Abstract
Previous studies have demonstrated that NT2N neurons derived from a human embryonal carcinoma cell line (NT2) constitutively process the endogenous wild-type β-amyloid precursor protein (APP) to amyloid β peptide in an intracellular compartment. These studies indicate that other proteolytic fragments generated by intracellular processing must also be present in these cells. Here we show that the NH2-terminal fragment of APP generated by β-secretase cleavage (APPβ) is indeed produced from the endogenous full length APP (APPFL). Pulse–chase studies demonstrated a precursor–product relationship between APPFL and APPβ as well as intracellular and secreted APPβ fragments. In addition, trypsin digestion of intact NT2N cells at 4°C did not abolish APPβ recovered from the cell lysates. Furthermore, the production of intracellular APPβ from wild-type APP appears to be a unique characteristic of postmitotic neurons, since intracellular APPβ was not detected in several non-neuronal cell lines. Significantly, production of APPβ occurred even when APP was retained in the ER/ intermediate compartment by inhibition with brefeldin A, incubation at 15°C, or by expression of exogenous APP bearing the dilysine ER retrieval motif.
Amyloid β (Aβ)1 peptides are the building blocks of the amyloid fibrils found in neuritic plaques and vascular deposits that accumulate in the brains of patients with Alzheimer's disease (AD; Selkoe, 1994). Aβ is derived from proteolytic processing of one or more isoforms of the amyloid precursor protein (APP; Kang et al., 1987). APP isoforms are alternatively spliced type I transmembrane glycoproteins that are encoded by a single gene on human chromosome 21 (Kang et al., 1987; St. George-Hyslop et al., 1987). The 39–43-amino acid-long Aβ sequence begins in the ectodomain of APP and extends into the transmembrane region (see Fig. 1). Of the three major Aβ-containing isoforms encoded by the APP gene (i.e., APP695, APP751, and APP770; Kang et al., 1987; Kitaguchi et al., 1988; Ponte et al., 1988; Tanzi et al., 1988), APP695 is expressed almost exclusively by neurons of the central and peripheral nervous systems (Golde et al., 1990; Kang and Müller-Hill, 1990; Arai et al., 1991).
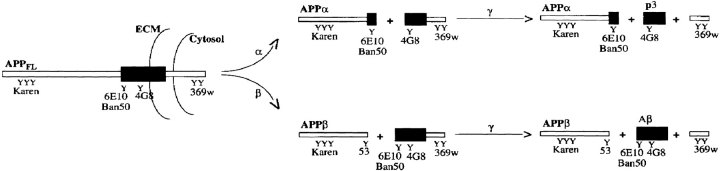
Figure 1.
Proteolytic processing of APPFL. The diagram depicts APP fragments generated by both the α- and β-secretase pathways. A large, secreted ectodomain called APPα is generated by the putative α-secretase(s) that cleaves APPFL within the Aβ domain. A second cleavage by the γ-secretase(s) releases a subfragment of Aβ known as p3. Alternative cleavage by the β-secretase(s) generates a similarly large ectodomain fragment known as APPβ. After the subsequent γ-secretase cleavage, Aβ is released. This schematic also shows the epitope location of the antibodies used in this study to identify the different proteolytic fragments.
Newly synthesized APP matures in the endoplasmic reticulum and the Golgi apparatus, acquiring N- and O-linked carbohydrates, tyrosine sulfates (Weidemann et al., 1989; Oltersdorf et al., 1990), and phosphates (Oltersdorf et al., 1990; Suzuki et al., 1992; Knops et al., 1993). Several pathways of APP metabolism have been described in cultured cells, and evidence suggests that the relative importance of each pathway depends on the cell type. For example, non-neuronal cells preferentially process APP by the α-secretase pathway, which cleaves APP within the Aβ sequence, thereby precluding the formation of Aβ (Esch et al., 1990; Sisodia et al., 1990). The putative α-secretase enzyme(s) is active at or near the cell surface, causing the NH2-terminal fragment (APPα) to be quickly secreted. In contrast, neuronal cells process a much larger portion of APP by the β-secretase pathway(s), which generate intact Aβ by the combined activity of two enzyme classes. The β-secretase(s) cleaves APP at the NH2 terminus of the Aβ domain releasing a distinct NH2-terminal fragment (APPβ). In addition, the γ-secretase(s) cleaves APP at alternative sites of the COOH terminus, generating species of Aβ that are either 40 (Aβ40) or 42 amino acids long (Aβ42; Seubert et al., 1993; Suzuki et al., 1994; Turner et al., 1996).
Although the identities of the putative α-, β-, and γ-secretases remain speculative, and the precise subcellular localization of their activity is poorly understood, in vitro studies have suggested the existence of at least two β-secretase pathways. In the endosomal/lysosomal pathway, APP targeted to the cell surface is endocytosed and delivered to endosomes and lysosomes where β- and γ-cleavages can occur (Golde et al., 1992; Haass et al., 1992a ; Nordstedt et al., 1993; Koo and Squazzo, 1994; Lai et al., 1995; Perez et al., 1996). The alternative β-secretory pathway is predicted to generate Aβ in Golgi-derived vesicles, most likely secretory vesicles, before secretion (Haass et al., 1995a ; Higaki et al., 1995; Perez et al., 1996; Thinakaran et al., 1996b ). Whether these pathways operate in the same or different cell types is not known, nor is the biological importance of each pathway for the production of Aβ in vivo understood.
Recently, we showed that both Aβ40 and Aβ42 are produced intracellularly from endogenous wild-type APP695 by cultured postmitotic central nervous system (CNS) neuronal cells (NT2N) that are induced to differentiate from a human teratocarcinoma cell line (NT2) by treatment with retinoic acid (Pleasure et al., 1992; Pleasure and Lee, 1993; Wertkin et al., 1993; Turner et al., 1996). To date, the human-derived NT2N neuron is the only cell line documented to generate intracellular Aβ40 and Aβ42 before their eventual release into the medium (Turner et al., 1996). Because neurons are the cell type most adversely affected by AD, the NT2N neurons represent a unique system for the study of intracellular β-secretase pathways in a human neuronal model. An essential first step in the analysis of such pathways is the identification of the proteolytic fragments that are the products of these cleavages. We report here that in addition to Aβ40 and Aβ42, the NH2-terminal fragment generated by β cleavage (i.e., APPβ) is produced intracellularly in NT2N neurons before secretion. More significantly, we demonstrate that novel β-secretase activity occurs in the ER/intermediate compartment (IC) of neuronal cells using inhibition with Brefeldin A (BFA), incubation at 15°C, and expression of exogenous APP bearing the dilysine ER-retrieval motif.
Materials and Methods
Cell Culture
NT2 cells derived from a human embryonal carcinoma cell line (Ntera 2/c1.D1) were grown and passaged twice weekly in Opti-Mem (Life Technologies, Inc., Gaithersburg, MD) supplemented with 5% FBS and penicillin/streptomycin (P/S) as described previously (Pleasure et al., 1992; Pleasure and Lee, 1993). To begin differentiation, 2.5 × 106 cells were seeded in a 75-cm2 (T75) flask and fed with DME HG (Life Technologies, Inc.) containing 10 μM retinoic acid, 10% FBS, and P/S twice weekly for 5 wk. The cells in a single T75 flask were then replated at a lower density in 2 × 225 cm2 (T225) flasks for 10 d (Replate 1 cells). Greater than 99% pure NT2N neurons were then obtained by enzymatic treatment and mechanical dislodegment of Replate 1 cells and replated at a density of 6 × 106 cells/10-cm dish previously coated with polylysine and Matrigel (Pleasure et al., 1992). The NT2N neurons were maintained in medium consisting of one part conditioned medium and one part DME HG containing 10% FBS and P/S. For experiments involving the incubation of NT2N neurons at 15°C for 16 h, regular medium containing DME HG and 10% FBS was replaced by DME HG containing 25 mM Hepes, 10% FBS, and P/S. Cultures of NT2N neurons were used for experiments when they were between 3 to 4 wk old. CHO695 cells, a gift from Dr. S. Sisodia (Johns Hopkins University School of Medicine, Baltimore, MD), were grown and passaged three times per week in α-MEM (Life Technologies, Inc.) supplemented with 10% FBS and P/S. M17 cells were grown and passaged once per week in Opti-Mem (Life Technologies, Inc.) containing 10% iron-enriched calf serum and P/S.
Metabolic Labeling, Gel Electrophoresis, Immunoblotting, and Quantitation
Cultured NT2N neurons were starved in methionine-free DME HG (Life Technologies, Inc.) for 30 min before incubation in fresh, methionine-free DME HG containing 0.5 mCi/ml of [35S]methionine (sp act 1,000 Ci/ mmol; NEN-Du Pont, Boston, MA). For steady-state labeling studies, NT2N neurons were labeled with [35S]methionine continuously for 16 h. For pulse–chase studies, cells were labeled with [35S]methionine for 1 h, washed twice with methionine-containing DME, and then chased in the same medium for 0 to 24 h. APPFL, APPα, and APPβ were separated on 7.5% Laemmli SDS-PAGE gels, and Aβ and p3 were separated on 10/ 16.5% step-gradient Tris-tricine gels. These gels were either stained with Coomassie brilliant blue R (Pierce, Rockford, IL) and dried or transferred to nitrocellulose membranes and dried before exposure on PhosphorImager plates (Molecular Dynamics, Sunnyvale, CA) for 3–5 d. The nitrocellulose replicas containing the immunoprecipitates were further probed with different antibodies, as described previously (Wertkin et al., 1993). Quantitation of bands in the autoradiogram was performed using the ImageQuant software (Molecular Dynamics) as described previously (Turner et al., 1996). Radiolabeled proteins in SDS-PAGE gels and nitrocellulose replicas were also analyzed by standard autoradiographic methods. All experiments were repeated between three to six times.
Sample Preparation and Serial Immunoprecipitations
Cell lysates were prepared as described elsewhere (Golde et al., 1992). Protein concentration was determined by the bicinchoninic acid procedure (Pierce). Media were centrifuged at 100,000 g for 1 h at 4°C before immunoprecipitation. Both cell lysates and media were precleared with protein A-Sepharose (Pharmacia Fine Chemicals, Piscataway, NJ) in RIPA for 1 h at 4°C. After recentrifugation at 15,000 g for 1 min, the supernatants were rocked overnight at 4°C with fresh protein A-Sepharose and the appropriate primary antibody. After collecting the immunoprecipitates by recentrifugation at 15,000 g for 1 min, the supernatants were used in a second round of immunoprecipitation with fresh protein A-Sepharose and a different primary antibody.
Trypsin Treatment of NT2N Neurons
NT2N neurons were metabolically labeled with 0.5 mCi/ml [35S]methionine for 16 h, as described above. After rinsing the cultures twice with PBS, the NT2N neurons were incubated on ice for 20 min with PBS, with 10 μg/ml of trypsin in PBS alone (Life Technologies, Inc.), or with 10 μg/ml trypsin and 0.1% Triton X-100 in PBS. After this treatment, trypsin was inactivated by the addition of 100 μg/ml soybean trypsin inhibitor. The cells were then washed with PBS, scraped into cell lysis buffer, and processed for immunoprecipitation, as described above.
BFA Treatment of NT2N Neurons and Deglycosylation of Immunoprecipitated APPβ
NT2N neurons were pretreated with 20 μg/ml of BFA for 1 h before the addition of 0.5 mCi/ml of [35S]methionine to the cultures for 16 h in the absence or presence of BFA. The cell lysates and media were processed for immunoprecipitation as described above. For deglycosylation of APPβ, the immunoprecipitates containing APPβ were washed twice in sodium phosphate buffer (20 mmol/liter, pH 7.2) and boiled for 2 min in 10 μl of 1% SDS. The samples were then boiled for an additional 2 min after adding 90 μl of the sodium phosphate buffer with sodium azide (10 mmol/ liter), EDTA (50 mmol/liter), and n-Octylglucoside (0.5% wt/vol). After the denaturation step as described, deglycosylation was initiated by the addition of 2 mU neuraminidase (Arthrobacter; Boehringer Mannheim, Indianapolis, IN), 2.5 mU O-Glycosidase (Boehringer Mannheim), and 0.4 U n-Glycosidase F (Boehringer Mannheim). The samples were then incubated at 37°C for 18 h, and deglycosylated APPβ was run on 7.5% SDS-PAGE gels as described above. For endoglycosidase H (Endo H) sensitivity test, cell lysates and media were immunoprecipitated with Karen as described. The immunoprecipitates were then recovered in 100 μl 60 mM phosphate buffer, pH 5.7, with 1% SDS. The samples were then split in half (50 μl each) and incubated with 4 μl Endo H (Boehringer Mannheim) or vehicle at 37°C for 18 h. The samples were then run on 7.5% SDS-PAGE gels as described above.
Antibodies for Immunoprecipitation and Immunoblotting
The antibodies used in this study and their epitope specificities are summarized in Fig. 1. Briefly, Karen is a goat polyclonal antisera raised to the large, secreted NH2-terminal fragment of APP, and antibody 53 is a rabbit polyclonal antisera raised to a synthetic peptide corresponding to the amino acid sequence SEVKM. Antibody 53 binds specifically to the free COOH terminus of APPβ (Howland et al., 1995). Antibody 369W is a rabbit polyclonal antiserum raised to a synthetic peptide corresponding to the last 45 amino acid residues at the COOH terminus of APP and was generously donated by Dr. Sam Gandy (Cornell University School of Medicine, New York, NY). Also used in this study were three mAbs to Aβ that are specific for residues 1–17 (6E10; Kim et al., 1988, residues 1–10 (Ban50; Suzuki et al., 1994), and residues 18–25 (4G8; Kim et al., 1988).
Preparation of SFV-bearing pSFV-1(APP695) and pSFV-1(APP695Δ KK)
The dilysine motif was introduced into APP695 by standard PCR site- directed mutagenesis of pSFV-1(APP695) using primers 5 ′-CGAAAACCACCGTGGAGCTCC TT-3′ and 5′-TTAACCCGGGCTAGTTCTGCTTCTTCTCAAAGAACTTGT-3′. The mutation-containing PCR fragment was isolated by digestion with BsmI and XmaI and then ligated into pSFV(APP695) to yield pSFV(APP695ΔKK). All pSFV-1 constructs, including a pSFV helper plasmid with SFV structural genes, were linearized by digestion with SpeI and then used as a template for RNA synthesis with SP6 RNA polymerase. Coelectroporation of RNA from the expression and helper plasmids into BHK cells yielded an infectious, replication-defective virus that was harvested 24 h later (Liljestrom and Garoff, 1991). Accurate determination of viral stock titers was made as described elsewhere (Cook et al., 1996). For all infection experiments, ∼1 × 106 NT2N neurons per 35-mm dish were infected in serum-free medium at a multiplicity of infection (MOI) of 7–10. When called for, 20 μg/ml BFA was added after the completion of the infection step.
Results
NT2N Neurons Exhibit Intracellular β-Secretase Activity
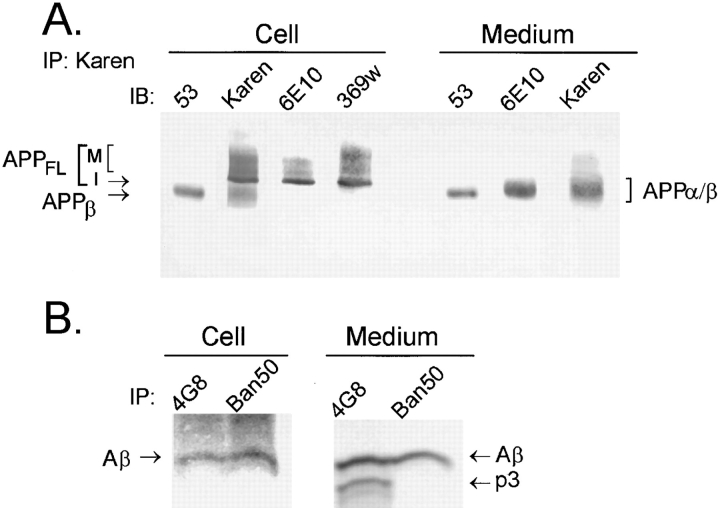
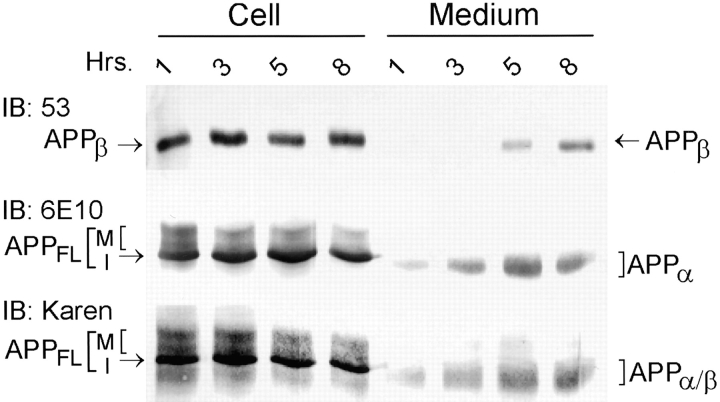
Our previous studies have demonstrated that NT2N cells produce intracellular Aβ (Wertkin et al., 1993; Turner et al., 1996). To determine if intracellular APPβ (Fig. 1) can also be recovered from these cells, samples of cell lysate were immunoprecipitated with Karen (an antiserum raised to the NH2-terminal region of APP). Then, the presence of APPβ in the immunoprecipitate was determined by immunoblot analysis using 53 (a polyclonal antibody specific for the free COOH terminus of APPβ). We found that 53 detects a single band of ∼95 kD (Fig. 2 a). That this 95-kD APP fragment is indeed APPβ, cleaved at the β-secretase site, was further substantiated by (a) the inability of 369W, an antibody specific for the COOH terminus of APP, to recognize this fragment; (b) the inability of 6E10, an antibody specific for the first 10 amino acid residues of Aβ, to detect this fragment; (c) the binding of Karen, an antibody that recognizes all APP species, to this fragment; (d) the fact that this intracellular APP fragment is ∼11–12 kD smaller than APPFL (Fig. 2 a); and (e) the detection of the same 95-kD APP fragment using a different antibody specific for APPβ (i.e., 192; Seubert et al., 1992; and data not shown). To determine if APPβ is secreted, media from NT2N neurons were again immunoprecipitated with Karen and subsequently immunoblotted with various antibodies (Fig. 2 a). We found that APPβ was readily detected in the media of NT2N neurons and that it comigrated with APPβ recovered from the cell lysates. However, as expected, APPβ migrated slightly faster than the product of α-secretase cleavage (APPα), which was also recovered from the media.
Figure 2.
NT2N neurons produce intracellular APPβ and Aβ. To demonstrate the presence of APPβ, samples of cell lysate and medium were collected from NT2N cultures and processed for immunoprecipitation (IP) with Karen, a polyclonal antibody that recognizes epitopes within the large ectodomain of APP. The presence of APPβ, APPα, APPα/β, and APPFL was detected by immunoblotting (IB) with the corresponding antibodies (A). To show that Aβ but not p3 is produced intracellularly, NT2N neurons were radiolabeled with [35S]methionine for 16 h. The cell lysate and the medium were then processed for immunoprecipitation with 4G8, a mAb that binds to both Aβ and p3, or Ban50, a mAb that recognizes only Aβ (B). Immunoprecipitates of Aβ and p3 were separated by electrophoresis in 10/16.5% step-gradient Tris-tricine gels. M, mature APPFL; I, immature APPFL.
The detection of intracellular APPβ and Aβ in NT2N neurons is consistent with our view that both β- and γ-secretase activities occur in an intracellular compartment. The absence of intracellular APPα, however, suggests that the majority or all of the α-secretase activity occurs at a different site. To further confirm that the β-secretase pathway, but not the α-secretase pathway, occurs inside these cells, we examined the cell lysate of NT2N neurons for the products of these respective pathways: Aβ, which is generated by β- and γ-secretase cleavages; and p3, a product of α- and γ-secretase cleavages. To do this, we immunoprecipitated the cell lysates of metabolically labeled NT2N neurons with mAbs that can distinguish between these peptides: 4G8 recognizes both Aβ and p3; Ban50, however, binds only to Aβ and not p3 (Fig. 2 b). Our data clearly demonstrate that Aβ, but not p3, is produced intracellularly. The p3 fragment was not detected in cell lysates even after prolonged exposure of the film. By contrast, both Aβ and p3 were readily recovered from the media. This observation supports previous findings that the α-secretase pathway occurs at or near the plasma membrane (Haass et al., 1992a , 1995b ; Sisodia, 1992).
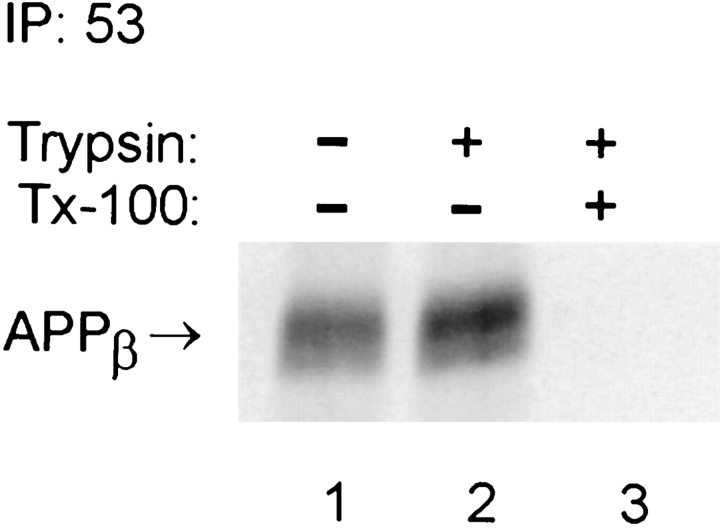
To determine if the recovery of APPβ from the cell lysates reflects its intracellular origin or its association with the cell surface, we treated cultures of NT2N neurons with trypsin at 4°C. Under such conditions, cell surface-associated but not intracellular APPβ should be proteolyzed. Fig. 3 shows that a similar amount of APPβ was recovered from NT2N neurons regardless of trypsin treatment (Fig. 3, compare lanes 1 and 2). By contrast, when the NT2N neurons were treated with trypsin and 0.1% Triton X-100, intracellular APPβ was completely eliminated (Fig. 3, lane 3). This experiment provides evidence that the APPβ recovered from the NT2N cell lysate is indeed produced in an intracellular compartment.
Figure 3.
APPβ is produced intracellularly in NT2N neurons. Culture dishes containing >99% pure NT2N cells were metabolically labeled with [35S]methionine for 16 h. Cells were rinsed twice with PBS and then incubated on ice for 20 min with PBS alone (lane 1), with 10 μg/ml trypsin (lane 2), or with 10 μg/ml trypsin and 0.1% Triton X-100 (lane 3). The cells were processed for immunoprecipitation with the anti-APPβ antibody 53, as described in Materials and Methods.
Intracellular APPβ Derived from Wild-Type APP Is Detected Only in Cells with a CNS Phenotype
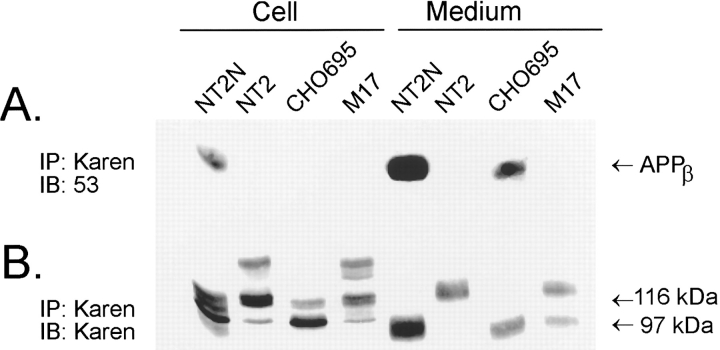
To determine if other cell types are capable of producing intracellular APPβ, the following cell lines were included in this study for comparison: (a) retinoic acid-naive NT2 cells, the undifferentiated precursors of the NT2N neurons that express high levels of the APP751 and APP770 isoforms; (b) Chinese hamster ovary (CHO) cells stably transfected with APP695; and (c) human M17 neuroblastoma cells. Approximately 800 μg of total protein collected in the cell lysates of each cell type was first immunoprecipitated with Karen and then immunoblotted with either antibody 53 to detect APPβ (Fig. 4 a) or Karen to detect all forms of APP (Fig.4 b). We found that while all four cell types synthesized similar amounts of APP, the NT2N neuron was the only cell type capable of producing detectable levels of intracellular APPβ (Fig. 4 a). However, both NT2N neurons and stably transfected CHO cells expressing APP695, but neither NT2 cells nor the M17 neuroblastoma cells, secreted APPβ, raising the possibility that secretion of APPβ may be isoform specific. While our data does not preclude low levels of intracellular β-secretase activity or faster rate of APPβ secretion in these cell lines, the evidence clearly indicates that the fraction of APP processed by β-secretase(s) as well as the subcellular site(s) of this activity may be strongly cell-type dependent.
Figure 4.
Intracellular APPβ is observed only in NT2N neurons. Samples of cell lysate and medium collected from cultures of NT2N, NT2, M17, and CHO cells stably expressing APP695 (CHO695) were processed for immunoprecipitation with the antibody Karen. The immunoprecipitates were separated by SDS-PAGE gels and transferred onto nitrocellulose replicas. APPβ present in the cell lysates and the media were detected by immunoblotting with the anti-APPβ antibody 53 (A). After stripping the nitrocellulose replica in A with 0.1% SDS, the blot was reprobed with Karen to detect all APP ectodomain species (B).
NT2N Neurons Produce Intracellular APPβ Before Secretion
The experiments shown in Figs. 2–4 demonstrated that intracellular APPβ can be detected in NT2N neurons. These data suggest that APPβ may be generated inside the cell before secretion. To demonstrate unequivocally that a precursor–product relationship exists between intracellular and secreted APPβ, we adopted the following approaches. In our first approach, NT2N neurons were washed with fresh medium, and then the amount of intracellular as well as secreted APPβ and APPα were measured over an 8-h period. This was accomplished by immunoprecipitation of cell lysates and media with Karen followed by immunoblotting with either antibody 53 (for APPβ) or 6E10 (for APPα). As shown in Fig. 5, secreted APPβ was first detected in 3 to 5 h, and its accumulation in the medium continued over the 8-h incubation period. By contrast, APPα was detected in 1 h, suggesting that APPα is produced at a faster rate than APPβ. As seen with APPβ, APPα accumulated in the conditioned media over time. Finally, our data also show that intracellular APPβ is produced constitutively, since a steady state level of APPβ is recovered from NT2N cell lysates prepared from parallel cultures over a period of 8 h (Fig. 5). These findings are consistent with the idea of APPβ being generated inside NT2N neurons before secretion.
Figure 5.
NT2N neurons produce intracellular APPβ before secretion. Cultures of NT2N neurons were washed and fresh medium was replenished before measuring the amount of intracellular and secreted APPβ over an 8-h period. Cell lysate and medium collected at the times indicated were immunoprecipitated with Karen. The immunoprecipitates were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. APPβ was identified in immunoblots using the antibody 53. APPα was detected using the antibody 6E10. APPFL and APPα/β were recognized by Karen.
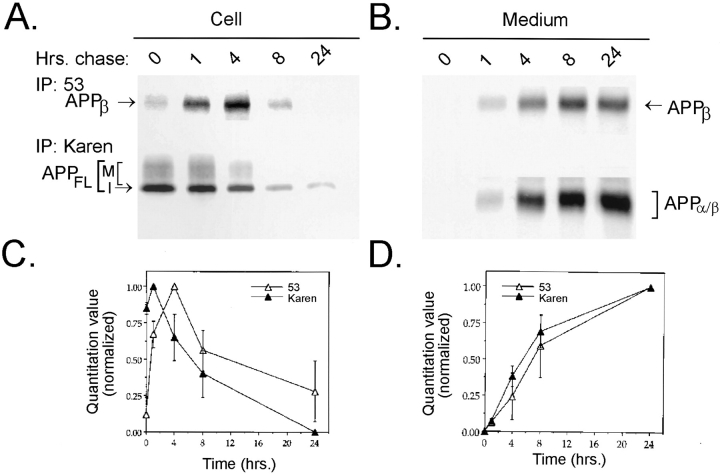
We next employed a pulse–chase paradigm to study more rigorously the temporal relationship between intracellular and secreted APPβ. To this end, NT2N cultures were pulsed with [35S]methionine for 1 h and then chased for different lengths of time (Fig. 6). We found that after 1 h of chase time, full length APP (APPFL) immunoprecipitated from the cell lysate began to decline, while the intracellular level of APPβ continued to increase until 4 h, after which it also declined (Fig. 6, a and c). This lag in maximum production of intracellular, radiolabeled APPβ supports the idea that APPβ is produced intracellularly from APPFL by β-secretase cleavage. Finally, the 1-h delay in the secretion of APPβ into the medium as well as the accumulation of this fragment with increasing chase time supports a temporal relationship between APPβ that is produced intracellularly and APPβ that is secreted into the medium (Fig. 6, b and d). Therefore, we conclude that APPβ is produced in an intracellular compartment in NT2N neurons before secretion.
Figure 6.
Pulse–chase labeling demonstrates that intracellular APPβ is produced in an intracellular compartment before secretion in NT2N neurons. NT2N neurons were pulse labeled with [35S]methionine for 1 h and chased for 0, 1, 4, 8, and 24 h. Radiolabeled cell lysates (A) or media (B) were immunoprecipitated sequentially with antibody 53 (for APPβ) followed by Karen (for APPFL in the cell lysates and APPα/β in the media). Radiolabeled immunoprecipitates were used to expose PhosphorImager plates (72 h) or X-ray film (3 wk) for visualization. C and D summarize the quantitation of experiments shown in A and B. Counts from three different experiments were normalized to percentage of maximum and plotted as shown (mean ± standard error).
Intracellular β-Cleavage in NT2N Neurons Occurs in a pre-Golgi Compartment
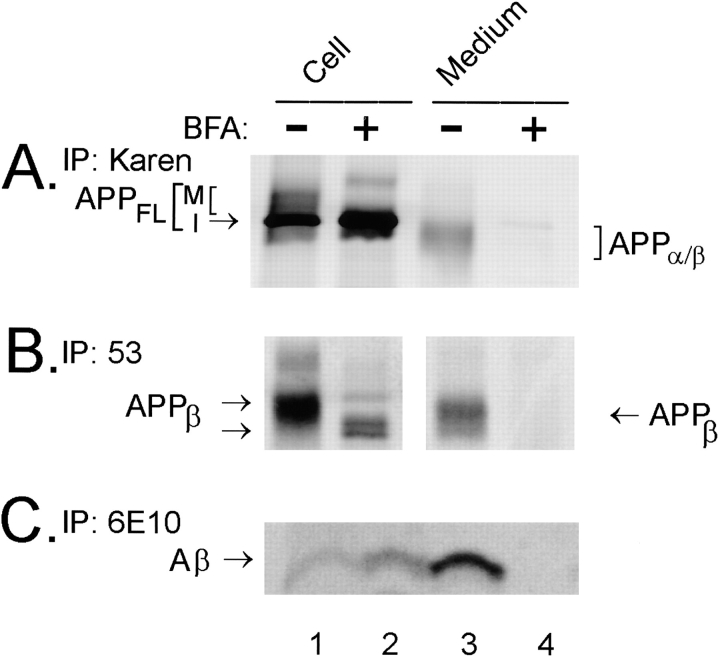
Since APPβ is produced in an intracellular compartment in NT2N neurons, we sought to identify the subcellular site(s) of β-secretase cleavage. Therefore, NT2N neurons were metabolically labeled with [35S]methionine in the presence or absence of 20 μg/ml BFA (Fig. 7). BFA is a pharmacological agent that causes a redistribution of the Golgi into the ER (Doms et al., 1989; Lippincott-Schwartz, 1989; Pelham, 1991). In the absence of BFA, APPFL, APPβ, and Aβ were recovered from the cell lysates, while APPα, APPβ, and Aβ were detected in the media of NT2N neurons (Fig. 7, a–c, lanes 1 and 3). Surprisingly, in the presence of BFA, not only APPFL but also APPβ and Aβ continued to be recovered from NT2N cell lysates (Fig. 7, a–c, lane 2). The effectiveness of BFA was verified by the fact that the secretion of APPα, APPβ, and Aβ into the medium was completely abolished in its presence (Fig. 7, a–c, lane 4). Furthermore, we found that APPβ recovered from BFA-treated cells (Fig. 7 b, lane 2) migrate with an accelerated electrophoretic mobility compared to APPβ from nontreated cells (Fig. 7 b, lane 1), suggesting that this fragment may have been derived from immature APP. Indeed, the faster mobility of mature APPFL in the presence of BFA (Fig. 7 a, compare M of lanes 1 and 2) indicates that this agent blocks APP from acquiring at least some of the posttranslational modifications. Thus, Aβ may be generated from immature as well as mature forms of APP.
Figure 7.
Intracellular β and γ cleavages occur in a pre-Golgi compartment in NT2N neurons. Cultures of NT2N cells were first preincubated with 20 μg/ml BFA for 1 h before radiolabeling with [35S]methionine for 16 h in the continuous presence of 20 μg/ml BFA. Control cultures were processed similarly, except that BFA was absent in the medium. Radiolabeled proteins from BFA-treated and untreated cell lysates and media were immunoprecipitated with Karen (for APPFL in the cell lysates and APPα/β in the media as shown in A), with antibody 53 (for APPβ in B), and with the mAb 6E10 (for Aβ in C). Note that APPβ and Aβ were recovered in the cell lysate but not in the medium of BFA-treated cells. (M, mature APPFL; I, immature APPFL.
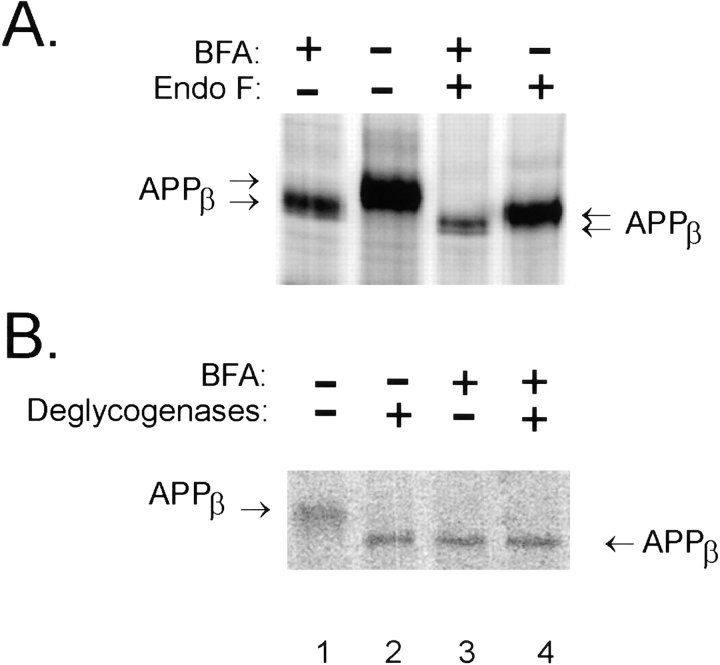
We sought next to determine if incomplete maturation of APP is indeed the cause of the shift in electrophoretic mobility of the APPβ fragment generated in the presence of BFA. Therefore, NT2N cells were metabolically labeled with [35S]methionine in the presence or absence of BFA, and APPβ immunoprecipitated from the cell lysate was incubated with N-glycosidase F (Nglyc F), an enzyme that removes N-linked carbohydrate chains. As shown, APPβ from BFA-treated NT2N neurons (Fig. 8 a, lane 1) migrated more quickly than APPβ recovered from untreated cells (Fig. 8 a, lane 2). After digestion with Nglyc F, APPβ demonstrated a mobility downshift in SDS-PAGE (Fig. 8 a, compare lanes 2 and 4). However, APPβ from BFA-treated cells (Fig. 8 a, lane 3) still migrated faster than APPβ from nontreated cells (Fig. 8 a, lane 4) despite enzymatic removal of all N-linked carbohydrate chains. Thus, the increased electrophoretic mobility of APPβ in the presence of BFA cannot be accounted for solely by differences in N-linked carbohydrate processing.
Figure 8.
APPβ generated in the presence of BFA is partially glycosylated. Cultures of NT2N neurons were metabolically labeled as in Fig. 7 in the presence or absence of 20 μg/ml BFA. The cell lysates were then immunoprecipitated with the antibody 53. (A) Samples in lanes 3 and 4 were treated with Nglyc F for 16 h to remove N-linked sugars, whereas immunoprecipitates in lanes 1 and 2 were treated with the vehicle. (B) Samples in lanes 2 and 4 were deglycosylated with a combination of Nglyc F, neuraminidase, and O-glycosidase for 16 h to remove both N- and O-linked chains (lanes 2 and 4); lanes 1 and 3 represent samples that were mock digested.
In addition to N-linked glycosylation, however, APP undergoes a variety of posttranslational modifications, including the addition of O-linked carbohydrate chains. Therefore, we removed both N- and O-linked carbohydrate chains from immunoprecipitated APPβ by simultaneous digestion with Nglyc F, O-glycosidase, and neuraminidase. As shown, fully deglycosylated APPβ (Fig. 8 b, lane 2) comigrated with APPβ recovered from BFA-treated NT2N neurons (Fig. 8 b, lane 3). Furthermore, combined BFA inhibition and deglycosylation (Fig. 8 b, lane 4) did not induce a greater mobility shift than either of these treatments alone (Fig. 8 b, lanes 2 and 3). Taken together, these results suggest that APPβ generated from BFA-treated NT2N neurons may represent β-secretase processing of immature (nonglycosylated) APPFL in a pre-Golgi compartment.
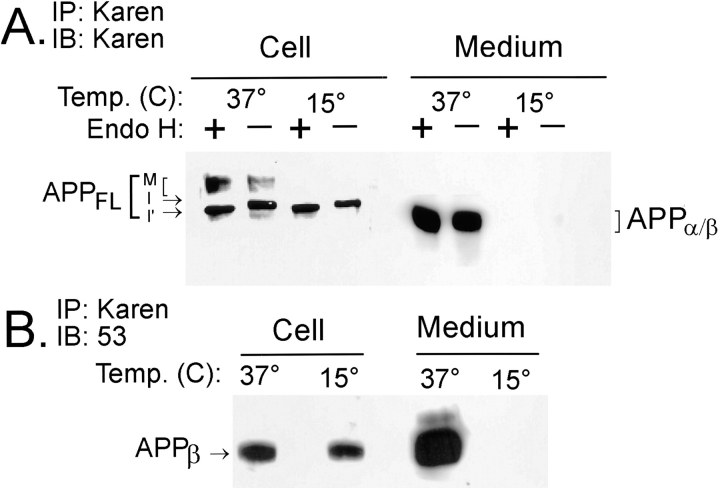
To further verify that β-secretase cleavage indeed occurs early in the biosynthetic pathway of NT2N neurons, we employed an alternative nonpharmacological method to block protein transport from the ER to the Golgi. Incubation of cultured cells at 15°C has been shown to inhibit newly synthesized proteins from exiting the intermediate compartment (Saraste and Kuismanen, 1984; Saraste et al., 1986; Schweizer et al., 1990). To this end, NT2N cells were incubated at 15°C for 16 h. Fig. 9 a shows that only the immature form of APPFL was present after a 16-h incubation at 15°C, as indicated by its sensitivity to Endo H digestion, suggesting that it is not transported to the Golgi apparatus under these conditions (Fig. 9 a, lanes 3 and 4). By contrast, incubation of the NT2N cells at 37°C yielded both immature and fully processed APPFL (Fig. 9 a, lanes 1 and 2). As expected, the immature APPFL was Endo H sensitive, while the mature forms of APPFL, having acquired posttranslational modifications after exiting the ER, were Endo H resistant. In addition, secreted forms of APP were not detected in cells maintained at 15°C, further substantiating the effectiveness of the temperature block. Significantly, continuous production of intracellular APPβ was observed at 15°C, despite the fact that the secretion of APP ectodomain is completely abolished (Fig. 9 b). Taken together, these data support the ER/IC of NT2N neurons as a β-cleavage site.
Figure 9.
APPβ is generated in the ER/IC of NT2N neurons. Approximately 6 × 106 NT2N neurons were incubated at either 15° or 37°C for 16 h. The cell lysates and media were harvested and immunoprecipitated with Karen. (A) The immunoprecipitates were then split, and half of the samples was treated with Endo H for 18 h, while the other half was mock digested. Subsequent to this step, the immunoprecipitates were separated by SDS-PAGE, transferred onto nitrocellulose replicas, and probed with the antibody Karen. The following observations serve to verify the effectiveness of the temperature block: (a) immature forms of APPFL (I and I′) in the cell lysate retain Endo H sensitivity at 15°C; (b) mature glycosylated forms of APPFL (M) in the cell lysate are not detected at 15°C; and (c) secreted fragments are not detected in the conditioned medium at 15°C. (B) Immunoprecipitates were separated by SDS-PAGE, transferred onto nitrocellulose replicas, and probed with antibody 53. APPβ continued to be produced intracellularly despite the effective temperature block. However, secreted APPβ was not detected in the medium at 15°C. Note that splitting intracellular APPβ samples recovered at 15°C for Endo H digestion decreased the yield to below the level of detection by this assay (data not shown). M, mature APPFL; I, immature APPFL; I′, immature APPFL demonstrating a mobility shift due to Endo H sensitivity.
A third approach was adopted to confirm that β-secretase cleavage indeed occurs in a pre-Golgi compartment of NT2N neurons. To accomplish this, we compared the processing of wild-type APP695 and APP695 bearing an ER-retrieval motif (APP695ΔKK; Jackson et al., 1990, 1993) in the NT2N cells. We used recombinant Semliki Forest virus (SFV) vectors to express APP695ΔKK, in which the third and fourth amino acids from the COOH terminus of APP are changed to lysines (i.e., APP695ΔKK). Our previous studies have shown that despite high levels of SFV-mediated APP expression, SFV-infected NT2N cells display a high degree of fidelity in processing APP (Wertkin et al., 1993; Turner et al., 1996; Cook et al., 1997). Furthermore, we have found that cytopathic effects of SFV infection in NT2N cells as measured by LDH release do not develop until >48 h after infection (data not shown). Importantly, all if not a significant majority of APP695ΔKK colocalize with calnexin, the ER marker, by immunofluorescence upon expression in NT2N neurons (Cook et al., 1997).
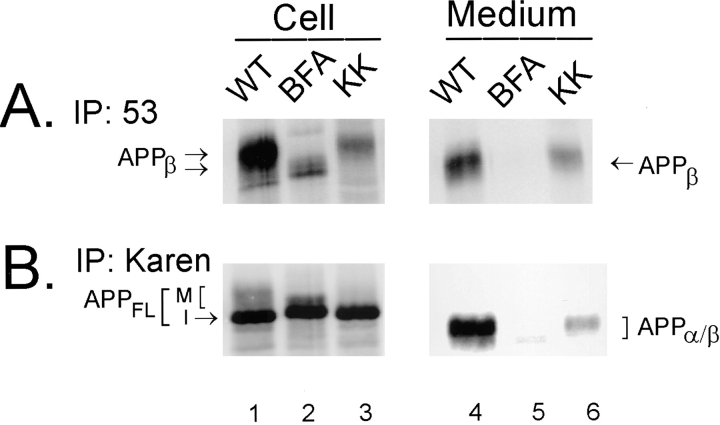
To determine whether or not APPβ can be produced from APP695ΔKK, wild-type APP695 and APP695ΔKK were separately expressed in NT2N neurons by infection with SFV vectors bearing these constructs. After infection, duplicate wells containing wild-type APP695-infected cells were also treated with 20 μg/ml BFA. The [35S]methionine- labeled cell lysates and the media were then sequentially immunoprecipitated with the antibodies 53 and Karen. Only the immature form of APPFL was detected from cells expressing APP695ΔKK (Fig. 10 b, compare lanes 1 and 3). Significantly, intracellular production and secretion of APPβ was not affected by genetic targeting of APP to the ER (Fig. 10 a, lanes 3 and 6). Furthermore, we found that unlike inhibition with BFA that eliminates transport of all proteins from the ER to the Golgi, specific retrieval of full length APP695ΔKK to the ER allowed the APPβ fragment generated in the ER/IC to be transported to the Golgi complex for modification before secretion (Fig. 10 a, compare lanes 2 and 3 and lanes 5 and 6). This suggests that once the ER retention motif is cleaved from the APPβ fragment, it can then be transported to the Golgi complex for further maturation and subsequent secretion.
Figure 10.
APPβ is generated from APPFL that is concentrated in the ER. NT2N cultures of ∼1 × 106 cells were infected with recombinant SFV containing either wild-type APP695 or APP695ΔKK constructs. The dilysine motif concentrates APPFL to the ER by an efficient retrieval mechanism. Duplicate cultures infected with wild-type APP695 were treated with 20 μg/ml BFA for comparison. Under these conditions, the cells were metabolically labeled with [35S]methionine for 16 h. Radiolabeled cell lysates and media were then immunoprecipitated with antibody 53 (for APPβ, A) and Karen (for APPFL and APPα/β, B). Radiolabeled immunoprecipitates were used to expose PhosphorImager plates (72 h) for visualization of bands. Unlike APPβ produced under BFA inhibition, APPβ derived from APP695ΔKK was modified and secreted into the medium.
Discussion
APP serves as a substrate for a variety of proteolytic processing pathways, only some of which result in the production of Aβ (Selkoe, 1994). However, Aβ is the major component of senile plaques in the AD brain. Moreover, mutations in the APP gene associated with Familial Alzheimer's disease alter APP processing and Aβ production in vitro (Citron et al., 1992; Cai et al., 1993; Suzuki et al., 1994). Thus, it will be important to determine the proteolytic events that lead to Aβ production and to identify the proteases responsible for each step as well as the sites of their action. In addition, it will be important to consider the cell type in which these processes occur. Non-neuronal cells favor the nonamyloidogenic α-secretase pathway. By contrast, neuronal cells exhibit increased β-secretase activity (Busciglio et al., 1993; Wertkin et al., 1993). To better understand APP processing in neurons, we have used the NT2N system for this study. We have previously shown that NT2N neurons express the isoform of APP expressed almost exclusively in the CNS (i.e., APP695) and that they constitutively produce intracellular and secreted Aβ. In this study, we have identified and characterized some of the intracellular β-secretase activities that cleave on the NH2 terminus side of Aβ by using specific antibodies to APPβ and to other proteolytic fragments. More significantly, however, we have used three independent approaches to document novel β- and γ-secretase activities that occur in a pre-Golgi compartment.
Several lines of evidence presented here demonstrate that APPβ is derived from APPFL within the cell before secretion. First, APPβ was recovered from NT2N cell lysates even after intact NT2N neurons were treated with trypsin. Such treatment would eliminate cell surface-associated APPβ but not intracellular APPβ. Indeed, the loss of APPβ after trypsin treatment of detergent-permeabilized NT2N neurons further confirms the intracellular origin of APPβ in NT2N neurons. Second, the continuous presence of steady state levels of APPβ in NT2N neurons, together with a delay in the detection of APPβ in freshly replenished medium, suggested that APPβ is generated intracellularly before secretion. Third, pulse–chase experiments demonstrated that the turnover of intracellular APPβ lags behind the turnover of newly synthesized APPFL, thereby confirming that APPβ is generated from APPFL inside NT2N neurons before secretion.
The detection of APPβ in the cell lysate of NT2N neurons, together with the presence of Aβ40 and Aβ42 (Turner et al., 1996), firmly established that an intracellular β-secretase pathway(s) must exist in these cells. At present, no other cell line has been reported to produce detectable levels of intracellular APPβ from endogenous or over-expressed wild-type APP (Seubert et al., 1993; Haass et al., 1995a ; Thinakaran et al., 1996b ). Only human kidney 293 cells stably transfected with APPsw cDNA yield the related APPβsw fragment from the cell lysates (Haass et al., 1995a ; Martin et al., 1995). In these non-neuronal cells, however, treatment with BFA completely eliminates APPβsw and Aβ production (Haass et al., 1995a ; Martin et al., 1995; Essalmani et al., 1996). In contrast, NT2N neurons continue to produce APPβ and Aβ during treatment with BFA, implying that the subcellular site(s) of the β-secretase pathway is cell-type specific. Furthermore, this lack of inhibition of APPβ and Aβ production by BFA in NT2N cells suggests that at least one of the β-secretase pathways is localized to the ER/IC. Two additional independent means of testing this hypothesis (i.e., the use of 15°C temperature block and expression of APP bearing the dilysine ER retrieval signal) yielded consistent results.
Our data also suggest that the β-secretase pathway, but not the α-secretase pathway, occurs inside NT2N neurons. This view is based on the absence of APPα and p3 fragments in NT2N cell lysates. Of course, this observation alone cannot rule out the possibility of their presence below the level of detection by our assay. Nevertheless, these results imply that at least in this regard, NT2N neurons are similar to almost all other cell lines in which the enzymes of the α-secretase pathway are active at or near the cell surface. The uniqueness of intracellular processing in postmitotic neuronal cells such as the NT2N neurons lies in the fact that unlike non-neuronal cells, the amyloidogenic β-secretase pathway(s) is preferred. Accordingly, the level of Aβ secretion is much higher than that of p3 in postmitotic NT2N neurons.
The effect of the Swedish mutation on APP processing is interesting. Overexpression of APPsw in transfected, non-neuronal cells results in a 5–10-fold increase in Aβ secretion (Citron et al., 1992; Cai et al., 1993). Concomitant with this change, intracellular APPβsw is also detected in non-neuronal cells stably transfected with APPsw (Haass et al., 1995a ; Thinakaran et al., 1996b ). Transfection of wild-type APP695 in non-neuronal cells, however, fails to produce intracellular APPβ and results in the secretion of more p3 than Aβ (Thinakaran et al., 1996b ). Thus, it appears that the introduction of the Swedish mutation shifts APP processing away from the α-secretase pathway to the β-secretase pathway. However, unlike NT2N neurons that may use multiple β-secretase pathways to produce both intracellular Aβ and APPβ, APPsw expressing non-neuronal cells use primarily the endosomal/lysosomal pathway or the Golgi-derived vesicles to generate intracellular Aβ and APPβsw, since treatment of these cells with BFA completely inhibits APPβsw and Aβ production (Haass et al., 1995a ; Martin et al., 1995).
In view of the foregoing, three potential β-secretase pathways have been identified to date. Of these three, the endosomal/lysosomal pathway, which processes APP targeted to the cell surface after its reinternalization into endosomes and lysosomes, is the most ubiquitous. Both primary cultures of neuronal and non-neuronal cells, as well as multiple cell lines, use this pathway to produce Aβ. However, the contribution of endosomal/lysosomal processing to the overall production of Aβ is relatively minor since non-neuronal cells transfected with wild-type APP produce mostly p3 and very little Aβ (Haass et al., 1992a , b ; Koo and Squazzo, 1994; Lai et al., 1995; Thinakaran et al., 1996b ). In contrast, an alternative β-secretase pathway that produces Aβ in Golgi-derived vesicles is the most important for the production of Aβ in cells transfected with APPsw. Consistent with this view, transfection of an APPsw construct lacking the cytoplasmic tail, which eliminates reinternalization of cell surface APPsw, does not reduce the secretion of Aβ (Haass et al., 1995a ; Essalmani et al., 1996). It is likely that the neuron-like NT2N cells also use this β-secretase pathway since neuronal cells (including hippocampal neurons and NT2N neurons) produce much higher levels of Aβ than p3. Finally, the third β-secretase pathway localized to the ER/IC appears to be preferentially used by postmitotic neuronal cells, since intracellular APPβ was not detected in several non-neuronal cell lines when treated with BFA.
The possibility of Aβ generation in the ER of NT2N neurons identifies these cells as a unique system in which to test the hypothesis that amyloidogenic processing of APP within that compartment plays an important role in the pathogenesis of AD. There is now strong evidence that mutations in both the APP gene and the recently identified presenilin genes cause AD by altering APP processing in ways that lead to the production of more amyloidogenic form of Aβ (i.e., Aβ42; Scheuner et al., 1996). Recently, in both non-neuronal and neuronal cells (including the NT2N neurons used in this study), the presenilin proteins have been localized to the ER (Cook et al., 1996; Kovacs et al., 1996; Thinakaran et al., 1996a ). Thus, the identification of amyloidogenic processing that may occur within the ER of neurons raises the formal possibility that direct or indirect interaction may occur between the presenilins and APP. Furthermore, the mutations in the presenilin genes may alter this interaction in a manner that leads to increased production of Aβ42. Therefore, it will be particularly interesting to examine the effects of both Familial Alzheimer's disease-linked mutations occurring in the APP as well as the presenilin genes on the processing of APP in the ER.
Acknowledgments
We thank Drs. T.E. Golde and J.Q. Trojanowski for critical review of the manuscript. We also thank Drs. D. Schenk, S. Gandy, and N. Suzuki for providing us with the antibody 192, antibody 369W, and the mAb Ban50, respectively. C.D. Page is thanked for providing some of the NT2N cells used in this study.
This work was supported by National Institutes of Health NIA grant AG-11542.
Abbreviations used in this paper
- Aβ
amyloid β
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- BFA
brefeldin A
- CNS
central nervous system
- Endo H
endoglycosidase H
- IC
intermediate compartment
- Nglyc F
N-glycosidase F
- SFV
Semliki Forest virus
Footnotes
Please address all correspondence to Dr. Virginia M.-Y. Lee, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Third Floor Maloney, HUP, Philadelphia, PA 19104-4283. Tel.: (215) 662-6427; Fax: (215) 349-5909; E-mail: vmylee@mail.med.upenn.edu
References
- Arai H, Lee VM-Y, Messinger M, Greenberg BD, Lowery DE, Trojanowski JQ. Expression patterns of β-amyloid precursor protein (βAPP) in neural and nonneural human tissues from Alzheimer's disease and control subjects. Ann Neurol. 1991;30:686–693. doi: 10.1002/ana.410300509. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. Generation of β-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci USA. 1993;90:2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X-D, Golde TE, Younkin SG. Release of excess amyloid β protein from a mutant amyloid β protein precursor. Science (Wash DC) 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in Alzheimer's disease increases β-protein production. Nature (Lond) 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Cook DG, Sung JC, Golde TE, Felsenstein KM, Wojczyk RE, Tanzi RE, Trojanowski JQ, Lee VM-Y, Doms RW. Expression and analysis of presenilin 1 in a human neuronal system: localization in cell bodies and dendrites. Proc Natl Acad Sci USA. 1996;93:9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D.G., M. Forman, J.C. Sung, S. Leight, D.L. Kolson, T. Iwatsubo, V.M.-Y. Lee, and R.W. Doms. 1997. Alzheimer Aβ (42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat. Med. In press. [DOI] [PubMed]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ. Cleavage of amyloid β-peptide during constitutive processing of its precursor. Science (Wash DC) 1990;248:1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Essalmani R, Macq A-F, Mercken L, Octave J-N. Missense mutations associated with familial Alzheimer's disease in Sweden lead to the production of the amyloid peptide without internalization of its precursor. Biochem Biophy Res Commun. 1996;218:89–96. doi: 10.1006/bbrc.1996.0017. [DOI] [PubMed] [Google Scholar]
- Golde TE, Estus S, Usiak M, Younkin LH, Younkin SG. Expression of β amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer's disease using PCR. Neuron. 1990;4:253–267. doi: 10.1016/0896-6273(90)90100-t. [DOI] [PubMed] [Google Scholar]
- Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science (Wash DC) 1992;255:728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature (Lond) 1992a;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature (Lond) 1992b;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. The Swedish mutation causes early-onset Alzheimer's disease by β-secretase cleavage within the secretory pathway. Nat Med. 1995a;1:1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- Haass C, Koo EH, Capell A, Teplow DB, Selkoe DJ. Polarized sorting of β-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol. 1995b;128:537–547. doi: 10.1083/jcb.128.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J, Quon D, Zhong Z, Cordell B. Inhibition of β-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron. 1995;14:651–659. doi: 10.1016/0896-6273(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Howland DS, Savage MJ, Huntress FA, Wallace RE, Schwartz DA, Loh T, Melloni RH, Jr, Degennaro IJ, Greenberg BD, Simam R, et al. Mutant and native human β-amyloid precursor proteins in transgenic mouse brain. Neurobiol Aging. 1995;16:685–699. doi: 10.1016/0197-4580(95)00078-s. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Retrieval of transmembrane proteins to the endoplasmic reticulum. J Cell Biol. 1993;121:317–333. doi: 10.1083/jcb.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Müller-Hill B. Differential splicing of Alzheimer's disease amyloid A4 precursor RNA in rat tissues: PreA4(695) mRNA is predominantly produced in rat and human brain. Biochem Biophys Res Commun. 1990;166:1192–1200. doi: 10.1016/0006-291x(90)90992-v. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire H-G, Unterbeck A, Alsboum M, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature (Lond) 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kim KS, Miller DL, Sapienza VJ, Chen C-M, Bai C, Grundke-Iqbal I, Currie JR, Wisniewski HM. Production and characterization of monoclonal antibodies reactive to synthetic cerebrovascular amyloid peptide. Neurosci Res Commun. 1988;2:121–130. [Google Scholar]
- Kitaguchi N, Takahashi Y, Tokushima Y, Shiojiri S, Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature (Lond) 1988;331:530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Knops J, Gandy S, Greengard P, Lieberburg I, Sinhas S. Serine phosphorylation of the secreted extracellular domain of APP. Biochem Biophys Res Commun. 1993;197:380–385. doi: 10.1006/bbrc.1993.2490. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo S. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Kovacs DM, Fausett HJ, Page KJ, Kim T-W, Moir RD, Merriam DE, Hollister RD, Hallmark OG, Mancini R, Felsenstein KM, et al. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- Lai A, Sisodia S, Trowbridge IS. Characterization of sorting signals in the β-amyloid precursor protein cytoplasmic domain. J Biol Chem. 1995;270:3565–3573. [PubMed] [Google Scholar]
- Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (NY) 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz JL, Yuan LC, Banifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the endoplasmic reticulum in cells treated with Brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Schrader-Fischer G, Busciglio J, Duke M, Paganetti P, Yankner BA. Intracellular accumulation of β-amyloid in cells expressing the Swedish mutant amyloid precursor proteins. J Biol Chem. 1995;270:26727–26730. doi: 10.1074/jbc.270.45.26727. [DOI] [PubMed] [Google Scholar]
- Nordstedt C, Caporaso G, Thyberg J, Gandy S, Greengard P. Identification of the Alzheimer β/A4 amyloid precursor protein in clathrin-coated vesicles purified from PC12 cells. J Biol Chem. 1993;268:608–612. [PubMed] [Google Scholar]
- Oltersdorf T, Ward PJ, Henriksson T, Beattie EC, Neve R, Lieberburg I, Fritz LC. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990;265:4492–4497. [PubMed] [Google Scholar]
- Pelham HR. Multiple targets for Brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Perez RG, Squazzo SL, Koo EH. Enhanced release of amyloid β protein from codon-670/671 Swedish mutant β amyloid precursor protein occurs in both secretory and endocytic pathways. J Biol Chem. 1996;271:9100–9107. doi: 10.1074/jbc.271.15.9100. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Lee VM-Y. NTera 2 cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J Neurosci Res. 1993;35:585–602. doi: 10.1002/jnr.490350603. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Page C, Lee VM-Y. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P, Gonzalez-DeWhitt P, Schilling J, Miller J, Hsu D, Greenberg BD, Davis K, Wallace W, Lieberburg I, Fuller F, et al. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature (Lond) 1988;331:525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. Temperature-sensitive steps in the transport of secretory proteins through the Golgi complex in exocrine pancreatic cells. Proc Natl Acad Sci USA. 1986;83:6425–6429. doi: 10.1073/pnas.83.17.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of semliki forest virus membrane glycoproteins to the cell surface. Cell. 1984;38:535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Matter K, Kreis TE, Ginsel L, Hauri HP. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer's disease. Annu Rev Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch R, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantitation of soluble Alzheimer's β-peptide from biological fluids. Nature (Lond) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Seubert P, Oltersdorf T, Lee MG, Barbour R, Blomquist C, Davis DL, Bryant K, Fritz LC, Galasko D, Thal LJ, et al. Secretion of β-amyloid precursor protein cleaved at the amino terminus of the β-amyloid peptide. Nature (Lond) 1993;361:260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai X-D, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science (Wash DC) 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Sisodia SS. β-amyloid precursor protein cleavage by a novel membrane bound protease. Proc Natl Acad Sci USA. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL. Evidence that β-amyloid protein in Alzheimer's disease is not derived by normal processing. Science (Wash DC) 1990;248:492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- St. George-Hyslop PH, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers RH, Feldman RG, Pollen D, Drachman D, et al. The genetic defect causing familial Alzheimer's disease maps on chromosome 21. Science (Wash DC) 1987;235:885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Nairn AC, Gandy SE, Greengard P. Phosphorylation of Alzheimer amyloid precursor protein by protein kinase C. Neuroscience. 1992;48:755–761. doi: 10.1016/0306-4522(92)90264-3. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai X-D, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science (Wash DC) 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, McClatchey AI, Lamperti ED, Villa-Komaroff LL, Gusella JF, Neve RL. Protease inhibitor domain encoded by an amyloid precursor mRNA associated with Alzheimer's disease. Nature (Lond) 1988;331:528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky F, Davenport F, Nordstedt C, Seeger M, et al. Endoproteolysis of Presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996a;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Teplow BD, Siman R, Greenberg B, Sisodia SS. Metabolism of the “Swedish” amyloid precursor protein variant in Neuro 2a (N2a) cells. J Biol Chem. 1996b;271:9390–9397. doi: 10.1074/jbc.271.16.9390. [DOI] [PubMed] [Google Scholar]
- Turner RS, Suzuki N, Chyung ASC, Younkin SG, Lee VM-Y. Amyloids β40 and β42are generated intracellularly in cultured human neurons and their secretion increases with maturation. J Biol Chem. 1996;271:8966–8970. doi: 10.1074/jbc.271.15.8966. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Konig G, Bunke D, Fischer P, Salbaum JM, Masters C, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's Disease A4 Amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wertkin AM, Turner RS, Pleasure SJ, Golde TE, Younkin SG, Trojanowski JQ, Lee VM-Y. Human neurons derived from a teratocarcinoma cell line express solely the 695-amino acid amyloid precursor protein and produce intracellular β-amyloid or A4 peptides. Proc Natl Acad Sci USA. 1993;90:9513–9517. doi: 10.1073/pnas.90.20.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]