.1159/000246983. [DOI] [PubMed] [Google Scholar]
- Burgeson, R.E. 1993A Dermal-epidermal adhesion in skin: An example of a novel attachment complex. In Molecular and Cellular Aspects of Basement Membranes (D.H. Rohrbach and R. Timpl, editors) Academic Press, Inc., Orlando, FL. 49–56.
- Burgeson RE. B. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101:252–255. doi: 10.1111/1523-1747.ep12365129. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Lunstrum GP, Rokosova B, Rimberg CS, Rosenbaum LM, Keene DR. The structure and function of type VII collagen. Ann NY Acad Sci. 1990;580:32–43. doi: 10.1111/j.1749-6632.1990.tb17915.x. [DOI] [PubMed] [Google Scholar]
- Champliaud M-F, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote epithelial-stromal attachment. J Cell Biol. 1996;132:1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DLA, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping specificities of the α3Aβ1 and α6Aβ1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shah S, Hynes RO. α3β1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J Cell Sci. 1995;106:2321–2336. doi: 10.1242/jcs.108.6.2321. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu Q-C, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion, and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison J, Garrod DR. Anchoring filaments of the amphibian epidermal-dermal junction transverse the basal lamina entirely from plasma membranes of hemidesmosomes to the dermis. J Cell Sci. 1984;72:163–172. doi: 10.1242/jcs.72.1.163. [DOI] [PubMed] [Google Scholar]
- Fretto LJ, Fowler WE, McCaslin DR, Erickson HP, McKee PA. Substructure of Human von Willebrand Factor. J Biol Chem. 1986;261:15679–15689. [PubMed] [Google Scholar]
- Gache YS, Chavanas JP, Lacour JP, Wiche G, Owaribe K, Meneguzzi G, Ortonne J-P. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest. 1996;97:2289–2298. doi: 10.1172/JCI118671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dietich A, Le Meur M. Absence of integrin a6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Gerecke, D.R., M.K. Gordon, D.W. Wagman, M.F. Champliaud, and R.E. Burgeson. 1994a Hemidesmosomes, anchoring filaments, and anchoring fibrils: components of a unique attachment complex. In Extracellular Matrix Assembly and Structure. Academic Press, Inc., Orlando, FL. 417–439.
- Gerecke, D.R., Wagman, D.W., Champliaud, M.F., and Burgeson, R.E. 1994b The complete primary structure for a novel laminin chain, the laminin B1K chain. J. Biol. Chem. 269:11073–11080. [PubMed]
- Guo L, Degenstein L, Dowling J, Yu Q-C, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, Jong MC, Heeres K, Pas HH, van der Meer JB, Owaribe K, Martinez-Velasco AM, Niessen CM, Sonnenberg A. 180-kD bullous pemphigoid antigen (BP180) is deficient in generalized atrophic bullous epidermolysis bullosa. J Clin Invest. 1995;95:1345–1352. doi: 10.1172/JCI117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki P, Sainio K, Eddy R, Byers M, Kallunki T, Sariola H, Beck K, Hirvonen H, Shows TB, Tryggvason K. A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignment. J Cell Biol. 1992;119:679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE. Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol. 1987;104:611–621. doi: 10.1083/jcb.104.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko S, McGrath JA, Aberdam D, Baudoin C, Ciatti S, Dunnill MGS, McMillan JR, Eady RAJ, Ortonne J-P, Meneguzzi G, et al. A homozygous nonsense mutation in the α3 chain gene of laminin 5 (LAMA3) in lethal (Herlitz) junctional epidermolysis bullosa. Hum Mol Genet. 1995;4:959–962. doi: 10.1093/hmg/4.5.959. [DOI] [PubMed] [Google Scholar]
- Lunstrum GP, Sakai LY, Keene DR, Morris NP, Burgeson RE. Large complex globular domains of type VII procollagen contribute to the structure of anchoring fibrils. J Biol Chem. 1986;261:9042–9048. [PubMed] [Google Scholar]
- Lunstrum GP, Kuo H-J, Rosenbaum LM, Keene DR, Glanville RW, Sakai LY, Burgeson RE. Anchoring fibrils contain the carboxyl-terminal globular domain of type VII procollagen, but lack the amino-terminal globular domain. J Biol Chem. 1987;262:13706–13712. [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Keene DR, Burgeson RE. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992a;119:695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992b;267:17900–17906. [PubMed] [Google Scholar]
- Mayer U, Poschl E, Gerecke DR, Wagman DW, Burgeson RE, Timpl R. Low nidogen affinity of laminin-5 can be attributed to two serine residues in EGF-like motive γ2III4. FEBS (Fed Eur Biochem Soc) Letts. 1995;365:129–132. doi: 10.1016/0014-5793(95)00438-f. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Gatalica B, Christiano AM, Owaribe K, McMillan JR, Eady RA, Uitto J. Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat Genet. 1995;11:83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Darling T, Gatalica B, Pohliagnbo G, Hintner H, Christiano AM, Yancy K, Uitto J. A homozygous deletion mutation in the 180-kD bullous pemphigoid antigen gene (BP180) in a family with generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 1996;106:771–774. doi: 10.1111/1523-1747.ep12345821. [DOI] [PubMed] [Google Scholar]
- McLean WHI, Pulkkinen L, Smith FJD, Rugg EL, Lane EB, Bullrich F, Burgeson RE, Amano S, Hudson DL, Owaribe K, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organisation. Genes Dev. 1996;10:1724–1735. doi: 10.1101/gad.10.14.1724. [DOI] [PubMed] [Google Scholar]
- Morris NP, Keene DR, Glanville RW, Burgeson RE. The tissue form of type VII collagen is an antiparallel dimer. J Biol Chem. 1986;261:5638–5644. [PubMed] [Google Scholar]
- Niessen CM, Hogervorst F, Jaspars LH, De Melker AA, Delwel GO, Hulsman EHM, Kuikman I, Sonnenberg A. The α6β4 integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Paulsson M, Aumailley M, Deutzmann R, Timpl R, Beck K, Engel J. Laminin-nidogen complex: extraction with celating agents and structural characterization. Eur J Biochem. 1987;166:11–19. doi: 10.1111/j.1432-1033.1987.tb13476.x. [DOI] [PubMed] [Google Scholar]
- Parente MG, Chung LC, Ryynanen J, Woodley DT, Wynn KC, Bauer EA, Mattei MG, Chu ML, Uitto J. Human type VII collagen: cDNA cloning and chromosomal mapping of the gene. Proc Natl Acad Sci USA. 1991;88:6931–6935. doi: 10.1073/pnas.88.16.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K, Uitto J. Mutations in the γ2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet. 1994a;6:293–298. doi: 10.1038/ng0394-293. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, Christiano AM, Gerecke DR, Burgeson RE, Pittelkow MR, Uitto J. A homozygous nonsense mutation in the β3 chain gene of laminin 5 (LAMB3) in Herlitz junctional epidermolysis bullosa. Genomics. 1994b;24:357–360. doi: 10.1006/geno.1994.1627. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, McGrath JA, Christiano AM, Uitto J. Detection of sequence variants in the gene encoding the β3 chain of laminin 5 (LAMB3) Hum Mutat. 1995;6:77–84. doi: 10.1002/humu.1380060115. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integin receptors. J Cell Biol. 1994;125:205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Golbick R, van der Rest M, Aumailley M. Structural requirement for cell adhesion to kalinin (laminin 5) J Biol Chem. 1995;270:13766–13770. doi: 10.1074/jbc.270.23.13766. [DOI] [PubMed] [Google Scholar]
- Ruggiero F, Petit B, Ronziere MC, Hartmann D, Herbage D. Composition and organization of the collagen network produced by fetal bovine chondrocytes cultured at high density. J Histochem Cytochem. 1993;41:867–875. doi: 10.1177/41.6.8315278. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Tizard R, VanDevanter DR, Carter WG. Cloning of the Lam A3 gene encoding the α3 chain of the adhesive ligand epiligrin. J Biol Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Burgeson RE. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986;103:1577–1586. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR. Fibrillin: monomers and microfibrils. Methods Enzymol. 1994;245:29–52. doi: 10.1016/0076-6879(94)45004-8. [DOI] [PubMed] [Google Scholar]
- Schittny JC, Yurchenco PD. Terminal short arm domains of basement membrane laminin are critical for its self-assembly. J Cell Biol. 1990;110:825–832. doi: 10.1083/jcb.110.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FJD, Eady RAJ, McMillan JR, Leigh IM, Geddes JF, Rugg EL, Kelsell DP, Bryant SP, Spurr NK, Kirtschig G, et al. Plectin deficiency: hereditary basis for muscular dystrophy with epidermolysis bullosa. Nature Genet. 1996;13:450–457. doi: 10.1038/ng0896-450. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, Christiano AM. Molecular basis of the dystrophic and junctional forms of epidermolysis bullosa: mutations in type VII collagen and kalinin (laminin 5) genes. J Invest Dermatol. 1994;103:39–46. doi: 10.1111/1523-1747.ep12398967. [DOI] [PubMed] [Google Scholar]
- Vailly J, Pulkkinen L, Miquel C, Christiano AM, Gerecke DR, Burgeson RE, Uitto J, Ortonne J-P, Meneguzzi G. Identification of a homozygous one basepair deletion in exon 14 of the LAMB3 gene in a patient with Herlitz junctional epidermolysis bullosa and prenatal diagnosis in a family at risk for recurrence. J Invest Dermatol. 1995;104:462–466. doi: 10.1111/1523-1747.ep12605898. [DOI] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Vidal F, Aberdam D, Miquel C, Christiano AM, Pulkkinen L, Uitto J, Ortonne J-P, Meneguzzi G. Integrin beta 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genet. 1995;10:229–234. doi: 10.1038/ng0695-229. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H. Binding of laminin to type IV collagen: a morphological study. J Biol Chem. 1985;260:7636–7644. [Google Scholar]
- Yurchenco PD, Cheng Y-S, Colognato H. Laminin forms an independent network in basement membranes. J Cell Biol. 1992;117:1119–1133. doi: 10.1083/jcb.117.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]